Abstract
Evidence accumulated over more than two decades has implicated Ca2+ dysregulation in brain aging and Alzheimer's disease (AD), giving rise to the Ca2+ hypothesis of brain aging and dementia. Electrophysiological, imaging, and behavioral studies in hippocampal or cortical neurons of rodents and rabbits have revealed aging-related increases in the slow afterhyperpolarization, Ca2+ spikes and currents, Ca2+ transients, and L-type voltage-gated Ca2+ channel (L-VGCC) activity. Several of these changes have been associated with age-related deficits in learning or memory. Consequently, one version of the Ca2+ hypothesis has been that increased L-VGCC activity drives many of the other Ca2+-related biomarkers of hippocampal aging. In addition, other studies have reported aging- or AD model-related alterations in Ca2+ release from ryanodine receptors (RyR) on intracellular stores. The Ca2+-sensitive RyR channels amplify plasmalemmal Ca2+ influx by the mechanism of Ca2+-induced Ca2+ release (CICR). Considerable evidence indicates that a preferred functional link is present between L-VGCCs and RyRs which operate in series in heart and some brain cells. Here, we review studies implicating RyRs in altered Ca2+ regulation in cell toxicity, aging, and AD. A recent study from our laboratory showed that increased CICR plays a necessary role in the emergence of Ca2+-related biomarkers of aging. Consequently, we propose an expanded L-VGCC/Ca2+ hypothesis, in which aging/pathological changes occur in both L-type Ca2+ channels and RyRs, and interact to abnormally amplify Ca2+ transients. In turn, the increased transients result in dysregulation of multiple Ca2+-dependent processes and, through somewhat different pathways, in accelerated functional decline during aging and AD.
Keywords: CICR, hippocampus, imaging, IP3, L-type Ca2+ channels, ryanodine receptor
Introduction
It has been over 20 years since it was initially proposed that altered Ca2+ regulation might play a role in brain aging and Alzheimer's disease (AD) (Landfield, 1983, 1987; Khachaturian, 1984, 1989; Gibson & Peterson, 1987; Disterhoft et al., 1994). In brain neurons from aging rodents and rabbits, as compared to neurons from younger animals, Ca2+ influx associated with action potentials induces a larger Ca2+-dependent afterhyperpolarization (AHP) (Landfield & Pitler, 1984; Kerr et al., 1989; Moyer et al., 1992; Potier et al., 1992; Disterhoft et al., 1996, 2004; Stutzmann et al., 2006) and impairs short-term synaptic plasticity (Landfield et al., 1986; Thibault et al., 2001). Furthermore, these findings have been reinforced by studies showing that pharmacologically isolated Ca2+ action potentials (Pitler & Landfield, 1990; Disterhoft et al., 1996), whole-cell Ca2+ currents (Campbell et al., 1996), and Ca2+ transients during repetitive spike trains also are larger in hippocampal neurons from aged animals (Thibault et al., 2001; Hemond & Jaffe, 2005). Conversely, Ca2+ influx via ligand-gated N-methyl-D-aspartate (NMDA) receptor channels appears reduced in aged animals (Barnes et al., 1997; Magnusson, 1998; Shankar et al., 1998).
Our studies on this general Ca2+ dysregulation hypothesis have focused primarily on apparent excess Ca2+ influx via voltage-gated Ca2+ channels (VGCC) (Landfield, 1996; Thibault et al., 1998). Studies of the L-type VGCC (L-VGCC) antagonist suggested that the aging-related increase in Ca2+-mediated responses might depend on greater activity through L-VGCC (Moyer et al., 1992; Campbell et al., 1996). Increased L-VGCC activity with aging was confirmed directly by single channel recording in partially dissociated hippocampal slices (Thibault & Landfield, 1996). Moreover, changes in L-VGCCs appear to be functionally relevant, as L-VGCC antagonists improve learning and memory in aged animals (Deyo et al., 1989; Disterhoft et al., 2004) and some AD patients (Forette et al., 2002). Furthermore, the increase in L-VGCC density is positively correlated with cognitive impairment in aged animals (Thibault & Landfield, 1996).
In addition to the accumulating evidence of increased Ca2+ influx through L-VGCCs, there is also recent evidence that altered function of intracellular organelles might play a critical role in Ca2+ regulation during aging or AD (Toescu & Verkhratsky, 2003). In particular, changes in intracellular Ca2+ release from the endoplasmic reticulum (ER) appear likely to contribute to brain Ca2+ dyshomeostasis, and have been associated with changes in [Ca2+]i. Therefore, in this review, we summarize several lines of evidence implicating altered release from intracellular stores in aging and AD, and attempt to integrate this evidence with the role of Ca2+ influx in aging-related Ca2+ dysregulation.
Interactions between L-VGCCs and Ca2+-induced Ca2+ release from the endoplasmic/sarcoplasmic reticulum
Several comprehensive reviews have recently considered mechanisms associated with Ca2+ sequestration and release by the ER in both peripheral cells (Bootman et al., 2001; Berridge, 2002; Carafoli, 2002; Fill & Copello, 2002) and in neurons (Paschen & Mengesdorf, 2005; Verkhratsky, 2005). Accordingly, only the points most relevant to ER function in brain aging are briefly recapitulated here. Two distinct intracellular Ca2+ release channels are present in several types of muscle and brain cells, the inositol 1,4,5-trisphospate receptor (IP3R) and the ryanodine receptor (RyR), each having multiple isoforms in different tissues. These receptor channels function to amplify or trigger Ca2+ rises initiated by either plasmalemmal Ca2+ influx or ligand binding, thereby inducing Ca2+ signaling cascades. Amplification is achieved through either the actions of Ca2+-induced Ca2+ release (CICR), provided by RyR, or actions of IP3-induced Ca2+ release (IICR) through IP3Rs.
Originally described in skeletal and cardiac muscle cells, RyRs in the membrane of the sarcoplasmic reticulum are an integral and essential Ca2+ source for excitation-contraction coupling (Endo, 1977; Fill et al., 1989; Takeshima et al., 1989; Meissner, 1994). Furthermore, an apparent direct physical interaction, which favors alignment between L-VGCCs and RyRs, enables L-VGCCs to function as a preferred source of extracellularly derived Ca2+ in triggering CICR from RyRs and amplifying Ca2+ transients (Lu et al., 1994; Cheng et al., 1996; Wang et al., 2001). In the brain, similar Ca2+ amplification functions of RyRs have been identified, again mediated in part by a close juxtaposition to L-VGCCs (Chavis et al., 1996; Empson & Galione, 1997; Borde et al., 2000; Fagni et al., 2000; Sukhareva et al., 2002).
The other major source of intracellular Ca2+ occurs in response to stimulation of IP3Rs by IP3 generated from activation of a number of metabotropic G-protein-coupled receptors. In some cases IP3Rs can also trigger Ca2+-sensitive K+ channels and hyperpolarize neurons (Sawada et al., 1987; Fink et al., 1988; Furuichi et al., 1989; Zhang et al., 1990; Berridge, 1993; Khodakhah & Ogden, 1995; Irving & Collingridge, 1998; Taylor et al., 1999; Johenning et al., 2002; Rossi & Taylor, 2004). Moreover, IP3Rs are also sensitive to Ca2+ concentrations (Bezprozvanny et al., 1991; Missiaen et al., 1992; Tsukioka et al., 1994; Hagar et al., 1998) and, depending on the cell type studied, it appears that IP3R may also be favorably aligned with L-VGCCs or metabotropic glutamate receptors (mGluR), through interactions with the scaffold protein Homer 1a (Tu et al., 1998; Fagni et al., 2000; Yamamoto et al., 2005).
