Abstract
Brain aging is associated with a progressive imbalance between antioxidant defenses and intracellular concentrations of reactive oxygen species (ROS) as exemplified by increases in products of lipid peroxidation, protein oxidation, and DNA oxidation. Oxidative conditions cause not only structural damage but also changes in the set points of redox-sensitive signaling processes including the insulin receptor signaling pathway. In the absence of insulin, the otherwise low insulin receptor signaling is strongly enhanced by oxidative conditions. Autophagic proteolysis and sirtuin activity, in turn, are downregulated by the insulin signaling pathway, and impaired autophagic activity has been associated with neurodegeneration. In genetic studies, impairment of insulin receptor signaling causes spectacular lifespan extension in nematodes, fruit flies, and mice. The predicted effects of age-related oxidative stress on sirtuins and autophagic activity and the corresponding effects of antioxidants remain to be tested experimentally. However, several correlates of aging have been shown to be ameliorated by antioxidants. Oxidative damage to mitochondrial DNA and the electron transport chain, perturbations in brain iron and calcium homeostasis, and changes in plasma cysteine homeostasis may altogether represent causes and consequences of increased oxidative stress. Aging and cognitive decline thus appear to involve changes at multiple nodes within a complex regulatory network.
Keywords: oxidative brain aging, brain aging, aging, autophagy, cognitive aging, insulin signaling, sirtuins
Introduction
Aging research is currently dominated by two competing concepts. The spectacular lifespan extension in certain mutants of nematodes, fruit flies, or mice suggested the possibility that aging may be a regulated process under the control of a few ‘aging genes’, notably genes involved in the insulin signaling pathway and in the expression of certain ‘silent information – regulating proteins’ or sirtuins (Kenyon et al., 1993; Dorman et al., 1995; Kimura et al., 1997; Hekimi & Guarente, 2003; Howitz et al., 2003; Sauve et al., 2006). On the other hand, there is a growing body of evidence supporting the ‘free-radical theory of aging’ (Harman, 1956), which predicts that the lifespan of an organism could be increased by augmenting antioxidant defenses. In this review, we summarize recent findings suggesting that the age-related increase in oxidative stress may have a direct impact on the insulin signaling pathway and downregulate sirtuin proteins. This review focuses mainly on the central nervous system although it is understood that many of the principles outlined may be germane to aging in non-neural tissues.
The decline in cognitive and motor functions is one of the hallmarks of ‘normal aging’ (Bartus, 1990; Kluger et al., 1997). It includes conspicuous changes in learning and memory, which have been linked to the hippocampus, as well as a decline in strength, balance, and coordination, which map to the basal ganglia and cerebellum (Joseph, 1992; Bickford, 1993; McDonald & White, 1994; Devan et al., 1996; Oliveira et al., 1997; Foster, 1999). In contrast to neurodegenerative diseases, the cognitive decline in ‘normal aging’ may not be associated with a significant loss of neurons (Gallagher et al., 1996).
Evidence for an age-related increase in oxidative damage and decrease in antioxidant levels
The putative role of oxygen radicals and radical-derived reactive oxygen species (ROS) in neurodegeneration and cognitive decline has been reviewed previously (Gallagher et al., 1996; Berr et al., 2000; Serrano & Klann, 2004). The age-related increase in oxidative brain damage is best exemplified by products of lipid peroxidation (Gupta et al., 1991; Cini & Moretti, 1995; Murray & Lynch, 1998; O'Donnel & Lynch, 1998; Calabrese et al., 2004; Richwine et al., 2005; Zhu et al., 2006), protein oxidation (Forster et al., 1996; Mohsen et al., 2005; Sigueira et al., 2005; Poon et al., 2006), and oxidative modifications in nuclear and mitochondrial DNA (Hamilton et al., 2001). An increase in protein carbonyl levels has been demonstrated for various brain regions including the hippocampus (Mohsen et al., 2005, Siqueira et al., 2005).
Age-related memory impairment is correlated with a decrease in brain and plasma antioxidants (Perrig et al., 1997; Perkins et al., 1999; Berr et al., 2000; Rinaldi et al., 2003). Intracellular glutathione (GSH) concentration as well as the ratio of glutathione : glutathione disulfide (GSH : GSSG ratio) were found to decrease with age in different animal models (Chen et al., 1989; Iantomasi et al., 1993; Oubidar et al., 1996; Pallardo et al., 1999; Sasaki et al., 2001; Liu, 2002; Sandhu & Kaur, 2002; Rebrin et al., 2003; Wang et al., 2003; Suh et al., 2004, 2005). This decrease in GSH level and/or GSH : GSSG ratio was found in all mammalian brain regions tested including the hippocampus (Calabrese et al., 2004; Balu et al., 2005; Donahue et al., 2006; Zhu et al., 2006). As cysteine is the most limiting precursor for intracellular GSH biosynthesis, it is reasonable to hypothesize that the decrease in intracellular GSH may be mechanistically related to the age-related decrease in post-absorptive plasma cysteine that has been found in humans between the third and the ninth decade of life (Hack et al., 1998). Most cells and tissues have relatively strong membrane transport activity for reduced cysteine and weak or no transport activity for its relatively large oxidized derivative, cystine.
Age-related derangement of redox-regulated signals
As ROS production by various tightly regulated NAD(P)H oxidase, isoenzymes is known to play a role in many redox-sensitive signaling pathways, any increase in ROS levels and/or any oxidative shift in the thiol-disulfide redox status may cause a shift in the set points of numerous physiological processes (reviewed in Dröge, 2002). The age-related increase in the steady state level of interleukin 6 (IL-6) mRNA and IL-6 production in the brain (Ye & Johnson, 1999; Richwine et al., 2005) is only one case in point. In vivo and in vitro studies indicate that this increase results from enhanced binding of the redox-sensitive transcription factor nuclear factor κ B (NF-κB) to the IL-6 promoter in microglial cells (Ye & Johnson, 1999, 2001). The age-related increase in IL-6 is reminiscent of the systemic increase in the concentration of the inflammatory cytokine, tumor necrosis factor α (TNF-α), which is also controlled by NF-κB. The increase in TNF-α was ameliorated by treatment with the GSH prodrug N-acetyl cysteine (NAC) (reviewed in Dröge, 2005b).
