The Keystone Symposium on Immunologic Memory took place between 3 and 8 March 2007, in Santa Fe, New Mexico, USA, and was organized by R.A. Seder, S.L. Swain, R. Ahmed and A. Lanzavecchia.
Introduction
A characteristic of the immune system is its ability to remember past encounters with a pathogen and to mount an altered—usually enhanced—response to a subsequent encounter with the same pathogen. This ‘immunological memory' is the basis of vaccination, and is of considerable interest to both immunologists and vaccinologists. During the past few years, many studies have contributed to our understanding of the cellular and molecular basis of immunological memory, and there is a sense of excitement about this rapid rate of progress. It was in this context that the Keystone Symposium on Immunologic Memory took place, with the aim of understanding how T and B cells are programmed to elicit and sustain immunity. Here, I review some of the highlights of this meeting.
Vaccines: memory versus protective efficacy
The meeting opened with a keynote address by P. Doherty (Melbourne, Vic, Australia), who reminded us of the history of vaccination and its relationship to immunological memory. He pointed out that most effective vaccines operate through the production of antibodies; however, many clinically important pathogens evade humoral immunity and are instead controlled by cellular immune responses. Unfortunately, it has proven difficult to produce effective vaccines against these types of pathogens, presumably owing to the difficulties associated with eliciting appropriate cellular immune responses.
One way to prime durable cellular immunity is to use live vectors that express a target protein in the context of an infection. In this regard, D. Barouch (Boston, MA, USA) discussed the use of adenovirus vectors and the problem of pre-existing immunity to them. He described the development of new strains of adenovirus vaccine from rare serotypes that retain immunogenicity, and discussed their use in a monkey model. Cellular immune responses can be improved by using prime–boost vaccination strategies, whereby the immune system of individuals primed with one vectored vaccine are boosted with a different vector encoding the same insert. The power of this strategy was illustrated by A. Hill (Oxford, UK), who has used attenuated vaccinia virus vaccines to elicit long-lived immunity to tuberculosis and malaria. In particular, antigen-specific T cells in these studies were highly polyfunctional, expressing tumour necrosis factor-α (TNFα), interferon-γ (INFγ), macrophage inflammatory protein-1β (MIP1β) and interleukin-2 (IL-2).
Clearly this approach does more than simply boost the numbers of memory cells that are specific for the target pathogen. Indeed, both R. Seder and R. Koup (Bethesda, MD, USA) showed that T cells generated by these vaccination strategies are heterogeneous in their ability to produce cytokines (Foulds et al, 2006). Of particular interest was the fact that the presence of T cells that simultaneously express multiple cytokines, such as IFNγ, TNFα and IL-2, correlated more strongly with protection than the presence of cells that secrete IFNγ alone. Furthermore, these multi-cytokine-producing cells also produced the highest levels of cytokines, indicating that there is a correlation between functional diversity and the strength of the effector response. These findings represent a substantial advance in understanding T-cell memory and emphasize the importance of developing multiparametric single-cell assays to evaluate the efficiency of vaccination protocols. In addition, they highlight the importance of determining how different adjuvants can elicit effector and memory T-cell populations with distinct functional properties.
S. Swain (Saranac Lake, NY, USA) presented data showing that CD4+ T cells provide good protection against influenza virus infection by mediating perforin-dependent cytotoxicity of infected lung epithelial cells and increasing antibody production. This indicates that influenza vaccines should be modified to boost CD4+ T-cell priming. A. Lanzavecchia (Bellinzona, Switzerland) discussed the generation of IL-17-producing T-helper 17 (Th17) CD4+ T cells, which represent a third arm of the Th1/Th2 model—that is, the general division of CD4+ T cells into IFNγ producers (Th1) and IL-4 producers (Th2). Interestingly, IL-6 and IL-1β, both of which are produced by monocytes, promoted the development of Th17 cells in vitro. In addition, these cells could be distinguished from Th1 cells on the basis of CC chemokine receptor 4 (CCR4) and CXC chemokine receptor 3 (CXCR3) expression—Th17 cells express CCR4, whereas Th1 cells express CXCR3—and seem to have differential responsiveness to bacterial and fungal antigens.
