Abstract
The objective of this paper is twofold: (i) to show how Multislice Computed Tomography (MSCT) data sets bring the information required for Cardiac Resynchronisation Therapy (CRT) planning; (ii) to demonstrate the feasibility of 3-D navigation into the veins where Left Ventricular leads have to be placed. The former has been achieved by exploring and labelling the cardiac structures of concern, the latter has been performed by using the concept of virtual navigation with high resolution surface detection and estimation algorithms.
Keywords: Cardiac Output, Low; therapy; Defibrillators, Implantable; Feasibility Studies; France; Heart Catheterization; Humans; Imaging, Three-Dimensional; instrumentation; Preoperative Care; Tomography, X-Ray Computed; methods
Keywords: Medical Imaging, Heart, Image guided intervention, cardiac resynchronization therapy, navigation, virtual reality.
1. Introduction
Cardiac Resynchronization Therapy (CRT) is aimed at the restoration of the contractile coordination in hearts with severe heart failure (HF), sinus rhythm and ventricular conduction delay. It is performed by stimulating both the right and the left ventricles, pacing them simultaneously or with a small delay. Several clinical trials have shown that this technique is beneficial for acute as well as chronic disorders, improving the heart’s performance, the capacity of the patient for exercise and reducing the mortality from heart failure [1–3]. However, these studies point out that the main issue remains to identify and assess the most effective pacing sites in order to reduce the percentage of non-responding patients which may reach up to 25 to 30% of recipients. They have shown that LV-lead positioning (either LV-only pacing or biventricular pacing) is without contest the most challenging one to implant.
Advances in instrumentation, implantation techniques and selection of appropriate candidates have to be achieved to increase the success rate of CRT. They can make use of tissue Doppler, MR tagged imaging or Multislice CT scanner (MSCT). The latter is the only imaging modality that brings a full 4-D morphological and functional insight into the heart. The availability of catheter-based 3D non fluoroscopic contact (Carto, Biosense) and noncontact mapping (Ensite 3000, Endocardial Solutions, Inc) techniques allows in vivo assessment of the activation sequence with a relatively high spatial resolution [4–6]. Recent technological breakthroughs of implantable devices have been achieved: they concern biventricular pacing (or CRT), cardioverter-defibrillator (ICD, Implantable Cardioverter Defibrillator) or joint device (CRT-ICD). These systems have several advanced functionalities including electrical potential sensing, electrical pulse stimulation, reprogramming through bidirectional wireless communication, event recording, etc. Major efforts were carried out for miniaturization, energy consuming reduction and therefore higher autonomy, robustness to environmental perturbations.
Improvements can also be expected from minimally invasive computer assisted implantation which has been recently proposed [7], based on a direct left ventricular epicardial approach. However the consequence of chest insuflation and single-lung ventilation which are used must be evaluated to a larger scale.
The conjunction of electrical data, morphological and mechanical behaviour is very likely a source of additional progress: insights into electromechanical coupling should improve the understanding of regional and global abnormalities.
The implantation of such pacing device is image-guided (X-ray angiography) and this is the target of this work: is it possible, based on MSCT, to better plan this procedure? This paper is addressing a part of the problem, the pre-operative phase of the whole procedure. It deals with:
- the labelling of the venous structures of the heart into which the lead implantation may take place
- the virtual 3-D navigation inside these vessels in order to define the possible paths that can be followed.
Section 2 brings more clues on the CRT. The approach that has been followed is described section 3. Some illustrations of heart labelling are provided section 4 and samples of virtual images issued from a navigation into the veins are given section 5. They are discussed in section 6 before concluding with some remarks and prospects.
2. Problem statement
In 1998, Daubert et al [8], from the Hospital of Rennes, proposed a way to overcome this problem by using a transvenous technique, permitting to stimulate the left ventricle on a long-term basis. The procedure consists in positioning epicardial leads in the right atrium and right ventricle, the left ventricle being paced via a lead passed through the coronary sinus to an epicardial vein on the free wall of the left ventricle (Fig. 1).
Fig. 1.