Redox sensitivity of the insulin signaling pathway and the role of autophagy and sirtuin proteins
The insulin receptor is expressed throughout the brain, including the hippocampus (Dou et al., 2005; Wada et al., 2005; Benedict et al., 2006). The importance of the insulin signaling pathway in aging has been convincingly demonstrated by genetic studies of nematodes, fruit flies, and mice. In all of these species, a dramatic lifespan extension was often associated with an impairment of the insulin receptor signaling pathway or with an increased expression of sirtuin family proteins, which are negatively regulated by this signaling cascade (Kenyon et al., 1993; Dorman et al., 1995; Kimura et al., 1997; Hekimi & Guarente, 2003, Howitz et al., 2003; Rogina & Helfand, 2004; Sauve et al., 2006). A substantial lifespan extension of Caenorhabditis elegans, for example, was achieved by a mutation in daf-2, which encodes an insulin receptor ortholog. Lifespan was negatively affected by the target of rapamycin (TOR), which functions downstream in the insulin receptor signaling pathway (Kapahi et al., 2004). By activating TOR or its mammalian counterpart mTOR, the insulin receptor signaling pathway downregulates the expression of proteins of the sirtuin family and the autophagic mechanism of proteolysis (schematically illustrated in Fig. 1; reviewed in Dröge, 2005a).
Fig. 1.
Oxidative upregulation of the insulin receptor signaling cascade in the absence of insulin. Akt, serine/threonine kinase PKB; IRS-1, insulin-receptor substrate; INS-RK, insulin receptor tyrosine kinase; PDK1, phosphoinositide-dependent protein kinase-1; PI3K, phosphatidylinositol-3 kinase; PTEN, phosphatase and tensin homolog on chromosome 10; PTP1B, protein tyrosine phosphatase 1B; PI(3,4)P2, phosphatidylinositol(3,4) diphosphate; PI(4,5)P2, phosphatidylinositol(4,5) diphosphate; PI(3,4,5)P3, phosphatidylinositol (3,4,5) triphosphate; SHIP2, SH2-domain-containing inositol phosphatase; TOR/mTOR, (mammalian) target of rapamycin. The question mark indicates that H2O as a product of this reaction has not been formally proven (for other details see text).
The autophagic mechanism of proteolysis plays an important role in the maintenance of cellular integrity by removing (damaged) mitochondria and other organelles (reviewed in Dröge, 2003). Not unexpectedly, autophagy was shown to be required for lifespan extension in longevity mutants of C. elegans (Melendez et al., 2003). Mice genetically engineered to lack either of the autophagy genes Atg5 or Atg7 in neuronal cells during later stages of embryogenesis develop signs of neurodegeneration and eventually show inclusion bodies akin to those seen in various human aging-related neurodegenerative disorders (Hara et al., 2006; Komatsu et al., 2006). In line with this finding, there is ample evidence implicating proteolytic stress in a wide range of neurodegenerative diseases (Ciechanover & Brundin, 2003; Bossy-Wetzel et al., 2004). Emerging evidence supports the view that induction of autophagy is a neuroprotective response and that inadequate or defective authophagy, rather than excessive autophagy, promotes neuronal cell death in most neurodegenerative disorders (Nixon, 2006; Rubinsztein, 2006). Signs of excessive autophagy have been reported in patients with Alzheimer's disease (AD) but are uncommon in brains devoid of AD pathology (Nixon et al., 2005).
Sirtuin proteins are a family of protein deacetylases, which regulate a diverse set of pathways implicated in the aging process (reviewed in Hekimi & Guarente, 2003). Certain sirtuins were found to regulate glucose and fat metabolism in mammals (Guarente & Picard, 2005; Rodgers et al., 2005) and to enhance mitochondrial biogenesis in liver and muscle through the transcriptional coactivator peroxisome proliferator-activator receptor-γ coactivator 1α (PGC-1α) (Rodgers et al., 2005; Lerin et al., 2006), and cell survival by deactivating the tumor surpressor, p53 (Smith, 2002). In view of these multiple effects, the exact role of sirtuin proteins in the aging process remains obscure. The plant-derived polyphenol, resveratrol, was found to increase the catalytic (deacetylase) activity of certain sirtuin proteins and to extend the lifespan of yeast by 70% (Howitz et al., 2003). In the short-lived fish, Nothobranchius furzeri, resveratrol extended the maximum lifespan by 60%, delayed the decay of locomotor activity and cognitive performance, and attenuated neurofibrillary degeneration in the brain (Valenzano et al., 2006). Middle-aged mice on a high-calorie diet that were treated with resveratrol for the remainder of their life showed a significantly increased lifespan, increased postprandial insulin sensitivity, AMP-activated protein kinase (AMPK), and PGC-1α activity, increased mitochondrial numbers, and improved motor function (Baur et al., 2006). Whether sirtuin proteins similarly influence human aging is not known.
As autophagy and sirtuin expression are down-regulated by the insulin signaling cascade, it is inferred that autophagy and sirtuin activity are optimally expressed when circulating insulin levels are low, that is, in the post-absorptive state during the night (see Fig. 2). This important point remains to be confirmed. The insulin-independent basic activity of the insulin receptor signaling pathway (i.e. its activity in the post-absorptive state during the night) is weak but subject to redox regulation (see Fig. 1). The signaling cascade is negatively controlled by several phosphatases, including protein tyrosine phosphatase 1B (PTB 1B), phosphatase and tensin homolog on chromosome 10 (PTEN) and SH2-domain-containing inositol phosphatase (SHIP2), all of which are inactivated under moderately oxidative conditions. In addition, the basic insulin receptor tyrosine kinase (Ins-RK) activity is strongly increased by low concentrations of hydrogen peroxide or by an oxidative shift in the GSH redox status (Schmitt et al., 2005). In the presence of adenosine 5’-triphosphate (ATP) and hydrogen peroxide, the insulin receptor kinase domain is phosphorylated at its catalytic site and thereby rendered catalytically active in the absence of insulin (schematically illustrated in Fig. 3). The phosphatases, in turn, contain a redox-sensitive cysteine moiety in their catalytic site, which is converted by hydrogen peroxide into a sulfenic acid moiety, which renders the phosphatase inactive (Fig. 3). The balance between kinase and phosphatase activities determines the rate of phosphorylation of the insulin receptor kinase domain and several downstream targets including the phosphatidylinositol phosphates, the serine/threonine kinase Akt1 and the TOR. Ultimately, this balance controls the aging-related functions of autophagy and sirtuin proteins (Fig. 1). The redox sensitivity of the basic insulin receptor signaling activity in vivo has been confirmed in a clinical study of nondiabetic obese persons in whom basic (i.e. post-absorptive) insulin receptor signaling was decreased after supplementation with relatively small doses of NAC (Hildebrandt et al., 2004). It is therefore conceivable that autophagic proteolysis and sirtuin activity may be compromised by the oxidative conditions that prevail in old age as schematically illustrated in Fig. 2. This important prediction remains to be confirmed.
