Abstract
Deferasirox is a once-daily, oral iron chelator developed for treating transfusional iron overload. Preclinical studies indicated that the kidney was a potential target organ of toxicity. As patients with sickle cell disease often have abnormal baseline renal function, the primary objective of this randomised, open-label, phase II trial was to evaluate the safety and tolerability of deferasirox in comparison with deferoxamine in this population. Assessment of efficacy, as measured by change in liver iron concentration (LIC) using biosusceptometry, was a secondary objective. A total of 195 adult and paediatric patients received deferasirox (n = 132) or deferoxamine (n = 63). Adverse events most commonly associated with deferasirox were mild, including transient nausea, vomiting, diarrhoea, abdominal pain and skin rash. Abnormal laboratory studies with deferasirox were occasionally associated with mild non-progressive increases in serum creatinine and reversible elevations in liver function tests. Discontinuation rates from deferasirox (11·4%) and deferoxamine (11·1%) were similar. Over 1 year, similar dose-dependent LIC reductions were observed with deferasirox and deferoxamine. Once-daily oral deferasirox has acceptable tolerability and appears to have similar efficacy to deferoxamine in reducing iron burden in transfused patients with sickle cell disease.
Keywords: deferasirox, ICL670, Exjade, sickle cell disease, iron overload
The majority of patients with sickle cell anaemia have received repeated blood transfusions by adulthood (Styles & Vichinsky, 1994; Wanko & Telen, 2005). Transfusion therapy is likely to further increase in paediatric patients because of recent evidence indicating its ability to prevent organ injury and improve the outcome of complications (Adams et al, 1998; The Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP2) Trial Investigators, 2005). However, because the body has no physiological mechanism to actively excrete excess iron, repeated blood transfusions lead to increased body iron burden, including iron deposition into the liver, heart and endocrine organs (McLaren et al, 1983). While studies are limited, progressive iron loading and subsequent tissue injury in sickle cell disease appears similar to other transfused populations (Ballas, 2001; Manci et al, 2003; Vichinsky et al, 2005). Guidelines from consensus conferences have therefore recommended daily treatment with deferoxamine in iron-overloaded patients (National Institutes of Health, 2002).
Effective administration of iron chelation therapy has been limited by the route of its administration. Although deferoxamine is effective in removing iron from the body, due to very poor oral bioavailability and a short half-life it must be administered by subcutaneous or intravenous infusion, usually over 8–12 h on 5–7 d/week (Porter, 2001). Compliance with this regimen is often poor (Treadwell et al, 2005). The availability of a once-daily, oral alternative would potentially facilitate improved compliance, and thereby reduce morbidity and mortality from iron overload.
Deferasirox is an orally absorbed iron chelator that has been developed for the management of transfusional iron overload. Its safety, tolerability and efficacy in reducing body iron burden have been demonstrated in patients with β-thalassaemia major and in other chronic transfusion-dependent anaemias (Cappellini et al, 2006). Over a 1-year period, deferasirox was demonstrated to maintain or reduce body iron burden in regularly transfused patients who had baseline liver iron concentration (LIC) values of at least 7 mg Fe/g dry weight (dw) with an acceptable side effect profile.
Individuals with sickle cell disease often have abnormal baseline renal function. Therefore, it was of note that preclinical studies conducted with deferasirox in animals indicated that a potential target organ of toxicity was the kidney. High doses of deferasirox were found to be associated with renal tubular damage in rats and marmosets, particularly in those that did not have iron overload (Vanorden & Hagemann, 2006). This effect was felt most likely, but not definitively, to be related to the pharmacological effect of deferasirox chelating iron. Based on these preclinical data, simple extrapolation of the safety profile of deferasirox from a population of patients with β-thalassaemia major to individuals with sickle cell disease was not deemed to be appropriate. In addition, whether or not treatment with deferasirox might trigger or exacerbate sickle cell crises was unknown. Thus, the primary objective of this randomised, open-label, phase II trial was to evaluate the safety and tolerability of deferasirox in comparison with deferoxamine in this population.