Release of Ca2+ from these two intracellular channels is regulated in part by the Ca2+ concentration gradient present between luminal ER Ca2+ and cytoplasmic Ca2+ (Alonso et al., 1999; Kiryushko et al., 2002; Solovyova et al., 2002) and is, thus, also dependent on the Ca2+-refilling function of sarcoplasmic/endoplasmic reticulum Ca2+-ATPases (SERCA). Sarcoplasmic/endoplasmic reticulum Ca2+-ATPases maintain the relatively high levels of Ca2+ in the ER (hundreds of µm) that serve CICR, and IICR, and, in the process, contribute to the control and reduction of cytosolic Ca2+ (Thastrup et al., 1990; MacLennan et al., 1997; Mogami et al., 1998; Meldolesi, 2001; Berridge, 2002; Verkhratsky, 2004).
Dysregulated Ca2+ and ER function in models of ischemia and toxicity
Although cell culture models of Ca2+-dependent cell death are generally not viewed as clear models of brain aging, or even AD, they are often employed in studies of ischemic events. These events increase in frequency with advancing age, and it is also possible that neuronal vulnerability from such events increases with aging. Therefore, examining the role of Ca2+ release from intracellular stores in cell death models may help elucidate implications of aging-related alterations in intracellular release. In particular, delayed toxicity after exposure to high glutamate (GLU) in cell culture (excitotoxicity) is a common model used to mimic a wide range of neurological insults, including anoxia/ischemia, head and spinal cord trauma, and even chronic neurodegenerative diseases such as AD. Dysregulated Ca2+ homeostasis and altered Ca2+ influx through NMDA receptors were identified as primary contributors to neuronal cell death early in the study of excitotoxicity (Rothman & Olney, 1986; Choi et al., 1987; Wahl et al., 1989; Regan & Choi, 1991; Randall & Thayer, 1992; Dubinsky, 1993; Lu et al., 1994; Marks et al., 1996; Tymianski & Tator, 1996; Toescu, 1998; Lee et al., 1999; Limbrick et al., 2001; Lipton, 2004). In excitotoxicity models, Ca2+ dysregulation is frequently manifested as an irreversible Ca2+ rise or slowed Ca2+ clearance, and is ultimately associated with neuronal death.
Several investigations of excitotoxicity have focused on a potential role of the ER in sustained Ca2+ elevations. These studies have found that blocking CICR with high concentrations of ryanodine, which lock RyRs in a low conductance state (Bezprozvanny et al., 1991; Coronado et al., 1994; Humerickhouse et al., 1994), or irreversibly inhibiting SERCA function and passively emptying ER stores with thapsigargin prior to GLU exposure, reduces sustained Ca2+ plateaus, as well as other indices associated with neuronal cell death (e.g. lactate dehydrogenase (LDH) release) (Frandsen & Schousboe, 1991; Segal & Manor, 1992; Leski et al., 1999; Clodfelter et al., 2002). Similar results have been noted in models of stroke and ischemia, particularly in astrocyte preparations (Duffy & MacVicar, 1996; Kuwabara et al., 1996; Verkhratsky et al., 1998; Aley et al., 2006). Somewhat paradoxically, while short-term ER Ca2+ depletion prior to an insult appears protective against necrotic (excitotoxic) cell death, long-term depletion of ER Ca2+ induces apoptosis, as indicated by elevations of apoptotic markers, stress responses and disturbance in protein synthesis, and/or massive cell death (Doutheil et al., 1999; Mengesdorf et al., 2001; Verkhratsky & Petersen, 2002; Paschen, 2003; Verkhratsky & Toescu, 2003; Lindholm et al., 2006).
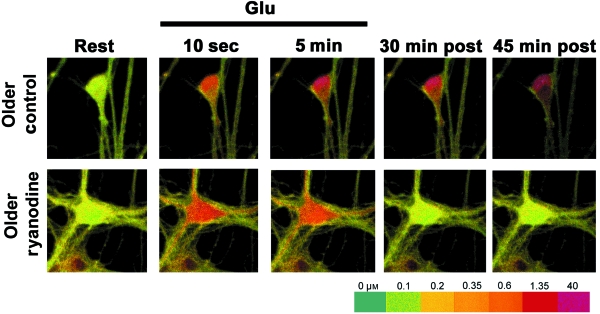
Thus, excessive release of Ca2+ from the ER may play an important role in excitotoxicity. Moreover, evidence suggests that such excessive release may be dependent on the relative maturity of the cells. It is well established that embryonic cortical/hippocampal neurons become increasingly vulnerable to GLU toxicity after a few weeks in culture (Choi, 1992; Toescu & Verkhratsky, 2000), an age in culture that coincides with the emergence of sustained Ca2+ plateaus following GLU insult (Attucci et al., 2002). Interestingly, ryanodine is particularly effective in reversing the Ca2+ plateau and in providing neuroprotection in older cultures (Fig. 1) (Clodfelter et al., 2002). Moreover, recent evidence suggests that the lethal Ca2+ plateau may be maintained by sustained Ca2+ influx via depolarized NMDA receptors (Norris et al., 2006). Together, these data indicate that the plateau may be sustained by CICR. Although age in culture is clearly not equivalent to brain aging, it is associated with increasing vulnerability and Ca2+ influx, which may model some aspects of normal aging (Porter et al., 1997). Conceivably therefore, if Ca2+ release from ER is altered with aging, this alteration may develop in parallel with altered Ca2+ influx (Clodfelter et al., 2002).
Fig. 1.
Ryanodine protection of older cultured hippocampal neurons from excitotoxicity. Following a glutamate insult, older cultured neurons exhibit a sustained [Ca2+]i elevation leading to cell death. Confocal indo-1 Ca2+ imaging shows ryanodine facilitated the recovery (decline) of the Ca2+ plateau and protected older neurons following glutamate insult (modified from Clodfelter et al. copyright 2002 with permission from Elsevier).
Ca2+ release from ER in models of AD
With the increasing development of transgenic (Tg) mouse models of AD, numerous studies testing the view that altered Ca2+ homeostasis might play a role in AD have recently emerged. Initial studies in fibroblasts from AD patients (Gibson et al., 1996) or in cells bearing the human presenilin 1 (PS1) AD mutation (Begley et al., 1999; Guo et al., 1999; Leissring et al., 1999; LaFerla, 2002; Stutzmann, 2005) found evidence of abnormal Ca2+ release through IP3R pathways (Leissring et al., 1999). Interestingly, abnormal IP3-mediated Ca2+ elevations in fibroblasts have also been seen in asymptomatic members of AD families (Etcheberrigaray et al., 1998).
Several studies also have implicated RyRs as being responsible for enhanced intracellular release in PS1 mutated animals (Chan et al., 2000; Mattson et al., 2000; Schneider et al., 2001; Popescu & Ankarcrona, 2004; Stutzmann et al., 2006). Smith and colleagues (2005) examined cultured cortical neurons from mice bearing a transgene containing three AD-related mutations (3×Tg mice), which develop both plaques and tangles, and observed an increase in RyR expression along with greater Ca2+ efflux from the stores in response to caffeine (an agonist at the RyR) (Smith et al., 2005). A recent study combining electrophysiological and Ca2+ imaging methods in cortical slice neurons from Tg mice bearing the PS1 mutation alone, or the 3×Tg transgene, or nontransgenic control animals, assessed the effects of aging vs. those of the PS1 mutation on ER release (Stutzmann et al., 2006). This study found that the PS1 mutation is a critical calciopathic mutation and that increased RyR expression is likely a major factor in the AD mutation-mediated enhancement of ER release. Although photolysis of IP3 was shown to evoke larger Ca2+ transients and Ca2+-dependent hyperpolarizations in Tg mice, the increase in IP3 effects was mediated by CICR from RyRs, triggered in response to IICR. However, some puzzling results also were seen. The enhanced IP3-mediated Ca2+ release and resulting hyperpolarization was larger in Tgs than in non-Tgs at all ages and did not change with aging in any Tg or non-Tg model. Conversely, the AHP induced by trains of spikes and VGCC activation increased with aging in all three model strains but did not differ between Tg and non-Tg mice (Stutzmann et al., 2006).
