Abstract
Aims
Genetically influenced aspects of adolescent behaviour can play a role in alcohol use and peer affiliation. We explored the correlations between friends' alcohol use and adolescent own use with a genetically sensitive design.
Design
Genetic and environmental factors were estimated on adolescent reports of their friends' alcohol use and their own use and problem use of alcohol. The correlations between the genetic and environmental factors that influence friends' alcohol use and adolescent own alcohol use and problem use were also estimated.
Participants
A total of 862 twin pairs aged 11–17 years sampled from the UK population-based Cardiff Study of All Wales and North-west of England Twins (CaStANET).
Measurements
Data on adolescent own alcohol use and problem use and the alcohol use of their three best friends were obtained using self-report questionnaires.
Findings
A significant genetic influence was found on adolescent friends' alcohol use (about 30%). Significant correlations of 0.60 and 0.70 were found between the genetic influences on friends' alcohol use and adolescents' own use and problem use of alcohol. Common environmental influences were almost completely correlated for friends' alcohol use and adolescents' own alcohol use and problem use (0.91 and 0.94).
Conclusions
There is considerable overlap in the common environmental and genetic factors that contribute to the relationship between adolescents' own alcohol use and that of their friends. These findings contribute to understanding of the mechanisms by which friends' alcohol use influences adolescent drinking behaviour.
Keywords: Adolescent, alcohol use, friends, genetic, gene-environment influences, peers, twin study
INTRODUCTION
Alcohol is the most prevalent form of substance use during adolescence [1]. Heavy use is linked with numerous negative health consequences and adverse social outcomes, including liver damage, mouth and throat cancer, sexually transmitted disease, antisocial behaviour and poor school performance [1]. Rates of alcohol use and problem use in the United Kingdom are high compared to most other European countries [2], with many adolescents regarding heavy alcohol use as normative [1]. Better understanding of the risk factors for alcohol problem use is important for the development of the most effective prevention and intervention approaches.
Both genetic factors [3] and peer influences [4–7] have been implicated in adolescent alcohol use. Twin studies indicate that 20–30% of the variation in liability to adolescent alcohol use is genetic in origin [3]. In the first UK-based twin study of substance use, we have found previously that genetic influences accounted for around 30% of the variation in the initiation and 50–60% in the progression of alcohol use [8]. Peer alcohol use (usually assessed by adolescent report rather than directly) has been reported to be one of the strongest predictors ofadolescent alcohol use [5–7], while perception of peers' attitudes to alcohol use is another important risk factor [7,9].
There are several possible reasons for the strong relationship between peer alcohol use and adolescent alcohol use. Peers may influence adolescent drinking behaviour by serving as role models and by influencing attitudes towards alcohol. They can also facilitate access to and encouragement of alcohol use [10,11]. Alternatively, given that adolescents are involved actively in selecting and maintaining friendships, they may select friends with similar drinking habits to themselves (peer selection [12,13]). It is likely that the association between friends' and adolescent alcohol use is due to both peer selection and peer influence. Other factors, including cultural norms, neighbourhood influences, school and larger peer networks, will also have an impact [5,14,15].
A possible explanation for the association is gene–environment correlation (rGE). Conceptually, rGE concerns the co-occurrence of genetic influences increasing risk for alcohol use in adolescents with risk environments (e.g. involvement with substance-using peers). Three types of rGE have been posited: passive (where the parental genes cause the correlation), active (where the subject's own genes make him/her seek certain environments) and evocative (where a subject's own genes increase the chances he/she is selected by others) [16,17]. It has been hypothesized that rGE plays an important role in the continuity of devious behaviour over time [18]. The study of gene–environment correlation is important because it can identify the environments that put individuals with a genetic liability for alcohol problem use at an increased risk. Twin studies using bivariate genetic analyses represent an important first step towards disentangling the mechanisms of gene–environment correlation. Genetic influences have been reported for adolescent alcohol use [3], peer behaviour [19] and friendship maintenance and formation [20]. It seems plausible that the correlation between peers and adolescent alcohol use is explained partially by an overlap in genetic factors. To date, a US-based study of 14-year old twins [4] is the only twin study to examine peer influences and adolescent alcohol use. The study used a measure of peer deviance and found that the association with alcohol use was explained by common environmental influences (i.e. environmental influences that make the twins more similar), with no evidence for genetic influences. Peer alcohol use was assessed as part of a broader index of peer deviance. Given the strong association identified between peer and adolescent alcohol use, it may be an important risk factor in its own right [5–7]. Further, genetic influences have been reported for a combined measure of peer alcohol and cigarette use [19] but not for peer deviance [4], suggesting that their aetiology may be different.
There is evidence to suggest that while frequency of alcohol use is associated with a range of negative outcomes, there are distinct risks involved in binge drinking and drunkenness, such as increased aggressive and antisocial behaviour [21,22]. A definition of binge drinking of five or more drinks in a row has been used previously to define problem drinking in epidemiological research and in reports used to inform UK policy decisions [1,2]. The relationship between close friends' alcohol use with adolescent use of alcohol versus problem use may differ and it is potentially informative to explore this.