Vaccine strategies were also discussed with respect to humoral immunity. S. Crotty and A. Sette (San Diego, CA, USA) undertook an extensive analysis of neutralizing epitopes on vaccinia virus using a protein library. Their main finding was an apparent link between antibody and helper epitopes on the same proteins. This was unexpected as viral complexes that comprise multiple proteins should allow T-cell and B-cell interactions to be mediated through different proteins within the same complex. There was considerable discussion as to whether this reflected a degradation of the virus during the immune response, such that each protein was handled as an independent entity, thereby linking the specificity of the T and B cells, or some other mechanism.
Trouble on the transgenic front
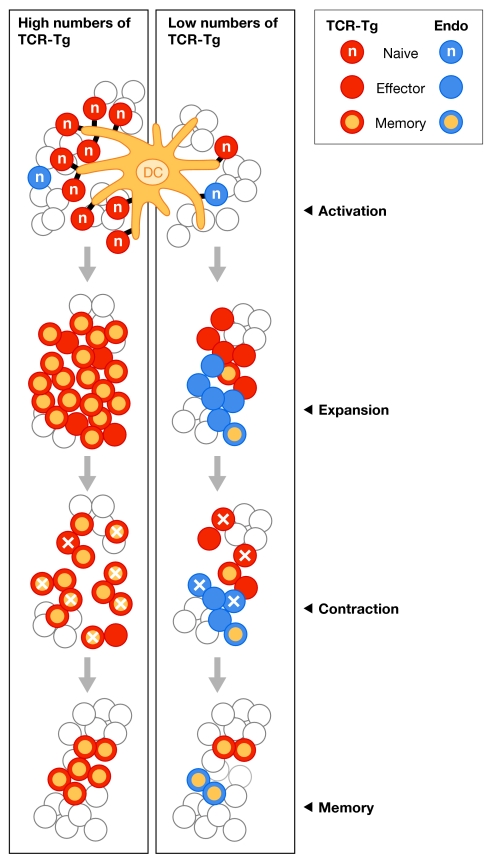
Doherty pointed out that recent advances in the field were made possible by the advent of techniques for studying immune responses in vivo, including the use of transgenic mice that express T-cell receptors (TCRs) that are specific for a single antigen. Indeed, TCR transgenic mice have been useful in adoptive-transfer models, in which investigators track a cohort of antigen-specific T cells in a highly controlled and accessible manner as they progress from naive through to effector and finally memory cells (Kearney et al, 1994). However, this approach has some limitations, which were highlighted at the meeting by M. Jenkins (Minneapolis, MN, USA). Jenkins showed that a large number (1 × 105) of monoclonal TCR transgenic T cells divided less, peaked early and fell below the starting level compared with a small number of identical T cells. This idea was further supported by data from J. Harty (Iowa City, IA, USA), who studied the response of different numbers of transgenic CD8+ T cells specific to an ovalbumin epitope expressed by a recombinant Listeria monocytogenes. In terms of kinetics, phenotype, function and memory generation, the donor transgenic T-cell response was most similar to the host T-cell response when small numbers of cells—typically fewer than 50—were transferred. By comparison, the transfer of larger numbers of donor T cells, in the range used in many earlier studies, resulted in responses that were subtly different from the endogenous host response. Furthermore, Harty showed that as few as 14 transferred transgenic T cells were sufficient to generate a response to a Listeria infection, and pointed out that every TCR transgenic system would have a distinct limit. The take-home message from this presentation was that input numbers of TCR transgenic T cells that are sufficient to inhibit the endogenous response to the same epitope will lead to aberrant responses of the transgenic T cells (Fig 1). P. Marrack (Denver, CO, USA) also pointed out that only a few transgenic T cells are needed to generate a potent T-cell response in vivo and that transferring too many cells results in suppression of the host response. As has been proposed by others, she argued that the optimal number of transgenic T cells generates a response that is one-half that of the total antigen-specific response.