While little is known regarding underlying mechanisms, it appears that altered CICR, perhaps in combination with IICR, confer some of the phenotypes of disrupted Ca2+ homeostasis in neurons from 3×Tg mice. Still, other sources and mechanisms likely also contribute. The PS1 mutation (which, alone, does not induce amyloid plaques), in combination with amyloid precursor protein (APP) mutations, increases Aβ production (Mullan & Crawford, 1993; Price & Sisodia, 1994; Tanzi et al., 1996; Holcomb et al., 1998; Selkoe, 1998). Some studies have found that Aβ production can exacerbate Ca2+ responses to NMDA or GLU exposure (Mattson, 1997). Furthermore, Aβ toxicity has been attributed, in part, to effects on VGCCs (Davidson et al., 1994; Weiss et al., 1994; Ueda et al., 1997; Ramsden et al., 2002; Bobich et al., 2004; Webster et al., 2006), which could trigger CICR from IP3Rs or RyRs (Koizumi et al., 1998; Ferreiro et al., 2004). However, APP proteolysis (γ-secretase activity) alone does not appear sufficient, because the PS1 mutation (rather than other more amyloidogenic mutations) must be present for the Ca2+ dysregulation to occur (Stutzmann et al., 2006). A possible alternative mechanism suggests that presenilins form Ca2+ leak channels in ER membranes of mouse fibroblasts, independently of γ-secretase activity. Mutations in presenilin interfere with this leak function, and result in greater Ca2+ filling and release from ER (Tu et al., 2006). Furthermore, a gene microarray study conducted in autopsied hippocampal tissue from human AD patients (Blalock et al., 2004) found that multiple genes encoding proteins involved in ER receptor function, or in protein folding and chaperoning, which are also mediated in part by the ER, were down-regulated in incipient AD. These widespread changes may reflect ER membrane/receptor instability in sporadic AD as well.
In addition, it should be noted that effects of PS1 mutations on Ca2+ dysregulation have been observed to occur via other processes, including capacitative Ca2+ entry (Yoo et al., 2000; Smith et al., 2002; Herms et al., 2003; Zatti et al., 2006), changes in mitochondrial potential (Begley et al., 1999; Ankarcrona & Hultenby, 2002; Chan et al., 2002; Behbahani et al., 2006), and L-VGCCs (Cook et al., 2005). Clearly therefore additional work will be needed to resolve the relative contributions of the different sources to the Ca2+dysregulation seen in various models of neurodegenerative diseases.
Neuronal ER release in normal aging
Electrophysiological markers of brain aging have been extensively characterized in the hippocampal formation (Landfield & Pitler, 1984; Moyer et al., 1992; Barnes, 1994; Thibault et al., 1998; Norris et al., 1998; Disterhoft et al., 2004; Burke & Barnes, 2006), a region well-established to be important for memory processes and highly vulnerable to deleterious/degenerative changes with aging. Many of the consistent biomarkers of aging, such as the slow AHP (sAHP), are Ca2+-dependent or Ca2+-mediated. However, it is important to assess the degree to which the ER contributes to the established biomarkers of aging. Both CICR and IICR pools exist within the ER of hippocampal CA1 and CA3 pyramidal neurons. The amount of Ca2+ released via CICR and IICR depends on binding of intracellular ligands including Ca2+, cyclic ADP ribose (cADPR), nicotinic acid adenine dinucleotide phosphate (NAADP) or IP3 (Verkhratsky, 2005), and also depends on the Ca2+ sequestering capacity of the ER, which determines ER Ca2+ content ([Ca2+]L) (Verma et al., 1992; Murayama & Ogawa, 1996; Dawson, 1997; Garaschuk et al., 1997). Solovyova and colleagues using a dual indicator loading technique (low affinity indicator for imaging Ca2+ in the ER, and high affinity indicator for imaging Ca2+ in the cytosol) were able to show that the resting [Ca2+]L in sensory neurons is in the range of 200–300 µm, and high concentrations of IP3 or caffeine result in approximately a 40% decrease in luminal Ca2+ (Solovyova et al., 2002). Depolarization induced [Ca2+]L release was less effective, ranging from 5 to 30 µm. Other techniques for imaging Ca2+ within the ER include the use of aequorin or cameleons. However, there are limitations with these techniques, as the Ca2+ reporting proteins must be genetically engineered and selectively targeted to the ER (Miyawaki et al., 1997; Alonso et al., 1998; Solovyova & Verkhratsky, 2002). In addition, they require long incubation times for transfecting and loading and, thus, preclude their use in acute brain slices.
Consequently, there have been only a handful of studies in neurons examining the effects of aging on ER Ca2+ concentration and release, or on RyR expression. Studies focusing on measures of ER Ca2+ content have generally relied on the use of single wavelength indicators to measure changes in [Ca2+]i transients activated by caffeine, and have found varying results, depending on the experimental approach or preparation. In an early study, no net change in ER Ca2+ release with aging was reported in synaptosomes from the whole brain (Martinez-Serrano et al., 1992). More recently, acute dissociation of several brain regions (cerebellar, basal forebrain, and hippocampal neurons) from aged animals found that CICR magnitude was reduced and that Ca2+ transients recovered more slowly (Verkhratsky et al., 1994; Kirischuk & Verkhratsky, 1996; Murchison & Griffith, 1999; Xiong et al., 2002; Alshuaib et al., 2006). In studies focusing on RyR expression, no clear pattern or consistent changes have been seen in neurons of normal aging rats and mice. Two studies reported no change in brain RyR expression during aging (Martini et al., 1994; Stutzmann et al., 2006), although a recent study of peripheral neurons found a transient elevation in protein levels (RyR3) in mid-aged rats (Vanterpool et al., 2006).
Another approach to the investigation of the possible role of the ER in brain aging is to examine the effects of aging on Ca2+-dependent processes that are modulated, in part, by intracellular Ca2+ release. In CA1 neurons, postsynaptic injection of IP3 or of RyR inhibitors prevents the induction of long-term potentiation and attenuates paired-pulse facilitation (Wang & Kelly, 1997). Similarly, bath application of thapsigargin or cyclopiazonic acid (blockers of SERCA) prevents the induction of long-term depression in both single neurons and in field potential measures (Reyes & Stanton, 1996). High concentrations of ryanodine also selectively reduce the sAHP and spike-frequency accommodation (Borde et al., 2000; Shah & Haylett, 2000). While examining the effect of aging on long-term depression induction (Norris et al., 1998), Foster and colleagues recently reported that cyclopiazonic acid, thapsigargin or ryanodine (agents that reduce CICR) all prevented long-term depression in aged neurons (Kumar & Foster, 2005). However, long-term potentiation, which tends to be decreased with aging (Burke & Barnes, 2006), was enhanced by high ryanodine concentrations in aged slices (Kumar & Foster, 2004). Ca2+-dependent processes mediated largely by IICR and mGluRs activation also have been shown to change with aging. Compared to younger animals, type 1 mGluR activation results in a reduced phosphoinositide turnover in aged rats, perhaps mediated by a reduction in phospholipase C activity (Nicolle et al., 1999). Similarly, protein kinase C (PKC) was also reported to show reduced activity in aging neurons (Araki et al., 1994; Pascale et al., 1998).
