Abstract
Dissimilatory reduction of metal (e.g. Fe, Mn) (hydr)oxides represents a challenge for microorganisms, as their cell envelopes are impermeable to metal (hydr)oxides that are poorly soluble in water. To overcome this physical barrier, the Gram-negative bacteria Shewanella oneidensis MR-1 and Geobacter sulfurreducens have developed electron transfer (ET) strategies that require multihaem c-type cytochromes (c-Cyts). In S. oneidensis MR-1, multihaem c-Cyts CymA and MtrA are believed to transfer electrons from the inner membrane quinone/quinol pool through the periplasm to the outer membrane. The type II secretion system of S. oneidensis MR-1 has been implicated in the reduction of metal (hydr)oxides, most likely by translocating decahaem c-Cyts MtrC and OmcA across outer membrane to the surface of bacterial cells where they form a protein complex. The extracellular MtrC and OmcA can directly reduce solid metal (hydr)oxides. Likewise, outer membrane multihaem c-Cyts OmcE and OmcS of G. sulfurreducens are suggested to transfer electrons from outer membrane to type IV pili that are hypothesized to relay the electrons to solid metal (hydr)oxides. Thus, multihaem c-Cyts play critical roles in S. oneidensis MR-1- and G. sulfurreducens-mediated dissimilatory reduction of solid metal (hydr)oxides by facilitating ET across the bacterial cell envelope.
Introduction
c-Type cytochromes (c-Cyts) are ubiquitous in nearly all living organisms, where they play vital roles in mediating electron transfer (ET) reactions associated with respiration. Although their amino acid sequences differ greatly, all c-Cyts possess at least one haem that is covalently bound through amino acid side-chains of the proteins to position and orient the haem moiety and thereby facilitate efficient reactions. The haem moieties are commonly co-ordinated through two thioester bonds to proximal cysteines in the protein, where the signature motif of most c-Cyts is CX2CH (other common motifs include CX3−4CH, CX2CK and A/FX2CH). These motifs with covalently bound haems are the key components used to constitute the haem-containing domains whose diverse functions range from binding of O2 and catalysis to electron transfer and accumulation (Stevens et al., 2004; Rodrigues et al., 2006). c-Cyts have been extensively investigated, and several excellent reviews have been dedicated to the structures, chemistry and biogenesis of c-Cyts (Stevens et al., 2004; Mowat and Chapman, 2005). This review focuses on the unique features of bacterial c-Cyts with multiple haems and their roles in bacteria-mediated dissimilatory reduction of solid metal (hydr)oxides.
The unique features of bacterial c-Cyts with multiple haems
During the last decade, high-throughput DNA sequencing of bacterial genomes has revealed that some facultatively or strictly anaerobic bacteria, such as the dissimilatory metal-reducing bacteria (DMRB) Shewanella oneidensis MR-1 and Geobacter sulfurreducens, contain numerous c-Cyts (predicted 42 and 111, respectively, compared with only 7 in Escherichia coli) (Heidelberg et al., 2002; Methe et al., 2003). The c-Cyts are essential for the versatile anaerobic respiration capabilities of S. oneidensis MR-1 because mutants defective in the c-Cyt maturation system are unable to produce functional c-Cyts and consequently fail to grow when fumarate, dimethyl sulphoxide (DMSO) or trimethylamine N-oxide (TMAO) is used as the terminal electron acceptor (Bouhenni et al., 2005). Genome sequence analysis has also revealed that most of these c-Cyts polypeptides found in DMRB possess more than one CX2CH motif, and that one of these putative c-Cyts in G. sulfurreducens has as many as 27 CX2CH motifs, in sharp contrast to the c-Cyts found in eukaryotes, which typically contain only one haem (Heidelberg et al., 2002; Methe et al., 2003). Some multihaem c-Cyts found in DMRB are located in the outer membrane, where they are positioned to interact with extracellular substrates, whereas most membrane c-Cyts found in other bacteria, including multihaem c-Cyts in sulphate respiring bacteria such as Desulfovibrio, are associated with the cytoplasmic or inner membranes (Heidelberg et al., 2004).
Although their overall three-dimensional (3-D) structures vary considerably, one of the unique features found in most bacterial multihaem c-Cyts whose 3-D structures have been solved is the arrangement of haem groups. In these multihaem c-Cyts, all haem groups are positioned in such way that each is in close proximity to at least one of the other haems, and the porphyrin rings of two adjacent haems are positioned either parallel or perpendicular to each other. These arrangements are thought to facilitate rapid ET with considerable specificity among the haem groups that form a continuous ‘electric wire’ (Mowat and Chapman, 2005). When protein complexes are formed among multihaem c-Cyts, at least one haem group in one c-Cyt subunit is usually positioned close to a haem group in another c-Cyt subunit, again permitting rapid and specific inter-ET between the proximal subunits (Rodrigues et al., 2006). Formation of protein complexes among multihaem c-Cyts and the close arrangement of haem groups within and between multihaem c-Cyts make it possible to transfer electrons rapidly over relatively long distances. The c-Cyts quinol dehydrogenase (NrfH)/nitrite reductase (NrfA) complex of Desulfovibrio vulgaris consists of two NrfHs and four NrfAs with a total of 28 haems that are used to form the entire ET network of the NrfH/NrfA complex. The longest distance that electrons could flow from the haem possibly used for quinol oxidation in one of the NrfH subunits to the haem for nitrite reduction in an NrfA subunit along the haem network (centre-to-centre) is ∼98 Å (or 9.8 nm), in which 10 haems are involved (Cunha et al., 2003; Rodrigues et al., 2006).