Figure 1.
Initial T-cell receptor-transgenic precursor frequency dictates crucial aspects of the CD8+ T-cell response to infection. When the input number of TCR-transgenic T cells is sufficiently high to inhibit the endogenous CD8+ T-cell response to the same epitope, most aspects of the CD8+ T-cell response—including kinetics (earlier peak of the expansion), proliferation (fewer divisions), surface-molecule expression (early upregulation of ‘memory' CD8+ T-cell markers such as CD62L and CD127), effector function (interleukin-2 production), and efficiency of expansion and memory CD8+ T-cell generation—are substantially altered. By contrast, when the input number of TCR-transgenic T cells reflects only a fraction of the endogenous CD8+ T-cell frequency, TCR-transgenic T cells exhibit the kinetic, phenotypic and functional properties associated with the corresponding endogenous CD8+ T-cell response. DC, dendritic cell; Endo, endogenous; TCR-Tg, transgenic T-cell receptor.
Although there are some limitations to transgenic T-cell models, it is also clear that these approaches are powerful and have played a crucial role in advancing our understanding of how T-cell responses are initiated. In particular, these approaches allow investigators to track relatively large numbers of cells during the earliest stages of T-cell activation in vivo. For example, Swain and others have used this approach to characterize the early T-cell response to intranasal influenza virus infection (Roman et al, 2002). Interestingly, it is now becoming possible to study these early events with non-transgenic polyclonal T-cell populations. Jenkins showed that polyclonal naive CD4+ T cells could be isolated by major histocompatibility complex (MHC) class II tetramers and magnetic-bead enrichment, and suggested that this might be a more appropriate method for studying the induction of immunological memory. In addition, L. Lefrancois (Farmington, CT, USA) beautifully illustrated the fact that endogenous CD8+T-cell responses can be readily followed by in situ tetramer staining, despite the fact that few antigen-specific CD8+T cells are initially present. By using ovalbumin-expressing L. monocytogenes, he found that during the primary response to infection, tetramer-positive T cells are initially observed at the interface of the T-cell and B-cell zones, as well as in the marginal zones and the B-cell zones of the spleen, and subsequently migrate to the central T-cell zone in association with the dendritic cell network. These cells then form clusters on the edges of the T-cell and B-cell zones, and apparent marginal zones in the bridging channels that connect the white pulp to the marginal zone. Cluster formation could be blocked by the inclusion of an antibody that recognizes the ovalbumin peptide bound to the MHC. Interestingly, after the infection has been cleared, memory CD8+T cells are located predominantly in the B-cell areas of the spleen, and move rapidly into the T-cell area during a recall response.
Making lasting memories
It is well established that only a small fraction of the T cells generated in an acute response develop into long-lived memory T cells; however, the mechanisms that underlie this process are not known. Are cells predetermined to become memory cells or do they divert into a memory pathway at some point during the response? To begin to address this question, B. Rocha (Paris, France) has developed a sensitive multiplexed approach to analysing the expression of messenger RNA (mRNA) in individual cells. She was able to measure mRNA expression in the range of two to two billion molecules per cell, and subsequently showed that T cells initially express inflammation-associated genes before acquiring effector functions, such as cytotoxicity. As cells become memory cells, they tend to lose their effector functions. Interestingly, the overall response is heterogeneous on the level of the individual cell with no common differentiation pathway of memory generation. Furthermore, after boosting, the expression of effector genes becomes permanent. Therefore, similar to B cells, CD8+ T cells eventually revert into long-lived effectors cells that persist in vivo in the absence of antigens. An explanation for the heterogeneity of the T-cell response was offered by provocative findings from S. Reiner (Philadelphia, PA, USA), who showed that the first division of a cell in response to an antigen is characterized by an unequal partitioning of proteins associated with cell fate (Chang et al, 2007). This asymmetric partitioning results from sustained interaction between the T cell and the antigen-presenting cell, and produces daughter cells with distinct phenotypic and functional characteristics.