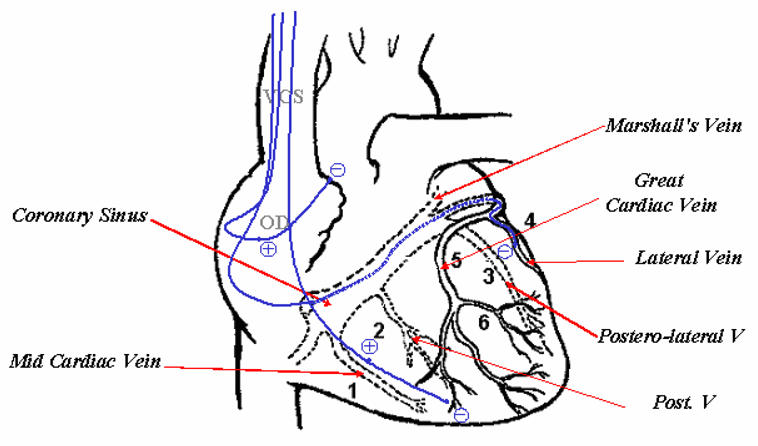
Lead paths and positions in transvenous biventricular pacing.
This technique, applied today worldwide for biventricular pacing, has however some limitations [9,10]:
-
The implant success rate is around 85% to 92%1, mainly due to:
Incorrect pacing site or electrode displacements (5–7%)
The impossibility to access the target vein
The dissection of the coronary sinus (3%)
The difficulty to attain an optimal pacing site.
a typical implant procedure for a biventricular pacemaker can be lengthy (about 1.5 to 3 hours) with, in average, 0.5 hours of X-ray,
The main objective is thus to assist health professionals in improving and securing the implant techniques. From a clinical standpoint, it will rely on the study of the patient’s coronary anatomy to define the target veins, to confirm their accessibility and to minimize the implant time. Pre-operative assistance, as defined in Computer Assisted Therapy [13] and in Virtual Navigation [14], will consist here to:
Study the anatomy of the patient’s coronary venous tree so as to define the optimal access path for the pacing leads.
Define which catheter and guide type should be used (with different diameters and curvatures, for example).
Better define the optimal pacing site, based on the anatomo-functional information and on the electro-mechanical models of the cardiac activity.
Up to now, there was no image-based planning of the implant procedure because no imaging source was capable to provide a full, 3-D, time image sequence access to the heart. CRT is still directly performed by using 2-D venous X-ray coronarography which leads to a partial and limited access to 3-D anatomy.
The availability of MSCT is dramatically changing this situation: this 4-D functional imaging CT Scanners can be used to obtain the basic structural and functional features required to achieve an optimal CRT planning.
The LV can be paced transvenously through a subclavian vein, going successively via the cava vein, the right atrium, the coronary sinus and the great vein. The target location is a lateral or posterolateral vein. If lateral vein catheterization failed or in the case of poor pacing threshold, the LV lead is inserted into the great cardiac vein to pace the anterobasal wall or into the mid cardiac vein to pace the inferoapical region (see appendix for more details). Specifically designed coronary sinus leads are used. The injection of contrast medium allows viewing the venous tree to be explored but it remains difficult to visually analyse due to the backward blood flow.
3. Method
The MSCT data provide detailed and effective insights into the structures of the heart but also new means to track and characterize its hemodynamic behaviour over time. This advantage has a challenging counterpart: the high complexity of the scene to be analysed both visually by the physician, and quantitatively through image processing techniques. This is not true, of course, for the study of the main components (cavities, major vessels, etc.) but it becomes critical for the complex-shaped objects, with reduced size and low contrast.
Basically, the same difficulties encountered for all slice-based descriptions are found here. This presentation mode requires a 3-D mental reconstitution of the 3-D shapes from the 2-D planes. They have motivated the development of 3-D segmentation, rendering and manipulation techniques for more than 30 years (refer to [15] for recent surveys). However, in many clinical applications, there is a low number of structures of interest with contrast characteristics such that simple and global solutions (thresholding) can be used for their rough discrimination. They do not lead to any labelling of the objects but they provide a way for the radiologist to view, locate, and identify the anatomical structures.