Thus, the evidence on the nature of altered CICR or IICR in neurons of normally aging mammals is somewhat inconsistent, perhaps reflecting the type of preparation, cell or brain region specificity, or the difficulty in imaging Ca2+ and its sources within the intact hippocampal slice (Brown & Jaffe, 1994). Recently therefore we sought to systematically test the contributions of CICR to aging changes in one of the brain regions studied most extensively in relation to aging (hippocampus). Specifically, we tested the key prediction that, if increased CICR plays a major role in normal brain aging, then blocking it with high concentration ryanodine should reduce the aging differences in multiple Ca2+ biomarkers of aging.
More broadly, in fact, several other important tenets of the overall Ca2+ hypothesis have, for some time, required adequate testing. These tenets and predictions include: (i) if a common mechanism of Ca2+ dysregulation underlies many aspects of brain aging, then multiple Ca2+-dependent biomarkers of aging in the hippocampus should emerge at approximately the same age in adulthood; and (ii) if Ca2+ dysregulation is a major factor in cognitive decline then Ca2+ biomarkers should precede or coincide with the earliest age of cognitive impairment, which in some studies of rats has been as early as 12-months old (approximately mid-life). To test these predictions and the involvement of CICR on the emergence of Ca2+-related biomarkers, we recently conducted an extensive age course study combining electrophysiological and Ca2+ imaging techniques in hippocampal slices from male rats. Animals at five age points were used to identify the age of onset for three Ca2+-mediated markers of aging, the sAHP, spike accommodation, and the synaptically activated Ca2+ transient. A subset of hippocampal slices received a high dose of ryanodine to block the contribution of CICR to the overall Ca2+ response. In this study, we also employed the least invasive procedures available (sharp intracellular electrodes instead of patch clamping electrodes, nondissociated slices) to minimize interactions of preparation trauma and age.
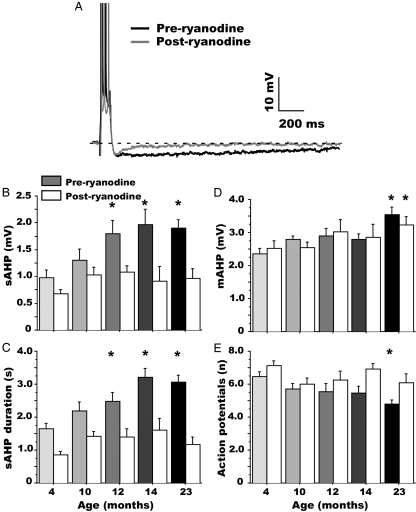
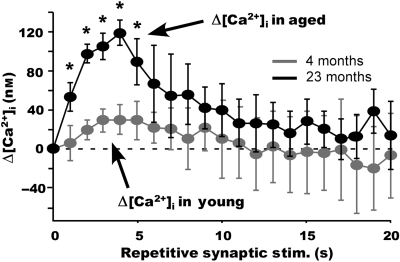
Results were consistent with the above predictions. That is, ryanodine essentially eliminated aging differences in the three markers (e.g. the sAHP, Fig. 2), and the three biomarkers were first detectable simultaneously and at 12 months of age (Fig. 2), an age range early enough to account for cognitive decline. The ryanodine-sensitive component of the Ca2+ response (i.e. CICR) during a 20-s train of synaptic spikes appears to be minimal in young neurons compared to aged neurons and, notably, CICR contributed most to the [Ca2+]i elevation during the first few seconds of the train (Fig. 3). This rapid ‘booster’ action of CICR on Ca2+ responses is consistent with its strong effect on the AHP (Fig. 2) (Gant et al., 2006).
Fig. 2.
Ryanodine reduces the slow afterhyperpolarization (AHP) in an age-dependent manner. (A) Representative example of the blocking effect of 20 µm ryanodine on the AHP of a 23-month-old rat CA1 neuron. (B) Age dependence of slow AHP (sAHP) amplitude, before and following ryanodine application. (C) Age dependence of slow AHP duration, pre- and postryanodine. (D) Age dependence of medium AHP (mAHP) amplitude, pre- and postryanodine. (E) Age-dependence measures of spike-frequency accommodation, pre- and postryanodine. * indicates a significant difference from the 4-month-old group (P < 0.05). Note that aging changes in sAHP markers emerge at 12 months of age (preryanodine group), and ryanodine completely eliminates the aging effects (B and C), indicating a selective blockade of the aging-related increase in Ca2+-induced Ca2+ release (CICR). The initial mAHP is not modulated by CICR (A) and its age dependence was not altered by ryanodine (D). Action potential accommodation changes generally followed the sAHP pattern, but the aging effect at 12 months was not significant in this subset of cells (mean ± SEM) (from Gant et al. copyright 2006 with permission from the Society for Neuroscience).
Fig. 3.
Ryanodine-sensitive component of the [Ca2+]i rise during repetitive synaptic stimulation. Ca2+-induced Ca2+ release (CICR) contribution to the [Ca2+]i rise was determined by subtracting [Ca2+]i measures following ryanodine from those before ryanodine application (Δ[Ca2+]i), in neurons from 4- and 23-month-old animals during 20-s trains of 7 Hz suprathreshold synaptic stimulation. Values shown represent only the (CICR) component of the Ca2+ response that was blocked by ryanodine. Note that the ryanodine-sensitive component of [Ca2+]i is significantly greater in aged rat neurons and contributes to the Ca2+ response primarily during the first 5 s of stimulation. * indicates a significant difference from the 4-month-old group (P < 0.05). (mean ± SEM).
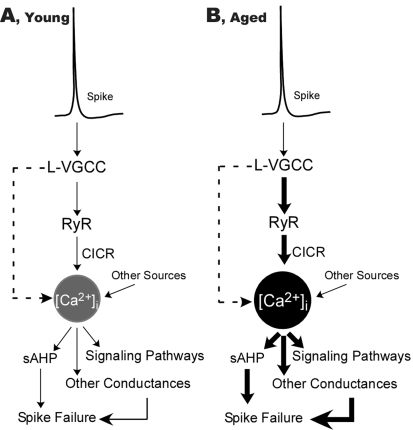
Thus, results of this large study provide considerable support for the proposition that in the hippocampus, an aging-related increase in CICR is necessary, from the onset, for the development of aging changes in several Ca2+-related processes. Moreover, the findings may help to resolve some of the contradictions in the literature by elucidating the conditions under which the contributions of CICR are most prominent. However, one apparent paradox is that similar kinds of evidence support a critical role for L-VGCCs in aging-related Ca2+ dysregulation (Thibault et al., 1998; Disterhoft et al., 2004). Nevertheless, these two lines of evidence are not necessarily contradictory, given that L-VGCCs and RyRs appear to operate in series in many cell types. In this view, then, Ca2+ influx via L-VGCCs may be the preferred source for triggering elevated CICR in aging. Together, the data suggest that aging changes in both types of channel may be part of the same pathway of dysregulation, in turn, suggesting the utility of expanding this version of the Ca2+ hypothesis to incorporate the results on Ca2+ release from intracellular stores (Fig. 4).
Fig. 4.
Schematic model of alterations in L-type voltage-gated Ca2+ channels (L-VGCC) and Ca2+-induced Ca2+ release (CICR) that drive other Ca2+-related hippocampal biomarkers of aging. With aging, increased L-VGCC activity and enhanced CICR operate in series, amplifying the impact of Ca2+ influx on multiple Ca2+-dependent functions. The thickness of arrows schematically represents the activity of Ca2+ flux or signaling pathways in aged rat neurons (B) relative to young (A). These pathways are increased at several stages despite equivalent spike amplitudes and durations. Dashed arrows indicate a possible direct parallel contribution of L-VGCCs to [Ca2+]i (From Gant et al. copyright 2006 with permission from the Society for Neuroscience).