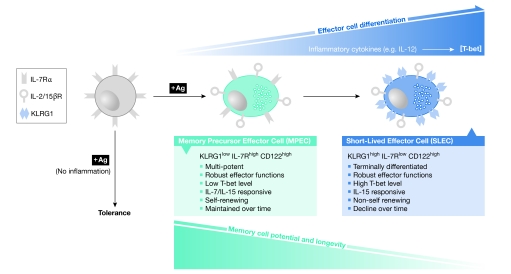
One marker that might have an important role in the development of memory T cells is the IL-7 receptor-α (IL-7Rα; also known as CD127). T cells that express high levels of CD127 (CD127high) have the ability to respond to survival signals that might facilitate their maintenance in the pool of memory T cells. However, it is not known how the diversity of CD127high and CD127low is initially generated during the acute response. S. Kaech (New Haven, CT, USA) reported that a subset of CD127low effector T cells express the killer cell lectin-like receptor G1 (KLRG1), which is a marker of terminally differentiated effector cells (Fig 2). Adoptive-transfer studies revealed that KLRG1low T cells developed into both KLRG1high and KLRG1low T cells, whereas KLRG1high T cells retained their KLRG1high (terminal effector) phenotype. In addition, the induction of KLRG1high CD127low effector CD8+ T cells seemed to be regulated by inflammatory signals, such as IL-12, rather than antigen load. These findings do not rule out the possibility that the level or duration of antigenic stimulation influences effector cell fate, but they do suggest that inflammatory signals have a more profound impact.
Figure 2.
Model for the development of KLRG1high/IL-7Rlow short-lived effector cells and KLRG1low/IL-7Rhigh memory precursor effector cells during infection. During CD8+ T-cell activation, exposure to inflammatory cytokines, such as interleukin-12 (IL-12), directly influences their expression of the transcription factor T-bet and their longevity. CD8+ T cells activated in the absence of inflammation become tolerant. During acute infection, CD8+ T cells activated under conditions of low inflammation develop robust effector functions, but contain lower levels of T-bet (indicated by light-blue shading) and retain IL-7 receptor-α (IL-7Rα) expression. These effector cells, which are referred to as MPECs, maintain a high potential to develop into long-lived memory CD8+ T cells. These cells are equally responsive to both IL-7 and IL-15, can undergo homeostatic turnover and are maintained over the long term. By contrast, CD8+ T cells that are activated under higher inflammatory conditions, for example, higher levels of IL-12, develop robust effector functions, express higher levels of T-bet (indicated by darker blue shading), increase expression of the natural killer inhibitory receptor KLRG1 and downregulate expression of IL-7R. These effector cells, which are referred to as SLECs, have a reduced potential to become long-lived memory CD8+ T cells. These KLRG1high SLECs are dependent on IL-15, but do not undergo homeostatic turnover and decline over time (in the absence of antigen). KLRG1, killer cell lectin-like receptor G1; MPECs, memory precursor effector cells; SLECs, short-lived effector cells.
Interestingly, antigen stimulation is not an essential prerequisite for memory T-cell development. S. Sarkar and V. Kalia (Atlanta, GA, USA) presented similar results showing that fate decisions about memory lineage are made early during expansion. Their studies further indicate that the duration of the infectious period and the resulting inflammation drives the terminal differentiation of the short-lived KLRG1high effector cells. In an interesting presentation, S. Jameson (Minneapolis, MN, USA) described how his group had investigated the homeostatic proliferation and activation of naive T cells after their transfer into a lymphopenic host. Using a transgenic system, he showed that these ‘activated' transgenic naive T cells exhibited all of the properties of authentic memory cells, including protective efficacy. This raises the question: to what extent are these ‘pseudo memory' T cells present under normal conditions? In this regard, Jameson pointed out that there are several examples when naive T cells enter a relatively lymphopenic environment, including the transient lymphopaenia that occurs during infection or in neonates.