Methods
Study design and patient population
Patient eligibility
Patients with sickle cell disease ≥2 years of age and with iron overload from repeated blood transfusions were enroled in this trial. Patients receiving regular blood transfusions or those sporadically transfused who received at least 20 units of packed red blood cells or equivalent were eligible. Prior chelation therapy was permitted but was not mandatory. The serum ferritin level for entry into the screening period of this study was ≥1000 μg/l. Patients eligible for entry into the screening period subsequently had biosusceptometry performed (see below) in order to determine that they had a sufficiently high body iron burden to be eligible for treatment on the trial.
Patients were excluded if they had a serum creatinine above the upper limit of normal (ULN), if they had significant proteinuria (as indicated by a urinary protein:creatinine ratio of ≥0·5 confirmed at two visits), or if they had active hepatitis B or C. Active hepatitis B was defined as liver function tests above the normal range, together with a positive antigen (hepatitis B e antigen, hepatitis B surface antigen) test or positive IgM core antibody test in conjunction with a negative hepatitis B surface antibody test. Active hepatitis C was defined as liver function tests above the normal range in the presence of a positive hepatitis C antibody test and detectable hepatitis C RNA levels. Other exclusion criteria were second and third atrioventricular block, QT interval prolongation, or therapy with digoxin or similar medications. Treatment with β-blockers or angiotensin-converting enzyme inhibitors was permitted. Patients with chelation therapy-associated ocular toxicity were excluded.
After screening requirements were met and informed consent was obtained, eligibility based on the presence of iron overload was confirmed by non-invasive determination of LIC using Superconducting QUantum Interference Device (SQUID) biosusceptometry (Brittenham et al, 1982). Patients receiving simple transfusions needed to have a LIC of ≥2 mg Fe/g dw, and those receiving exchange transfusions needed to have a LIC of ≥5 mg Fe/g dw at study entry. Biosusceptometry was performed at three centres under a standardised operational procedure (Turin, Italy; Hamburg, Germany; Oakland, USA). For consistency, all SQUID biosusceptometry assessments for a given subject were conducted at the same site.
In vivo LIC values by SQUID biosusceptometry (approximately wet weight) were converted into dry weight LIC values using the widely adopted wet-to-dry weight ratio of 3·33 (Olivieri & Brittenham, 1997). Studies performed as part of another clinical trial that was initiated just before the current trial and completed prior to it found that a more precise conversion factor of twice the adopted factor was applicable to all three sites (Fischer et al, 2006). Because of these findings, adjusted LIC values are presented unless otherwise noted.
Randomisation
Patients were randomised to receive deferasirox or deferoxamine in a 2:1 manner. The randomisation was performed using an interactive voice response system and was stratified according to the following age groups: 2 to <6 years, 6 to <12 years, 12 to <16 years and 16 years and older. The randomisation sequence included permuted block groups of six patients for each of the three age strata.
Treatment plan
The study duration was 52 weeks. The initial 24 patients enroled were randomised to receive deferasirox 10 mg/kg or deferoxamine at recommended doses of 20–60 mg/kg based on initial LIC. Subsequently, additional safety information became available for deferasirox suggesting a need to modify the starting dose (Cappellini et al, 2006). Therefore, following the enrolment of the first 24 patients, the study was amended so that all subsequent patients randomised to deferasirox were dosed at 10–30 mg/kg according to baseline LIC. Deferasirox was given once daily each morning as a dispersed solution in water, half-an-hour before breakfast. The dose of deferasirox was reduced by one dose level and not re-escalated for patients 15 years and older if serum creatinine increased >33% above baseline on two consecutive occasions. For children less than 15 years of age, the dose was only decreased if these values were also above the age-appropriate ULN. Deferasirox was interrupted for moderate or severe skin rash and reinstituted at half the initial dose, and dose re-escalation was permitted. Deferoxamine was administered as a slow subcutaneous infusion over 8–12 h using electronic Microject Chrono® (Medical Technology, Turin, Italy) infusion pumps on 5–7 d a week. In order to facilitate comparison of different schedules, all deferoxamine doses reported were normalised to administration for 5 d/week (i.e. 50 mg/kg administered 7 d/week would be reported as 70 mg/kg).