Conclusions and a new model of Ca2+ dysregulation in hippocampal aging
The work summarized above points to the following basic conclusions:
Extensive evidence supporting the hypothesis that Ca2+ dysregulation contributes in part to brain aging and AD that has accumulated for more than 20 years, some of it implicating a larger Ca2+-dependent AHP and increased activity of L-type Ca2+ channels in the functional and cognitive decline seen with normal aging in mammals.
Elevated Ca2+ release from RyRs appears to contribute importantly to cell death and vulnerability in several models of toxicity, which may have relevance to aging-associated ischemic events or other degenerative conditions.
Some types of AD mutations (e.g. presenilins), but not all, appear to alter RyR expression. Under some conditions, (e.g. IP3 stimulation and consequent CICR), this can result in elevated intracellular Ca2+ release and greater hyperpolarization of cortical neurons from transgenic mice of all ages. Surprisingly, however, in the triple transgenic AD model, the aging-related increase in spike train-induced AHP did not differ from the aging change in the AHP seen in wild-type mice.
The observed contributions of altered CICR to Ca2+ dysregulation in neurons during normal aging have been somewhat inconsistent, apparently depending, in part, on cell type and preparation, regional localization and possibly species. However, our recent studies in hippocampal slices from rats of increasing age (five age points) indicate that elevated CICR, beginning at about 12 months of age, may be an important underlying factor in the emergence of multiple Ca2+-related biomarkers of brain aging in rats.
The apparent strong evidence linking both L-VGCCs and RyRs to dysregulated hippocampal Ca2+ homeostasis during aging, rather than being contradictory, may instead suggest an expanded model of the Ca2+ dysregulation pathway in brain aging and, perhaps in AD (as shown in Fig. 4). In this new model, L-VGCCs and RyRs operate in series and aging changes in both (or either) contribute to the aberrant amplification of Ca2+ transients.
Acknowledgments
We thank Dr. Nada Porter for her valuable input and editorial comments on the manuscript. Our research described here was supported by grants R37-AG04542, PO1-AG10836, and T32-AG00242 from the National Institute on Aging and P20-RR15592 from the National Institutes of Health.
References
- Aley PK, Murray HJ, Boyle JP, Pearson HA, Peers C. Hypoxia stimulates Ca2+ release from intracellular stores in astrocytes via cyclic ADP ribose-mediated activation of ryanodine receptors. Cell Calcium. 2006;39:95–100. doi: 10.1016/j.ceca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Alonso MT, Barrero MJ, Carnicero E, Montero M, Garcia-Sancho J, Alvarez J. Functional measurements of [Ca2+] in the endoplasmic reticulum using a herpes virus to deliver targeted aequorin. Cell Calcium. 1998;24:87–96. doi: 10.1016/s0143-4160(98)90076-8. [DOI] [PubMed] [Google Scholar]
- Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo I, Garcia AG, Garcia-Sancho J, Montero M, Alvarez J. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J. Cell Biol. 1999;144:241–254. doi: 10.1083/jcb.144.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshuaib WB, Cherian SP, Hasan MY, Fahim MA. Modulation of neuronal [Ca2+]i by caffeine is altered with aging. Int. J. Dev. Neurosci. 2006;24:389–394. doi: 10.1016/j.ijdevneu.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Hultenby K. Presenilin-1 is located in rat mitochondria. Biochem. Biophys. Res. Commun. 2002;295:766–770. doi: 10.1016/s0006-291x(02)00735-0. [DOI] [PubMed] [Google Scholar]
- Araki T, Kato H, Kanai Y, Kogure K. Age-dependent changes in second messenger and rolipram receptor systems in the gerbil brain. J. Neural Transm. Gen. Sect. 1994;97:135–147. doi: 10.1007/BF01277949. [DOI] [PubMed] [Google Scholar]
- Attucci S, Clodfelter GV, Thibault O, Staton J, Moroni F, Landfield PW, Porter NM. Group I metabotropic glutamate receptor inhibition selectively blocks a prolonged Ca(2+) elevation associated with age-dependent excitotoxicity. Neuroscience. 2002;112:183–194. doi: 10.1016/s0306-4522(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol. Aging. 1997;18:445–452. doi: 10.1016/s0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Begley JG, Duan W, Chan S, Duff K, Mattson MP. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J. Neurochem. 1999;72:1030–1039. doi: 10.1046/j.1471-4159.1999.0721030.x. [DOI] [PubMed] [Google Scholar]
- Behbahani H, Shabalina IG, Wiehager B, Concha H, Hultenby K, Petrovic N, Nedergaard J, Winblad B, Cowburn RF, Ankarcrona M. Differential role of Presenilin-1 and -2 on mitochondrial membrane potential and oxygen consumption in mouse embryonic fibroblasts. J. Neurosci. Res. 2006;84:891–902. doi: 10.1002/jnr.20990. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc. Natl Acad. Sci. USA. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobich JA, Zheng Q, Campbell A. Incubation of nerve endings with a physiological concentration of Abeta1-42 activates CaV2.2(N-Type)-voltage operated calcium channels and acutely increases glutamate and noradrenaline release. J. Alzheimers Dis. 2004;6:243–255. doi: 10.3233/jad-2004-6305. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling – an overview. Semin. Cell Dev. Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- Borde M, Bonansco C, de Sevilla F, Le Ray D, Buno W. Voltage-clamp analysis of the potentiation of the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. Hippocampus. 2000;10:198–206. doi: 10.1002/(SICI)1098-1063(2000)10:2<198::AID-HIPO9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Brown TH, Jaffe DB. Calcium imaging in hippocampal neurons using confocal microscopy. Ann. N. Y. Acad. Sci. 1994;747:313–324. doi: 10.1111/j.1749-6632.1994.tb44419.x. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Campbell LW, Hao SY, Thibault O, Blalock EM, Landfield PW. Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. J. Neurosci. 1996;16:6286–6295. doi: 10.1523/JNEUROSCI.16-19-06286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Calcium signaling: a tale for all seasons. Proc. Natl Acad. Sci. USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Culmsee C, Haughey N, Klapper W, Mattson MP. Presenilin-1 mutations sensitize neurons to DNA damage-induced death by a mechanism involving perturbed calcium homeostasis and activation of calpains and caspase-12. Neurobiol. Dis. 2002;11:2–19. doi: 10.1006/nbdi.2002.0542. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- Cheng G, Liu BF, Yu Y, Diglio C, Kuo TH. The exit from G(0) into the cell cycle requires and is controlled by sarco(endo)plasmic reticulum Ca2+ pump. Arch. Biochem. Biophys. 1996;329:65–72. doi: 10.1006/abbi.1996.0192. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J. Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J. Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clodfelter GV, Porter NM, Landfield PW, Thibault O. Sustained Ca2+-induced Ca2+-release underlies the post-glutamate lethal Ca2+ plateau in older cultured hippocampal neurons. Eur. J. Pharmacol. 2002;447:189–200. doi: 10.1016/s0014-2999(02)01843-5. [DOI] [PubMed] [Google Scholar]
- Cook DG, Li X, Cherry SD, Cantrell AR. Presenilin 1 deficiency alters the activity of voltage-gated Ca2+ channels in cultured cortical neurons. J. Neurophysiol. 2005;94:4421–4429. doi: 10.1152/jn.00745.2005. [DOI] [PubMed] [Google Scholar]
- Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. Am. J. Physiol. 1994;266:C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Davidson RM, Shajenko L, Donta TS. Amyloid β-peptide (A β P) potentiates a nimodipine-sensitive L-type barium conductance in N1E-115 neuroblastoma cells. Brain Res. 1994;643:324–327. doi: 10.1016/0006-8993(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Dawson AP. Calcium signalling: how do IP3 receptors work? Curr. Biol. 1997;7:R544–R547. doi: 10.1016/s0960-9822(06)00277-6. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Moyer JR, Jr, Thompson LT. The calcium rationale in aging and Alzheimer's disease. Evidence from an animal model of normal aging. Ann. N. Y. Acad. Sci. 1994;747:382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Jr, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Wu WW, Ohno M. Biophysical alterations of hippocampal pyramidal neurons in learning, ageing and Alzheimer's disease. Ageing Res. Rev. 2004;3:383–406. doi: 10.1016/j.arr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Doutheil J, Treiman M, Oschlies U, Paschen W. Recovery of neuronal protein synthesis after irreversible inhibition of the endoplasmic reticulum calcium pump. Cell Calcium. 1999;25:419–428. doi: 10.1054/ceca.1999.0042. [DOI] [PubMed] [Google Scholar]
- Dubinsky JM. Intracellular calcium levels during the period of delayed excitotoxicity. J. Neurosci. 1993;13:623–631. doi: 10.1523/JNEUROSCI.13-02-00623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, MacVicar BA. In vitro ischemia promotes calcium influx and intracellular calcium release in hippocampal astrocytes. J. Neurosci. 1996;16:71–81. doi: 10.1523/JNEUROSCI.16-01-00071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empson RM, Galione A. Cyclic ADP-ribose enhances coupling between voltage-gated Ca2+ entry and intracellular Ca2+ release. J. Biol. Chem. 1997;272:20967–20970. doi: 10.1074/jbc.272.34.20967. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govoni S, Racchi M, Tanzi RE, Alkon DL. Calcium responses in fibroblasts from asymptomatic members of Alzheimer's disease families. Neurobiol. Dis. 1998;5:37–45. doi: 10.1006/nbdi.1998.0176. [DOI] [PubMed] [Google Scholar]
- Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- Ferreiro E, Oliveira CR, Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-β peptide. J. Neurosci. Res. 2004;76:872–880. doi: 10.1002/jnr.20135. [DOI] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Fill M, Ma JJ, Knudson CM, Imagawa T, Campbell KP, Coronado R. Role of the ryanodine receptor of skeletal muscle in excitation-contraction coupling. Ann. N. Y. Acad. Sci. 1989;560:155–162. doi: 10.1111/j.1749-6632.1989.tb24092.x. [DOI] [PubMed] [Google Scholar]
- Fink LA, Connor JA, Kaczmarek LK. Inositol trisphosphate releases intracellularly stored calcium and modulates ion channels in molluscan neurons. J. Neurosci. 1988;8:2544–2555. doi: 10.1523/JNEUROSCI.08-07-02544.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, Bossini A, Fagard R, Gil-Extremera B, Laks T, Kobalava Z, Sarti C, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Birkenhager WH. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch. Intern. Med. 2002;162:2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- Frandsen A, Schousboe A. Dantrolene prevents glutamate cytotoxicity and Ca2+ release from intracellular stores in cultured cerebral cortical neurons. J. Neurochem. 1991;56:1075–1078. doi: 10.1111/j.1471-4159.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J. Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Yaari Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J. Physiol. 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. (Pt 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiol. Aging. 1987;8:329–343. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Zhang H, Toral-Barza L, Szolosi S, Tofel-Grehl B. Calcium stores in cultured fibroblasts and their changes with Alzheimer's disease. Biochim. Biophys. Acta. 1996;1316:71–77. doi: 10.1016/0925-4439(96)00002-6. [DOI] [PubMed] [Google Scholar]
- Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat. Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemond P, Jaffe DB. Caloric restriction prevents aging-associated changes in spike-mediated Ca2+ accumulation and the slow afterhyperpolarization in hippocampal CA1 pyramidal neurons. Neuroscience. 2005;135:413–420. doi: 10.1016/j.neuroscience.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Herms J, Schneider I, Dewachter I, Caluwaerts N, Kretzschmar H, Van Leuven F. Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. J. Biol. Chem. 2003;278:2484–2489. doi: 10.1074/jbc.M206769200. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Humerickhouse RA, Bidasee KR, Gerzon K, Emmick JT, Kwon S, Sutko JL, Ruest L, Besch HR., Jr High affinity C10-Oeq ester derivatives of ryanodine. Activator-selective agonists of the sarcoplasmic reticulum calcium release channel. J. Biol. Chem. 1994;269:30243–30253. [PubMed] [Google Scholar]
- Irving AJ, Collingridge GL. A characterization of muscarinic receptor-mediated intracellular Ca2+ mobilization in cultured rat hippocampal neurones. J. Physiol. 1998;511:747–759. doi: 10.1111/j.1469-7793.1998.747bg.x. (Pt 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johenning FW, Zochowski M, Conway SJ, Holmes AB, Koulen P, Ehrlich BE. Distinct intracellular calcium transients in neurites and somata integrate neuronal signals. J. Neurosci. 2002;22:5344–5353. doi: 10.1523/JNEUROSCI.22-13-05344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DS, Campbell LW, Hao SY, Landfield PW. Corticosteroid modulation of hippocampal potentials: increased effect with aging. Science. 1989;245:1505–1509. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Scientific challenges and opportunities related to Alzheimer's disease. Clin. Pharm. 1984;3:522–523. [PubMed] [Google Scholar]
- Khachaturian ZS. The role of calcium regulation in brain aging: reexamination of a hypothesis. Aging (Milano) 1989;1:17–34. doi: 10.1007/BF03323872. [DOI] [PubMed] [Google Scholar]
- Khodakhah K, Ogden D. Fast activation and inactivation of inositol trisphosphate-evoked Ca2+ release in rat cerebellar Purkinje neurones. J. Physiol. 1995;487:343–358. doi: 10.1113/jphysiol.1995.sp020884. (Pt 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Verkhratsky A. Calcium homeostasis in aged neurones. Life Sci. 1996;59:451–459. doi: 10.1016/0024-3205(96)00324-4. [DOI] [PubMed] [Google Scholar]
- Kiryushko DV, Savtchenko LP, Verkhratsky AN, Korogod SM. Theoretical estimation of the capacity of intracellular calcium stores in the Bergmann glial cell. Pflugers Arch. 2002;443:643–651. doi: 10.1007/s00424-001-0739-z. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Ishiguro M, Ohsawa I, Morimoto T, Takamura C, Inoue K, Kohsaka S. The effect of a secreted form of β-amyloid-precursor protein on intracellular Ca2+ increase in rat cultured hippocampal neurones. Br. J. Pharmacol. 1998;123:1483–1489. doi: 10.1038/sj.bjp.0701712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. J. Neurophysiol. 2004;91:2437–2444. doi: 10.1152/jn.01148.2003. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Intracellular calcium stores contribute to increased susceptibility to LTD induction during aging. Brain Res. 2005;1031:125–128. doi: 10.1016/j.brainres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Kuwabara K, Matsumoto M, Ikeda J, Hori O, Ogawa S, Maeda Y, Kitagawa K, Imuta N, Kinoshita T, Stern DM, Yanagi H, Kamada T. Purification and characterization of a novel stress protein, the 150-kDa oxygen-regulated protein (ORP150), from cultured rat astrocytes and its expression in ischemic mouse brain. J. Biol. Chem. 1996;271:5025–5032. doi: 10.1074/jbc.271.9.5025. [DOI] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat. Rev. Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Mechanisms of altered neural function during aging. In: Gipsen WH, Traber J, editors. Aging of the Brain. Amsterdam, The Netherlands: Elsevier; 1983. pp. 51–57. [Google Scholar]
