The natural environment is in a state of constant flux and living organisms are perpetually challenged to adapt to these changes. Yet the mechanisms of adaptation, which lead to the development of new characteristics or behaviours, have troubled philosophers and scientists since the days of ancient Greece. In fact, it was not until 1859, when Charles Darwin (1809–1882) published The Origin of Species that part of the puzzle was solved. Darwin developed his theory of natural selection to explain the enormous diversity and adaptability of living organisms. He theorized that organisms of the same species develop subtle differences in their phenotypes that make them more or less able to survive and reproduce, and that those differences, which improve survival and reproduction, are passed on to future generations.
…despite its success, Darwin came to regard The Origin of Species as an incomplete explanation of his theory of evolution
But Darwin did not address the question of how the variety, on which natural selection acts, arises in the first place. This piece of the puzzle was supplied seven years later, in 1866, when Gregor Mendel (1822–1884) published his laws of inheritance. Mendel provided a mathematical model that explained how the phenotype of an organism is dependant on its genotype, and that genotypes are passed on from parents to their progeny and recombine to create new variations. It was later, in 1953, that Darwin's and Mendel's explanations were fully completed, when Francis Crick and James Watson published the structure of DNA, which explained the mechanism of how genes are copied and inherited.
Yet, despite its success, Darwin came to regard The Origin of Species as an incomplete explanation of his theory of evolution (Darwin, 1859). Later in his career, he spent considerable time studying the underlying causes of the variations that he believed were subject to natural selection and the laws of inheritance. He published his insights in a two-volume book, The Variation of Animals and Plants under Domestication (Darwin, 1868), in which he developed his ‘provisional hypothesis of pangenesis'. This theory attempted to explain how the changes in the physiology of an organism resulting from its environment—so-called, acquired characteristics—could also be passed on to its progeny, even without genetic information encoding them. In addition, it would also explain many other observations pertaining to variation, heredity and development. However, although the scientific community widely accepted Darwin's theory of natural selection and its explanation of evolution, his theory of pangenesis was largely regarded as wrong and ignored by geneticists.
Even now, more than a century after Darwin's death, whether phenotypes that are not encoded in the genome can be transmitted across generations, and how this is possible, still remain unanswered questions. Furthermore, if the inheritance of such acquired characteristics occurs at all, does it play a significant role in evolution?
This question has been the subject of heated controversy for more than 2,000 years and has attracted renowned scientists and philosophers to both sides of the debate. Rather anecdotally, one of the earliest proponents was Hippocrates of Cos II (ca. 460–370 BC), ‘the father of medicine', who firmly believed in the inheritance of acquired characteristics, based on his observations of the somewhat mythical race of people, the Macrocephali. He wrote of their elongated heads: “The characteristic was thus acquired at first by artificial means, but, as time passed, it became an inherited characteristic and the practice was no longer necessary” (Adams, 1891).
…if the inheritance of such acquired characteristics occurs at all, does it play a significant role in evolution?
More famously, Jean-Baptiste Lamarck (1744–1829), who coined the term ‘biology', devoted a chapter of his book, Philosophie Zoologique, published in 1809, to the influence of the environment on the activities and habits of animals. He wrote that environmental changes in “situation of climate, food, habits of life, etc., lead to corresponding changes in animals and plants in size, shape, proportion of parts, color, consistency, swiftness and skill”, which can be passed on to the next generation (Lamarck, 1809).
Indeed, Darwin also linked the cause of some variation with changes in the environment. He favoured the view that “variations of all kinds and degrees are directly or indirectly caused by the conditions of life to which each being, and more especially its ancestors, have been exposed […] if it were possible to expose all the individuals of a species during many generations to absolutely uniform conditions of life, there would be no variability” (Darwin, 1868).
However, various early attempts to provide scientifically satisfying proof for the inheritance of acquired characteristics all failed. Most geneticists eventually took the view that characteristics acquired as a result of environmental influences are rarely inherited and that any exceptions to this are of little importance, either for understanding the mechanisms of evolution or for commercial breeders to consider in their pedigrees.
