Abstract
The treatment of patients who have cardiac tumors can be challenging, because these tumors occur infrequently and in variable locations, and surgical resection is often technically difficult. We report the cases of 2 patients, each of whom underwent successful surgical excision of large melanomas that had metastasized to the right cardiac ventricle. Although the presence of widespread disease and a limited life expectancy usually prevent surgical therapy for patients who have metastatic cardiac tumors, removal of such masses greatly facilitated subsequent medical management of these 2 patients and improved their quality of life. (Tex Heart Inst J 2003;30:218–20)
Key words: Heart ventricle, heart neoplasms/diagnosis/secondary/surgery, melanoma/diagnosis/secondary/surgery
Melanoma is a neoplasm with a dismal prognosis, mainly due to its propensity for metastasizing early with few symptoms. Primary melanoma is predominantly cutaneous, and the primary tumor is rarely the cause of severe morbidity or mortality. 1 Approximately 30% of patients who have melanoma will develop metastatic disease requiring systemic therapy. 2 The most common sites of visceral metastases identified antemortem are the lungs, liver, brain, and bones. Although 50% of patients with metastatic malignant melanoma are found to have had cardiac involvement postmortem, 3,4 tumors are found in less than 2% of patients antemortem. 1 Melanoma is commonly referred to as the neoplasm with the greatest propensity for cardiac involvement.
The discrepancy between ante- and postmortem diagnoses may be a result of a variability in symptoms. Impairment of cardiac function occurs only in approximately 30% of patients with cardiac metastases, 5 meaning that most patients do not experience symptoms that would link their presentation with cardiac involvement. Glancy and Roberts 3 reported that the extent of cardiac involvement revealed at autopsy correlates poorly with the presence of clinical features such as tachycardia, dyspnea, and edema—most of which either were absent or were attributed to noncardiac disease. Others have also reported a poor correlation between clinical features and cardiac tumors. 6,7 However, refinements in conventional computed tomographic (CT) scanning, echocardiography, and magnetic resonance imaging (MRI) have improved diagnostic capabilities; these imaging techniques can provide important anatomic information about the tumor's extension to adjacent vascular and mediastinal structures and the potential for surgical resection.
Surgical management of patients with cardiac tumors is highly individualized because of the large number of cases and the variability and extension of the tumor sites. Cardiac involvement often coincides with extensive extracardiac disease, and, in most patients, the presence of such widespread disease and a limited life expectancy usually prevent a more aggressive surgical approach. However, surgical removal of solitary or localized metastases can facilitate subsequent medical management and allow meaningful survival. We describe the cases of 2 patients who underwent successful removal of a large metastatic melanoma to the right ventricle.
Case Reports
The 1st patient was a 50-year-old woman with a history of treated melanoma who presented with leg swelling and shortness of breath. A chest radiograph showed the presence of a mass within the right ventricle. The 2nd patient was a 41-year-old man who had shortness of breath and a long history of heart murmur. In each case, the presence of a mass within the right ventricle was confirmed by dynamic cine MRI with multiple projections, before and after administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) (Patient 1: Figs. 1A and 1B; Patient 2: Figs. 2A and 2B). Both patients underwent surgery for removal of the mass.
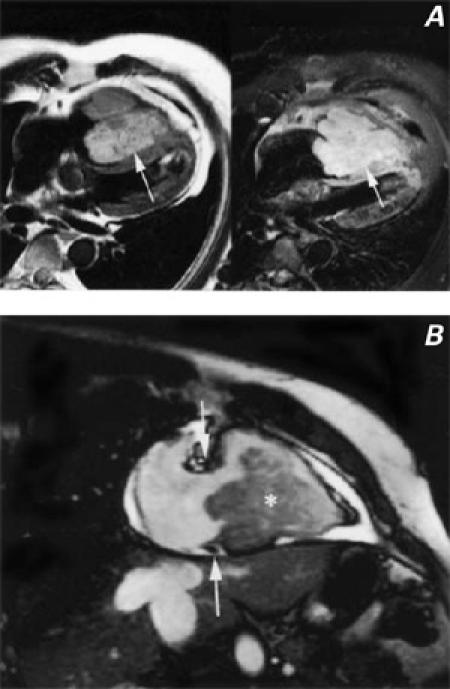
Fig. 1 A) Spin echo T2-weighted (left) and contrast-enhanced T1-weighted (right) images show a large mass (arrows) nearly filling the right ventricular cavity. The mass has lobulated borders and is adherent to and infiltrating the distal interventricular system. B) Cine magnetic resonance image obtained in the 2-chamber long-axis projection through the right ventricle. The mass (asterisk) is filling the right ventricular cavity and projecting posteriorly through the tricuspid valve (the tricuspid annulus is between the white arrows).