The aims of the present study were to examine:
the relationship between adolescent alcohol use and problem use and their best friends' alcohol use in a UK population-based sample of twins;
the genetic and environmental influences on adolescent alcohol use and problem use and their friends' alcohol use; and
whether the relationship between friends' and adolescent alcohol use is influenced by genetic or environmental factors.
Because alcohol use and peer influences may differ, depending on gender and age [23–25], this was also evaluated in our analyses.
METHOD
Sample
This study used data from the fourth wave (2004) of data collection of the longitudinal Cardiff Study of All Wales and North-west of England Twins (CaStANET), a population-based twin register, including families with twins born between 1976 and 1991 in the Cardiff area of South Wales and between 1980 and 1991 for the rest of Wales and the North-west of England [26,27]. Previous waves of data collection (1991, 1997, 2000) have focused on indices of child psychological adjustment [including attention deficit hyperactive disprder (ADHD), conduct problems, depression and chronic fatigue] using child and/or parent and teacher reports [26]. In 2004, questionnaires were mailed to families taking part in CaStANET with twins aged 11–19 years, which assessed substance use and associated risk factors, including family functioning, parent health and twin psychological adjustment. The sample is representative of the general UK population in terms of socio-economic status and ethnicity [27,28]. Zygosity was assigned in an earlier wave of the study [23] using a parent-rated twin similarity questionnaire [29,30]. Non-responders were initially sent reminder postcards and were then re-mailed the questionnaire. Of 1755 families contacted, at least one individual from 1214 families returned questionnaires (a response rate of 69%; the individual response rate was 61%). Families received gift vouchers as a token of appreciation.
Alcohol can be purchased legally in the United Kingdom from 18 years of age. For the present paper, we analysed data on adolescents under the legal age. Our sample therefore consisted of 862 twin pairs (142 male, 185 female MZ twin pairs and 108 male, 141 female, 286 opposite sex DZ twin pairs) and 1629 individuals (complete data on both twins were not always available) aged 11–17 years (mean = 15.33 years; SD = 1.41).
Measures
For the current paper, all measures were from adolescent self-report. The measures used to assess own and close friends' alcohol use were derived from the Add Health questionnaire [31]. The Add Health study is the largest survey of adolescent health undertaken to date. All the measures used in the present study have been piloted extensively in the Add Health study [32].
Quantity of alcohol use
This was assessed using the question: ‘Think of all the alcoholic drinks you had during the past 12 months. How many drinks did you have during a typical week’? Responses ranged from 1 (never had a drink in my life) to 7 (more than 30 in a typical week). For the present analysis, individuals were recoded into those who had never drunk alcohol, light drinkers (less than six drinks in a typical week) and heavier drinkers (six or more drinks in a typical week). A drink was defined as a glass of wine, a can or half pint of beer or lager, a bottle (e.g. Bacardi Breezer), or a single measure of spirits.
Problem alcohol use
This was assessed as: ‘over the past 12 months, on how many days did you drink five or more alcoholic drinks in a row?’ and ‘over the past 12 months, on how many days have you got drunk on alcohol?’. Both indices of drinking behaviour have been used to define problem drinking in previous research [1,2,31]. Response options ranged from 1 (never) to 5 (most days). Scores were strongly associated (Spearman's r = 0.69), and combined whereby the highest endorsement from either behaviour was taken as the measure of problem alcohol use. For the present analysis, individuals were recoded into those who had never drunk alcohol in their life, those who had not engaged in problem alcohol use more than twice in the last year and those who had engaged in problem alcohol use more than twice in the last year (i.e. drinking until drunk or drinking five or more drinks in a row in the last year).
Friend's alcohol use
Friend's alcohol use was assessed with the question: ‘of your three best friends (apart from your twin), how many drink alcohol at least once a month?’. Response options ranged from 0 (none) to 3 (all three).
Finally, we created a composite score of twin-sharing of best friends by averaging the responses of both twins to the question: ‘how many of your three best friends are also your twin’s best friends?'. Possible responses to these questions ranged from 0 (none) to 3 (all 3). There was a significant association between twins as to the number of shared friends (r = 0.77, P < 0.001). This combined measure provided an index of shared friends for each twin pair.
Statistical analysis
Initial analyses were conducted to examine whether friends' alcohol use was associated with an increase in adolescent quantity of alcohol use and problem alcohol use. As each twin is part of a twin pair, when conducting epidemiological analyses reduced variance due to the correlation between twins' scores can cause high false positive rates [33]. To account for this the data were treated as equivalent to a two-stage cluster design, with twin pairs as the primary sampling unit [34], using the survey analyses procedure in STATA (release 9) [35].