R. Kedl (Denver, CO, USA) pointed out that isolated toll-like receptor (TLR) ligands elicit a less-effective immune response than infections with whole virus. This deficiency seems to be due to the lack of appropriate co-stimulatory signals, because the addition of anti-CD40 to the TLR substantially increased the development of CD8+ T-cell memory. Interestingly, this memory was also effectively generated in CD4+-deficient hosts. This raises the interesting questions of how and why CD4+ T-cell help is essential for the full differentiation of memory CD8+ T cells. M. Bevan (Seattle, WA, USA) linked this effect to IL-2 signals delivered during the initial programming phase of the primary response. He also concluded that CD4+ T cells have an active role in maintaining CD8+ T cells, and that CD4+ T-cell interactions with self antigens are required. S. Schoenberger (La Jolla, CA, USA) went on to show that memory CD8+ T cells that develop in the absence of help express increased levels of TNF-related apoptosis-inducing ligand (TRAIL), have an increased propensity to apoptose and can be rescued by caspase inhibitors. Consistent with this, memory CD8+ T cells that develop in the absence of T-cell help were able to undergo a strong secondary expansion in TRAIL-deficient mice. By contrast, Bevan found no role for TRAIL in determining the fate of ‘unhelped' memory CD8+ T cells in the context of a recall response. In view of data from the Harty group indicating that CD4+ help consists of both TRAIL-dependent and TRAIL-independent components, more studies need to be performed to clarify this crucial aspect of T-cell-memory development and maintenance (Badovinac et al, 2006).
Both L. Selin and R. Welsh (Worcester, MA, USA) discussed the fact that the establishment of T-cell memory is strongly influenced by the prior antigenic experience of the host. Memory T cells specific for previously encountered pathogens can cross-react with a newly encountered pathogen, and modify the specificity, function and efficacy of the immune response to the new pathogen. By contrast, non-cross-reactive memory T cells can be lost, resulting in a decline in pre-existing memory. S. Mueller (Atlanta, GA, USA) also discussed the factors regulating the immune response to a pathogen, and reported that expression of the lymphoid chemokines CC ligand 21 (CCL21) and CXC ligand 13 (CXCL13) is transiently downregulated during the response, which results in impaired priming of new T cells, and might preferentially promote memory and ongoing effector immune responses.
Chronic infections: memory or not?
Many pathogens have the ability to persist in the host and to mediate either chronic or latent infection. These infections are characterized by the persistent expression of antigens, and several speakers addressed the impact of this on the T-cell response. Pathogens that replicate continuously in the host, such as human immunodeficiency virus (HIV), place a considerable burden on the immune system. Antigen-specific T cells can become chronically stimulated under these conditions, and enter a state of ‘exhaustion' in which they lose function and subsequently die. Because understanding the regulation of T-cell responses under these conditions is a priority, considerable excitement has been generated by the recent finding that the activation marker programmed death-1 (PD1) is a major regulator of T-cell exhaustion (Barber et al, 2006).