In addition to their role in ET, some of the bacterial c-Cyts with multiple haems are believed to function as capacitors to accumulate electrons (Rodrigues et al., 2006). One of the possible reasons to accumulate electrons is that some of the chemical reactions catalysed by these c-Cyts require multiple electrons (e.g. six-electron reduction of nitrite to ammonia by NrfA). Accumulation of a sufficient number of electrons might help complete the chemical reactions (Rodrigues et al., 2006). Thus, the ability to pack multiple haem groups densely in c-Cyts through their covalent attachment to the protein backbone, as opposed to the more frequently found Cyts with non-covalently bound haem (e.g. b-type Cyts) (Stevens et al., 2004), not only facilitates efficient ET over a relatively long distance, but might also be well suited for efficient accumulation of electrons for catalysing specific chemical reactions.
Dissimilatory reduction of solid metal (hydr)oxides: a challenge for electron transfer by microbial cells
The ability of bacteria to use oxidized metals, such as iron [Fe(III)] or manganese [Mn(III, IV)] (hydr)oxides, as terminal electron acceptors to generate energy for biosynthesis and cell maintenance (i.e. dissimilatory metal reduction), was originally discovered in the Gram-negative bacteria S. oneidensis MR-1 and Geobacter metallireducens nearly two decades ago (Lovley and Phillips, 1988; Myers and Nealson, 1988). Since then, the dissimilatory metal reduction activity has been observed not only in other Gram-negative bacteria, but also in Gram-positive bacteria and Archaea (Lovley, 2006; Weber et al., 2006). This metal reduction activity plays an important role in: (i) global carbon and nutrient cycling, (ii) the weathering and formation of minerals, (iii) the remediation of metal contaminants such as uranium (U), technetium and chromium, and (iv) harvesting electricity generated from bacterial-mediated biomass conversion involving waste treatment (Gorby et al., 2006; Lovley, 2006; Marshall et al., 2006; Xiong et al., 2006).
Reduction of Fe(III)/Mn(III, IV) (hydr)oxides, however, requires specific mechanisms to overcome physical limitations associated with ET to the (hydr)oxides because: (i) Fe(III)/Mn(III, IV) (hydr)oxides are poorly soluble in water at neutral pH and in the absence of strong complexing ligands [e.g. 10−18 M for Fe(III)] (Schröder et al., 2003), and (ii) nearly all DMRB contain cell walls and outer membranes in the case of the Gram-negative bacteria that physically separate cytoplasmic membranes, where electrons are usually accumulated, from insoluble and extracellular Fe(III)/Mn(III, IV) (hydr)oxides. The distance that electrons need to cross from the inner membrane to the bacterial cell surface is at least 8 nm (Prescott et al., 1996), too great a distance for electrons to cross via a tunnelling mechanism that is limited to < 1.4 nm (Leys and Scrutton, 2004). To overcome these physical obstacles, several ET strategies have evolved among different DMRB, including (i) physical transfer of electrons from the cytoplasmic membrane across the cell envelope to the surface of solid metal (hydr)oxides external to the cell, (ii) production of organic complexing ligands such as siderophores that are excreted to the extracellular milieu to solubilize the metal (hydr)oxides (Weber et al., 2006), and (iii) production of low-molecular-weight redox-active organic molecules that shuttle electrons between oxidized and reduced compounds (Hernandez and Newman, 2001). Although detailed information on the underlying mechanisms are lacking, bacterial multihaem c-Cyts are known to be the principal components directly involved in facilitating ET from the inner membrane to the surface of solid metal (hydr)oxides.
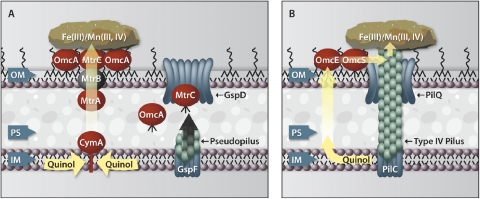
Considerable work has been done in S. oneidensis MR-1, where a range of different multihaem c-Cyts provide the structural and electrochemical means to mediate ET from the quinone/quinol pool of the inner membrane, to the periplasm, to the outer membrane and finally to the Fe(III)/Mn(III, IV) (hydr)oxides outside the cell (Fig. 1A). This conceptual model differs considerably from that proposed for G. sulfurreducens, which has been suggested to transfer electrons from c-Cyts in the outer membrane to solid metal (hydr)oxides through an elongated type IV pili (T4P), or geopili, that is electrically conductive (Reguera et al., 2005; Lovley, 2006) (Fig. 1B). The latter model, however, currently lacks a mechanistic explanation for how electrons are transferred via pilin proteins.
Fig. 1.
Proposed models depicting electron transfer pathways for S. oneidensis MR-1 (A) and G. sulfurreducens (B) during dissimilatory reduction of solid metal (hydr)oxides. For simplicity, the quinone-reducing portion of respiratory chain, the peptidoglycan layer and the individual components of the type II secretion system (T2S) and type IV pilus (T4P) biogenesis machine (other than GspD/PilQ, GspF/PilC and pseudopilus/pilus apparatus) are omitted from these models. Identified multihaem c-type cytochromes (c-Cyts) are in red. Yellow arrows indicate the proposed electron transfer (ET) path.
A. As a member of NapC/NirT family of quinol dehydrogenases, inner membrane (IM) c-Cyt CymA of S. oneidensis MR-1 is capable of oxidizing quinol at IM and reducing the redox proteins, such as c-Cyt MtrA, at periplasm (PS). MtrA might also interact with the outer membrane (OM) protein MtrB. Although it is not a c-Cyt, MtrB is speculated to facilitate ET across OM to MtrC, an OM c-Cyt. Pseudopilus apparatus of T2S, whose formation is regulated by a protein complex in the IM, where only GspF is shown, pushes MtrC and OmcA (another OM c-Cyt) from PS through GspD to the surface of bacterial cells where MtrC and OmcA form a functional complex. The cell surface MtrC and OmcA are capable of directly reducing solid Fe(III)/Mn(III, IV) (hydr)oxides.