Univariate genetic analysis
As the alcohol use variables were ordinal, a threshold model was used whereby the underlying liability to each variable is considered as normally, or approximately normally, distributed in the population. This model assumes that behaviour, e.g. problem alcohol use, will occur only if an individual's position on the liability distribution for that behaviour is above a certain liability threshold [36]. In univariate analyses, twin data allowed us to estimate the relative contribution of genetic and environmental influences on quantity of alcohol use and problem use. By conducting similar analyses on twin reports of friends' alcohol use, it is also possible to test whether there are genetic influences on the adolescent that are correlated with friend's alcohol use. The twin method is based on comparisons of monozygotic (MZ) twins, who share 100% of their genes in common, with dizygotic (DZ) twins, who share on average 50% of their genes. For genetically influenced behaviour we would expect greater MZ than DZ similarity. In the basic (ACE) model, variation is assumed to arise from three sources: (1) additive genetic effects (a2); (2) common environmental effects (c2); and (3) unique environmental effects (e2). Common environmental effects serve to make twins more similar to one another, while unique environmental effects tend to make the individuals in a twin pair less similar [37].
Genetic analyses using a structural equation modelling approach and full information maximum likelihood estimation with raw ordinal data were undertaken with the software package Mx [38]. Conventional tests of fit are not appropriate for this type of analysis; however, the relative difference in the fit of minus twice the log-likelihood between submodels is approximately distributed as χ2. Thus, it is possible to compare more complex models with simpler models, for example a model including gender differences in aetiology with one that includes no gender differences, and assess on the grounds of parsimony which is the best-fitting model. To test whether a model represents a good fit of the data, the model is compared to a ‘perfect’ fitting model, referred to as a saturated model, in which the variance and covariance of the data is freely modelled. If the model is not significantly different from the saturated model, it represents a good fit to the data [39].
When investigating the genetic influences on friends' behaviour it is possible that MZ twins share more friends than DZ twins. This would represent a violation of the ‘equal environments assumption’, which assumes that MZ and DZ twins are correlated equally in their exposure to environmental factors of importance for the trait under study and would result in an inflated heritability estimate for friends' alcohol use [40]. In addition, the extent to which friends' alcohol use is correlated with adolescent own alcohol use could lead to a greater resemblance between MZ twins and an inflated heritability estimate for this variable. Based on the significant correlation between increased sharing of best friends and peer alcohol use (r = 0.17, P < 0.0001) and increased sharing of best friends in MZ twins (Mann–Whitney U = 36804.5, P > 0.0001), we controlled for sharing of best friends when conducting the genetic analyses by allowing the threshold to vary as a function of the number of shared friends [41]. The threshold is modelled as a simple linear function: ti = t + shared best friendsi ts, where t is the population baseline threshold (for individuals with 0 shared best friends), ts models the regression of the threshold on the number of shared best friends, and shared best friends i is the number of shared best friends of the individual i [41]. The same approach was used to control for possible age effects (see below).
It is plausible, particularly with substance use such as alcohol use, that the behaviour of a twin may have an impact on the same behaviour in the other twin. This is termed ‘sibling interaction’ and can take two forms, sibling competition and sibling cooperation [34]. In traits measured on an ordinal scale we would expect a sibling interaction to result in differences in threshold levels and in the prevalence of individuals in extreme categories for MZ and DZ twins [37]. This can be tested by assessing whether the MZ and DZ twin thresholds can be equated without a significant drop in fit. We found that MZ and DZ thresholds could be equated for all variables, suggesting that there was no significant effect of sibling interaction (results available from the corresponding author upon request).
With a sample of twins reared together that includes opposite sex DZ pairs, it is possible to test for qualitative and quantitative gender differences in the aetiology of behaviour [37]. A sex limitation model that includes qualitative gender differences is referred to as a ‘general effects model’ and can be specified to assume a (partially) different genetic or common environmental aetiology for males and females. A sex limitation model that includes quantitative gender differences but no qualitative gender differences (i.e. has the same genetic and environmental aetiology but differences in magnitude for males and females) is referred to as the ‘common effects model’. A model with no gender differences (i.e. the genetic and environmental estimates for males and females are equated) is referred to as a ‘sex homogeneity’, or ‘no sex effects model’ [37]. By comparing the fit of minus twice the log-likelihood and the change in degrees of freedom between these models it is possible to test for significant gender differences in aetiology. However, the two alternate forms of the general effects sex limitation model (i.e. qualitative differences in genetic versus common environmental influence) are not nested and therefore comparisons of alternative indices of fit such as Aikake's information criterion (AIC) [42] are necessary when selecting the most appropriate model [37].
Given the likelihood of an association between age and the level of alcohol use and friends' alcohol use, an age correction was employed which adjusts the threshold for each twin according to his or her age on the distribution of liability to substance use, using the same method as described for controlling for sharing of best friends [41]. We also tested for quantitative differences in aetiology due to age by assessing whether the genetic and environmental estimates can be equated for individuals from different age groups without a significant drop in fit. For this analysis, the sample was divided into early (12–14 years old, 175 twin pairs), middle (14–16 years old, 386 twin pairs) and late (16–18 years old, 315 twin pairs) adolescent age groups. Although dividing the sample in this way will lead to a reduction of power to detect differences, we can conclude tentatively that there was no evidence for quantitative differences in aetiology due to age (results available from the corresponding author upon request).