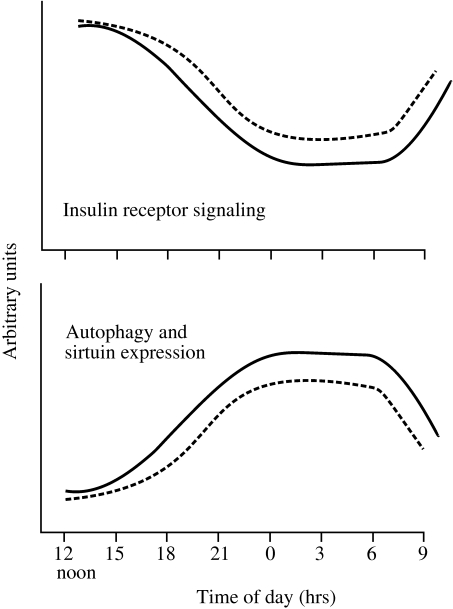
Fig. 2.
Interrelated changes of insulin receptor signaling, autophagy, and sirtuin expression during the day (hypothetical scheme). The solid lines illustrate the temporal changes in young healthy subjects. During the night, that is, in the post-absorptive state, insulin receptor signaling is low and autophagy and sirtuin activity are accordingly high. In the aging organism (dashed lines), the age-related oxidative shift in redox status leads to an increased basic signaling activity from the insulin receptor in spite of low insulin levels; autophagy and sirtuin expression are accordingly suppressed.
Fig. 3.
Oxidative activation of insulin receptor kinase and inactivation of phosphatases (schematic illustration). Upper panel: in the absence of insulin, basic insulin receptor kinase (Ins-RK) activity is relatively low. Hydrogen peroxide oxidizes cysteine sulfhydryl (SH) groups into sulfenic acid moieties (S-OH). This oxidative process allows adenosine 5’-triphosphate (ATP) to phosphorylate the insulin receptor kinase at its catalytic site and to render the kinase catalytically active. Lower panel: phosphatases typically contain an SH group at their catalytic center. Upon oxidation by hydrogen peroxide the phosphatase is converted into a catalytically inactive intermediate and other inactive derivatives. The question marks indicate that these reaction products have not been formally proven.
Effects of GSH enhancing antioxidants on oxidative damage and functional decline – suggestive evidence for a cause–effect relationship
N-acetyl cysteine is widely used both in clinical medicine and in experimental animal studies to increase the availability of cysteine and intracellular GSH, that is, the quantitatively most important cellular antioxidant. Feeding of NAC-containing pellets (0.3% w/w) to 48-week-old mice for 24 weeks caused a significant decrease in protein carbonyls in synaptic mitochondria of the brain in comparison with untreated controls (Banaclocha et al., 1997). Chronic dietary administration of NAC was also shown to preserve mitochondrial proteins involved in oxidative phosphorylation in the liver of senescent mice (Miquel et al., 1995). Feeding of NAC-containing pellets (0.3% w/w) to mice from 12 months until killed at 28 months resulted in a significant increase in ADP-stimulated respiration in brain mitochondria. In addition, NAC treatment reversed the age-related decline in cytochrome c content and the decrease in cytosolic GSH in the brain (Cocco et al., 2005).
The age-related decline in memory function was also ameliorated by NAC in a recent study on rats. Again, this was associated with diminished levels of protein oxidation and lipid peroxides in brain synaptic mitochondria (Martinez et al., 2000). Furthermore, a diet containing 7.2 mg NAC per day significantly attenuated the accelerated, age-related cognitive dysfunction observed in transgenic mice overexpressing growth hormone (Lemon et al., 2003). The excitatory amino acid carrier 1 (EAAC1) serves as a transporter for glutamate, aspartate and reduced L-cysteine. Although EAAC1 is not the only cysteine transporter, EAAC1 deficient mice (EAAC1−/–) have relatively low neuronal (hippocampal) GSH levels and, with advancing age, develop brain atrophy and behavioral changes. Increasing cysteine availability in these animals by intraperitoneal injections of 150 mg kg−1 of NAC normalized brain GSH concentrations and decreased the extent of neuronal death after oxidant exposure (Aoyama et al., 2006). Collectively, these data attest to the important contribution of the thiol redox status in the preservation of healthy cognition in aging rodents.
(R)-α-Lipoic acid (LA) is a coenzyme involved in mitochondrial redox processes. The reduced form of LA chelates iron and copper and recycles several antioxidants including GSH and vitamins C and E (Packer et al., 1997; Moini et al., 2002). Feeding of LA (0.2% w/w for 2 weeks) to 24- to 28-month-old rats reversed the age-related increase in iron levels and the age-related decrease in GSH and ascorbic acid levels in the brain (Suh et al., 2005). Several reports described positive effects of LA in combination with acetyl-L-carnitine on memory and brain mitochondrial functions in old rats (Liu et al., 2002a,b). Rats that were fed LA exhibited improved spatial learning and memory (Stoll et al., 1994).
Other experimental strategies to ameliorate oxidative damage
The radical scavenger α-phenyl-tert-butyl-nitrone (PBN) has potent salutary effects in certain aging-related diseases (reviewed in Floyd et al., 2002). Intraperitoneal administration of PBN in aged gerbils for 14 days caused a significant decrease in brain levels of oxidized protein and improved performance in a temporal and spatial memory test when compared to age-matched controls. The same treatment had no effect on young adult gerbils (Carney et al., 1991). Similar results have been obtained in mice (Fredriksson & Archer, 1996). Chronic systemic administration of synthetic catalytic scavengers of ROS (i.e. superoxide dismutase/catalase mimetics) to mice for 3 months between 8 and 11 months of age almost completely reversed not only the increase in brain oxidative damage but also the cognitive deficits (Liu et al., 2003). It remains to be determined whether these antioxidative treatments may have a GSH sparing effect and indirectly elevate cellular GSH levels.
Supportive evidence for a role of oxidative stress in aging has also been obtained in animal studies of calorie restriction. A restriction of dietary calories results in significant lifespan extension in diverse organisms (Sohal & Weindruch, 1996; Weindruch & Walford, 1997). In several independent studies of mice, rats, and fruit flies, the increased survival of calorically restricted animals was associated with a decrease in tissue oxidative damage (Hamilton et al., 2001; Sohal et al., 1994; Zheng et al., 2005). Also, in a series of long-lived strains of worms, flies, and mice, the increased lifespan was correlated with increased oxidative stress resistance (Orr & Sohal, 1994; Lin et al., 1998; Parkes et al., 1998; Honda & Honda, 1999; Lee et al., 1999; Migliaccio et al., 1999; Taub et al., 1999). Again, GSH levels have not been reported.