B. In G. sulfurreducens, OM c-Cyts OmcE and OmcS are suggested to transfer electrons to the T4P apparatus, which then transfers electrons directly to solid Fe(III)/Mn(III, IV) (hydr)oxides. The structural components that mediate ET from the IM to OmcE/OmcS in the OM during reduction of solid metal (hydr)oxides have yet to be identified experimentally.
Because of their critical roles in ET to solid metal (hydr)oxides in S. oneidensis MR-1, we will first discuss in detail the current knowledge regarding the multihaem c-Cyts of S. oneidensis MR-1, which are directly involved in the ET to solid metal (hydr)oxides. We will then discuss the possible roles of multihaem c-Cyts of G. sulfurreducens in ET to solid metal (hydr)oxides and current evidence suggesting functional similarities and differences in the reduction of extracellular metal (hydr)oxides by these DMRB.
The multihaem c-Cyts directly involved in S. oneidensis MR-1-mediated dissimilatory reduction of solid metal (hydr)oxides
CymA: an inner membrane c-Cyt that serves as an entry point for electron transfer from the inner membrane quinone/quinol pool to the periplasm
CymA (SO_4591) of S. oneidensis MR-1 is a tetrahaem c-Cyt that shares some sequence similarity to the members of NapC/NirT family of quinol dehydrogenases (Table 1). Like other members of the NapC/NirT family, CymA contains a short N-terminal region that is anchored in the inner membrane and a long C-terminal region that binds four haems and protrudes into the periplasm (Fig. 1A). Deletion of the gene encoding CymA significantly impedes the ability of S. oneidensis MR-1 (i.e. 80–100%) to use a range of substrates as terminal electron acceptors, including Fe(III)/Mn(IV) oxides, fumarate, nitrate, nitrite and DMSO (Myers and Myers, 1997; 2000; Schwalb et al., 2003; Lies et al., 2005). The residual Fe(III)/Mn(IV) oxides reduction activity of cymA-inactivation mutant indicates that other proteins whose function is similar to that of CymA might also be involved in reduction of solid metal oxides.
Table 1.
The multihaem c-type cytochromes directly involved in Shewanella oneidensis MR-1- or Geobacter sulfurreducens-mediated reduction of solid Fe(III)/Mn(IV) oxides.
| Species | Cyt/locus tag | No. of haems | MM (kDa) | Location |
|---|---|---|---|---|
| Shewanella oneidensis MR-1 | CymA/SO_4591 | 4 | 20 | PS side of IM |
| MtrA/SO_1777 | 10a | 36 | PS | |
| MtrC/SO_1778 | 10a | 71 | ES side of OM | |
| OmcA/SO_1779 | 10a | 78 | ES side of OM | |
| Geobacter sulfurreducens | OmcE/GSU0618 | 4 | 27 | ES side of OMb |
| OmcS/GSU2504 | 6 | 51 | ES side of OMb | |
| OmcT/GSU2503 | 6 | 45 | Unknown |
The numbers of haem group per polypeptide of these Cyts were determined experimentally (Pitts et al., 2003; Shi et al., 2006).
Their locations need to be validated in future experiments.
Cyt, cytochrome; MM, molecular mass; PS, periplasm; IM, inner membrane or cytoplasmic membrane; ES, extracellular space; OM, outer membrane.
CymA is believed to serve as an ET intermediate between the quinone/quinol pool of the inner membrane and the terminal reductases for fumarate, nitrate and nitrite, which are located in the periplasm, as well as the periplasmic proteins involved in delivering electrons across periplasm to the outer membrane to reduce solid Fe(III)/Mn(IV) oxides and DMSO (Gralnick et al., 2006). Consistent with this periplasmic function, truncated CymA lacking an N-terminal membrane-anchoring domain retains the ability to transfer electrons directly to FccA (formerly known as Fcc3), the respiratory fumarate reductase of S. oneidensis MR-1, with a second-order rate constant of 19 μM−1 s−1 (Maier et al., 2003; Schwalb et al., 2003). In contrast to FccA, no ET is observed from CymA to cytochrome c3, a periplasmic tetrahaem c-Cyt of S. oneidensis MR-1, demonstrating that the ET from CymA to FccA is specific (Schwalb et al., 2003). In some members of ε- and δ-proteobacteria, such as Wolinella and Desulfovibrio, NrfH, a CymA homologue, forms a stable complex with NrfA (Rodrigues et al., 2006). Given that deletion of cymA abolishes the ability of S. oneidensis MR-1 to reduce nitrite (Schwalb et al., 2003) and that CymA is a homologue of NrfH, CymA is believed to interact directly with NrfA (SO_3980) of S. oneidensis MR-1.
MtrA: a periplasmic c-Cyt that transfers electron across the periplasm?
The gene encoding MtrA (SO_1777) is located within a four-gene cluster that also includes genes encoding three outer membrane proteins: MtrB (SO_1776), MtrC or OmcB (SO_1778) and OmcA (SO_1779). All have been linked to dissimilatory reduction of insoluble forms of Fe(III) and/or Mn(IV) oxides (Beliaev and Saffarini, 1998; Beliaev et al., 2001; Myers and Myers, 2001; 2002a; Lies et al., 2005; Gorby et al., 2006). MtrA is a soluble c-Cyt with 10 haems (Table 1) that is localized to the periplasm (Pitts et al., 2003). Compared with wild-type (wt) S. oneidensis MR-1, a mutant lacking MtrA exhibits attenuated reduction of Fe(III)-citrate and MnO2, while its ability to reduce fumarate, nitrate, nitrite, DMSO, TMAO, thiosulphate and sulphide remains normal, demonstrating its specific role in reducing metals (Beliaev et al., 2001). The role of MtrA in reduction of solid Fe(III) (hydr)oxides remains to be determined. Because of its periplasmic location, MtrA is believed to play a critical role in transferring electrons from CymA across the periplasm to the outer membrane proteins such as MtrB (Fig. 1A).