Bivariate genetic analysis
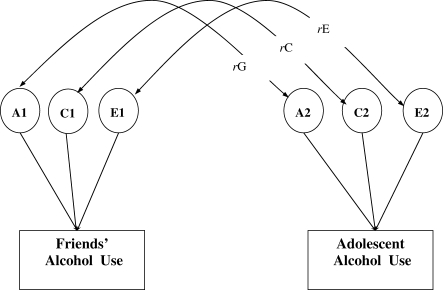
In bivariate analyses, we tested whether genetic and environmental influences on the twins' alcohol use and problem use were correlated with those influencing their friends' use. Given that in the literature there is evidence of both friends influencing an adolescent's alcohol use and vice versa [6], and as the data used in the current analyses were cross-sectional, it was not possible to assume a direction of effect between these variables. As such, a bivariate correlated factors model was fitted to the data (see Fig. 1). This is equivalent to a bivariate Cholesky decomposition model but makes no assumptions about the direction of causation [43]. It gives estimates of the genetic (rG), common environment (rC) and unique environment (rE) correlation between two variables of interest. These correlations are independent of the size of the estimates of genetic and environmental influences on the variables of interest.
Figure 1.
Correlated factors model. Additive genetic, common environment and unique environment variance contributions to friends' alcohol use and adolescent alcohol use are indicated by A1, C1, E1 and A2, C2, E2, respectively. rG, rC and rE represent the correlations between genetic, common environment and unique environment contributions to peer alcohol use and adolescent alcohol use. For clarity of presentation, the figure depicts one member of a twin pair
RESULTS
Figures for quantity of alcohol use showed that 69% of adolescents drank alcohol less than six times in a typical week. Sixteen per cent of adolescents had never had a drink of alcohol, while 15% reported consuming more than six drinks in a typical week. Figures for problem alcohol use indicated that of the 84% of adolescents who drank alcohol, 24% reported drinking until drunk or consuming five or more drinks in a row more than twice in the past 12 months. Adolescent reports of the number of close friends who used alcohol showed that 19% had one close friend who drank alcohol at least once a month, 17% had two close friends who drank alcohol at least once a month and 33% had three close friends who drank alcohol at least once a month. Thirty per cent of adolescents reported that none of their close friends drank alcohol at least once a month. Each variable was associated with age (polyserial correlations r = 0.40–0.50). Friends' alcohol use was associated with adolescent own quantity of alcohol use (polychoric correlation r = 0.66) and alcohol problem use (polychoric correlation r = 0.72). Ordered logistic regression was used to examine the associations between friends' alcohol use and adolescent own alcohol use. Friends' alcohol use predicted both quantity of alcohol use (b = 1.087, t = 13.95 P < 0.001) and alcohol problem use (b = 1.111, t = 14.10 P < 0.001) in analyses controlling for age, gender and zygosity.
Univariate genetic model fitting
Table 1 presents the results of the model fits for the different gender-specific models. It indicates that the no sex effects (sex homogeneity) model provides the best fit for all variables, suggesting that there were no quantitative or qualitative gender difference in aetiology. This model also equates the thresholds for males and females, suggesting that there was no difference in prevalence for males and females. Estimates of a2, c2, e2 appear in Table 2 as part of the bivariate analysis. The results for friends' alcohol use indicate significant genetic (approximately 28%), common environment (approximately 36.5%) and unique environment (approximately 35.5%) influences. For quantity of alcohol use the majority of the variance was explained by genetic influences (61%), although both common environment (20%) and unique environment (19%) were also significant. Similarly, for problem alcohol use, genetic influences explained the largest proportion of variance (46%) although common environment (29%) and unique environment (25%) were also significant. The best-fitting models were not significantly different from the fully saturated models (see Table 1), suggesting that they represented a good fit of the data.
Table 1.
Model fit statistics for univariate genetic analysis.
