Abstract
Transcatheter closure of patent ductus arteriosus is routinely performed using various devices. After the withdrawal of the Rashkind umbrella device from the market, the wide window-type patent ductus arteriosus has been difficult to close percutaneously. Two patients with this condition are presented: a 17-year-old man and a 62-year-old woman with short (4.5- and 5.2-mm-diameter, respectively) ductus. We successfully implanted a 17-mm CardioSEAL® device in the 1st patient and a 23-mm STARFle® in the 2nd, with no residual shunt at follow-up (22 and 8 months, respectively). Although these devices (which follow the general design of the Rashkind umbrella) are usually applied to atrial septal defect closure, we believe that CardioSEAL and STARFlex implants could be the treatment of choice in percutaneous treatment of window-type patent ductus arteriosus. (Tex Heart Inst J 2003;30:236–9)
Key words: Blood vessel prosthesis implantation/methods; heart catheterization/methods; ductus arteriosus, patent/therapy
Closure of patent ductus arteriosus (PDA) by interventional catheterization has become the standard procedure for correction. In cases when devices such as coils, the Amplatzer Duct Occluder, and the Gianturco-Grifka Vascular Occlusion Device are available, surgical repair of PDA has become a historical procedure and nowadays is performed only in ventilator-dependent premature babies. The Rashkind PDA Occluder System, which was used extensively in the early 1990s, was especially useful in correcting large and very short PDAs. Unfortunately, it disappeared from the market when the far cheaper and equally effective coils were introduced. 1 Its successors, the CardioSEAL® and STARFlex® occluding devices* (NMT Medical, Inc.; Boston, Mass), are commonly used for other interventional catheterizations—mainly percutaneous closure of atrial septal defect (ASD) and patent foramen ovale (PFO). 2,3 We describe our results in 2 patients, a 17-year-old man and a 62-year-old woman, whose wide window-type PDAs were treated successfully with the CardioSEAL and STARFlex devices. To our knowledge, this is the first published report of PDA application of these devices, although the possibility of such treatment has been mentioned occasionally at scientific meetings.
Case Reports
Patient 1 was a 17-year-old man (weight, 72 kg; height, 173 cm) who was known to have a PDA. He was doing well and had no complaints. He presented at our center in October 2000 for consultation. Palpation demonstrated an active precordial area with a left ventricular impulse. There was also the classic machinery murmur of PDA at the left mid sternum and upper sternal border, with bounding peripheral pulses. Electrocardiography showed left ventricular hypertrophy, and chest radiography revealed a prominent pulmonary artery segment and cardiac enlargement. Echocardiography demonstrated left atrial (LA) and left ventricular (LV) enlargement: LA, 3.1 cm; and LVIDd (LV internal diastolic dimension), 5.3 cm. On color-flow Doppler, there was evidence of PDA, with left-right shunting.
Patient 2 was a 62-year-old woman (weight, 64 kg; height, 160 cm), who presented in January 2002 with fatigability and had auscultatory, echocardiographic (LA, 3.6 cm; and LVIDd, 5.8 cm), and radiologic findings similar to those of our 1st patient. Previously, she had refused surgery.
For the procedure, both patients were consciously sedated (without intubation) and administered antibiotic prophylaxis (cephazolin). At cardiac catheterization, slightly elevated pulmonary artery pressures were found: 35/20/28 mmHg in Patient 1 and 38/12/24 mmHg in Patient 2, with Qp/Qs of 1.9 and 2.6, respectively. Angiography demonstrated short PDA in both patients (Figs. 1A and 2A), with diameters of 4.6 mm and 5.2 mm, respectively (type B, according to the Kirchenko classification 4).
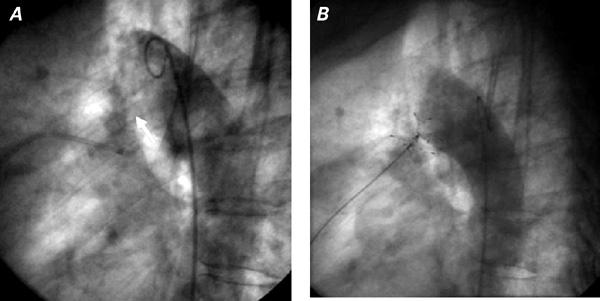
Fig. 1 Patient 2: Aortogram in lateral view shows the window-type patent ductus arterious (arrow) A) before and B) after implantation of a STARFle® device, which is still attached to the delivery system.