- Landfield PW. ‘Increased calcium-current’ hypothesis of brain aging. Neurobiol. Aging. 1987;8:346–347. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Aging-related increase in hippocampal calcium channels. Life Sci. 1996;59:399–404. doi: 10.1016/0024-3205(96)00318-9. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA, Applegate MD. The effects of high Mg2+-to-Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. J. Neurophysiol. 1986;56:797–811. doi: 10.1152/jn.1986.56.3.797. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Paul BA, Parker I, Cotman CW, LaFerla FM. Alzheimer's presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J. Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- Leski ML, Valentine SL, Coyle JT. L-type voltage-gated calcium channels modulate kainic acid neurotoxicity in cerebellar granule cells. Brain Res. 1999;828:27–40. doi: 10.1016/s0006-8993(99)01270-6. [DOI] [PubMed] [Google Scholar]
- Limbrick DD, Jr, Pal S, DeLorenzo RJ. Hippocampal neurons exhibit both persistent Ca2+ influx and impairment of Ca2+ sequestration/extrusion mechanisms following excitotoxic glutamate exposure. Brain Res. 2001;894:56–67. doi: 10.1016/s0006-8993(00)03303-5. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Xu L, Meissner G. Activation of the skeletal muscle calcium release channel by a cytoplasmic loop of the dihydropyridine receptor. J. Biol. Chem. 1994;269:6511–6516. [PubMed] [Google Scholar]
- MacLennan DH, Rice WJ, Green NM. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J. Biol. Chem. 1997;272:28815–28818. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. The aging of the NMDA receptor complex. Front. Biosci. 1998;3:e70–80. doi: 10.2741/a368. [DOI] [PubMed] [Google Scholar]
- Marks JD, Friedman JE, Haddad GG. Vulnerability of CA1 neurons to glutamate is developmentally regulated. Brain Res. Dev. Brain Res. 1996;97:194–206. doi: 10.1016/s0165-3806(96)00149-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Serrano A, Blanco P, Satrustegui J. Calcium binding to the cytosol and calcium extrusion mechanisms in intact synaptosomes and their alterations with aging. J. Biol. Chem. 1992;267:4672–4679. [PubMed] [Google Scholar]
- Martini A, Battaini F, Govoni S, Volpe P. Inositol 1,4,5-trisphosphate receptor and ryanodine receptor in the aging brain of Wistar rats. Neurobiol. Aging. 1994;15:203–206. doi: 10.1016/0197-4580(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J. Neurosci. 2000;20:1358–1364. doi: 10.1523/JNEUROSCI.20-04-01358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Meldolesi J. Rapidly exchanging Ca2+ stores in neurons: molecular, structural and functional properties. Prog. Neurobiol. 2001;65:309–338. doi: 10.1016/s0301-0082(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Mengesdorf T, Althausen S, Oberndorfer I, Paschen W. Response of neurons to an irreversible inhibition of endoplasmic reticulum Ca(2+)-ATPase: relationship between global protein synthesis and expression and translation of individual genes. Biochem. J. 2001;356:805–812. doi: 10.1042/0264-6021:3560805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Droogmans G, Casteels R. Luminal Ca2+ controls the activation of the inositol 1,4,5-trisphosphate receptor by cytosolic Ca2+ J. Biol. Chem. 1992;267:22961–22966. [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Mogami H, Tepikin AV, Petersen OH. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 1998;17:435–442. doi: 10.1093/emboj/17.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J. Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Mullan M, Crawford F. Genetic and molecular advances in Alzheimer's disease. Trends Neurosci. 1993;16:398–403. doi: 10.1016/0166-2236(93)90007-9. [DOI] [PubMed] [Google Scholar]
- Murayama T, Ogawa Y. Properties of Ryr3 ryanodine receptor isoform in mammalian brain. J. Biol. Chem. 1996;271:5079–5084. doi: 10.1074/jbc.271.9.5079. [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. Age-related alterations in caffeine-sensitive calcium stores and mitochondrial buffering in rat basal forebrain. Cell Calcium. 1999;25:439–452. doi: 10.1054/ceca.1999.0048. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Colombo PJ, Gallagher M, McKinney M. Metabotropic glutamate receptor-mediated hippocampal phosphoinositide turnover is blunted in spatial learning-impaired aged rats. J. Neurosci. 1999;19:9604–9610. doi: 10.1523/JNEUROSCI.19-21-09604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Blalock EM, Thibault O, Brewer LD, Clodfelter GV, Porter NM, Landfield PW. Electrophysiological mechanisms of delayed excitotoxicity: positive feedback loop between NMDA receptor current and depolarization-mediated glutamate release. J. Neurophysiol. 2006;96:2488–2500. doi: 10.1152/jn.00593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J. Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A, Govoni S, Battaini F. Age-related alteration of PKC, a key enzyme in memory processes: physiological and pathological examples. Mol. Neurobiol. 1998;16:49–62. doi: 10.1007/BF02740602. [DOI] [PubMed] [Google Scholar]
- Paschen W. Endoplasmic reticulum: a primary target in various acute disorders and degenerative diseases of the brain. Cell Calcium. 2003;34:365–383. doi: 10.1016/s0143-4160(03)00139-8. [DOI] [PubMed] [Google Scholar]
- Paschen W, Mengesdorf T. Cellular abnormalities linked to endoplasmic reticulum dysfunction in cerebrovascular disease – therapeutic potential. Pharmacol. Ther. 2005;108:362–375. doi: 10.1016/j.pharmthera.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Landfield PW. Aging-related prolongation of calcium spike duration in rat hippocampal slice neurons. Brain Res. 1990;508:1–6. doi: 10.1016/0006-8993(90)91109-t. [DOI] [PubMed] [Google Scholar]
- Popescu BO, Ankarcrona M. Mechanisms of cell death in Alzheimer's disease: role of presenilins. J. Alzheimers Dis. 2004;6:123–128. doi: 10.3233/jad-2004-6203. [DOI] [PubMed] [Google Scholar]
- Porter NM, Thibault O, Thibault V, Chen KC, Landfield PW. Calcium channel density and hippocampal cell death with age in long-term culture. J. Neurosci. 1997;17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier B, Rascol O, Jazat F, Lamour Y, Dutar P. Alterations in the properties of hippocampal pyramidal neurons in the aged rat. Neuroscience. 1992;48:793–806. doi: 10.1016/0306-4522(92)90267-6. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer's disease and animal models. Annu. Rev. Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Henderson Z, Pearson HA. Modulation of Ca2+ channel currents in primary cultures of rat cortical neurones by amyloid beta protein (1–40) is dependent on solubility status. Brain Res. 2002;956:254–261. doi: 10.1016/s0006-8993(02)03547-3. [DOI] [PubMed] [Google Scholar]
- Randall RD, Thayer SA. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J. Neurosci. 1992;12:1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan RF, Choi DW. Glutamate neurotoxicity in spinal cord cell culture. Neuroscience. 1991;43:585–591. doi: 10.1016/0306-4522(91)90317-h. [DOI] [PubMed] [Google Scholar]
- Reyes M, Stanton PK. Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J. Neurosci. 1996;16:5951–5960. doi: 10.1523/JNEUROSCI.16-19-05951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AM, Taylor CW. Ca2+ regulation of inositol 1,4,5-trisphosphate receptors: can Ca2+ function without calmodulin? Mol. Pharmacol. 2004;66:199–203. doi: 10.1124/mol.104.002592. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic – ischemic brain damage. Ann. Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Sawada M, Ichinose M, Maeno T. Ionic mechanism of the outward current induced by intracellular injection of inositol trisphosphate into Aplysia neurons. J. Neurosci. 1987;7:1470–1483. doi: 10.1523/JNEUROSCI.07-05-01470.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I, Reverse D, Dewachter I, Ris L, Caluwaerts N, Kuiperi C, Gilis M, Geerts H, Kretzschmar H, Godaux E, Moechars D, Van Leuven F, Herms J. Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J. Biol. Chem. 2001;276:11539–11544. doi: 10.1074/jbc.M010977200. [DOI] [PubMed] [Google Scholar]