MtrB is not a c-Cyt but is nonetheless required for metal reduction (Beliaev and Saffarini, 1998; Lies et al., 2005). Based on its amino acid sequence, MtrB is predicted to be a transmembrane protein (Beliaev and Saffarini, 1998). The exact function of MtrB is currently unknown but there is evidence to suggest that it is involved in the proper localization and insertion of MtrC and OmcA into the outer membrane (Myers and Myers, 2002b).
Interestingly, the MtrA polypeptide can be divided into two pentahaem domains, each of which shares sequence similarity with NrfB of E. coli (Beliaev and Saffarini, 1998; Clarke et al., 2004). Like NrfH (see above), NrfB functions as a physiological electron donor to NrfA (Clarke et al., 2004). In addition, heterologously expressed MtrA in E. coli is able to serve as an electron donor to the NrfA of E. coli (Pitts et al., 2003). All these results suggest that mtrA most likely evolved from an nrfB homologue by gene duplication and fusion (Clarke et al., 2004). Given that MtrA and CymA are capable of interacting and carrying out intermolecular ET with NrfA, it is reasonable to speculate that NrfA might function as an ET intermediate between CymA and MtrA during the dissimilatory reduction of solid Fe(III) and Mn(III, IV) (hydr)oxides.
MtrC and OmcA: two cell surface c-Cyts that can serve as terminal reductases towards solid metal (hydr)oxides
Both MtrC and OmcA are lipoproteins that each polypeptide contains 10 haems, and they are located on the extracellullar site of the outer membrane (Table 1) (Myers and Myers, 2003a; 2004). Disruption of the genes encoding OmcA or MtrC does not have any effect on the ability of S. oneidensis MR-1 to reduce many soluble electron acceptors, including nitrate and nitrite, but mutants defective in either mtrC or omcA have reduced rates of MnO2 reduction (Beliaev et al., 2001; Myers and Myers, 2001; 2002a). Inactivation of mtrC lowers the capacity of S. oneidensis MR-1 to reduce solid Fe(III) oxides as well (Myers and Myers, 2002a; Lies et al., 2005). Likewise, the reduction rate of insoluble silica (Si)-hydrous ferric oxide (HFO) by a ΔmtrC/omcA double-deletion mutant is only 30% of that of wt (Gorby et al., 2006). Overproduction of MtrC can also restore the ability of a ΔomcA mutant to reduce MnO2 (Myers and Myers, 2003b). This functional redundancy of MtrC and OmcA emphasizes their important roles in dissimilatory reduction of solid Fe(III)/Mn(IV) oxides.
Like CymA, MtrC and OmcA interact with other proteins. MtrC is thought to assist in the proper localization of OmcA to the outer membrane (Myers and Myers, 2001). Recombinant OmcA purified from wt S. oneidensis MR-1 cells contains endogenous MtrC. The recombinant MtrC and OmcA that are independently purified from ΔmtrC/omcA mutant cells form a stable complex in vitro with Kd < 500 nM. Because the stoichiometry of MtrC : OmcA is 1:2, this complex contains at least 30 haems. Each purified protein can reduce Fe(III)-nitrilotriacetic acid (NTA) but, when mixed, their combined activity is greater than the sum of the individual activities, suggesting that MtrC and OmcA interact cooperatively (Shi et al., 2006). Consistent with these observations, MtrC and OmcA can be cross-linked in vivo (Tang et al., 2007).
In addition to soluble Fe(III)-NTA, both purified MtrC and OmcA bind to and reduce crystalline Fe(III) oxide haematite (α-Fe2O3) (Xiong et al., 2006; Lower et al., 2007). The turnover numbers of MtrC and OmcA to haematite are 0.26 s−1 and 0.11 s−1, respectively (Xiong et al., 2006; L. Shi, unpubl. data), which are at least three orders of magnitudes slower than those of cytochrome c oxidases (COX) to O2 (650 s−1 for COX of bovine heart and 1230 s−1 for COX of Rhodobacter sphaeroides) (Han et al., 2005; Riegler et al., 2005). The extremely low-turnover numbers of MtrC and OmcA towards solid haematite indicate that the ET from proteins to solid minerals might be the rate-limiting step of the ET pathways of S. oneidensis MR-1 for dissimilatory reduction of insoluble Fe(III)/Mn(III, IV) (hydr)oxides, although other factors such as potential energy associated with the mineral surfaces may also be important.
Under anaerobic conditions, S. oneidensis MR-1 produces extracellular structures that are associated with nano-sized particles of uraninite (UO2). MtrC and OmcA, the major components of these structures, might serve as the extracellular terminal reductases to uranyl carbonate complexes, because (i) double deletion of mtrC/omcA genes considerably lowers the amount of extracellular UO2 nano-particles formed, (ii) MtrC and OmcA are colocalized with the UO2 nano-particles and (iii) MtrC reduces uranyl carbonate complexes in vitro (Marshall et al., 2006). Under O2-limited conditions, S. oneidensis MR-1 cells produce extracellular materials that were termed nanowires, which could conduct a tunnelling current and therefore could be imaged by scanning tunnelling microscopy (STM). Nanowires have been implicated in S. oneidensis MR-1-mediated reduction of Si-HFO and generation of electrical current in microbial fuel cells (MFC). MtrC and OmcA were proposed to have a functional role in these materials, as the ΔmtrC/omcA mutant produces extracellular materials that are not readily imaged by STM and has impaired abilities either to reduce Si-HFO or to generate the current in MFC (Gorby et al., 2006). Whether MtrC or OmcA is structural components of nanowires and the properties of nanowires that facilitate electron tunnelling remain to be determined.