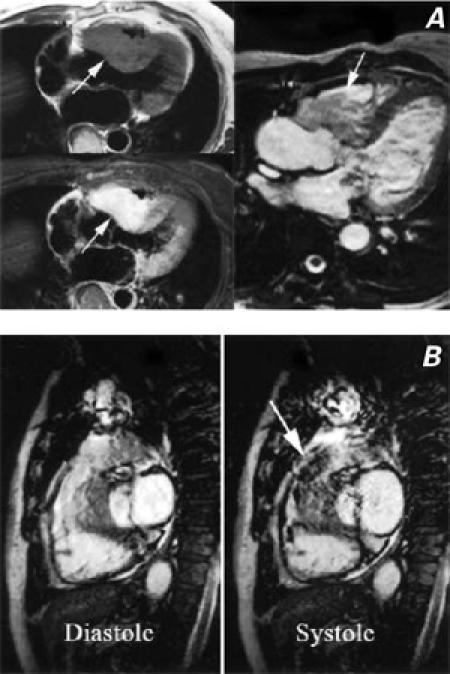
Fig. 2 A) Spin echo T1-weighted non-enhanced (upper left) and contrast-enhanced (lower left) images, and a contrast-enhanced cine magnetic resonance image (MRI) (right). All of the images show a large mass infiltrating the myocardium of both the right ventricular outflow tract and the proximal interventricular septum. The mass narrows the right ventricular outflow tract but not the left. B) Cine MRI images in diastole (left) and systole (right), demonstrate dynamic stenosis of the right ventricular outflow tract. The arrow (right) highlights the turbulent jet, caused by stenosis, extending into the main pulmonary artery.
Cardiopulmonary bypass was instituted through a median sternotomy incision, with aortic and bicaval cannulation. Moderate hypothermia was induced. The aorta was cross-clamped, and antegrade and retrograde cardioplegia were used for myocardial protection. The right ventricle was approached through a vertical incision that extended from the lower mid portion of the anterior wall to the outflow tract. The incision was extended to just below the insertion of the pulmonary valve annulus.
In the 1st patient, the large intraventricular mass was found to extend from the trabecular portion of the ventricular septum back to the inlet portion, just below the septal leaflet of the tricuspid valve and up to the infundibular septum. Although the mid and lower portions of the left side of the ventricular septum were free of tumor, the superior portions of both sides of the infundibular septum had been infiltrated by the mass. The tumor partially invaded the aortic valve annulus and the right sinus of Valsalva and the ostium of the right coronary artery. The aortic valve was removed, and a Konno-type incision was extended from the aortic annulus into the infundibular septum in order to completely remove the tumor. The 2nd patient had a similar pathology in that the mass was adherent to the inlet septum. In these patients, the resulting ventricular septal defects were repaired with a bovine pericardial patch. A size 23 Medtronic stentless aortic valve prosthesis (Medtronic, Inc.; Minneapolis, Minn) was sutured to the aortic annulus and the pericardial patch. The left coronary ostium was sutured as a button, and the remainder of the repair was completed as an aortic “mini-root” replacement. The superior portion of the right ventricular outflow tract was repaired with a pericardial patch. During rewarming, the 1st patient underwent a right coronary artery bypass, because the mass had partially obstructed the origin of the right coronary artery.
Both patients had uneventful recoveries. Postoperative echocardiograms showed good biventricular function and no ventricular septal defects, outflow tract obstruction, or valvular regurgitation.
Discussion
Surgical excision of cardiac tumors can be useful for improving quality of life and ameliorating symptoms. Gerbode and co-authors 8 described the surgical treatment of locally benign tumors of the heart in 1967 and stated that removal of such tumors usually carries with it a good prognosis. Although there have been reports of the surgical management of myxomas 8,9 and the successful removal of metastatic melanomas from the atria, 10–12 we did not find any reference in the English medical literature to complete surgical resection of a large metastatic melanoma involving the ventricle.