- Segal M, Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. J. Physiol. 1992;448:655–676. doi: 10.1113/jphysiol.1992.sp019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- Shah M, Haylett DG. Ca(2+) channels involved in the generation of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurons. J. Neurophysiol. 2000;83:2554–2561. doi: 10.1152/jn.2000.83.5.2554. [DOI] [PubMed] [Google Scholar]
- Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J. Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- Smith IF, Boyle JP, Vaughan PF, Pearson HA, Cowburn RF, Peers CS. Ca(2+) stores and capacitative Ca(2+) entry in human neuroblastoma (SH-SY5Y) cells expressing a familial Alzheimer's disease presenilin-1 mutation. Brain Res. 2002;949:105–111. doi: 10.1016/s0006-8993(02)02970-0. [DOI] [PubMed] [Google Scholar]
- Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3×Tg-AD mouse model of Alzheimer's disease. J. Neurochem. 2005;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- Solovyova N, Verkhratsky A. Monitoring of free calcium in the neuronal endoplasmic reticulum: an overview of modern approaches. J. Neurosci. Methods. 2002;122:1–12. doi: 10.1016/s0165-0270(02)00300-x. [DOI] [PubMed] [Google Scholar]
- Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A. Ca(2+) dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca(2+)-induced Ca(2+) release triggered by physiological Ca(2+) entry. EMBO J. 2002;21:622–630. doi: 10.1093/emboj/21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE. Calcium dysregulation, IP3 signaling, and Alzheimer's disease. Neuroscientist. 2005;11:110–115. doi: 10.1177/1073858404270899. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J. Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhareva M, Smith SV, Maric D, Barker JL. Functional properties of ryanodine receptors in hippocampal neurons change during early differentiation in culture. J. Neurophysiol. 2002;88:1077–1087. doi: 10.1152/jn.2002.88.3.1077. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, Numa S. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Kovacs DM, Kim TW, Moir RD, Guenette SY, Wasco W. The gene defects responsible for familial Alzheimer's disease. Neurobiol. Dis. 1996;3:159–168. doi: 10.1006/nbdi.1996.0016. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Weber ML, Gaughan CL, Lehning EJ, LoPachin RM. Oxygen/glucose deprivation in hippocampal slices: altered intraneuronal elemental composition predicts structural and functional damage. J. Neurosci. 1999;19:619–629. doi: 10.1523/JNEUROSCI.19-02-00619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl Acad. Sci. USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J. Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Thibault O, Porter NM, Chen KC, Blalock EM, Kaminker PG, Clodfelter GV, Brewer LD, Landfield PW. Calcium dysregulation in neuronal aging and Alzheimer's disease: history and new directions. Cell Calcium. 1998;24:417–433. doi: 10.1016/s0143-4160(98)90064-1. [DOI] [PubMed] [Google Scholar]
- Toescu EC. Apoptosis and cell death in neuronal cells: where does Ca2+ fit in? Cell Calcium. 1998;24:387–403. doi: 10.1016/s0143-4160(98)90062-8. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. Neuronal ageing in long-term cultures: alterations of Ca2+ homeostasis. Neuroreport. 2000;11:3725–3729. doi: 10.1097/00001756-200011270-00027. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. Neuronal ageing from an intraneuronal perspective: roles of endoplasmic reticulum and mitochondria. Cell Calcium. 2003;34:311–323. doi: 10.1016/s0143-4160(03)00142-8. [DOI] [PubMed] [Google Scholar]
- Tsukioka M, Iino M, Endo M. pH dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in permeabilized smooth muscle cells of the guinea-pig. J. Physiol. 1994;475:369–375. doi: 10.1113/jphysiol.1994.sp020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Tator CH. Normal and abnormal calcium homeostasis in neurons: a basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery. 1996;38:1176–1195. doi: 10.1097/00006123-199606000-00028. [DOI] [PubMed] [Google Scholar]
- Ueda K, Shinohara S, Yagami T, Asakura K, Kawasaki K. Amyloid β protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: a possible involvement of free radicals. J. Neurochem. 1997;68:265–271. doi: 10.1046/j.1471-4159.1997.68010265.x. [DOI] [PubMed] [Google Scholar]
- Vanterpool CK, Vanterpool EA, Pearce WJ, Buchholz JN. Advancing age alters the expression of the ryanodine receptor 3 isoform in adult rat superior cervical ganglia. J. Appl. Physiol. 2006;101:392–400. doi: 10.1152/japplphysiol.00167.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. Endoplasmic reticulum calcium signaling in nerve cells. Biol. Res. 2004;37:693–699. doi: 10.4067/s0716-97602004000400027. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol. Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Petersen OH. The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur. J. Pharmacol. 2002;447:141–154. doi: 10.1016/s0014-2999(02)01838-1. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Shmigol A, Kirischuk S, Pronchuk N, Kostyuk P. Age-dependent changes in calcium currents and calcium homeostasis in mammalian neurons. Ann. N. Y. Acad. Sci. 1994;747:365–381. doi: 10.1111/j.1749-6632.1994.tb44423.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Toescu EC. Endoplasmic reticulum Ca(2+) homeostasis and neuronal death. J. Cell. Mol. Med. 2003;7:351–361. doi: 10.1111/j.1582-4934.2003.tb00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Hirsch DJ, Snyder SH. Calcium pools mobilized by calcium or inositol 1,4,5-trisphosphate are differentially localized in rat heart and brain. Mol. Biol. Cell. 1992;3:621–631. doi: 10.1091/mbc.3.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P, Schousboe A, Honore T, Drejer J. Glutamate-induced increase in intracellular Ca2+ in cerebral cortex neurons is transient in immature cells but permanent in mature cells. J. Neurochem. 1989;53:1316–1319. doi: 10.1111/j.1471-4159.1989.tb07430.x. [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. Attenuation of paired-pulse facilitation associated with synaptic potentiation mediated by postsynaptic mechanisms. J. Neurophysiol. 1997;78:2707–2716. doi: 10.1152/jn.1997.78.5.2707. [DOI] [PubMed] [Google Scholar]
- Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- Webster NJ, Ramsden M, Boyle JP, Pearson HA, Peers C. Amyloid peptides mediate hypoxic increase of L-type Ca2+ channels in central neurones. Neurobiol. Aging. 2006;27:439–445. doi: 10.1016/j.neurobiolaging.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Pike CJ, Cotman CW. Ca2+ channel blockers attenuate β-amyloid peptide toxicity to cortical neurons in culture. J. Neurochem. 1994;62:372–375. doi: 10.1046/j.1471-4159.1994.62010372.x. [DOI] [PubMed] [Google Scholar]
- Xiong J, Verkhratsky A, Toescu EC. Changes in mitochondrial status associated with altered Ca2+ homeostasis in aged cerebellar granule neurons in brain slices. J. Neurosci. 2002;22:10761–10771. doi: 10.1523/JNEUROSCI.22-24-10761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sakagami Y, Sugiura S, Inokuchi K, Shimohama S, Kato N. Homer 1a enhances spike-induced calcium influx via L-type calcium channels in neocortex pyramidal cells. Eur. J. Neurosci. 2005;22:1338–1348. doi: 10.1111/j.1460-9568.2005.04278.x. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Zatti G, Burgo A, Giacomello M, Barbiero L, Ghidoni R, Sinigaglia G, Florean C, Bagnoli S, Binetti G, Sorbi S, Pizzo P, Fasolato C. Presenilin mutations linked to familial Alzheimer's disease reduce endoplasmic reticulum and Golgi apparatus calcium levels. Cell Calcium. 2006;39:539–550. doi: 10.1016/j.ceca.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang ET, Hansen AJ, Wieloch T, Lauritzen M. Influence of MK-801 on brain extracellular calcium and potassium activities in severe hypoglycemia. J. Cereb. Blood Flow Metab. 1990;10:136–139. doi: 10.1038/jcbfm.1990.18. [DOI] [PubMed] [Google Scholar]