R. Ahmed (Atlanta, GA, USA) reviewed the current state of the field with regard to PD1 and the ability of anti-PD1 to reverse T-cell exhaustion. Previous studies in a mouse model of persistent lymphocytic choriomeningitis virus (LCMV) infection had shown that T cells specific for viral antigens became ‘exhausted' and expressed PD1. Ahmed reported that a natural ‘escape mutant' LCMV virus, in which a specific T-cell epitope had been deleted, resulted in a loss of PD1 expression and a regain of function by T cells specific for that epitope. Evidence was also presented that PD1 expression has a role in the regulation of both CD4+ and CD8+ T-cell responses to HIV. Ahmed also discussed the role of the PD1 ligands PDL1 and PDL2: the former is expressed on most cells and is upregulated during an infection, whereas the latter is selectively expressed on dendritic and other antigen-presenting cells. Studies with mice that are deficient in the expression of either of these ligands indicate that blockade of PDL2 could be a more refined approach to modulating immune responses to chronic infections.
R.P. Sekaly (Montreal, QC, Canada) showed that PD1 is upregulated on HIV-specific CD8+ T cells, and that this activity is correlated with high viral load and a reduced ability to produce cytokines. Furthermore, memory CD4+ T cells from long-term HIV ‘non-progressors' are protected from apoptosis to some extent by the inactivation of the forkhead box O3a (FOXO3a) transcription pathway. S. Suvas (Knoxville, TN, USA) added the interesting observation that the basal levels of PD1 expression on CD4+ and CD8+ T cells increase with age, which might explain some of the declining function of the ageing immune response.
Some pathogens, such as viruses that establish latent infections, persist in a more quiescent state in the host. Although these pathogens constantly challenge the immune system of the host, lower levels of antigen are expressed and antigen-specific T cells are not typically driven to a state of exhaustion. A. Hill (Portland, OR, USA) used the mouse model of cytomegalovirus (CMV) infection to show that memory T cells specific for different viral antigens exhibit different patterns of response. Interestingly, the numbers of T cells specific for some epitopes increased over time, which is a phenomenon referred to as memory inflation. Data from K. Cho (Portland, OR, USA) indicate that this inflation seems to be driven, at least in part, by the development of new memory cells from the pool of naive cells.
Peripheral memory
One surprising aspect of the meeting was the relatively limited discussion of peripheral versus systemic T-cell memory. It is generally accepted that T-cell memory is heterogeneous and can be divided into two main categories: effector-memory cells, which localize primarily in the periphery; and central-memory T cells, which localize primarily in the secondary lymphoid organs (Sallusto et al, 1999). However, the mechanisms that direct the generation of these cells and their lineage relationships remain unclear. V. Venturi (Kensington, NSW, Australia) used mathematical approaches to show that the TCR diversity of central-memory CD8+ T cells is greater than that of effector-memory CD8+ T cells. Interestingly, this difference is stable over time, despite the fact that there are progressive changes in the absolute numbers of effector-memory and central-memory T cells. A. Goldrath (San Diego, CA, USA) showed that the inhibitor of DNA-binding 2 (Id2) has a crucial role in mediating a robust immune response, generating effector-memory T cells and enhancing the survival of these cells in a Listeria model of infection. In Id2-deficient mice, a normal acute response was generated to Listeria infection; however, this declined and the memory that developed had a predominantly central-memory phenotype. D. Woodland (Saranac Lake, NY, USA) discussed the fact that effector-memory CD8+ T cells persist in the lung airways following resolution of a respiratory virus infection. Maintenance of these cells depends on a dynamic process of recruitment of memory cells from the circulation, but is independent of any form of persistent antigen. Maintenance of memory cells in the airways also depends on their ability to survive in the relatively harsh environment of the lung airways. In this regard, D. Topham (Rochester, NY, USA) showed that influenza virus-specific memory CD8+ T cells in the lung airways express CD49a (very late antigen-1), which allows them to bind to type IV collagen. Interestingly, these CD49a-positive cells exhibit reduced signs of apoptosis, which suggests that they are actively maintained at this site. Furthermore, collagen and TNFα synergized to protect the cells against Fas-induced apoptosis in vitro. J. McDyer (Baltimore, MD, USA) extended these studies to the clinical situation by showing that many CMV-specific effector-memory CD8+ T cells are recruited and persist in the airways of lung-transplant recipients during primary viral infection.