Although the mechanism by which MtrC and OmcA are translocated across outer membranes has yet to be determined, the bacterial type II secretion system (T2S) seems to play a critical role. Insertional inactivation of gspE, a gene encoding a key component of the T2S, impairs the ability of Shewanella putrefaciens strain 200 to reduce solid Fe(III)/Mn(IV) oxides, demonstrating for the first time the direct involvement of T2S in reduction of solid metal oxides (DiChristina et al., 2002). Likewise, a S. oneidensis MR-1 mutant lacking functional GspD exhibits a similar phenotype to a ΔmtrC/omcA mutant with respect to the diminished ability to reduce Si-HFO and to generate the current in MFC (Gorby et al., 2006). Also functioning as a major component of bacterial T2S, GspD is an outer membrane protein through which certain proteins of Gram-negative bacteria are translocated across the outer membrane into the extracellular space (Fig. 1A). The phenotypic similarity between ΔmtrC/omcA and insertionally inactivated gspD mutants indicates that MtrC and OmcA, which are synthesized in the cytoplasm and matured in the periplasm, might be translocated to the extracellular space through GspD. Consistent with this expectation, examination of Shewanella cells cultured under aerobic conditions by atomic force microscopy reveals that their surfaces are relatively smooth. In contrast, cells grown under electron acceptor-limited conditions exhibit the protruding structures or domains, whose height is 18–25 nm with lateral dimension of 35–50 nm, formed on the cell surfaces. Examination with confocal surface-enhanced Raman scattering spectroscopy also suggests that c-Cyts are associated with these cell surface domains. Inactivation of gspD resulted in the absence of c-Cyts on these domains, indicating a critical role played by GspD for translocation of cell surface c-Cyts (Biju et al., 2007). Our recent comparative proteomic analysis of the proteins released to the growth medium under anaerobic conditions by wt and ΔgspD mutant revealed that: (i) MtrC and OmcA were the major c-Cyts in the medium, and (ii) their abundances in the medium were significantly reduced by the gspD deletion. This result was further substantiated by haem-staining and Western blot analyses (L. Shi, unpubl. data). Thus, in the absence of O2 as the terminal electron acceptor, S. oneidensis MR-1 cells most likely use the T2S to facilitate the translocation of MtrC and OmcA across the outer membrane to the bacterial surface (Fig. 1A).
Extracellular MtrC and OmcA function as metal reductases with ability to bind and reduce haematite. Because MtrC and OmcA form a high-affinity complex and one of their common functions is to reduce solid metal (hydr)oxides, the extracellular MtrC/OmcA complex should be considered as a single functional unit for metal reduction. Many important questions regarding extracellular MtrC/OmcA complex remain unanswered, such as how it receives the electrons from the periplasm and how it functions at the molecular scale in terms of engaging with and transferring electrons to the substrates. It is clear, however, that S. oneidensis MR-1 cells are able to reduce solid metal (hydr)oxides via direct contact between MtrC/OmcA complex and surface of solid metal (hydr)oxides (Fig. 1A).
Formation of nanoscale extracellular structures that contain MtrC/OmcA and appear flexible (Marshall et al., 2006; Biju et al., 2007) suggests that S. oneidensis MR-1 cells might be able to reduce the solid metal (hydr)oxides that are located in regions of sediments and soils where pore sizes limit direct access by the whole cells. The extracellular structures could be extended into small pores where they may contact the (hydr)oxides, consistent with previous results showing that MtrC is involved in the reduction of solid Fe(III) oxide trapped inside porous glass beads (Lies et al., 2005).
Multihaem c-Cyts involved in G. sulfurreducens-mediated dissimilatory reduction of solid metal (hydr)oxides
All available functional data suggest that multihaem c-Cyts play a critical role in mediating the reduction of extracellular metal oxides by G. sulfurreducens. When insoluble Mn(IV) oxide is used as the terminal electron acceptor in G. sulfurreducens cultures, the two c-Cyts OmcE (GSU0618) and OmcS (GSU2504) are identified in culture supernatants after subjecting cells to shear forces generated by blending for 2 min at low speed. Both OmcE and OmcS are predicted to be outer membrane proteins with four and six CX2CH motifs respectively (Table 1). Because they are readily released into the solution phase by weak shearing force, OmcE and OmcS seem to be loosely associated with the cell surface. Deletion of either gene decreases the ability of G. sulfurreducens to reduce Mn(IV) as well as Fe(III) oxides. A gene located immediately downstream of omcS, omcT (GSU2503), also encodes a hexahaem c-Cyt that is 62% identical to OmcS (Table 1). The omcS gene is either transcribed by itself or co-transcribed with omcT, while omcT is only co-transcribed with omcS. Deletion of omcT affects the expression of omcS and lowers the rates of Mn(IV) and Fe(III) oxides reduction by G. sulfurreducens. Deletion of omcE, omcS or omcT, however, does not have any effect on the ability of G. sulfurreducens to reduce soluble Fe(III)-citrate. These results indicate that OmcE, OmcS and OmcT are likely to be directly involved in reduction of insoluble Fe(III)/Mn(IV) oxides by G. sulfurreducens (Mehta et al., 2005).