The trial was conducted in accordance with the Declaration of Helsinki. Institutional Review Board approval was obtained at each participating institution and written informed consent was obtained from all patients or guardians prior to participation in any study procedures. Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA) coordinated the design and execution of this trial and contributed to the analysis and interpretation of the trial data. Novartis Pharmaceuticals Corporation also collaborated with the external authors to assist in the development and approval of the manuscript for publication.
Safety assessments
Laboratory assessments were performed at least monthly and included complete blood counts with differential counts. Biochemistry testing included electrolytes, glucose, liver function tests, gamma-glutaryl-transferase, lactate dehydrogenase, cholesterol, triglycerides, uric acid, total protein, C-reactive protein, copper and zinc levels. Iron parameters included total iron, transferrin, transferrin saturation and ferritin. Urinary testing performed on random collections included determination of creatinine, total protein and albumin. Physical examinations, electrocardiograms (ECGs), audiometry and ophthalmological tests were performed at baseline, 12, 24, 36 and 52 weeks. In patients less than 16 years of age, additional assessments included growth velocity and pubertal stage.
Efficacy assessments
Liver iron concentration was determined by SQUID biosusceptometry at baseline, 24 and 52 weeks. The 24-week assessment was performed primarily for safety purposes, and the change in LIC was calculated between baseline and 52 weeks. Serum ferritin was assessed monthly during the study and the change was determined using the baseline and final ferritin level.
Compliance
For deferasirox, compliance was assessed by counting the number of tablets returned in bottles at each visit. For deferoxamine, the numbers of vials returned at each visit were counted.
Statistical analysis
The data were analysed under supervision of the trial statistician and were reviewed by the investigators. The sample size was calculated based on the number of deferasirox-treated patients required to detect adverse experiences having an underlying event rate of at least 4% with 95% confidence, according to the method of Hanley and Lippman-Hand (1983). The assessment of safety was based mainly on the frequency of adverse events and on the number of laboratory values that fell outside the predetermined ranges. Other safety data (e.g. ECGs, vital signs and other tests) were considered as appropriate. All safety data are reported for patients who received at least one dose of study medication.
The main efficacy endpoint was the change in LIC from baseline assessed at 52 weeks, adjusted according to the transfusion category (simple, exchange, simple and exchange). Descriptive statistics are presented throughout where appropriate. Testing for statistical significance for differences between baseline and end-of-study for each treatment group was performed using Student's t-test. Using a two-sided test, P-values <0·05 were considered to be statistically significant.
Results
Study population
Patients were recruited by investigators at 44 sites in the USA, France, Italy, UK and Canada. Of the 203 eligible patients randomised, 195 received treatment and are included in the safety population. The percentage of patients discontinuing deferasirox and deferoxamine was similar (11·4% vs. 11·1% respectively). Adverse events resulted in discontinuations in 5·3% of patients on deferasirox and 3·2% of patients on deferoxamine. The remainder of the discontinuations was due to patients lost to follow-up, withdrawal of consent and protocol violations. Six patients randomised to deferasirox and one patient randomised to deferoxamine withdrew consent. The reasons for withdrawal of consent were not included in the database. Three patients randomised to deferoxamine were unwilling to comply with drug administration and were discontinued due to protocol violations. Two patients randomised to deferasirox and one patient randomised to deferoxamine could not be located for follow-up. The two groups were well balanced for age, transfusion type and history of prior chelation therapy (Table I). Baseline LIC and ferritin values in the two groups were similarly elevated.
Table I.