In general, a learning curve is required for the physicians to read the volume data. A similar situation has to be dealt with for the coronary venous tree. The volume sets must be explored many times before being capable to name the structures. It is this motivation that brings us to anatomically label MSCT data sets. The procedure that has been worked out consists in selecting spatial sequences among 8 angio-scanners at our disposal (with minimal motion artefacts). The data were acquired on a Siemens Somatom volume zoom 4 detectors. Identical protocols were used with the following acquisition parameters: collimation of 0.6 mm, table displacement of 1.5 mm/rotation, reconstruction increment of 0.6 mm, size of the matrix 512 × 512 with about 200 slices and a pixel size from 0.33 × 0.33 to 0.4 × 0.4 mm. The resolution is 12 bits and the slice thickness of 1.25 mm. Labelling has been independently performed by a radiologist and an interventional cardiologist followed by a consensus decision. One labelled data set will be briefly commented throughout this paper2.
4. A labelled example
The 2-D slices, together with the labels of coronary veins, are displayed in figure 2. A subset of slices (over 193) has been selected interactively in order to depict the most relevant veins of concern. The traversal of the heart is done from top to bottom (but it should be noticed that the slices correspond to the axial section looked from below, that is, with the right and left inverted). These structures are of different natures (cavities, arteries, valves, etc.) and serve as landmarks to visually understand the 3-D locations and complex conformations of the veins.
Fig. 2.
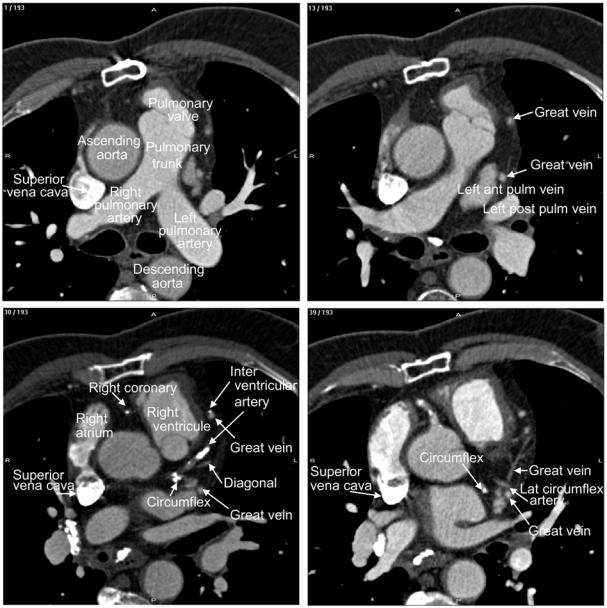
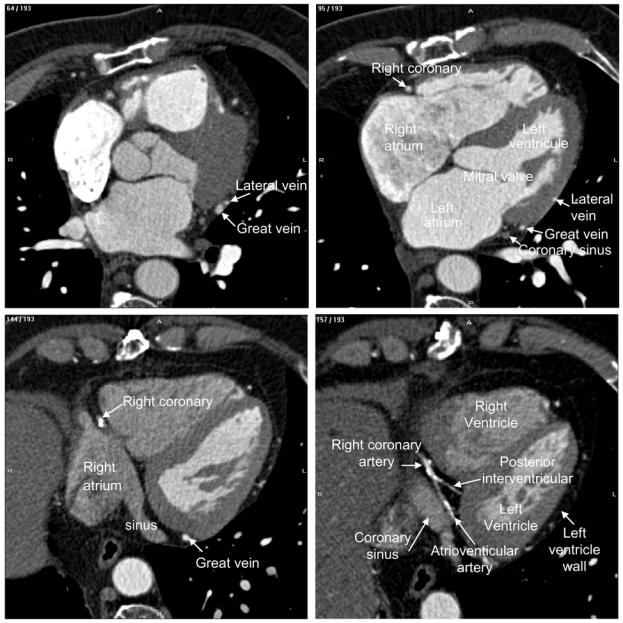
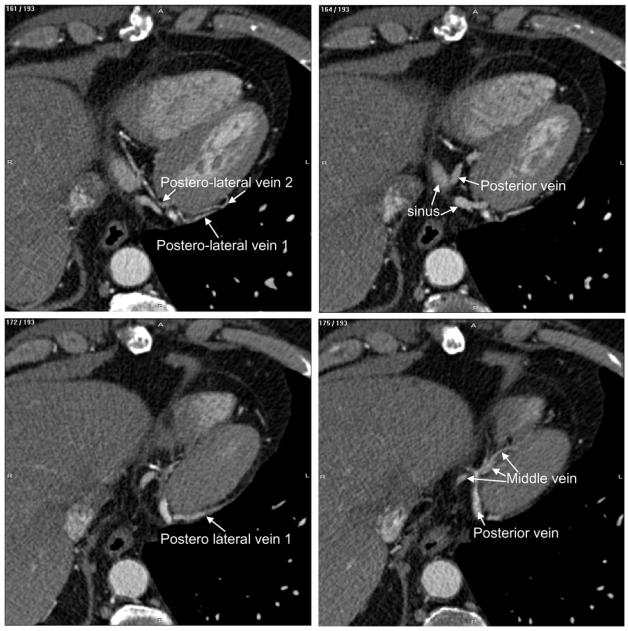
Labelling of heart structures in MSCT data. The dataset was acquired at 30% of the cardiac cycle. The subset of slices is composed of 12 labelled slices. The slices, selected to show different anatomical features, are numbered at the top left corner. The level window is between -100 HU and 700 HU. The letters A, P, R and L, that denote respectively anterior, posterior, right and left, are used to indicate the slice position relative to the patient’s body.
The ascending and descending Aorta, the pulmonary trunk and the pulmonary valves as well as the superior vena cava, right and left pulmonary arteries can be clearly recognized in the first slice (1/193). The right auricle, starting from the vena cava, on the left side of the aorta, and the great vein are displayed on the next slice. The latter, appearing twice due to its arch shape, has a much smaller size and its boundaries are blurred. Left anterior and posterior pulmonary veins (13/193) will join the right atrium when going down within the slices. Subsets of the arterial tree (with the circumflex, the interventricular, the right coronary and diagonal) together with the great vein can be localized within the slice 30. In continuation with the vena cava, the superior vena cava appears slice 39 with the lateral circumflex artery.