| Goodness-of-fit statistics | ||||||
|---|---|---|---|---|---|---|
| −2LL | d.f. | AIC | Δ −2LL | Δd.f. | ΔAIC | |
| Quantity of alcohol use | ||||||
| 1. Saturated model | 2263.127 | 1576 | −888.873 | |||
| 2. ACE No sex effects model | 2251.844 | 1582 | −912.156 | 11.283 | 6 | 23.283 |
| 3. ACE Common effects model | 2247.839 | 1577 | −906.161 | 4.005 | 5 | 5.995 |
| 4. ACE general effects model, rg free | 2242.666 | 1576 | −909.334 | 9.178 | 6 | 2.822 |
| 5. ACE general effects model, rc free | 2247.439 | 1576 | −904.561 | 4.405 | 6 | 7.595 |
| Problem alcohol use | ||||||
| 1. Saturated model | 2548.009 | 1606 | −663.991 | |||
| 2. ACE no sex effects model | 2554.788 | 1612 | −669.212 | 6.779 | 6 | 5.221 |
| 3. ACE common effects model | 2553.741 | 1607 | −660.259 | 1.047 | 5 | 8.953 |
| 4. ACE general effects model, rg free | 2551.809 | 1606 | −660.191 | 2.979 | 6 | 9.021 |
| 5. ACE general effects model, rc free | 2553.737 | 1606 | −658.263 | 1.051 | 6 | 10.949 |
| Friends' alcohol use | ||||||
| 1. Saturated model | 3779.201 | 1603 | 573.201 | |||
| 2. ACE no sex effects model | 3784.579 | 1612 | 560.579 | 5.378 | 9 | 12.622 |
| 3. ACE common effects model | 3777.080 | 1607 | 563.080 | 7.499 | 5 | 2.501 |
| 4. ACE general effects model, rg free | 3777.080 | 1606 | 565.080 | 7.499 | 6 | 4.501 |
| 5. ACE general effects model, rc free | 3776.375 | 1606 | 564.375 | 8.204 | 6 | 3.796 |
−2LL: minus twice the log likelihood; d.f.: degrees of freedom. The best-fitting model is shown in bold type. The ACE (additive genetic, common enviroment and unique enviroment effects) no sex effects model assumes no gender differences in the genetic and environmental estimate. This model (model 2) was compared to a saturated model (model 1) which estimates a covariance for each zygosity and the means and variances for each person-category. Models 3–5 were compared to the no sex effects model. These models assess sex differences in aetiology. The common effects model refers to a model assuming quantitative sex differences in the genetic and environmental estimates. The general effects model, rg free, refers to a model assuming qualitative sex differences in the genetic estimate while the general effects model, rc free, refers to a model assuming qualitative sex differences in common environment. AIC: Aikake's information criterion.
Table 2.
Bivariate genetic analysis.
| Model 1: Friends' alcohol use with quantity of alcohol use | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Friends' alcohol use | Quantity of alcohol use | Correlations | Model fit statistics | ||||||||||
| a2 | c2 | e2 | a2 | c2 | e2 | rg | rc | re | −2LL | d.f. | AIC | Δχ2from saturated model | Δd.f. from saturated model |
| 0.27* | 0.37* | 0.35* | 0.61* | 0.20* | 0.19* | 0.70* | 0.91* | 0.15 | 5770.204 | 3193 | −615.796 | 15.871 | 20 |
| (0.05–0.51) | (0.18–0.55) | (0.27–0.45) | (0.34–0.81) | (0.03–0.41) | (0.13–0.29) | (0.36–1.00) | (0.39–1.00) | (−0.08–0.36) | |||||
| Model 2: Friends' alcohol use with problem alcohol use | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Friends' alcohol use | Problem alcohol use | Correlations | Model fit statistics | ||||||||||
| a2 | c2 | e2 | a2 | c2 | e2 | rg | rc | re | −2LL | d.f. | AIC | Δχ2from saturated model | Δd.f. from saturated model |
| 0.29* | 0.36* | 0.36* | 0.46* | 0.29* | 0.25* | 0.60* | 0.94* | 0.34* | |||||
| (05–0.52) | (0.16–0.54) | (0.28–0.45) | (0.22–0.68) | (0.10–0.48) | (0.19–0.34) | (0.21–1.0) | (0.55–1.0) | (0.15–0.51) | 5982.081 | 3223 | −463.919 | 14.353 | 20 |
Full models with 95% confidence intervals are presented.
P < 0.05, a2, c2, e2 are the genetic, common environment and unique environment estimates. rg, rc and re are the correlations between the genetic, common environment and unique environment factors influencing the phenotypes. −2LL = minus twice the log likelihood, d.f. = degrees of freedom. AIC: Aikake's information criterion.
Bivariate genetic model fitting
Significant genetic and environmental correlations were found between friends' alcohol use with adolescent quantity of alcohol use and with problem use (see Table 2). The only exception was a non-significant unique environmental correlation between friends' alcohol use and adolescent own quantity of alcohol use. The bivariate analyses did not provide a significantly worse fit than the fully saturated model, suggesting that they represented a good fit to the data.
DISCUSSION
This is the first study to examine the genetic relationship between peer alcohol use and adolescent alcohol use. The study replicated previous findings of a strong association between peer and adolescent alcohol use [5–7]. The results indicated a high level of overlap between the genetic influences on both friends' drinking and adolescent alcohol use, as indicated by the significant genetic correlations (r = 0.6–0.7). However, the results also emphasize the importance of environmental factors with the majority of the common environmental factors being shared.