What about B cells and antibodies?
Owing to the recent Keystone meeting on B-cell biology in Banff (AB, Canada), comparatively few presentations focused on B-cell memory. Nevertheless, several interesting developments in this area were discussed. M. McHeyzer-Williams (La Jolla, CA, USA) examined the development of different B-cell compartments after vaccination. Interestingly, the route of vaccination affected the distribution of CXCR5+ follicular helper T cells that are specialized in their ability to provide help to B cells. These cells accumulated preferentially in lymphoid tissues near the vaccination site, and their localization at these sites might be driven by persisting antigens. A. Radbruch (Berlin, Germany) discussed the maintenance of plasma cells, and the role of chemokine receptors in regulating competition between new and pre-existing plasma cells. His data indicate that plasma cells specific for a given antigen will decrease at a rate of 0.1% per subsequent competitive immune response and are reduced by 1% per year. This allows the humoral immune response to incorporate new antigenic specificities into the memory pool while maintaining existing specificities at high levels. The issue of plasma cells was also addressed by L. Glimcher (Boston, MA, USA) who discussed X-box-binding protein 1 (XBP1), which is a transcription factor that is involved in the unfolded protein response and endoplasmic-reticulum stress. Interestingly, XBP1 is expressed at high levels in plasma cells, and its absence results in a failure to develop plasma cells and a lack of antibody in the serum. This transcription factor seems to protect plasma cells and other cells with high secretory activity from the considerable burden of high levels of protein production.
Summary
This much-needed meeting allowed investigators in the field of immunological memory to take stock of recent developments. It provided a solid review of the field and highlighted the impressive progress that has been made during the past few years. Of particular note are the exciting advances in the technologies that allow investigators to analyse responses in vivo. Assays that were previously performed in vitro, ranging from cytotoxicity to proliferation assays, can now be carried out in vivo. It is also possible to focus on specific subsets of T cells that are present in tiny numbers in situ. Furthermore, it is possible to manipulate many aspects of B-cell and T-cell responses in vivo with an impressive array of knockout, knock-in, inducible and transgenic mice. However, the meeting also highlighted crucial gaps in our understanding, and focused our attention on specific problems. It is clear that despite substantial advances, we still do not have the answers to the fundamental questions of T-cell and B-cell memory that have challenged the field for many years. How do memory cells develop from the acute response? What is the importance of memory T-cell heterogeneity? How is memory maintained over the long term? Santa Fe offered a beautiful setting, with fabulous weather and crystal clear air, in which to ponder these issues. The sense of the meeting was that the answers are close by.

David L. Woodland
Acknowledgments
I am deeply indebted to J. Harty, V. Badovinac, N.S. Joshi and S. Kaech for contributing figures to this review. I thank T. Strutt, M. Blackman and R. Dutton for critically reading the manuscript. Owing to space limitations and my own biases, not all of the presentations given at the meeting are discussed here; I apologize to all those participants whose work I have not mentioned. My work is supported by the Trudeau Institute and by grants from the US National Institutes of Health.
References
- Badovinac VP, Messingham KA, Griffith TS, Harty JT (2006) TRAIL deficiency delays, but does not prevent, erosion in the quality of ‘helpless' memory CD8 T cells. J Immunol 177: 999–1006 [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439: 682–687 [DOI] [PubMed] [Google Scholar]
- Chang JT et al. (2007) Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315: 1687–1691 [DOI] [PubMed] [Google Scholar]
- Foulds KE, Wu CY, Seder RA (2006) Th1 memory: implications for vaccine development. Immunol Rev 211: 58–66 [DOI] [PubMed] [Google Scholar]
- Kearney ER, Pape KA, Loh DY, Jenkins MK (1994) Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1: 327–339 [DOI] [PubMed] [Google Scholar]
- Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL (2002) CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med 196: 957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712 [DOI] [PubMed] [Google Scholar]