Major structures like the right and left atrium, left ventricle and coronary sinus are displayed when going top-down (slices 95 and 144). All the coronary veins flow into the coronary sinus and its size is enough to get a good rendering of its walls (section 5 below). The atrioventricular and posterior interventricular arteries, proceeding from the right coronary artery, are visible on the slice 157/193. The post-lateral veins (slice 161) and the posterior vein (draining the posterior surface of the left ventricle, slices 164 and 172) point out that, with their low diameter and contrast, their complex shapes, they will be difficult to render. This will also be the case of the middle cardiac vein (slice 175/193).
The main difficulties encountered during the travelling, the reading and the labelling process of the several data sets we have explored are mainly due to (i) the enhancement of all vessels (ii) the proximity of the arterial and venous trees and (iii) the complexity of 3-D shapes and geometries. The veins are in general large enough in their proximal segments but small in their distal parts. And these distal parts are of course the places where the pacemaker electrodes should be located. The information brought by MSCT may, of course, be used already to mentally sketch possible access to the veins reaching the LV but 3-D navigation can highly facilitate this task. These anatomical views can be considered as a preliminary step to read and understand the contents of MSCT data: they must be complemented by measurements (diameters, lengths, angles). Such task could be carried out on the way in preparing the intervention but the fact that the data are seen in 2-D does not lead to precise estimations.
5. Samples of virtual navigation through left coronary veins
Pre-operative planning can make use of virtual endoscopy techniques in order to define the candidate paths to reach the most appropriate sites for electrical receding and stimulation of the left ventricle. The basics of interactive and active navigation methods have been reported already in several papers [16–18]. In addition, local measures (lengths, diameters, angles) can be carried out as shown in [19]. These techniques are of major interest for pre-operative CRT because several difficulties may arise during the intervention among which:
- the insertion of the cannula into the coronary sinus: X-ray image guiding is often carried out by touching and the internal guide must be sometimes reshaped or changed.