Consistent with previous studies of adolescent alcohol use, genetic influences were found to be important and no gender differences in aetiology were found [3]. Genetic estimates were slightly higher than has been reported, but as the genetic aetiology of adolescent alcohol use has not been studied previously in UK-based samples, it is not clear whether this effect is specific to alcohol use within the CaStaNET sample or to the United Kingdom in general. However, the prevalence rates in CaStaNET were consistent with those reported in large studies of alcohol use in the United Kingdom [2]. Genetic influences were slightly stronger for quantity of alcohol use (a2 = 0.61) than alcohol problem use (a2 = 0.46). This may be because alcohol problem use was assessed as whether the problem use had occurred twice in the last year (rather than the regularity of it occurring), and this may be more influenced by environmental opportunities to drink to excess.
Peers have been referred to as an important environmental risk factor for adolescent substance use and problem use. However, our results indicate that the situation is more complicated. We found that genetic influences explained about 30% of the variation of twin reports of friends' alcohol use. This is compatible with a peer selection process whereby twins seek out particular peers or peers seek out the twin with whom to be friends, which is partly genetically influenced. The results could also be explained by the adolescents' individual characteristics, given that peer selection and friendship maintenance are influenced by attitudes, personality and behaviour [12,13]. Both attitudes and personality have been shown to be, at least in part, genetically influenced [44,45].
For both peer and adolescent alcohol use no age or gender differences in aetiology were found, nor evidence for sibling interaction for alcohol use. A strong correlation was found between the genetic factors influencing adolescent alcohol use and friends' alcohol use (r = 0.6–0.7). This is consistent with the possibility of rGE (gene–environment correlation). As mentioned previously, three types of rGE have been posited: passive (where the parental genes cause the correlation), active (where the subject's own genes make him/her seek certain environments) and evocative (where a subject's own genes make him/her more likely to be selected by others) [16,17]. The occurrence of both active and evocative rGE processes on drinking habits is plausible, where genes influence the choice of friends (who in turn may further affect liability to consume alcohol). Passive rGE is also possible whereby parental genes contribute to both the genotype and the environment of their children. Thus, for example, genetic factors influence parent–child relations [46] and parent–child relations influence peer selection (with poor parent–child relationships quality is associated with affiliation with deviant peers; [47]). Given that the relationship between peer and own alcohol use is potentially reciprocal [6], it is plausible that genetic factors create a risk environment which influences adolescent alcohol use. Given the cross-sectional nature of the present study, it was not possible to further distinguish between these processes. Peer influences are one of the strongest risk factors for adolescent alcohol involvement [3] and therefore a powerful potential domain for intervention. These results suggest individual differences in interactions with peers, which are influenced by genetic as well as environmental influences. It appears that to be most efficacious, peer-based interventions need to take into account these individual differences in addressing the relationship between peer influences and adolescent alcohol use.
The correlation between common environmental influences on friends' alcohol use and adolescent own alcohol use was almost one (r = 0.91–0.94). At face value, our results suggest that the common environmental influences on alcohol use overlap almost entirely with those that impact friends' alcohol use. Although rater effects may have contributed to these findings (see below), another study has provided similar results. Walden and colleagues [4] found that the relationship between peer deviance and alcohol use was explained by common environmental factors (although they were not examining peer alcohol use per se).
In the present study, the peer influences examined were those of the behaviour of the adolescent's three closest friends, hence the importance of peer selection as a possible mechanism. An important caveat is that peer relationships are not restricted to dyadic relationships with friends, but involve small and larger groups of friends [48] and fellow school pupils [4]. The peer group investigated (friends, classmates) and whether group or individual relationships are examined have potentially important implications for the derived pattern of results.
Limitations
There are several limitations of the present study. The same rater reported their own and their close friends' alcohol use, which may have led to shared method variance inflating covariance artificially between the two assessments. Because adolescent reports of peer drinking may include bias [19], as their accounts may measure imperfectly peer's actual use and reflect their own use [49], it is possible that the genetic influences on perceived peer use may index genetic influences on the adolescent's reporting, and this needs to be taken into account when interpreting our findings. Although very few studies have presented correlations between perceived and actual peer use, one study in adolescents has reported that the correlations were not perfect, but substantial (0.63–0.68) [24]. Furthermore, our results are in agreement with an earlier univariate twin study that was based on peers' own reports of a combined measure of alcohol and cigarette use [19] and also found evidence of genetic influences.
Although no quantitative age differences were observed, without longitudinal data it is impossible to discount the possibility of qualitative age differences (specific aetiological influences coming online at different ages). Finally, we took a conservative approach to control for twins sharing the same peers. However, this greater sharing of friends may be due to genetic influences on friendship formation and therefore represent a valid genetic influence on peer alcohol use. If this were the case, our estimates of genetic influences on peer alcohol use would be overly conservative.
The implications of these findings are that friends' alcohol use should not be considered an entirely environmental risk factor for adolescent alcohol use and problem use. Genetic influences can be expected to play a role in individual differences in alcohol use by adolescents as well as contribute to who their friends are and to what extent these friends use alcohol. These findings point to the possibility of gene–environment correlations in adolescent alcohol use. However, further research is required to increase understanding of the type and form such correlations take and the processes by which they exert their effects. The high common environment correlation also implies that environmental influences on twins that make them more similar in drinking behaviour are the same as those that make their friends' drinking behaviour more similar to their own. These findings contribute to understanding of the mechanisms by which friends' alcohol use influences adolescent drinking behaviour.