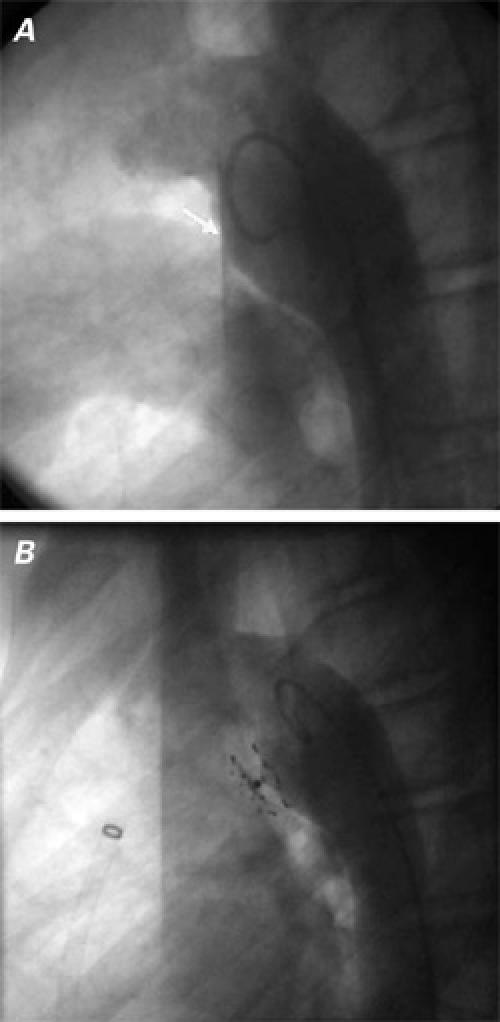
Fig. 2 Patient 1: Aortogram in lateral view shows the window-type patent ductus arterious (arrow) A) before and B) after complete closure of the PDA with a CardioSEAL® device.
Using right venous femoral access in both patients, we placed an exchange guidewire and an 11F (long) Mullins transseptal Check Flo Introducer® (William Cook Europe; Bjaeverskov, Denmark) through the PDA into the descending aorta. Intravenous heparin (100 U/kg) was administered before placement of the Mullins sheath. The delivery catheter with a loaded 17-mm CardioSEAL occluder (in Patient 1) or a 23-mm STARFlex (in Patient 2) was introduced into the previously positioned sheath and advanced to the area of the tricuspid valve. Then it was advanced carefully until the distal legs of the occluding device sprang open in the descending aorta. The sheath, delivery catheter, and delivery wire were then withdrawn as a single unit, to the aortic border of the duct. Adequate positioning of the devices was confirmed by aortography. With the delivery wire and the catheter fixed at this position, the sheath alone was withdrawn from the still-attached device, enabling the proximal legs of the device to open. After angiographic confirmation of good positioning of the device (Fig. 1B), it was released (Fig. 2B). Another aortogram was obtained, and it showed no residual shunt through the ducts and no evidence of coarctation. No vascular or other complications occurred during the procedures or during follow-up. After 2 days, both patients were discharged in good condition, on no medications. After 8 and 22 months of follow-up, respectively, the PDAs were seen by color-Doppler examination to be completely closed in both patients.