- the access to veins with an adverse angle onto a junction is still a problem and prior simulation can reduce the number of trials and, accordingly, the risk afferent to them (damage to vein walls).
- in order to decide if the instruments can go through or not, diameter measures are required.
- the selection of the optimal veins and sites for lead placement.
The implantation of the right leads do not represent the same difficulties in terms of access.
The detection of walls during navigation is performed in 3 steps [20]: (1) a standard ray casting is launched from the observer position through the image screen coupled with a rough detection algorithm; (2) when an object is detected, an interpolation around the corresponding voxel is worked out in order to get a higher local sampling of the volume; (3) a refined detection step is then applied which allows to get a better estimation of the surface position and orientation (required to have a good shading for 3-D rendering).
It is not possible of course to render in a paper the full navigation sequence performed on the computer. Fig. 3 shows some image samples computed during traveling into the heart and the venous tree. They provide the path to the major structures (right atrium, coronary sinus, great vein, posterolateral and lateral veins), their positions and shapes that the interventional cardiologist need to know. The detection threshold was set to 60 HU. The refined detection has been performed using a three-linear operator with 1/5 resolution. It can be easily seen that the cavity and the vein walls are clearly depicted even when small structures are explored.
Fig. 3.
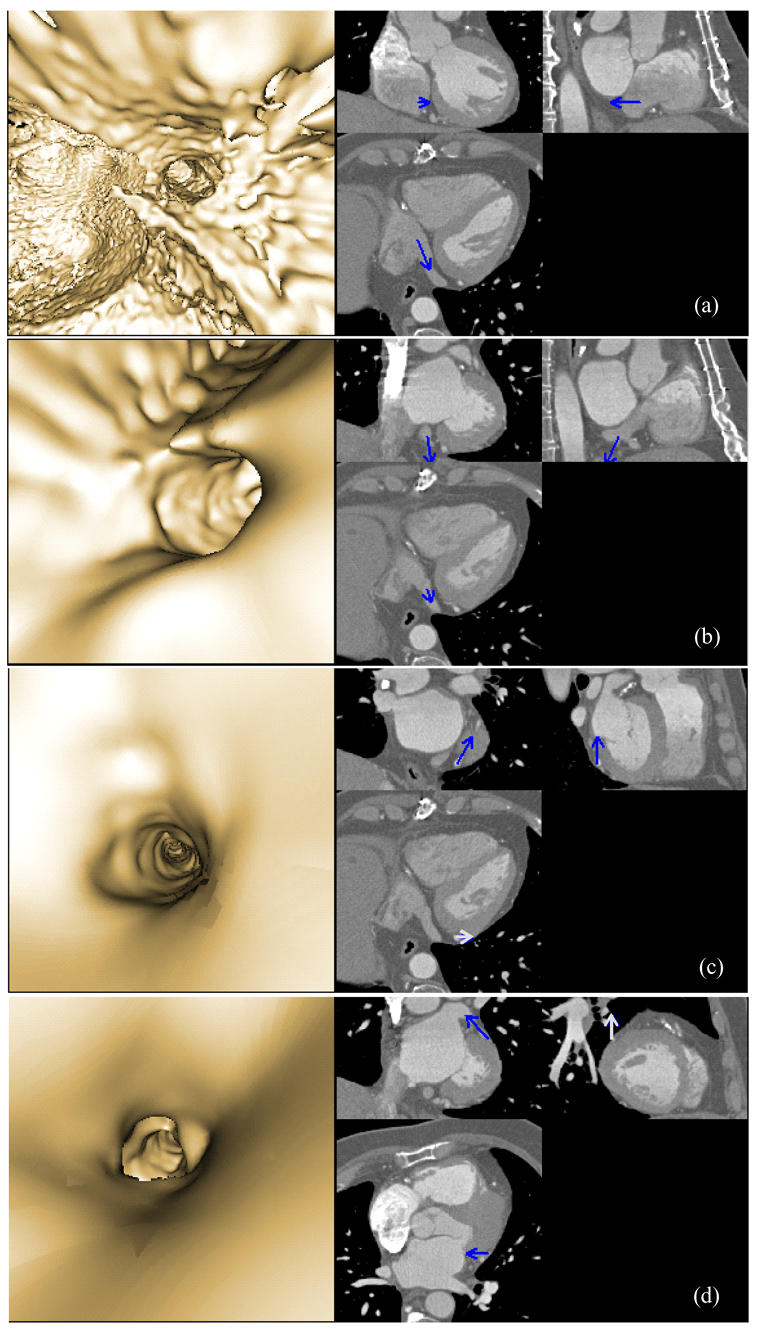
Virtual fly-through the heart venous structures. The sequence is composed of images selected at four different locations (a: inside the right atrium, close to the coronary sinus, b: at the coronary sinus entrance, c: inside the great vein, and d: inside the lateral vein). For each location, the left part shows the virtual navigation view and the right part shows an arrow representing the pose of the virtual endoscope in the volume image and superimposed on three orthogonal MSCT slices. The location of the MSCT slices corresponds to the origin of the arrow, whereas the viewing axis of the virtual endoscope is represented by the orientation of the arrow.