An example of this stance was Darwin's contemporary, August Weismann (1834–1914), who was one of the most influential evolutionary theorists during the nineteenth century. He entirely rejected the idea of the inheritance of acquired characteristics and instead explained heredity by his theory of the “continuity of the germ plasm”. He held that, from the very first cell divisions of the growing embryo, the so-called germ plasm was destined to become the cells that would later be passed on during sexual reproduction. Furthermore, he argued, this germ plasm would remain completely unaffected by the somatoplasm or environmental influences and would be a ‘safe' copy of the embryo's original genome. In an effort to disprove the idea of inheritable acquired characteristics, Weismann cut off the tails of male and female mice after birth to show that, even over many generations, tail chopping never produced tailless progeny (Weismann, 1889).
Most geneticists eventually took the view that characteristics acquired as a result of environmental influences are rarely inherited, and that any exceptions to this are of little importance…
However, critics pointed out that this experiment did not actually test the inheritance of acquired characteristics because cutting off a mouse's tail is an external modification. In fact, Lamarck distinguished between two types of acquired characteristics: directly acquired, such as removal of the tail; and indirectly acquired, in response to a change of habit or environment. In his view, only indirectly acquired characteristics could be passed on to progeny (Steele et al, 1998). Darwin made this same distinction: “a part or organ may be removed during several successive generations, and if the operation be not followed by disease, the lost part reappears in the offspring” (Darwin, 1868).
Thus the question remains unanswered: does the inheritance of acquired characteristics occur? As Otto Landman (1993) has pointed out, the inheritance of acquired characteristics is not often encountered in natural science, despite a substantial body of evidence—mostly in bacteria and other lower organisms—to support it. This evidence has been accumulated over the past 2,000 years, but most significantly, rigorous scientific evidence has replaced anecdotal evidence during the past century, resulting in a compelling case for reassessing the possibility of acquired inheritance.
By way of example, in 1964, Tchang Tso-Run and co-workers generated an artificial hypotrich doublet in the ciliate Stylonychia mytilus (Tchang et al, 1964). They isolated a fused macronucleus and some cytoplasm when the ciliate began to divide asexually, and the isolated piece developed into mirror-image doublets with the two ventral surfaces on the same plane, rather than the usual back-to-back configuration. These artificial doublets had a complete set of physiological and reproductive functions, and were heritable in the normal manner—that is, their progeny had the same phenotype. Using similar methods, Gray Grimes et al (1980) obtained the same result in Pleurotricha lanceolata.
In the 1950s, Pyotr Sopikov (1903–1977) claimed to have induced inheritable acquired characteristics in birds by performing repeated blood transfusion from black Australorp hens to White Leghorn hens. He found that the subsequent mating of the White Leghorn hens with White Leghorn roosters yielded progeny with a modified phenotype (Sopikov, 1954). Importantly, other researchers between 1950 and 1970 confirmed Sopikov's observations. For example, Maurice Stroun and co-workers reported that birds of the White Leghorn variety, which were repeatedly injected with blood from the gray guinea fowl, produced progeny with some gray or black-flecked feathers in the second and later generations (Stroun et al 1963).
There are also many records of graft-induced inheritable changes in plants and Darwin was the first to compile the available information on graft hybrid individuals produced from the cellular tissue of two different plants (Darwin 1868). Several famous plant breeders, including Luther Burbank (1849–1926) and Ivan Michurin (1855–1935), created plants with inheritable characteristics that were acquired from the tissues of both original plants. In addition, about 500 papers on these types of hybridization experiment were published in the Soviet Union during the 1950s, although Western geneticists largely ignored the literature and dismissed the work as based on fraudulent results. Over the past decades, however, independent scientists have repeatedly shown that graft-induced variant characteristics in plants are stable and inheritable (Liu, 2006a).