We believe that surgical management of cardiac metastases should be approached in a manner similar to that for primary disease, depending on the clinical circumstances. Complete surgical resection is indicated in patients with a preoperative Karnofsky performance status of more than 80%, 12 minimal extracardiac disease, or a deteriorating clinical picture due to cardiac symptoms. Previous embolic events, syncopal attacks, and echocardiographic evidence of a multilobulated mass are indications for urgent surgery. 13
The anatomic site and the extent of tumor growth are important factors in determining surgical procedure. If anatomically feasible, complete resection and accurate anatomic reconstruction should be the goal of surgery. Kosuga and colleagues 14 reported an early mortality rate of 12.5% and a late mortality rate of 100% in 8 patients who underwent incomplete surgical excision for treatment of malignant cardiac tumors. Gowdamarajan and Michler 15 reported that, in patients who underwent cardiac transplantation for inoperable cardiac tumors, the mean survival period was 46 months for those with benign tumors and 12 months for those with malignant tumors. These results indicate that poor outcomes may be due to the extensive extracardiac disease often seen in patients with malignant tumors. The prognosis for patients with any form of malignancy treated with surgical debulking or palliative therapy is poor; however, in many cases, aggressive surgery can improve symptoms and allow time for medical management of the extracardiac disease. In our patients, the removal of a large mass obstructing the right ventricle and the outflow tract prevented right ventricular hypertension, hypoxia, and congestive heart failure and greatly facilitated the subsequent medical management of the patients, improving their chance for recovery.
Footnotes
Address for reprints: O.H. Frazier, MD, Texas Heart Institute, MC3-147, P.O. Box 20345, Houston, TX 77225-0345
E-mail: knowlin@heart.thi.tmc.edu
References
- 1.Savoia P, Fierro MT, Zaccagna A, Bernengo MG. Metastatic melanoma of the heart. J Surg Oncol 2000;75:203–7. [DOI] [PubMed]
- 2.NIH Consensus Conference. Diagnosis and treatment of early melanoma. JAMA 1992;268:1314–9. [DOI] [PubMed]
- 3.Glancy DL, Roberts WC. The heart in malignant melanoma. A study of 70 autopsy cases. Am J Cardiol 1968;21:555–71. [DOI] [PubMed]
- 4.Klatt EC, Heitz DR. Cardiac metastases. Cancer 1990;65:1456–9. [DOI] [PubMed]
- 5.Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics 2001;21:439–49. [DOI] [PubMed]
- 6.Waller BF, Gottdiener JS, Virmani R, Roberts WC. The “charcoal heart;” melanoma to the cor. Chest 1980;77:671–6. [DOI] [PubMed]
- 7.Hanfling SM. Metastatic cancer to the heart: review of the literature and report of 127 cases. Circulation 1960;22:474–83. [DOI] [PubMed]
- 8.Gerbode F, Kerth WJ, Hill JD. Surgical management of tumors of the heart. Surgery 1967;61:94–101. [PubMed]
- 9.Bortolotti U, Maraglino G, Rubino M, Santini F, Mazzucco A, Milano A, et al. Surgical excision of intracardiac myxomas: a 20-year follow-up. Ann Thorac Surg 1990;49:449–53. [DOI] [PubMed]
- 10.Prabhakar G, Vasilakis A, Hill RC, Cruzzavala JL, Graeber GM, Gustafson RA, Murray GF. Right atrial metastatic melanoma in a patient with transient ischemic attacks. Ann Thorac Surg 1998;65:844–6. [DOI] [PubMed]
- 11.Merer DM, Dutcher JP, Mercando A, Brodman R, Suhrland MJ, Bhandari A, et al. Case report: clinical findings and successful resection of melanoma metastatic to the right atrium. Cancer Invest 1994;12:409–13. [DOI] [PubMed]
- 12.Chen RH, Gaos CM, Frazier OH. Complete resection of a right atrial intracavitary metastatic melanoma. Ann Thorac Surg 1996;61:1255–7. [DOI] [PubMed]
- 13.Hake U, Iversen S, Schmid FX, Erbel R, Oelert H. Urgent indications for surgery in primary or secondary cardiac neoplasms. Scand J Thorac Cardiovasc Surg 1989;23:111–4. [DOI] [PubMed]
- 14.Kosuga T, Fukunaga S, Kawara T, Yokose S, Akasu K, Tayama E, et al. Surgery for primary cardiac tumors. Clinical experience and surgical results in 60 patients. J Cardiovasc Surg (Torino) 2002;43:581–7. [PubMed]
- 15.Gowdamarajan A, Michler RE. Therapy for primary cardiac tumors: is there a role for heart transplantation? Curr Opin Cardiol 2000;15:121–5. [DOI] [PubMed]