6. Discussion
The previous results show that 3-D navigation into the labelled heart cavities and veins is feasible by using MSCT data volume. It is, to our knowledge, the first attempt to explore the possible paths prior to Cardiac Resynchronization Therapy. It must be emphasized that this method provides at the same time segmentation means for small, low contrasted structures despite the blurring effects caused by motion during the reconstruction. The segmentation tools developed previously for cardiac arterial trees [21], based on 3-D moment and level sets has been qualitatively compared to the present algorithm. They provide simultaneously the centerlines, the borders and the diameters of the vessels. However, if the quantitative features they provide are very close to those estimated here, the time computation obtained through 3-D navigation is more compatible with the interactive exploration requirements (about 0.5 second for each image to render using a PC with a 2-GHz CPU and 1-GB memory).
The scheme presented in this paper is only one step toward pre-operative assistance. Its interest is to provide new resources to the physician when preparing a CRT. A full support may include more simulation functionalities. In clinical setting, for instance, the physician is moving the probe backward and forward, rotating, twisting and bending it, all gestures that can be facilitated with pre-operative assistance. It may sometimes happen in practice that the introduction of the probe in the coronary sinus fails: such situation can be integrated in the simulation and consequently anticipated. A recent study performed on 20 men and women [24] shows that the radiation dose level was about 11 mSv in MSCT, compared to 2 mSv in conventional cardiac X-ray angiography. This difference is clearly a limiting factor but the technological improvements that can be expected in the future will reduce this exposure. Moreover, it can be anticipated that the pre-operative planning of CRT procedure will lead to a decrease in radiation dose during the interventions.
Another objective could be the construction of a quantitative, fully 3-D atlas of the heart, as it has been done for the brain. This atlas should include the inter-individual deviations (structural, morphological) with statistical values as well as diseases. A virtual representation, computer-based, of an average patient (and exceptions) could be then defined and have a great interest in training.
The future of such project will rely on generic methods for data collection and decision making, advanced image processing tools, sophisticated multimodal models (e.g mechanical, electrical, etc.), particularly to:
acquire and represent the specific physiological knowledge necessary to choose the most appropriate CRT for a given patient.
define new methods for automatic navigation on 3D volumes in a dynamic environment, so as to define an access path to the optimal pacing site and propose an electrophysiological exploration protocol.
model the pacemaker behaviour and to couple it with the cardiac model.
propose a way to assist operator-based navigation by coupling pre- and intra-operative images (or volumes).
model the pacemaker behaviour and to couple it with the cardiac model so as to simulate the hemodynamic benefit of biventricular pacing, with respect to the pacing site and to propose patient-specific solutions.
7. Summary
It has been shown throughout this paper that MSCT data sets offer a major contribution for preparing interventions dealing with Cardiac Resynchronization Therapy. They provide in depth insights into the main structures as well as the critical veins of concern. The complexity of the scene, due to the many entities that it contains, the complicated shapes and the inter-individual variations that may be observed, requires previous visual exploration and labelling, advanced image processing tools and efficient 3-D rendering techniques. These new resources, however, open the road to multiple research developments that can be of major importance to reduce the time of intervention, to make the procedure safer for the patient and finally to improve the success rate of CRT.