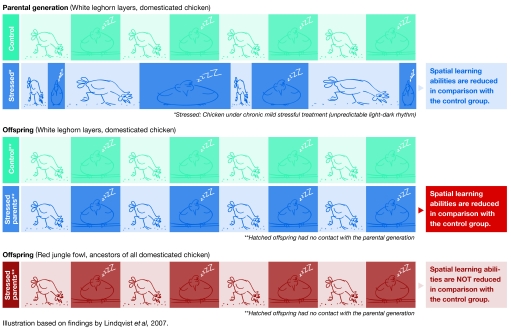
In addition to physical phenotypes, behavioural characteristics also appear to be inheritable. Swedish scientists recently produced substantial evidence when they raised Red junglefowl—the ancestors of modern chickens—and domesticated White Leghorn chickens in a stressful environment. They exposed the birds to an unpredictable rhythm of darkness and light that reduced their ability to solve a spatial learning task (Lindqvist et al, 2007). The progeny of stressed White Leghorn—but not Jungle Fowl—birds, raised without parental contact, had a reduced spatial learning ability compared with the progeny of non-stressed birds in a similar test. The progeny of the stressed White Leghorns were also more competitive and grew faster than the progeny of non-stressed parents, suggesting that behavioural stress responses were transmitted to the next generation.
The inheritance of acquired characteristics is not limited to physical and behavioural traits. In 1980, Gorczynski and Steele provided evidence that the inheritance of acquired characteristics plays a role in the developing immune system. They showed that neonatally acquired antigen-specific immune tolerance to foreign H-2 antigens in male mice is transmitted to a high proportion (50–60%) of first-generation offspring. Further incrossing and outcrossing of these first-generation mice showed that 20–40% of second-generation animals were again specifically tolerant or hyporesponders to the original H-2 antigen (Gorczynski & Steele, 1980). Several attempts to repeat these experiments yielded both positive and negative results, and produced a heated scientific controversy. Just two decades later, Hilmar Lemke et al (2004) suggested that the functional impact of maternally acquired IgG in the newborn is an example of non-genetic inheritance, and reveals a Lamarckian dimension to the immune system.
There is also evidence for the inheritance of non-Mendelian traits in humans. During the winter of 1945/46, there was a major famine in much of Europe caused by the devastation of the Second World War. Many pregnant women received less than 1,000 calories per day during the last trimester of their pregnancy. Researchers Ursula Kyle and Claude Pichard (2006) found that there was a clear correlation between the birth weight of these women's babies and maternal weight at parturition, in addition to other physiological and pathological changes in the next generation. In the Netherlands, researchers went further and examined the phenotypes of the next generation—who grew up with no food restrictions—and found a lingering relation between a mother's weight at her birth and the birth weights of her children (Susser & Stein, 1994).
 |
Similarly, Andreas Plagemann and colleagues showed that children of overweight or diabetic mothers have a higher risk of developing high blood pressure and diabetes later in life (Harder et al, 2001a). They explained this effect by suggesting that the body's ‘default' levels of insulin and other hormones are ‘set' during fetal and neonatal development; throughout life, the body's metabolism then tries to maintain or restore these ‘set' levels (Harder et al, 2001b). However, if this process is disturbed during the early stages of development through environmental influences—if the mother has abnormal hormone levels caused by diabetes or obesity—then the child's ‘default' levels will be set outside the normal range, with ensuing consequences for the overall metabolism and disease risk.
In summary, there is an increasing body of evidence for the inheritability of environmentally induced acquired characteristics; however, the problem that has historically hindered the acceptance of this theory is the lack of a theoretical framework to explain the mechanism by which acquired traits could be inherited. Although Lamarck took the inheritance of acquired characteristics for granted, he made no attempt to show how such transmission works. Conversely, Darwin theorized that the mechanism was through minute particles or molecules—that he called ‘gemmules'—which, he proposed, are expelled by cells that have changed in response their environment. These gemmules could then circulate the body and cause other cells to undergo similar changes—including cells of the germline.
A modern version of Darwin's pangenesis is the ‘somatic selection' hypothesis, which explains how mutant somatic information could be integrated into the germline. According to the theory, endogenous retroviral vectors would capture RNA from somatic cells and transduce them into germline cells. Once inside, the passenger RNA would be reverse-transcribed and spliced into the genome of the cell by recombination (Steele et al, 1998). In addition, Darwin's theoretical gemmules could in fact be circulating DNA, prions, mobile elements or as yet unknown molecules (Liu, 2006b). What seems clear, however, is that there might be multiple vectors for the transmission of environmentally induced changes to the progeny of an organism.