Acknowledgments
We would like to thank the families who participated in the Cardiff Study of All Wales and North-west of England Twins for their time and contribution to this project. The development of this manuscript was supported by grants awarded by the Wellcome Trust (GR073063) to Marianne van den Bree, Anita Thapar, Michael C. Neale, Gordon Harold and Jane Scourfield and by the European Research Advisory Board awarded to Marianne van den Bree, Anita Thapar and Jane Scourfield. Support was also provided by an NIH grant (DA-018673) awarded to Michael C. Neale.
References
- 1.British Medical Association (BMA) Board of Science and Education. Adolescent Health. London: BMA Publishing Unit; 2003. [Google Scholar]
- 2.Hibell B, et al. Alcohol and Other Drug Use Among Students in 35 European Countries. Stockholm: Sweden: Swedish Council for Information on Alcohol and Other Drugs (CAN) and the Pompidou Group at the Council of Europe; 2004. The ESPAD Report 2003. [Google Scholar]
- 3.van den Bree MB. Combining research approaches to advance our understanding of drug addiction. Curr Psychiatry Rep. 2005;7:125–32. doi: 10.1007/s11920-005-0009-4. [DOI] [PubMed] [Google Scholar]
- 4.Walden B. Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. J Abnorm Psychol. 2004;113:440–50. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland HH. The moderation of adolescent-to-peer similarity in tobacco and alcohol use by school levels of substance use. Child Dev. 2003;74:279–91. doi: 10.1111/1467-8624.00535. [DOI] [PubMed] [Google Scholar]
- 6.Curran PJ. The relation between adolescent alcohol use and peer alcohol use: a longitudinal random coefficients model. J Consult Clin Psychol. 1997;65:130–40. doi: 10.1037//0022-006x.65.1.130. [DOI] [PubMed] [Google Scholar]
- 7.Needle R. Familial, interpersonal, and intrapersonal correlates of drug use. a longitudinal comparison of adolescents in treatment, drug-using adolescents not in treatment, and non-drug-using adolescents. Int J Addict. 1988;23:1211–40. doi: 10.3109/10826088809058854. [DOI] [PubMed] [Google Scholar]
- 8.Fowler T, et al. Exploring the relationship between and genetic influences on initiation and progression of substance use. Addiction. 2007;102:413–22. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sale E. Risk, protection, and substance use in adolescents: a multi-site model. J Drug Educ. 2003;12:91–105. doi: 10.2190/LFJ0-ER64-1FVY-PA7L. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan HB. Pathways to adolescent drug use: self-derogation, peer influence, weakening of social controls, and early substance use. J Health Soc Behav. 1984;25:270–89. [PubMed] [Google Scholar]
- 11.Oetting ER. Peer cluster theory: drugs and the adolescent. J Counsel Dev. 1986;65:17–22. [Google Scholar]
- 12.Block J. Longitudinally foretelling drug usage in adolescence: early childhood personality and environmental precursors. Child Dev. 1988;59:126–355. doi: 10.1111/j.1467-8624.1988.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 13.Shedler J. Adolescent drug use and psychological health: a longitudinal inquiry. Am Psychol. 1990;45:612–30. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- 14.Ennett ST. School and neighborhood characteristics associated with school rates of alcohol, cigarette, and marijuana use. J Health Soc Behav. 1997;38:55–71. [PubMed] [Google Scholar]
- 15.Kadushin C. The substance use system. social and neighborhood environments associated with substance use and misuse. Subst Use Misuse. 1998;33:1681–710. doi: 10.3109/10826089809058950. [DOI] [PubMed] [Google Scholar]
- 16.Scarr S. How people make their own environments: a theory of genotype greater than environment effects. Child Dev. 1983;54:424–35. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 17.DeFries JC. Behavioural Genetics. 4. New York: Worth Publishers; 2001. [Google Scholar]
- 18.Moffitt T. E: The neuropsychology of conduct disorder. Dev Psychopathol. 1993;5:135–52. [Google Scholar]
- 19.Cleveland HH. Sources of exposure to smoking and drinking friends among adolescents: a behavioral-genetic evaluation. J Genet Psychol. 2005;166:153–69. [PubMed] [Google Scholar]
- 20.Iervolino AC. Genetic and environmental influences in adolescent peer socialization: evidence from two genetically sensitive designs. Child Dev. 2002;73:162–74. doi: 10.1111/1467-8624.00398. [DOI] [PubMed] [Google Scholar]
- 21.San Jose B. Drinking patterns and health outcomes: occasional versus regular drinking. Addiction. 2000;95:865–72. doi: 10.1080/09652140050031617. [DOI] [PubMed] [Google Scholar]