Putative mechanisms accounting for the age-related increase in oxidative damage: changes in antioxidant enzymes
Several authors suggested the possibility that the increase in oxidative stress may result, at least partly, from a relative decrease in antioxidant enzyme activities (Tian et al., 1998; Navarro et al., 2004; Sigueira et al., 2005). A decline in GSH peroxidase activity has been found in hippocampus and hypothalamus of aged rats (Sigueira et al., 2005). A decrease in the activities of Mn and CuZn superoxide dismutase isoenzymes and catalase has been found in the brain, heart, liver, and kidney of aging mice (Gupta et al., 1991; Navarro et al., 2004). A decrease in the apparent catalytic turnover of γ-glutamyl cysteine ligase (GCL) and/or GCL subunit expression has been found in old rats (Liu, 2002). However, it should be noted that an age-related decline in brain antioxidant enzyme activities has not been confirmed by other investigators and remains controversial (Serrano & Klann, 2004).
Oxidative mitochondrial damage as part of a vicious cycle leading to increased ROS production
Mitochondria are widely believed to play a key role in aging because they are a major source of oxygen radicals and arguably their most important targets. Mitochondrial DNA is particularly vulnerable to oxidative damage and shows a more than ten-fold greater mutation rate than nuclear DNA. Mutated mitochondrial DNA may code for abnormal cytochromes and may cause infidelity of the electron transport chain associated with increased superoxide radical production and a vicious cycle of progressively increasing oxidative stress (Linnane et al., 1992; Finkel & Holbrook, 2000; Sohal et al., 2002; Hekimi & Guarente, 2003; Sastre et al., 2003; Vina et al., 2003; Navarro & Boveris, 2004; Schipper, 2004; Balaban et al., 2005). Moreover, accumulation of peroxidation products in mitochondria leads to a decrease in ATP production and compromises the maintenance of cellular homeostasis (Chance et al., 1979). Mitochondrial dysfunction and mitochondria-derived ROS have been implicated in both normal brain aging and neurodegenerative diseases (Toescu & Verkhratsky, 2003; Lin & Beal, 2006). A study of rat brain mitochondria also revealed an age-related increase in mitochondrial fragility, volume, and water permeability (Navarro & Boveris, 2004). In the mammalian central nervous system (CNS), cellular senescence is often associated with the accumulation of mitochondria-derived cytoplasmic inclusions such as Gomori-positive glial granules and corpora amylacea (Brunk & Terman, 2002; Schipper, 2004). Evidence supporting the free radical-mitochondrial theory of aging has accrued from a broad range of in vitro studies and whole animal models from unicellular organisms to mammals. However, the theory is not immune from controversy and some investigators maintain that aging exerts no adverse effect on either the electron transport chain or oxidative phosphorylation (Maklashina & Ackrell, 2004).
Dysregulation of iron homeostasis as a potential cause of oxidative stress
Free iron is a potential source of oxidative stress because it catalyses the conversion of hydrogen peroxide into highly reactive hydroxyl radicals by the Fenton reaction. In addition, iron-dependent lipid peroxidation may also generate potentially toxic peroxyl/alkoxyl radicals (Gutteridge, 1982), and ferrous free iron may convert neutral catechols (e.g. dopamine) to neurotoxic o-semiquinone intermediates (Schipper, 2004). An age-related increase in iron content has been found in human brain tissue (Martin et al., 1998) as well as rat and mouse brain (Cook & Yu, 1998; Sohal et al., 1999; Donahue et al., 2006). In rats the concentrations of several metals, including iron, increase in the hippocampus and frontal cortex (Donahue et al., 2006). In the aging human brain, excessive iron accumulation associated with markers of free radical injury and mitochondrial DNA deletion are most prominent in the basal ganglia, hippocampus, and certain cerebellar nuclei (Soong et al., 1992). In rat astrocytes hydrogen peroxide was found to induce the heme-degrading enzyme heme oxygenase 1 (HO-1), which fosters the liberation of free cytosolic Fe++ (Mydlarski et al., 1993). HO-1 may therefore exacerbate brain aging processes by engaging in a neuropathological vicious cycle of ROS generation (Schipper, 2004). The most common cell type in the brain to stain for iron under normal conditions is the oligodendrocyte (Connor & Menzies, 1999). In humans, an age-related increase in iron staining and ferritin immunoreactivity was found in microglia and astrocytes in various brain regions including the hippocampus (Zecca et al., 2004). Thus, aging-related oxidative stress may participate in a vicious cycle of events characterized by dysregulation of cellular iron homeostasis, mitochondrial insufficiency, bioenergetic failure and augmented ROS generation by the electron transport chain. Iron chelators, free radical scavengers and inhibitors of glial HO-1 expression, alone or in combination, may be useful in the management of aging-related human neurodegenerative afflictions (Schipper, 2004). The observation that brain iron levels can be reduced in old rats by oral administration of the blood-brain barrier-permeable chelator, LA (Suh et al., 2005) may be an important step in this direction.
Dysregulation of calcium homeostasis and oxidative stress
Another potential vicious cycle may involve changes in the cytosolic and mitochondrial calcium concentration (Toescu & Verkhratsky, 2003; Foster, 2006). Well-controlled temporary elevations of cytosolic calcium concentrations play a role in numerous cellular signaling systems and are typically induced by the release of calcium from the endoplasmic reticulum (ER) (reviewed in Toescu & Verkhratsky, 2003). A growing body of evidence suggests that the calcium channels, which control calcium efflux from the ER in response to different biochemical signals, are also sensitive to small changes in ROS concentrations or changes in the intracellular thiol redox status, suggesting that these calcium channels serve as physiological redox sensors (Okabe et al., 2000; Pessah & Feng, 2000; reviewed in Camello-Almaraz et al., 2006; Zima & Blatter, 2006). Effects of hydrogen peroxide on calcium fluxes have been demonstrated in rat hippocampal cells, cortical astrocytes, and dentate granule cells (Jacobson & Duchen, 2002; Akaishi et al., 2004; Gonzalez et al., 2006). Robust elevation of cytosolic calcium causes calcium flux into the mitochondrial matrix, which, in turn, enhances mitochondrial ROS production, thereby completing the vicious cycle (Van de Water et al., 1994; Bindokas et al., 1996). Increased mitochondrial calcium concentrations combined with increased ROS production are characteristic of neurodegenerative conditions, such as AD and ischemic brain injury (Toescu & Verkhratsky, 2003; Starkov et al., 2004). It has been suggested that calcium-dependent mitochondrial ROS production may also play a role in normal cellular physiology (see Hongpaison et al., 2003). There is a strong possibility that the age-related oxidative shift in redox status may cause subpathological changes in calcium homeostasis and especially changes in the kinetics of calcium fluctuations (see Toescu & Verkhratsky, 2003). Whether these, in turn, may have a substantial effect on mitochondrial ROS production remains to be ascertained.