In contrast to S. oneidensis MR-1 cells that mainly use c-Cyts for ET to solid metal (hydr)oxides, it has been suggested that G. sulfurreducens uses T4P to transfer the electrons directly from the outer membrane to extracellular metal (hydr)oxides (Reguera et al., 2005; Lovley, 2006) (Fig. 1B). Because T4P are anchored to the inner membrane and extend across the periplasm and outer membrane into the extracellular space, the pilus of G. sulfurreducens might be able to receive electrons from the proteins associated with the inner membrane, periplasm and/or outer membrane. Therefore, OmcE and OmcS might transfer electrons to pili (Mehta et al., 2005). Like the model itself, however, no biochemical or biophysical data are available to substantiate this speculation.
Conceptual models and knowledge gaps
Because of their abilities to transfer electrons rapidly, to catalyse chemical reactions and their appropriate electrochemical properties, multihaem c-Cyts are the key structural components employed by both S. oneidensis MR-1 and G. sulfurreducens for transferring electrons accumulated at the inner membrane during respiration to extracellular terminal electron acceptors when soluble electron acceptors are absent. However, the proposed roles played by these multihaem c-Cyts in S. oneidensis MR-1 differ significantly from those in G. sulfurreducens.
As already discussed (see above), multihaem c-Cyts CymA, MtrA, MtrC and OmcA in S. oneidensis MR-1 all play leading roles in facilitating ET from the respiratory chain in the inner membrane, through the periplasm (i.e. CymA→MtrA) and, finally to the outer membrane protein complex MtrC/OmcA facing the extracellular space, which are capable of transferring electrons directly to solid metal (hydr)oxides (Fig. 1A). Indeed, MtrC and OmcA have been purified, reconstituted and demonstrated to function as terminal reductases of haematite. MtrB does not appear to contain any haems but might function as an organizing centre that facilitates ET from MtrA at the periplasm to the MtrC/OmcA at cell surface.
In contrast to our understanding of metal reduction in S. oneidensis MR-1, much less is known about the mechanisms underlying metal reduction in G. sulfurreducens. The multihaem c-Cyts OmcE and OmcS might play supporting roles to facilitate ET to the T4P structural complex, which then relays electrons directly to the surface of solid metal (hydr)oxides (Fig. 1B). This suggestion represents a new ET mechanism not previously observed in nature, as the pilin proteins in this structure lack obvious haem or other metal binding sites. Indeed, this model ‘contrasts with the nearly universal concept that outer-membrane cytochromes are the proteins that transfer electrons to Fe(III) oxides’ (Reguera et al., 2005). An important prediction is that the structural pilin proteins in the T4P complex can receive and transport electrons. However, isolation and characterization of these proteins need to be carried out to test whether they have the electrochemical properties that are consistent with this model. An alternative hypothesis is that, like S. oneidensis MR-1, G. sulfurreducens uses multihaem c-Cyts to mediate direct ET to solid metals, taking advantage of the nearly universal structural motifs commonly used in biological systems to mediate ET reactions. In this respect, it is interesting to note that the T2S and T4P biogenesis machines in Shewanella and Geobacter, respectively, evolved from a common ancestor (Filloux, 2004). Their involvement in S. oneidensis MR-1- and G. sulfurreducens-mediated dissimilatory reduction of solid metal (hydr)oxides, respectively, demonstrates a common biological function shared by these two systems. Furthermore, nearly all key structural components of the ET pathway used by S. oneidensis MR-1 to reduce solid metal (hydr)oxides have their putative counterparts in G. sulfurreducens (Lovley, 2006) and one of the T2Ss in G. sulfurreducens is required for reducing solid Fe(III)/Mn(IV) oxides (Mehta et al., 2006), suggesting that the general strategy for ET to solid metal (hydr)oxides might be well conserved in Shewanella and Geobacter.
Despite recent advances in understanding the roles of the multihaem c-Cyts in S. oneidensis MR-1-mediated dissimilatory reduction of solid metal (hydr)oxides, four important questions must be answered to provide a basic framework for understanding of the physiology of extracellular metal (hydr)oxide reduction by DMRB. Our primary question is how the T2S facilitates translocation of MtrC/OmcA across the outer membrane. To date, the pullulanase of Klebsiella oxytoca is probably the only outer membrane lipoprotein whose translocation via the T2S is well characterized (Francetic and Pugsley, 2005). If confirmed, MtrC/OmcA would provide another model system to investigate how lipoproteins are translocated across the outer membrane by T2S. A mechanistic understanding of the MtrC/OmcA translocation process will be the first step to unravel the molecular mechanisms that control the proper placement of MtrC/OmcA where they can directly contact solids outside the cells. Second, which domain elements in the MtrC/OmcA complex are associated with binding and reduction of solid metal (hydr)oxides? These proteins were the first isolated proteins for which the ability to bind and reduce solid metal (hydr)oxides was directly demonstrated. An understanding of their mechanisms has great potential both in understanding bacterial physiology and with respect to practical applications involving, for example, the construction of biological fuel cells whose greater efficiency will require the development of stable and long-lived protein-based materials. Third, the role of MtrB in promoting ET to the cell surface remains uncertain, and structure/function measurements are necessary to reveal the mechanisms by which MtrB facilitates, directly or indirectly, ET. Such investigations will need to identify any possible interactions between MtrB and the multihaem c-Cyts involved in the process of reduction of solid metal (hydr)oxides. This is critical, as MtrB is essential for S. oneidensis MR-1 to reduce the solid metal (hydr)oxides. Finally, it is unclear how the electrons are transferred from CymA to MtrA. Does CymA transfer electrons directly to MtrA or require NrfA and/or other periplasmic proteins as ET intermediates? Answers for these questions are critical to our understanding of how electrons are transferred across the periplasm.
In summary, although considerable gaps in our knowledge about the bacterial ET pathways used for dissimilatory reduction of solid metal (hydr)oxides remain, the common feature of these pathways found in S. oneidensis MR-1 and G. sulfurreducens is that multihaem c-Cyts, which are strategically located at the inner membrane, periplasm and outer membrane and are able to interact with other proteins, facilitate ET across the physical barrier of bacterial cell envelope to the surface of solid metal (hydr)oxides.