Discussion
Generally, it is not necessary to catheterize, for diagnostic purposes, children or adults who have isolated PDA. When a procedure is started in the catheterization laboratory for a PDA, it should be concluded with percutaneous closure. Small and moderate-sized PDAs are safely closed with coils. 5,6
In PDAs with diameters greater than 3 mm and well-developed aortic ampullae, the Amplatzer Duct Occluder or even large coils can be used with good results. 7 Unfortunately, in bigger, window-type PDAs, the use of any of the above-mentioned devices carries a risk of embolization, or of protrusion of part of the device in the aorta or pulmonary artery. Under such circumstances, the Rashkind umbrella was a good device, because of its low profile. It was successfully applied in a large number of patients, 8 including those with residual leak after a previous ligation. 9 Late follow-up of these patients was favorable. 10 Next-generation devices similar to the Rashkind umbrella are the CardioSEAL and STARFlex occluding devices. Similar to the large (17-mm) Rashkind umbrella, each of the disks has 4 spring-loaded arms. The CardioSEAL and STARFlex devices have joints in the arms, which makes them more flexible than the older Rashkind device. The distal and proximal disks are attached to each other at the center, giving each disk a “spider shape”—that is, the disks are arched toward each other. The metal skeleton is covered with a woven Dacron fabric to promote early endothelialization (in contrast to the Rashkind umbrella, which was covered by polyurethane). The extra hinges improve positioning of the device and enable better alignment with surrounding structures. The STARFlex device has, in addition, a self-centering mechanism, which helps to position the implant in the PDA. The delivery system and implant procedure, which proved to be safe and effective with the Rashkind device, 8 remains essentially unchanged in the CardioSEAL and STARFlex devices. However, the umbrella is now connected to the release wire in the delivery catheter by means of a pin-pin mechanism, which is a departure from the Rashkind umbrella's knuckle-eye mechanism. Disadvantages of the CardioSEAL and STARFlex devices for closure of PDAs are their relatively high price and the necessity of using a 10F or 11F delivery system, which is not as significant in adult patients but may play a role in small children. Although the usual application of these devices is to ASD or PFO closure, 2,3 they seem also to be ideal for percutaneous closure of the window-type PDA.
Footnotes
*In the United States, CardioSEAL has been approved by the Food and Drug Administration for selected patients with patent foramen ovale, ventricular septal defect, and fenestrated Fontan defect, under the humanitarian device exemption regulations. For atrial septal defects, the STARFlex is in government-approved clinical trials.
Address for reprints: Dr. Jacek Bialkowski, Chief, Congenital Heart Disease & Pediatric Cardiology Dept., Silesian Center for Heart Disease, ul. Szpitalna 2, 41800 Zabrze, Poland
E-mail: jabi_med@priv4.onet.pl
References
- 1.Gersony WM, Apfel HD. Patent ductus arteriosus and other aortopulmonary anomalies. In: Moller JH, Hoffman JI, editors. Pediatric cardiovascular medicine. New York: Churchill Livingstone; 2000. p. 323–34.
- 2.Kaulitz R, Paul T, Hausdorf G. Extending the limits of transcatheter closure of atrial septal defects with the double umbrella device (CardioSEAL). Heart 1998;80:54–9. [PMC free article] [PubMed]
- 3.Hung J, Landzberg MJ, Jenkins KJ, King ME, Lock JE, Palacios JF, Lang P. Closure of patent foramen ovale for parodoxical emboli: intermediate-term risk of recurrent neurological events following transcatheter device placement. J Am Coll Cardiol 2000;35:1311–6. [DOI] [PubMed]
- 4.Krichenko A, Benson LN, Burrows P, Moes CA, McLaughin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol 1989;63:877–80. [DOI] [PubMed]
- 5.Cambier PA, Kirby WC, Worham DC, Moore JW. Percutaneous closure of the small (less than 2.5 mm) patent ductus arteriosus using coil embolization. Am J Cardiol 1992;69:815–6. [DOI] [PubMed]
- 6.Bermudez-Canete R, Santoro G, Bialkowsky J, Herraiz I, Formigari R, Szkutnik, Ballerini L. Patent ductus arteriosus occlusion using detachable coils. Am J Cardiol 1998;82:1547–9,A8. [PubMed]
- 7.Faella HJ, Hijazi ZM. Closure of patent ductus arteriosus with amplatzer PDA device: immediate results of the international clinical trial. Catheter Cardiovasc Interv 2000;51:50–4. [DOI] [PubMed]
- 8.Rashkind WJ, Mullins CE, Hellenbrand WE, Tait MA. Nonsurgical closure of patent ductus arteriosus: clinical application of the Rashkind PDA Occluder System. Circulation 1987;75:583–92. [DOI] [PubMed]
- 9.Bialkowski J, Bermudez-Canete R, Ballerini L, Herraiz I, Casado J, Szkutnik M, et al. Percutaneous closure of the previously ligated arterial duct. Arch Inst Cardiol Mex 1997;67:286–9.
- 10.Formigari R, Toscano A, Herraiz I, Bialkowski J, Donti A, Pichip FM, et al. Late follow-up of occlusion of the patent ductus arteriosus with Rashkind device with emphasis on long-term efficacy and risk for infections. Am J Cardiol 2001;88:586–8. [DOI] [PubMed]