Age-related decrease in plasma cysteine concentration
Neurons exhibit relatively strong membrane transport activity for the small amino acid, cysteine but weak transport activity for its larger oxidized derivative cystine. Therefore, and because the plasma concentration of reduced cysteine is extremely low in comparison to most other amino acids, cysteine is the limiting amino acid for GSH biosynthesis in neural and most other cells (Bannai & Tateishi, 1986; Aoyama et al., 2006). Clinical studies have shown that the post-absorptive plasma cysteine concentration in healthy human subjects decreases significantly between the third and ninth decade of life (Hack et al., 1998; Dröge, 2005b). Supplementation with NAC or a cysteine-rich protein has been shown to improve muscular strength and other functional and biochemical parameters, which are typically compromised in old age (Hauer et al., 2003; reviewed in Dröge, 2005a,b).
Why the 70-year-old seemingly healthy subject has, on average, a substantially lower post-absorptive plasma concentration of reduced cysteine than a 20-year-old person with the same dietary protein intake remains enigmatic. The basic biochemical mechanisms of cysteine biosynthesis, utilization, and catabolism are well known, and yet the mechanisms responsible for the age-related changes in cysteine homeostasis remain obscure. These changes may be related to the facts that (i) in the postprandial state, a substantial proportion of the dietary cysteine is normally converted into hepatic GSH and proteins, which serve as a reservoir for cysteine (Cho et al., 1984; Cheema-Dhadli & Halperin, 1993), and (ii) the rate of postprandial protein synthesis per time unit decreases with age (Volpi et al., 2000; Dardevet et al., 2002; Combaret et al., 2005; Fujita & Volpi, 2006). As the hepatic concentration of free cysteine is a key regulator of its own catabolism into sulfate (Stipanuk et al., 1992; Lee et al., 2004), we tentatively assume that the post-absorptive cysteine concentration in old age decreases as a consequence of the age-related decrease in postprandial protein synthetic capacity and the resulting failure to rapidly clear dietary cysteine before it is catabolized into sulfate. To understand and to reverse these changes in cysteine homeostasis may be a worthwhile and achievable goal in the near future.
Conclusions
There is an impressive body of evidence (i) for an age-related increase in oxidative stress in both humans and experimental animals, (ii) for the redox sensitivity of the insulin signaling cascade as demonstrated by numerous molecular studies, and (iii) for the dramatic impact of the insulin receptor signaling cascade and insulin-controlled sirtuin proteins on the lifespan of nematodes, fruit flies, and mice as demonstrated by genetic studies. There is therefore a strong possibility that oxidative enhancement of the insulin receptor signaling pathway may have an even greater impact on aging than the direct oxidative damage to structural constituents. In spite of the persuasive mosaic of data, there are still many information gaps especially regarding the extrapolation of animal data to humans. With regard to insulin-controlled, aging-related functions, there is suggestive evidence that inadequate autophagic proteolysis does play a role including in human aging, but we still do not know whether sirtuins or resveratrol are capable of influencing fundamental aging processes in humans. Also, the predicted effects of the age-related oxidative stress on autophagy and sirtuin activity as well as the corresponding putative effects of antioxidants have not yet been demonstrated experimentally.
The notion that oxidative stress plays a causative role in age-related functional decline is not proven but it is already strongly supported by the effects of antioxidants on various correlates of aging. This review has been somewhat biased in favor of thiol containing and GSH enhancing antioxidants and may not have done justice to other types of antioxidants and ROS scavengers. One of the reasons for this bias is the large number of reports concerning the effects of NAC and the thiol compound α-LA. In patients with AD, NAC was shown to improve certain cognitive tasks (Adair et al., 2001). In our view, understanding and reversing age-related changes in plasma thiol (cysteine) homeostasis is an achievable goal with important implications for the amelioration of a broad spectrum of neurological and systemic conditions linked to the aging process.
The abundant evidence for an age-related increase in products of oxidative damage in the mammalian brain is in good agreement with findings in other tissues (reviewed in Beckman & Ames, 1998; Dröge, 2002). ROS-mediated mitochondrial damage, the increase in iron deposition, dysregulation of Ca++ homeostasis and post-absorptive plasma cysteine homeostasis, and impaired autophagy and sirtuin activity secondary to aberrant insulin receptor signaling may represent several pivotal mechanisms in a vicious cycle leading to progressively increasing intracellular ROS concentrations and oxidative stress.
Taken together, the evidence suggests that aging is not mediated by a single gene or by a single ‘master mechanism’ of oxidative damage but may rather result from progressive dysregulation in a complex functional network as illustrated schematically in Fig. 4. In thermodynamic terms this reflects an increase in entropy. Similar conclusions were recently expressed by Drachman (2006) with respect to the multiplicity of cellular and molecular mechanisms that contribute to senescence in the human brain. Certain genes or certain biochemical processes may, nevertheless, contribute more than others. If this concept is basically correct, a cocktail of multiple interventions that simultaneously targets several of the most important nodes in this network may be required to limit age-related dyshomeostasis.
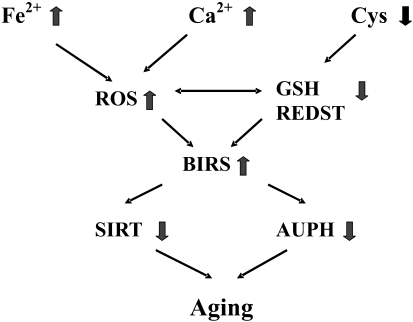
Fig. 4.
Pathomechanisms of aging: deregulation at key ‘nodes’ in a network of interrelated physiological processes. AUPH, autophagic mechanism of proteolysis involved in the turnover of (damaged) mitochondria and other cellular constituents. Autophagy is negatively regulated by insulin. BIRS, basal activity of the insulin receptor signaling pathway, that is, in the post-absorptive state.Ca2+, mitochondrial calcium concentrations (calcium homeostasis); Cys, post-absorptive plasma cysteine concentration (cysteine homeostasis); Fe2+, nontransferrin iron concentration (iron homeostasis); GSH, intracellular glutathione concentrations; REDST, glutathione redox status; ROS, intracellular reactive oxygen species; SIRT, sirtuin proteins impact lifespan and are negatively regulated by insulin.
Acknowledgments
We thank Ms. Clare Malbon and Mrs. Adrienne Liberman for excellent assistance with the preparation of this manuscript.