For example, environmentally induced genomic rearrangements might be enacted by transposable elements. Barbara McClintock (1902–1992), who received the 1983 Nobel Prize in Physiology or Medicine for the discovery of transposons, was convinced that environmental stressors could trigger inheritable changes in the genome: “I believe there is little reason to question the presence of innate systems that are able to restructure a genome. It is now necessary to learn of these systems and to determine why many of them are quiescent and remain so over very long periods of time only to be triggered into action by forms of stress, the consequences of which vary according to the nature of the challenge to be met” (McClintock, 1978).
There is sufficient evidence that this is at least the case in plants. Various research groups have shown that specific concentrations of certain mineral nutrients or temperature can cause plants to grow differently. These phenotypic changes are transmitted to the progeny and remain stable for several generations (Durrent, 1962). The DNA modifications associated with these environmentally induced changes have been extensively characterized (Cullis, 2005).
…observations of the inheritance of acquired characteristics are increasingly compatible with current concepts in molecular biology
For example, Gerhard Ries et al (2000) reported that UV-B radiation induces DNA rearrangements in Arabidopsis thaliana and tobacco plants, and that the effects of UV-B on genomic stability increased with each generation, suggesting that there were inheritable changes occurring in the expression of genes involved in DNA metabolism. Similarly, Jean Molinier et al (2006) showed that Arabidopsis plants treated with short-wavelength radiation or flagellin had increased somatic homologous recombination of a transgenic reporter. Furthermore, these increased levels of recombination persisted in subsequent, untreated generations. The authors concluded from their study that environmental factors led to increased genomic flexibility even in successive, untreated generations, perhaps as a mechanism to increase the potential of the plants to adapt to changes in environment.
During the past years, the scientific community has realized that prions—proteins that had already overthrown another scientific dogma: that only DNA-carrying particles can be infectious agents—are able to transmit phenotypic information. Susan Lindquist's work on the yeast prion sup35 revealed that the protein acts as a switch so that when the environmental conditions deteriorate sup35 switches to its prion state [PS1+], in which translation fidelity is decreased and the ribosome reads beyond nonsense codons. This in turn allows the expression of formerly silent genes and gene variants to create new phenotypes. [PS1+] is passed on to daughter cells where it self-replicates by imposing its conformation on normal sup35 proteins (Shorter & Lindquist, 2005). In an earlier paper, Yury Chernoff (2001) had postulated that prions could be a mechanism for the inheritance of acquired characteristics. Peter Maury (2006) has also proposed a mechanism by which prions store and transmit acquired information in specific β-sheet protein conformations. These can act as cytoplasmic molecular memories and can be transmitted to future generations utilizing their self-perpetuating potential.
Another possible mechanism that has drawn increasing attention in the past few years is epigenesis. Conrad Hal Waddington (1905–1975), who first defined ‘epigenetics' as “…the interactions of genes with their environment which bring the phenotype into being” (Waddington, 1942), was a keen supporter of the inheritance of acquired characteristics. It seems that Waddington might have been right: Lindqvist et al (2007) concluded, from their experiments with chickens, that epigenetic modifications might be the mechanism of transmission of stress and physiological responses to the next generation. More generally, epigenetic mechanisms mediate a semi-independent non-Mendelian inheritance system, which enables environmentally induced phenotypes to be transmitted to the next generations (Jablonka & Lamb, 1998).
Experimental evidence for this comes from studies using mice. A maternal diet that supplements methyl-donors with folic acid, vitamin B12, choline and betaine, alters the fur colour of their progeny towards the brown pseudoagouti phenotype (Wolff et al, 1998; Waterland & Jirtle, 2003). This diet-induced change in colour distribution was shown to result from an increase in DNA methylation at sites in the upstream intracisternal A-particle transposable element (Waterland & Jirtle, 2003). Therefore, the effect of a mother's diet during pregnancy on the phenotype of her progeny was directly linked to DNA methylation (Cropley et al, 2006). Tessa Roseboom et al (2006) therefore suggested that epigenetic changes such as imprinting, which take place before conception, might help to explain the effects of the Dutch Famine on the next generation.