- 22.Midanik LT. Drunkenness, feeling the effects and 5+ measures. Addiction. 1999;94:887–97. doi: 10.1046/j.1360-0443.1999.94688711.x. [DOI] [PubMed] [Google Scholar]
- 23.Maccoby E. The Psychology of Sex Differences. Stanford, CA: Stanford University Press; 1974. [Google Scholar]
- 24.Wilks J. Parent, peer and personal determinants of adolescent drinking. Br J Addict. 1989;84:619–30. doi: 10.1111/j.1360-0443.1989.tb03477.x. [DOI] [PubMed] [Google Scholar]
- 25.School Health Education Unit (SHEU) Trends; Young People and Alcohol, Attitudes to Drinking. Exeter: SHEU; 2003. pp. 1983–2001. p. [Google Scholar]
- 26.van den Bree M, et al. The Cardiff Study of All Wales and North West of England Twins (CaStANET): a longitudinal research programme of child and adolescent development. Twin Res Hum Genet. in press. [DOI] [PubMed]
- 27.Rice F. Assessing the effects of age, sex and shared environment on the genetic aetiology of depression in childhood and adolescence. J Child Psychol Psychiatry. 2002;43:1039–51. doi: 10.1111/1469-7610.00231. [DOI] [PubMed] [Google Scholar]
- 28.Thapar A. Does the definition of ADHD affect heritability? J Am Acad Child Adolesc Psychiatry. 2000;39:1528–36. doi: 10.1097/00004583-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Nichols RC. The diagnoses of twin zygosity. Acta Genetica Statistica Med. 1966;16:265–75. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- 30.Cohen DJ. Reliably separating identical from fraternal twins. Arch Gen Psychiatry. 1975;32:1371–5. doi: 10.1001/archpsyc.1975.01760290039004. [DOI] [PubMed] [Google Scholar]
- 31.Resnick MD, et al. Protecting adolescents from harm: findings from the National Longitudinal Study on Adolescent Health. JAMA. 1997;278:823–32. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- 32.The National Longitudinal Study of Adolescent Health. [2007-April-20]. http://www.cpc.unc.edu/projects/addhealth.
- 33.Bland JM. Statistics notes. Trials randomised in clusters. BMJ. 1997;315:600. doi: 10.1136/bmj.315.7108.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonoff E, et al. Influences of age, sex, and impairment on rates of disorder. Arch Gen Psychiatry. 1997;54:801–8. doi: 10.1001/archpsyc.1997.01830210039004. The Virginia Twin Study of Adolescent Behavioral Development. [DOI] [PubMed] [Google Scholar]
- 35.Statacorp. STATA Statistical Software. College Station, TX: Statcorp LP; 2005. release 9. [Google Scholar]
- 36.Falcolner DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- 37.Neale MC. Methodology for Genetic Studies of Twins and Families. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 38.Neale MC. Mx: Statistical Modelling. 5. Richmond, VA: Department of Psychiatry; 1999. [Google Scholar]
- 39.Rijsdijk FV. Genetic and environmental influences on psychological distress in the population: General Health Questionnaire analyses in UK twins. Psychol Med. 2003;33:793–801. doi: 10.1017/s0033291703007451. [DOI] [PubMed] [Google Scholar]
- 40.Kendler KS. Parental treatment and the equal environment assumption in twin studies of psychiatric illness. Psychol Med. 1994;24:579–90. doi: 10.1017/s0033291700027732. [DOI] [PubMed] [Google Scholar]
- 41.Neale MC. Extensions to the modeling of initiation and progression. Behav General. 2006;36:507–24. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- 42.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–32. [Google Scholar]
- 43.Loehlin JC. The Cholesky approach: a cautionary note. Behav Gen. 1996;26:65–9. [Google Scholar]
- 44.Eaves LJ, et al. Genes, Culture, and Personality: an Empirical Approach. London: Oxford University Press; 1989. [Google Scholar]
- 45.Bouchard TJ., Jr Genes, environment, and personality. Science. 1994;264:1700–1. doi: 10.1126/science.8209250. [DOI] [PubMed] [Google Scholar]
- 46.McGue M. Perceptions of the parent–adolescent relationship: a longitudinal investigation. Dev Psychol. 2005;41:971–84. doi: 10.1037/0012-1649.41.6.971. [DOI] [PubMed] [Google Scholar]
- 47.Wood MD. Do parents still matter? Parent and peer influences on alcohol involvement among recent high school graduates. Psychol Addict Behav. 2004;18:19–30. doi: 10.1037/0893-164X.18.1.19. [DOI] [PubMed] [Google Scholar]
- 48.Deater-Deckard K. Annotation: recent research examining the role of peer relationships in the development of psychopathology. J Child Psychol Psychiatry. 2001;42:565–79. [PubMed] [Google Scholar]
- 49.Kandel DB. The parental and peer contexts of adolescent deviance: An algebra of interpersonal influences. J Drug Issues. 1996;26:289–315. [Google Scholar]