Acknowledgments
The authors are indebted to B. Le Bruno, from Siemens Medical Division, France, for her constant support during the collection of MSCT data sets.
Appendix
Schematically, this new procedure begins with placement of a coronary sinus (CS) catheter. A long sheath is then passed over this catheter and placed in the CS. The CS catheter is removed and a balloon-tipped catheter is inserted via the sheath into the CS. The balloon is inflated, contrast is injected and a CS venogram is made in both the right and left anterior oblique views. Using both views allows identification of possible target sites for the permanent pacing lead. The usual site chosen is a lateral coronary vein about midway between the apex and the base. Next, the balloon catheter is removed and the pacing lead is introduced through the sheath (soft tines at the tip of the lead enable successful passive fixation in the coronary sinus). It is manipulated into the target site and pacing measurements are made (threshold, R wave, slew rate, and impedance). If satisfactory, the lead is left in place. The optimal site for left ventricular pacing is in the lateral or posterolateral cardiac vein. This is because pacing from the mid lateral wall or posterior wall results in the best percentage increase in pulse pressure and left ventricular dP/dt. However, at times, they are too small for the lead to enter or do not result in a stable position and the lead is then positioned in the anterior great cardiac vein [22].
Once the left ventricular lead is secured, the right ventricular apex lead and right atrial lead are then implanted in the usual manner for a dual chamber pacemaker. Where possible, the RV and LV lead should however be anatomically as far apart as possible, so as to obtain maximal separation between the right and the left ventricular lead tip in both AP and LAO views. In patients with left ventricular leads in the great cardiac vein, or in the lateral or postero-lateral branches of the CS a mid-inferior wall position is chosen for the right ventricular lead. In those with a posterior or mi-cardiac vein position, the right ventricular lead is positioned in the right ventricular outflow tract or on the right ventricular septum. The right atrial leads are routinely positioned in the right atrial appendage [23].
Footnotes
This data set will be made publicly available at www.ltsi.univ-rennes1.fr with more detailed comments after publication
References
- 1.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular delay. New England Journal of Medicine. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 2.Leclercq C, Kass DA. Re-timing the failing heart: principles and current clinical status of cardiac resynchronization. Journal of the American College of Cardiology. 2002;39:194–201. doi: 10.1016/s0735-1097(01)01747-8. [DOI] [PubMed] [Google Scholar]
- 3.Kass DA. Predicting cardiac resynchronization response by GRS duration. Journal of the American College of Cardiology. 2003;42:2125–2127. doi: 10.1016/j.jacc.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Gepstein L, Hayan G, Ben-Haim SA. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart: in vitro and in vivo accuracy results. Circulation. 1997;95:1611–1622. doi: 10.1161/01.cir.95.6.1611. [DOI] [PubMed] [Google Scholar]
- 5.Schilling RJ, Peters S, Davies DW. Simultaneous endocardial mapping in the left ventricle using a noncontact catheter: comparison of contact and reconstructed electrograms during sinus rhythm. Circulation. 1998;98:887–898. doi: 10.1161/01.cir.98.9.887. [DOI] [PubMed] [Google Scholar]
- 6.De Bakker JMT, Hauer RNW, Simmens TA. Activation mapping: unipolar versus bipolar recording. In: Zipes DP, Jalife J, editors. Cardiac Electrophysioloy: from cell to bedsite. 3. Philadelphia: Saunders; 2000. pp. 1068–1078. [Google Scholar]
- 7.DeRose JJ, Ashton RC, Belsey S, et al. Robotically assisted left ventricular epicardial lead implantation for biventricular pacing. Journal of the American College of Cardiology. 2003;41:1414–1419. doi: 10.1016/s0735-1097(03)00252-3. [DOI] [PubMed] [Google Scholar]