Root Gorelick (2004) went even further and coined the term neo-Lamarckian medicine to describe the effects of epigenetic inheritance on diseases. Exposure to certain environmental pollutants can alter the methylation patterns of regulatory genes. This not only increases the risk of cancer, by up-regulating genes controlling cell division or down-regulating tumor suppressor genes, but might also underlie many other diseases. Such epigenetic changes could be inheritable, thus transmitting the increased risk of disease to future generations even if they are no longer exposed to the contaminant.
Horizontal gene transfer—the exchange of genes across mating barriers—has long been recognized as a major force in evolution, particularly among prokaryotes. However, there is increasing evidence that horizontal gene transfer also occurs between higher organisms. Ulfar Bergthorsson et al (2003) showed that mitochondrial genes are frequently transferred between distantly related flowering plants with various genomic outcomes, including gene duplication, the recapture of genes lost through transfer to the nucleus, and chimaeras. These results suggest the existence of a mechanism for unrelated plants to ‘swap' DNA. Recently, Jeffrey Mower et al (2004) described two new cases of horizontal gene transfer from parasitic flowering plants to their host plants, and presented phylogenetic and geographic evidence that this occurred as a result of direct physical contact. Their findings complement earlier discoveries that genes can be transferred in the opposite direction, from host to parasite plant (Davis & Wurdack, 2004).
In light of the mounting evidence, can we continue to ignore Darwin's theory of pangenesis, which provides a mechanistic explanation of how environmentally-induced variations are inherited?
Notably, Ivan Michurin's basic principle of plant breeding was to manipulate environmental conditions during the early developmental stage of a plant to induce phenotypic changes. He used grafting to ‘improve' plants, and stated that the younger the plant the more successful the experiment would be (Michurin, 1949). Recent grafting experiments showed that endogenous mRNAs use the phloem as a long-distance translocation system (Lucas et al, 2001). Furthermore, the transport of other macromolecules including proteins and nucleic acids between plant cells is most promiscuous in young, undifferentiated tissues and becomes more restricted as tissues age (Ueki & Citovsky, 2005). With the realisation that mRNA species can move around the plant, and the ability of retroviruses or retrotransposons to reverse transcribe mRNA into cDNA, it becomes clear that mechanisms exist for horizontal gene transfer from stock to scion—and vice versa—by grafting.
In a letter to Moritz Wagner, Darwin wrote: “In my opinion, the greatest error which I have committed, has been not allowing sufficient weight to the direct action of the environment, for example, food and climate, independently of natural selection. When I wrote The Origin, and for some years afterwards, I could find little good evidence of the direct action of the environment; now there is a large body of evidence” (Darwin, 1888). During the past decades, the evidence for the inheritance of acquired characteristics has been increasing in both quantity and quality, as have the number of hypotheses to explain the phenomenon at the molecular level. Consequently, observations of the inheritance of acquired characteristics are increasingly compatible with current concepts in molecular biology (Landman, 1991). Although this does not discredit the important contributions made by Weismann and Mendel, nor in any way revive the theories of Lamarck or Lysenko, it nevertheless sheds new light on the inheritance of acquired characteristics.
There are many precedents where once widely disregarded theories eventually made their way into the main body of scientific knowledge. In the early 1940s, Waddington coined the term epigenetics, which he derived from Aristotle's theory of epigenesis. Until the 1980s, epigenetics was barely mentioned in the scientific literature, yet was used abundantly from the 1990s onwards, as experimental evidence began to support its existence and importance. Similarly, Stanley Prusiner's discovery that prions are infectious agents was long disregarded by the scientific community, but is now generally accepted.
In light of the mounting evidence, can we continue to ignore Darwin's theory of pangenesis, which provides a mechanistic explanation of how environmentally induced variations are inherited? Do we in fact need to enrich and expand Darwin's pangenesis, and develop a modern theory of inheritance, which is broader in scope and consistent with the wealth of experimental evidence? A wider understanding of how acquired characteristics are inherited would not only indicate that there is much more to inheritance than genes and Mendelian genetics, but would also create new intellectual challenges and give a wider perspective of evolution.