Acknowledgments
We thank the support from the US Department of Energy (DOE) Office of Biological and Environmental Sciences (BER) under the W.R. Wiley Environmental Molecular Sciences Laboratory (EMSL) Biogeochemistry Scientific Grand Challenge Project, the Genomics: GTL Program and the Environmental Remediation Sciences Program. EMSL is a national scientific user facility sponsored by the DOE BER programme located at Pacific Northwest National Laboratory. Pacific Northwest National Laboratory is operated for the DOE by Battelle Memorial Institute under Contract DE-AC05-76RLO1830.
References
- Beliaev AS, Saffarini DA. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J Bacteriol. 1998;180:6292–6297. doi: 10.1128/jb.180.23.6292-6297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliaev AS, Saffarini DA, McLaughlin JL, Hunnicutt D. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol Microbiol. 2001;39:722–730. doi: 10.1046/j.1365-2958.2001.02257.x. [DOI] [PubMed] [Google Scholar]
- Biju V, Pan D, Gorby YA, Fredrickson J, McLean J, Saffarini D, Lu HP. Combined spectroscopic and topographic characterization of nanoscale domains and their distributions of a redox protein on bacterial cell surfaces. Langmuir. 2007;23:1333–1338. doi: 10.1021/la061343z. [DOI] [PubMed] [Google Scholar]
- Bouhenni R, Gehrke A, Saffarini D. Identification of genes involved in cytochrome c biogenesis in Shewanella oneidensis, using a modified mariner transposon. Appl Environ Microbiol. 2005;71:4935–4937. doi: 10.1128/AEM.71.8.4935-4937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TA, Dennison V, Seward HE, Burlat B, Cole JA, Hemmings AM, Richardson DJ. Purification and spectropotentiometric characterization of Escherichia coli NrfB, a decaheme homodimer that transfers electrons to the decaheme periplasmic nitrite reductase complex. J Biol Chem. 2004;279:41333–41339. doi: 10.1074/jbc.M407604200. [DOI] [PubMed] [Google Scholar]
- Cunha CA, Macieira S, Dias JM, Almeida G, Goncalves LL, Costa C, et al. Cytochrome c nitrite reductase from Desulfovibrio desulfuricans ATCC 27774. The relevance of the two calcium sites in the structure of the catalytic subunit (NrfA) J Biol Chem. 2003;278:17455–17465. doi: 10.1074/jbc.M211777200. [DOI] [PubMed] [Google Scholar]
- DiChristina TJ, Moore CM, Haller CA. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. J Bacteriol. 2002;184:142–151. doi: 10.1128/JB.184.1.142-151.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A. The underlying mechanisms of type II protein secretion. Biochim Biophys Acta. 2004;1694:163–179. doi: 10.1016/j.bbamcr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Francetic O, Pugsley AP. Towards the identification of type II secretion signals in a nonacylated variant of pullulanase from Klebsiella oxytoca. J Bacteriol. 2005;187:7045–7055. doi: 10.1128/JB.187.20.7045-7055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA. 2006;103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick JA, Vali H, Lies DP, Newman DK. Extracellular respiration of dimethyl sulfoxide by Shewanella oneidensis strain MR-1. Proc Natl Acad Sci USA. 2006;103:4669–4674. doi: 10.1073/pnas.0505959103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Morgan JE, Gennis RB. G204D, a mutation that blocks the proton-conducting d-channel of the aa3-type cytochrome c oxidase from Rhodobacter sphaeroides. Biochemistry. 2005;44:12767–12774. doi: 10.1021/bi051141m. [DOI] [PubMed] [Google Scholar]
- Heidelberg JF, Paulsen IT, Nelson KE, Gaidos EJ, Nelson WC, Read TD, et al. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat Biotechnol. 2002;20:1118–1123. doi: 10.1038/nbt749. [DOI] [PubMed] [Google Scholar]
- Heidelberg JF, Seshadri R, Haveman SA, Hemme CL, Paulsen IT, Kolonay JF, et al. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat Biotechnol. 2004;22:554–559. doi: 10.1038/nbt959. [DOI] [PubMed] [Google Scholar]
- Hernandez ME, Newman DK. Extracellular electron transfer. Cell Mol Life Sci. 2001;58:1562–1571. doi: 10.1007/PL00000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys D, Scrutton NS. Electrical circuitry in biology: emerging principles from protein structure. Curr Opin Struct Biol. 2004;14:642–647. doi: 10.1016/j.sbi.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl Environ Microbiol. 2005;71:4414–4426. doi: 10.1128/AEM.71.8.4414-4426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR. Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol. 2006;4:497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- Lovley DR, Phillips EJ. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lower BH, Shi L, Yongsunthon R, Droubay TC, McCready DE, Lower SK. Dynamic force spectroscopy of individual bonds that form between an iron oxide surface and outer membrane cytochromes OmcA and MtrC from Shewanella oneidensis. J Bacteriol. 2007. [WWW document]. URL http://jb.asm.org/cgi/reprint/JB.01518-06v1. [DOI] [PMC free article] [PubMed]
- Maier TM, Myers JM, Myers CR. Identification of the gene encoding the sole physiological fumarate reductase in Shewanella oneidensis MR-1. J Basic Microbiol. 2003;43:312–327. doi: 10.1002/jobm.200390034. [DOI] [PubMed] [Google Scholar]