- 8.Daubert J, Fitter P, Le Breton H, Gras D, Leclercq C, Lazarus A, Mugica J, Mabo P, Cazeau S. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. PACE. 1998;21:239–245. doi: 10.1111/j.1540-8159.1998.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 9.Abraham WT. Rationale and design of a randomized clinical trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with advanced heart failure: the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Journal of Cardiac Failure. 2000;6:369–380. doi: 10.1054/jcaf.2000.20841. [DOI] [PubMed] [Google Scholar]
- 10.Alonzo C, Leclercq C, d’Allones FR, et al. Six years experience of transvenous left ventricular lead implantation for permanent biventricular pacing in patients with advanced heart failure: technical aspects. Heart. 2001;86:405–410. doi: 10.1136/heart.86.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. The Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. New England Journal of Medicine. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 12.Abraham WT, Fisher WG, Smith AL, et al. for the MIRACLE Study Group. Cardiac Resynchronization in chronic heart failure. New England Journal of Medicine. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RH, Lavallée S, Burdea GC, Mösges R. Computer-Integrated Surgery, Technology and Clinical Applications. Cambridge; MIT press: 1996. [PubMed] [Google Scholar]
- 14.Roux C, Coatrieux JL. Contemporary perspectives on Three Dimensional Biomedical Imaging. Amsterdam: IOS Press; 1997. [PubMed] [Google Scholar]
- 15.Coatrieux JL, Roux C. IEEE EMBS Series. NJ: IEEE Press; 2002. Biomedical Imaging IV. [Google Scholar]
- 16.Haigron P, Bellemare ME, Acosta O, Goksu C, Kulik C, Rioual K, Lucas A. Depth-Map-Based Scene Analysis for Active Navigation in Virtual Endoscopy. IEEE Transactions on Medical Imaging. 2004;23(11):1380–1390. doi: 10.1109/TMI.2004.836869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellemare ME, Haigron P, Coatrieux JL. Toward an active three dimensional navigation system in medical imaging. Proceedings of Computer Vision, Virtual Reality, and Robotics in Medicine and Medical Robotics and Computer Assisted Surgery Grenoble (France): Springer LNCS. 1205;1997:337–346. [Google Scholar]
- 18.Coatrieux JL. Les bases scientifiques de l’endoscopie virtuelle. Bulletin de l’Académie Nationale de Médecine. 1999;183(3):455–464. [PubMed] [Google Scholar]
- 19.Acosta O, Moisan C, Haigron P, Lucas A. Evaluation of Virtual Exploratory Navigation for the Characterization of Stenosis in the Planning of Endovascular Interventions. In: Clough AV, Chen CT, editors. Proceedings of SPIE Medical Imaging San. Vol. 4683. Diego (California, USA): SPIE; 2002. pp. 42–53. [Google Scholar]
- 20.Haigron P, Le Berre G, Coatrieux JL. 3-D navigation in medicine. IEEE Engineering in Medicine and Biology. 1996;15(2):70–78. [Google Scholar]
- 21.Larralde A, Boldak C, Garreau M, Toumoulin C, Boulmier D, Rolland Y. Proceedings of Functional Imaging and Modeling of the Heart. Lyon(France): Springer LNCS 3674; 2003. Evaluation of a 3D Segmentation Software for the Coronary Characterization in Multi-slice Computed Tomography; pp. 39–51. [Google Scholar]
- 22.Teo WS, Kam R, Hsu LF. Treatment of Heart Failure - Role of Biventricular Pacing for Heart Failure Not Responding Well to Drug Therapy. Singapore Medical Journal. 2003;44(3):114–122. [PubMed] [Google Scholar]
- 23.Walker S, Levy T, Rex S, Brant S, Paul V. Initial United Kingdom experience with the use of permanent, biventricular pacemakers: Implantation procedure and technical considerations. Europace. 2000;2(3):233–239. doi: 10.1053/eupc.2000.0106. [DOI] [PubMed] [Google Scholar]
- 24.Willmann JK, Weishaupt D, Kobza R, Verdun FR, Seifert B, Marincek B, Boehm T. Coronary artery bypass grafts: ECG-gated multi-detector row CT angiography-influence of image reconstruction interval on graft visibility. Radiology. 2004;232(2):568–577. doi: 10.1148/radiol.2322030788. [DOI] [PubMed] [Google Scholar]