As Darwin wrote to Hooker: “You will think me very self-sufficient, when I declare that I feel sure if Pangenesis is now still-born it will, thank God, at some future time reappear, begotten by some other father, and christened by some other name. Have you ever met with any tangible and clear view of what takes place in generation, whether by seeds or buds, or how a long-lost character can possibly reappear; or how the male element can possibly affect the mother plant, or the mother animal, so that her future progeny are affected? Now all these points and many others are connected together, whether truly or falsely is another question, by Pangenesis. You see I die hard, and stick up for my poor child” (Darwin, 1988).

References
- Adams F (1891) The Genuine Works of Hippocrates (translated by Francis Adams). New York, NY, USA: William Wood [Google Scholar]
- Bergthorsson U, Adams KL, Thomason B, Palmer JD (2003) Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature 424: 197–201 [DOI] [PubMed] [Google Scholar]
- Chernoff YQ (2001) Mutation processes at the protein level: is Lamarck back? Mutat Res 488: 39–64 [DOI] [PubMed] [Google Scholar]
- Cropley JE, Suter CM, Beckman KB, Martin DI (2006) Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proc Natl Acad Sci USA 103: 17308–17312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis CA (2005) Mechanisms and control of rapid genomic changes in flax. Ann Bot 95: 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1859) On the Origin of Species by Means of Natural Selection; or, The Preservation of Favoured Races in the Struggle for Life. London, UK: John Murray [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1868) The Variation of Animals and Plants Under Domestication. London, UK: John Murray [Google Scholar]
- Darwin F (ed) (1888) The Life and Letters of Charles Darwin. London, UK: John Murray [Google Scholar]
- Davis CC, Wurdack KJ (2004) Host-to-parasite gene transfer in flowering plants: phylogenetic evidence from malpighiales. Science 305: 676–678 [DOI] [PubMed] [Google Scholar]
- Durrent A (1962) Induction, reversion and epitrophism of flax genotrophs. Nature 196: 1302–1304 [Google Scholar]
- Gorczynski RM, Steele EJ (1980) Inheritance of acquired immunological tolerance to foreign histocompatibility antigens in mice. Proc Natl Acad Sci USA 77: 2871–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick R (2004) Neo-Lamarckian medicine. Med Hypotheses 62: 299–303 [DOI] [PubMed] [Google Scholar]
- Grimes GW, McKenna ME, Goldsmith-Spoegler CM, Knaupp EA (1980) Patterning and assembly of ciliature are independent processes of hypotrich ciliates. Science 209: 281–283 [DOI] [PubMed] [Google Scholar]
- Harder T, Franke K, Plagemann A, Kohlhoff R (2001a) Early nutrition and later blood pressure: effect of maternal diabetes. J Pediatr 139: 905–906 [DOI] [PubMed] [Google Scholar]
- Harder T, Kohlhoff R, Dorner G, Rohde W, Plagemann A (2001b) Perinatal ‘programming' of insulin resistance in childhood: critical impact of neonatal insulin and low birth weight in a risk population. Diabet Med 18: 634–639 [DOI] [PubMed] [Google Scholar]
- Jablonka E, Lamb MJ (1998) Epigenetic inheritance in evolution. J Evol Biol 11: 159–183 [Google Scholar]
- Kyle UG, Pichard C (2006) The Dutch famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care 9: 388–394 [DOI] [PubMed] [Google Scholar]
- Lamarck J-B (1809) Philosophie Zoologique. Paris, France: JB Baillière [Google Scholar]
- Landman OE (1991) The inheritance of acquired characteristics. Annu Rev Genet 25: 1–20 [DOI] [PubMed] [Google Scholar]
- Landman OE (1993) Inheritance of acquired characteristics revisited. Bioscience 43: 696–705 [Google Scholar]