References
- Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer's disease. Neurology. 2001;57:1515–1517. doi: 10.1212/wnl.57.8.1515. [DOI] [PubMed] [Google Scholar]
- Akaishi T, Nakazawa K, Sato K, Saito H, Ohno Y, Ito Y. Hydrogen peroxide modulates whole cell Ca2+ currents through L-type channels in cultured rat dentate granule cells. Neurosci. Lett. 2004;356:25–28. doi: 10.1016/j.neulet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chem W, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Balu M, Sangeetha P, Murali G, Panneerselvam C. Age-related oxidative protein damages in central nervous system of rats: modulatory role of grape seed extract. Int. J. Devl. Neuroscience. 2005;23:501–507. doi: 10.1016/j.ijdevneu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Banaclocha MM, Hernandez AI, Martinez N, Ferrandiz ML. N-Acetylcysteine protects against age-related increase in oxidized proteins in mouse synaptic mitochondria. Brain Res. 1997;762:256–258. doi: 10.1016/s0006-8993(97)00493-9. [DOI] [PubMed] [Google Scholar]
- Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J. Membr. Biol. 1986;89:1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- Bartus RT. Drugs to treat age-related neurodegenerative problems. The final frontier of medical science? J. Am. Geriat Soc. 1990;38:680–695. doi: 10.1111/j.1532-5415.1990.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kaira A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Benedict L, Nelson CA, Schunk E, Sullwold K, Seaquist ER. Effect of insulin on the brain activity obtained during visual and memory tasks in healthy human subjects. Neuroendocrinology. 2006;83:20–26. doi: 10.1159/000093338. [DOI] [PubMed] [Google Scholar]
- Berr C, Balansard B, Arnaud J. Cognitive decline is associated with systemic oxidative stress: the EVA study. Etude du Viellissement Arteriel. J. Am. Geriatr. Soc. 2000;48:1285–1291. doi: 10.1111/j.1532-5415.2000.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Bickford P. Motor learning deficits in aged rats are correlated with loss of cerebellar noradrenergic function. Brain Res. 1993;620:133–138. doi: 10.1016/0006-8993(93)90279-v. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordan J, Lee CC. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat. Med. 2004;10(Suppl.):S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of ageing: accumulation of damaged mitrochondria as a result of imperfect autophagocytosis. Eur. J. Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Guffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech. Ageing Dev. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-α-phenylnitrone. Proc. Natl Acad. Sci. USA. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Cheema-Dhadli S, Halperin ML. Relative rates of appearance of nitrogen and sulphur: implications for postprandial synthesis of proteins. Can. J. Physiol. Pharmacol. 1993;71:120–127. doi: 10.1139/y93-017. [DOI] [PubMed] [Google Scholar]
- Chen TS, Richie JP, Lang CA. The effect of aging on glutathione and cysteine levels in different regions of the mouse brain. Proc. Soc. Exp. Biol. Med. 1989;190:399–402. doi: 10.3181/00379727-190-42879. [DOI] [PubMed] [Google Scholar]
- Cho ES, Johnson N, Snider BCF. Tissue glutathione as a cyst(e)ine reservoir during cystine depletion in growing rats. J. Nutr. 1984;114:1853–1862. doi: 10.1093/jn/114.10.1853. [DOI] [PubMed] [Google Scholar]
- Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol. Aging. 1995;16:53–57. doi: 10.1016/0197-4580(95)80007-e. [DOI] [PubMed] [Google Scholar]
- Cocco T, Sgobbo P, Clemente M, Lopriore B, Grattagliano I, Di Paola M, Villani G. Tissue-specific changes of mitochondrial functions in aged rats: effect of a long-term dietary treatment with N-acetylcysteine. Free Radic. Biol. Med. 2005;38:796–805. doi: 10.1016/j.freeradbiomed.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Rieu I, Pouch M-N, Béchet D, Taillandier D, Grizard J, Attaix D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J. Physiol. 2005;569:489–499. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Cellular management of iron in the brain. J. Neurol. Sci. 1999;134:33–44. doi: 10.1016/0022-510x(95)00206-h. [DOI] [PubMed] [Google Scholar]
- Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech. Ageing Dev. 1998;102:1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J. Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol. Learn. Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- Donahue AN, Aschner M, Lash LH, Syversen T, Sonntag WE. Growth hormone administration to aged animals reduces disulfide glutathione levels in hippocampus. Mech. Ageing Dev. 2006;127:57–63. doi: 10.1016/j.mad.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Dorman JB, Albinder B, Shroyer T, Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics. 1995;141:1399–1406. doi: 10.1093/genetics/141.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou J-T, Chem N, Dufour F, Alkon DL, Zhao W-Q. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn. Mem. 2005;12:646–655. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67:1340–1352. doi: 10.1212/01.wnl.0000240127.89601.83. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dröge W. Autophagy and aging – importance of amino acid levels. Mech. Ageing Dev. 2003;225:161–168. doi: 10.1016/j.mad.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Dröge W. Oxidative aging and insulin receptor signaling. J. Gerontol. A, Biol. Sci. 2005a;60:1378–1385. doi: 10.1093/gerona/60.11.1378. Med. Sci. [DOI] [PubMed] [Google Scholar]
- Dröge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos. Trans. R. Soc. B: Biol. Sci. 2005b;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K, Forster MJ, Kelleher-Anderson JA, Wood PL. Nitrones as Neuroprotectants and Antiaging Drugs. Ann. N. Y. Acad. Sci. 2002;959:321–329. doi: 10.1111/j.1749-6632.2002.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl Acad. Sci. USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res. Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Foster TC. Biological markers of age-related memory deficits. Treatment of senescent physiology. CNS Drugs. 2006;20:153–166. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Archer T. α-phenyl-tert-butyl-nitrone (PBN) reverses age-related maze learning performance and motor activity deficits in C57 BL/6 mice. Behav. Pharmacol. 1996;7:245–253. [PubMed] [Google Scholar]
- Fujita S, Volpi E. Amino Acids and Muscle Loss with Aging. J. Nutr. 2006;136:277S–280S. doi: 10.1093/jn/136.1.277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Landfield PW, McEwen B. Hippocampal neurodegeneration in aging. Science. 1996;274:484–485. doi: 10.1126/science.274.5287.484. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Granados MP, Pariente JA, Salido GM. H2O2 mobilizes Ca2+ from agonist- and thapsigargin-sensitive and insensitive intracellular stores and stimulates glutamate secretion in rat hippocampal astrocytes. Neurochem. Res. 2006;31:741–750. doi: 10.1007/s11064-006-9078-y. [DOI] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction – SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Gupta A, Hasan M, Chander R, Kapoor NK. Age-related elevation of lipid peroxidation products: diminution of superoxide dismutase activity in the central nervous system of rats. Gerontology. 1991;37:305–309. doi: 10.1159/000213277. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC. The role of superoxide and hydroxyl radicals in phospholipid peroxidation catalysed by iron salts. FEBS Lett. 1982;150:454–458. doi: 10.1016/0014-5793(82)80788-6. [DOI] [PubMed] [Google Scholar]