- Marshall MJ, Beliaev AS, Dohnalkova AC, Kennedy DW, Shi L, Wang Z, et al. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol. 2006;4:e268. doi: 10.1371/journal.pbio.0040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta T, Coppi MV, Childers SE, Lovley DR. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl Environ Microbiol. 2005;71:8634–8641. doi: 10.1128/AEM.71.12.8634-8641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta T, Childers SE, Glaven R, Lovley DR, Mester T. A putative multicopper protein secreted by an atypical type II secretion system involved in the reduction of insoluble electron acceptors in Geobacter sulfurreducens. Microbiology. 2006;152:2257–2264. doi: 10.1099/mic.0.28864-0. [DOI] [PubMed] [Google Scholar]
- Methe BA, Nelson KE, Eisen JA, Paulsen IT, Nelson W, Heidelberg JF, et al. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science. 2003;302:1967–1969. doi: 10.1126/science.1088727. [DOI] [PubMed] [Google Scholar]
- Mowat CG, Chapman SK. Multi-heme cytochromes – new structures, new chemistry. Dalton Trans. 2005;7:3381–3389. doi: 10.1039/b505184c. [DOI] [PubMed] [Google Scholar]
- Myers CR, Myers JM. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J Bacteriol. 1997;179:1143–1152. doi: 10.1128/jb.179.4.1143-1152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CR, Myers JM. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl Environ Microbiol. 2002b;68:5585–5594. doi: 10.1128/AEM.68.11.5585-5594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CR, Myers JM. Cell surface exposure of the outer membrane cytochromes of Shewanella oneidensis MR-1. Lett Appl Microbiol. 2003a;37:254–258. doi: 10.1046/j.1472-765x.2003.01389.x. [DOI] [PubMed] [Google Scholar]
- Myers CR, Myers JM. The outer membrane cytochromes of Shewanella oneidensis MR-1 are lipoproteins. Lett Appl Microbiol. 2004;39:466–470. doi: 10.1111/j.1472-765X.2004.01611.x. [DOI] [PubMed] [Google Scholar]
- Myers CR, Nealson KH. Bacterial manganese reduction and growth with manganese oxides as the sole electron acceptor. Science. 1988;240:1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- Myers JM, Myers CR. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J Bacteriol. 2000;182:67–75. doi: 10.1128/jb.182.1.67-75.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JM, Myers CR. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl Environ Microbiol. 2001;67:260–269. doi: 10.1128/AEM.67.1.260-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JM, Myers CR. Genetic complementation of an outer membrane cytochrome omcB mutant of Shewanella putrefaciens MR-1 requires omcB plus downstream DNA. Appl Environ Microbiol. 2002a;68:2781–2793. doi: 10.1128/AEM.68.6.2781-2793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JM, Myers CR. Overlapping role of the outer membrane cytochromes of Shewanella oneidensis MR-1 in the reduction of manganese(IV) oxide. Lett Appl Microbiol. 2003b;37:21–25. doi: 10.1046/j.1472-765x.2003.01338.x. [DOI] [PubMed] [Google Scholar]
- Pitts KE, Dobbin PS, Reyes-Ramirez F, Thomson AJ, Richardson DJ, Seward HE. Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA: expression in Escherichia coli confers the ability to reduce soluble Fe(III) chelates. J Biol Chem. 2003;278:27758–27765. doi: 10.1074/jbc.M302582200. [DOI] [PubMed] [Google Scholar]
- Prescott LM, Harley JP, Klein DA. Microbiology. 3. Dubuque, IA: Wm. C. Publishers; 1996. The prokaryotic cell wall; p. 51. [Google Scholar]
- Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- Riegler D, Shroye L, Pokalsky C, Zaslavsky D, Gennis R, Prochaska LJ. Characterization of steady-state activities of cytochrome c oxidase at alkaline pH: mimicking the effect of K-channel mutations in the bovine enzyme. Biochim Biophys Acta. 2005;1706:126–133. doi: 10.1016/j.bbabio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Oliveira TF, Pereira IA, Archer M. X-ray structure of the membrane-bound cytochrome c quinol dehydrogenase NrfH reveals novel haem coordination. EMBO J. 2006;25:5951–5960. doi: 10.1038/sj.emboj.7601439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder I, Johnson E, Vries S. Microbial ferric iron reductases. FEMS Microbiol Rev. 2003;27:427–447. doi: 10.1016/S0168-6445(03)00043-3. [DOI] [PubMed] [Google Scholar]
- Schwalb C, Chapman SK, Reid GA. The tetraheme cytochrome CymA is required for anaerobic respiration with dimethyl sulfoxide and nitrite in Shewanella oneidensis. Biochemistry. 2003;42:9491–9497. doi: 10.1021/bi034456f. [DOI] [PubMed] [Google Scholar]
- Shi L, Chen B, Wang Z, Elias DA, Mayer MU, Gorby YA, et al. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J Bacteriol. 2006;188:4705–4714. doi: 10.1128/JB.01966-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JM, Daltrop O, Allen JW, Ferguson SJ. C-type cytochrome formation: chemical and biological enigmas. Acc Chem Res. 2004;37:999–1007. doi: 10.1021/ar030266l. [DOI] [PubMed] [Google Scholar]
- Tang X, Yi W, Munske GR, Adhikari DP, Zakharova NL, Bruce JE. Profiling the membrane proteome of Shewanella oneidensis MR-1 with new affinity labeling probes. J Proteome Res. 2007;6:724–734. doi: 10.1021/pr060480e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KA, Achenbach LA, Coates JD. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol. 2006;4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Shi L, Chen B, Mayer MU, Lower BH, Londer Y, et al. High-affinity binding and direct electron transfer to solid metals by the Shewanella oneidensis MR-1 outer membrane c-type cytochrome OmcA. J Am Chem Soc. 2006;128:13978–13979. doi: 10.1021/ja063526d. [DOI] [PubMed] [Google Scholar]