- Lemke H, Coutinho A, Lange H (2004) Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol 25: 180–186 [DOI] [PubMed] [Google Scholar]
- Lindqvist C, Janczak AM, Natt D, Baranowska I, Lindqvist N, Wichman A, Lundeberg J, Lindberg J, Torjesen PA, Jensen P (2007) Transmission of stress-induced learning impairment and associated brain gene expression from parents to offspring in chickens. PLoS ONE 2: e364, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-S (2006a) The historical and modern genetics of plant graft hybridization. Adv Genet 56: 101–129 [DOI] [PubMed] [Google Scholar]
- Liu Y-S (2006b) Response to Till-Bottraud and Gaggiotti: going back to Darwin's works. TIPS 11: 472–473 [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Yoo B-C, Kragler F (2001) RNA as a long- distance information macromolecule in plants. Nat Rev Mol Cell Biol 2: 849–857 [DOI] [PubMed] [Google Scholar]
- Maury CP (2006) Molecular mechanism based on self-replicating protein conformation for the inheritance of acquired information in humans. Med Hypotheses 67: 1164–1169 [DOI] [PubMed] [Google Scholar]
- McClintock B (1978) Mechanisms that rapidly reorganize the genome. Stadler Symp 10: 25–48 [Google Scholar]
- Michurin IV (1949) Selected Works. Moscow, USSR: Foreign Languages Publishing House [Google Scholar]
- Molinier J, Ries G, Zipfel C, Hohn B (2006) Transgeneration memory of stress in plants. Nature 442: 1046–1049 [DOI] [PubMed] [Google Scholar]
- Mower JP, Stefanovic S, Young GJ, Palmer JD (2004) Gene transfer from parasitic to host plants. Nature 432: 165–166 [DOI] [PubMed] [Google Scholar]
- Ries G, Heller W, Puchta H, Sandermann H, Seidlitz HK, Hohn B (2000) Elevated UV-B radiation reduces genome stability in plant. Nature 406: 98–101 [DOI] [PubMed] [Google Scholar]
- Roseboom T, de Rooij S, Painter R (2006) The Dutch famine and its long-term consequences for adult health. Early Hum Dev 82: 485–491 [DOI] [PubMed] [Google Scholar]
- Shorter J, Lindquist S (2005) Prions as adaptive conduits of memory and inheritance. Nat Rev Genet 6: 435–450 [DOI] [PubMed] [Google Scholar]
- Sopikov PM (1954) Changes in heredity by the prenatal administration of blood. Agrobiogiia 6: 34–45 [Google Scholar]
- Steele EJ, Lindley RA, Blanden RV (1998) Lamarck's Signature: How Retrogenes Are Changing Darwin's Natural Selection Paradigm. New York, NY, USA: Perseus Books Group [Google Scholar]
- Stroun J, Stroun-Guttieres L, Rossi J, Stroun M (1963) Transfer to the progeny of alterations induced in the White Leghorn by repeated injections of heterologous blood. Arc Sci Geneve 16: 247–262 [Google Scholar]
- Susser M, Stein Z (1994) Timing in prenatal nutrition: a reprise of the Dutch famine study. Nutr Rev 52: 84–94 [DOI] [PubMed] [Google Scholar]
- Tchang T-R, Shi X-B, Pang Y-B (1964) An induced monster ciliate transmitted through three hundred and more generations. Sci Sin 13: 850–853 [Google Scholar]
- Ueki S, Citovsky V (2005) Control improves with age: intercellular transport in plant embryos and adults. Proc Natl Acad Sci USA 102: 1817–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH (1942) The epigenotype. Endeavour 1: 18–20 [Google Scholar]
- Waterland RA, Jirtle RL (2003) Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 23: 5293–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann A (1889) The supposed transmission of mutilations. In Essays upon Heredity and Kindred Problems, EB Poulton, S Schönland, AE Shipley (eds). Oxford, UK: Clarendon [Google Scholar]
- Wolff GL, Kodell RL, Moore SR, Cooney CA (1998) Maternal epigenetics and methy supplements affect agouti gene expression in Avy/a mice. FASEB J 12: 949–957 [PubMed] [Google Scholar]