- Hack V, Breitkreutz R, Kinscherf R, Röhrer H, Bartsch P, Taut F, Benner A, Dröge W. The redox state as a correlate of senescence and wasting and as a target for therapeutic intervention. Blood. 1998;92:59–67. [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc. Natl Acad. Sci. USA. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hauer K, Hildebrandt W, Sehl Y, Edler L, Oster P, Dröge W. Improvement in muscular performance and decrease in tumor necrosis factor level in old age after antioxidant treatment. J. Mol. Med. 2003;81:118–125. doi: 10.1007/s00109-002-0406-7. [DOI] [PubMed] [Google Scholar]
- Hekimi S, Guarente L. Genetics and the specificity of the ageing process. Science. 2003;299:1351–1354. doi: 10.1126/science.1082358. [DOI] [PubMed] [Google Scholar]
- Hildebrandt W, Hamann A, Krakowski-Roosen H, Kinscherf R, Dugi K, Sauer R, Lacher S, Nöbel N, Bodens A, Bellou V, Edler L, Nawroth P, Dröge W. Effect of thiol antioxidant on body fat and insulin reactivity. J. Mol. Med. 2004;82:336–344. doi: 10.1007/s00109-004-0532-5. [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Hongpaison J, Winters CA, Andrews SB. Calcium-dependent mitochondrial superoxide modulates nuclear CREB phosphorylation in hippocampal neurons. Mol. Cell. Neurosci. 2003;24:1103–1115. doi: 10.1016/j.mcn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Chung JP, Kisielewski A, Zhang L-L, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisia. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Iantomasi T, Favilli F, Marraccini P, Stio M, Treves C, Quatrone A, Capaccioli S, Vincenzini MT. Age and GSH metabolism in rat cerebral cortex, as related to oxidative and energy parameters. Mech. Ageing Dev. 1993;70:65–82. doi: 10.1016/0047-6374(93)90060-5. [DOI] [PubMed] [Google Scholar]
- Jacobson J, Duchen MR. Mitochondrial oxidative stress and cell death in astrocytes – requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell. Sci. 2002;115:1175–1188. doi: 10.1242/jcs.115.6.1175. [DOI] [PubMed] [Google Scholar]
- Joseph JA. The putative role of free radicals in the loss of neuronal functioning in senescence. Integ. Physiol. Behav. Sci. 1992;27:216–227. doi: 10.1007/BF02690894. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan by TOR signaling pathway in Drosophila. Curr. Biol. 2004;14:885–891. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. Daf-2, an insulin receptor-line gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kluger A, Gianutsos JG, Golomb J, Ferris SH, Goerge AE, Frannssen E, Reisberg B. Patterns of motor impairment in normal aging, mild cognitive decline, and early Alzheimer's disease. J. Gerontol. 1997;52:38–39. doi: 10.1093/geronb/52b.1.p28. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J-I, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Lee C-K, Klopp RG, Weindruch R, Prolla TA. Gene Expression Profile of Aging and Its Retardation by Caloric Restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee JI, Londono M, Hirschberger LL, Stipanuk MH. Regulation of cysteine dioxygenase and gamma-glutamylcysteine synthetase is associated with hepatic cysteine level. J. Nutr. Biochem. 2004;15:112–122. doi: 10.1016/j.jnutbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lemon JA, Boreham DR, Rollo CD. A dietary supplement abolishes age-related cognitive decline in transgenic mice expressing elevated free radical processes. Exp. Biol. Med. 2003;228:800–810. doi: 10.1177/15353702-0322807-05. [DOI] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin Y-J, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant Methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Zhang C, Baumer A, Nagley P. Mitochondrial DNA mutation and the ageing process: bioenergy and pharmacological intervention. Mutat. Res. 1992;275:195–208. doi: 10.1016/0921-8734(92)90023-i. [DOI] [PubMed] [Google Scholar]
- Liu R-M. Down-regulation of γ-glutamyl cysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J. Neurosci. Res. 2002;68:344–351. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- Liu J, Atmna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann. N. Y. Acad. Sci. 2002a;959:133–166. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Head G, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carntine and/or R-α-lipoic acid. Proc. Natl Acad. Sci. USA. 2002b;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl Acad. Sci. USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklashina E, Ackrell BAC. Is defective electron transport at the hub of aging? Aging Cell. 2004;3:21–27. doi: 10.1111/j.1474-9728.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- Martin WR, Ye FQ, Allen PS. Increasing striatal iron content associated with normal aging. Mov. Disord. 1998;13:281–286. doi: 10.1002/mds.870130214. [DOI] [PubMed] [Google Scholar]
- Martinez M, Hermandez AI, Martinez N. N-acetylcysteine delays age-associated memory impairment in mice: role in synaptic mitochondria. Brain Res. 2000;855:100–106. doi: 10.1016/s0006-8993(99)02349-5. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldis P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66she adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–319. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Miquel J, Ferrandiz ML, De Juan E, Sevila I, Martinez M. N-acetylcysteine protects against age-related decline of oxidative phosphorylation in liver mitochondria. Eur. J. Pharmacol. 1995;292:333–335. doi: 10.1016/0926-6917(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Mohsen MMAE, Iravani MM, Spencer JPE, Rose S, Fahim AT, Motawi TMK, Ismail NAF, Jenner P. Age-associated changes in protein oxidation and proteasome activities in rat brain: Modulation by antioxidants. Biochemical & Biophysical Res. Communications. 2005;336:386–391. doi: 10.1016/j.bbrc.2005.07.201. [DOI] [PubMed] [Google Scholar]
- Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of α-lipoic acid and dihydrolipoic acid. Toxicol. Appl. Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 β is a common trigger for age- and stress-induced impairments in long-term potentiation. J. Neurosci. 1998b;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydlarski MB, Liang JJ, Schipper HM. Role of the cellular stress response in the biogenesis of cysteamine-induced astrocytic inclusions in primary culture. J. Neurochem. 1993;61:1755–1765. doi: 10.1111/j.1471-4159.1993.tb09813.x. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1244–R1249. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Nurosci. 2006;29:528–535. doi: 10.1016/j.tins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- O'Donnell E, Lynch MA. Dietary antioxidant supplementation reverses age-related neuronal changes. Neurobiol. Aging. 1998;19:461–467. doi: 10.1016/s0197-4580(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Okabe E, Tsujimoto Y, Kobayashi Y. Calmodulin and cyclic ADP-ribose interaction in Ca2+ signaling related to cardiac sarcoplasmic reticulum: superoxide anion radical-triggered Ca2+ release. Antioxid. Redox Signal. 2000;2:47–54. doi: 10.1089/ars.2000.2.1-47. [DOI] [PubMed] [Google Scholar]
- Oliveira MGM, Bueno OFA, Pomarico AC, Gugliano EB. Strategies used by hippocampal- and caudate-putamen-lesioned rats in a learning task. Neurobiol. Learn. Mem. 1997;68:32–41. doi: 10.1006/nlme.1996.3761. [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Oubidar M, Boquillon M, Bouvier MC, Beley A, Bralet J. Effect of intracellular iron loading on lipid peroxidation of brain slices. Free Radic. Biol. Med. 1996;21:763–769. doi: 10.1016/0891-5849(96)00173-6. [DOI] [PubMed] [Google Scholar]
- Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radic. Biol. Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- Pallardo FV, Asensi M, Garcia de la Asuncion J, Anton V, Lloret A, Sastre J, Vina J. Late onset administration of oral antioxidants prevents age-related loss of motor co-ordination and brain mitochondrial DNA damage. Free Radic. Res. 1999;28:617–623. doi: 10.1080/10715769800300671. [DOI] [PubMed] [Google Scholar]
- Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat. Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- Perkins AJ, Hendrie HC, Callahan CM. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination survey. Am. J. Epidemiol. 1999;150:37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- Perrig WJ, Perrig P, Stahelin HB. The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc. 1997;45:18–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Feng W. Functional role of hyperreactive sulfhydryl moieties within the ryanodine receptor complex. Antioxid. Redox. Signal. 2000;2:17–25. doi: 10.1089/ars.2000.2.1-17. [DOI] [PubMed] [Google Scholar]
- Poon HF, Calabrese V, Calvani M, Butterfield DA. Proteomics Analyses of Specific Protein Oxidation and Protein Expression in Aged Rat Brain and Its Modulation by L-Acetylcarnitine: Insights Into the Mechanisms of Action of This Proposed Therapeutic Agent for CNS Disorders Associated with Oxidative Stress. Antioxid. Redox. Signal. 2006;8:381–394. doi: 10.1089/ars.2006.8.381. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of Age and Caloric Restriction on Glutathione Redox State in Mice. Free Radical Biology & Medicine. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richwine AF, Godbout JP, Berg BM, Chen J, Escobar J, Millard DK, Johnson RW. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain, Behav, Immun. 2005;19:512–520. doi: 10.1016/j.bbi.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rinaldi P, Polidori MC, Metasstasio A. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol. Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Cygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl Acad. Sci. USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Kaur G. Alterations in oxidative stress scavenger system in aging rat brain and lymphocytes. Biogerontology. 2002;3:161–173. doi: 10.1023/a:1015643107449. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Senda M, Kim S-N, Kojima S, Kubodera A. Age-related changes of glutathione content, glucose transport and metabolism, and mitochondrial electron transfer function in mouse brain. Nucl. Med. Biol. 2001;28:25–31. doi: 10.1016/s0969-8051(00)00180-3. [DOI] [PubMed] [Google Scholar]
- Sastre J, Pallardo FV, Vina J. The role of mitochondrial oxidative stress in aging. Free Radic. Biol. Med. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu. Rev. Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res. Rev. 2004;3:265–301. doi: 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Schmitt TL, Hotz-Wagenblatt A, Klein H, Dröge W. Interdependent regulation of insulin receptor kinase activity by ADP and hydrogen peroxide. J. Biol. Chem. 2005;280:3795–3801. doi: 10.1074/jbc.M410352200. [DOI] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res. Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sigueira IR, Fochesatto C, Lucena da Silva Torres I, Dalmaz C, Netto CA. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005;78:271–278. doi: 10.1016/j.lfs.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Smith J. Human Sir2 and the ‘silencing’ of p53 activity. Trends Cell Biol. 2002;12:404–406. doi: 10.1016/s0962-8924(02)02342-5. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lai H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of ageing: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sohal R, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–67. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Wennberg-Kirch E, Jaiswal K, Kwong LK, Forster MJ. Effect of age and caloric restriction on bleomycin-chelatable and nonheme iron in different tissues of C57BL/6 mice. Free Radic. Biol. Med. 1999;27:287–293. doi: 10.1016/s0891-5849(99)00052-0. [DOI] [PubMed] [Google Scholar]
- Soong NW, Hinton DR, Cortopassi G, Arnheim N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat. Genet. 1992;2:318–323. doi: 10.1038/ng1292-318. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Coloso RM, Garcia RA, Banks MF. Cysteine concentration regulates cysteine metabolism to glutathione, sulfate and taurine in rat hepatocytes. J. Nutr. 1992;122:420–427. doi: 10.1093/jn/122.3.420. [DOI] [PubMed] [Google Scholar]
- Stoll S, Rostock A, Bartsch R, Korn E, Meichelbock A, Muller WE. The potent free radical scavenger α-lipoic acid improves cognition in rodents. Ann. N. Y. Acad. Sci. 1994;717:122–128. doi: 10.1111/j.1749-6632.1994.tb12079.x. [DOI] [PubMed] [Google Scholar]
- Suh JH, Moreau R, Heath S-HD, Hagen TM. Dietary supplementation with (R)-α-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep. 2005;10:52–59. doi: 10.1179/135100005X21624. [DOI] [PubMed] [Google Scholar]
- Suh JH, Wang H, Liu R-M, Liu J, Hagena TM. (R)-α-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for zGSH synthesis. Arch. Biochem. Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub J, Lau JF, Ma C, Hahn JH, Hoque R, Rothblatt J, Chalfie M. A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and elk-1 mutants. Nature. 1999;399:162–166. doi: 10.1038/20208. [DOI] [PubMed] [Google Scholar]
- Tian L, Cai Q, Wei H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic. Biol. Med. 1998;24:1477–1484. doi: 10.1016/s0891-5849(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. Neuronal ageing from an intraneuronal perspective: roles of endoplasmic reticulum and mitochondria. Cell Calcium. 2003;34:311–323. doi: 10.1016/s0143-4160(03)00142-8. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Van de Water B, Zoeteweij JP, de Bont HJGM, Mulder GJ, Nagelkerke JF. Role of mitochondrial Ca2+ in the oxidative stress-induced dissipation of the mitochondrial membrane potential. J. Biol. Cehm. 1994;269:14546–14552. [PubMed] [Google Scholar]
- Vina J, Sastre J, Pallardo F, Borras C. Mitochondrial theory of aging: importance to explain why females live longer than males. Antioxid. Redox. Signal. 2003;5:549–556. doi: 10.1089/152308603770310194. [DOI] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A, Yokoo H, Yanagita T, Kobayashi H. New twist on neuronal insulin receptor signaling in health, disease, and therapeutics. J. Pharmacol. Sci. 2005;99:128–143. doi: 10.1254/jphs.crj05006x. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu H, Liu R-M. Gender difference in glutathione metabolism during aging in mice. Exp. Gerontol. 2003;38:507–517. doi: 10.1016/s0531-5565(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. Caloric intake and aging. N. Engl. J. Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J. Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor κ-B. J. Neuroimmunol. 2001;117:87–96. doi: 10.1016/s0165-5728(01)00316-2. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. 2004b;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zheng J, Mutcherson R, II, Helfand SL. Calorie restriction delays lipid oxidative damage in Drosophila melanogaster. Aging Cell. 2005;4:209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Carvey PM, Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006;1090:35–44. doi: 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]