Patient demographics and selected clinical parameters.
| Variable/statistic | Deferasirox (n = 132) | Deferoxamine (n = 63) | All patients (n = 195) |
|---|---|---|---|
| Age, years | |||
| Median | 15 | 16 | 15 |
| Range | 3–54 | 3–51 | 3–54 |
| Age group, % | |||
| <6 years | 3·0 | 4·8 | 3·6 |
| 6 to <12 years | 22·7 | 23·8 | 23·1 |
| 12 to <16 years | 25·0 | 20·6 | 23·6 |
| 16 to <50 years | 47·7 | 49·2 | 48·2 |
| 50 to <65 years | 1·5 | 1·6 | 1·5 |
| Sex, % | |||
| Female | 60·6 | 55·6 | 59·0 |
| Race, % | |||
| Caucasian | 6·1 | 4·8 | 5·6 |
| Black | 89·4 | 93·7 | 90·8 |
| Others | 4·5 | 1·6 | 3·6 |
| Ferritin, μg/l | |||
| Median | 3460 | 2834 | 3298 |
| Min–max | 1082–12 901 | 1015–15 578 | 1015–15 578 |
| Baseline ALT, % | |||
| ≤2·5 ULN | 83·3 | 92·1 | 86·2 |
| >2·5 ULN | 15·9 | 7·9 | 13·3 |
| Missing | 0·8 | – | 0·5 |
| History of hepatitis B and/or C, % | |||
| Present | 7·6 | 6·3 | 7·2 |
| Prior chelation therapy, % | |||
| Deferoxamine or deferiprone | 62·9 | 60·3 | 62·1 |
| Blood transfusions during study (units of packed red blood cells) | |||
| Median | 12 | 12 | 12 |
| Range | 0–24 | 1–22 | 0–24 |
ALT, alanine aminotransferase; ULN, upper limit of normal.
Drug administration and compliance
The doses of deferasirox and deferoxamine were based on the baseline LIC determined by SQUID biosusceptometry converted by the adopted wet-to-dry weight factor of 3·33. The percentage of patients falling into each dose category was similar for the two treatment arms (Table II). The mean doses of deferasirox administered were approximately half those of deferoxamine. During the study the ratios of the administered to intended doses of therapy were high (1·16 for deferasirox and 0·97 for deferoxamine), indicating high adherence to the prescribed treatment regimens.
Table II.
Dosing algorithm according to baseline liver iron concentration (LIC) groups (reported and adjusted) and average daily doses administered.
| Baseline LIC (mg Fe/g dw) | ||||
|---|---|---|---|---|
| Baseline LIC group | ≤3 | >3–7 | >7–14 | >14 |
| Deferasirox (n = 132) | (n = 4) | (n = 64) | (n = 46) | (n = 18) |
| Protocol assigned dose | 5 mg/kg | 10 mg/kg | 20 mg/kg | 30 mg/kg |
| Reported mean LIC ± SD* | 2·5 ± 0·4 | 7·9 ± 5·5 | 9·8 ± 1·9 | 17·5 ± 3·0 |
| Adjusted mean LIC ± SD | 5·0 ± 0·8 | 15·8 ± 11·0 | 19·6 ± 3·8 | 35·0 ± 6·0 |
| Deferasirox dose (mg/kg)† | 9·5 ± 3·2 | 13·0 ± 3·1 | 19·7 ± 2·1 | 28·0 ± 2·8 |
| Min–max deferasirox dose | 5·0–12·3 | 8·4–23·9 | 10·0–24·5 | 22·8–30·0 |
| Deferoxamine (DFO, n = 63) | (n = 6) | (n = 21) | (n = 20) | (n = 16) |
| Protocol assigned dose† | 20–30 mg/kg | 25–35 mg/kg | 35–50 mg/kg | ≥50 mg/kg |
| Reported mean LIC ± SD | 3·9 ± 3·5 | 5·2 ± 2·1 | 8·6 ± 3·0 | 14·3 ± 5·4 |
| Adjusted mean LIC ± SD | 7·8 ± 7·0 | 10·4 ± 4·2 | 17·2 ± 6·0 | 28·6 ± 10·8 |
| DFO dose (mg/kg)‡ | 22·9 ± 3·9 | 28·7 ± 3·2 | 36·6 ± 9·5 | 50·0 ± 7·3 |
| Min–max DFO dose | 20·0–29·5 | 21·6–34·4 | 7·0–52·6 | 32·4–62·0 |
| Deferasirox/DFO dose ratio | 1:2·4 | 1:2·2 | 1:1·85 | 1:1·8 |
For the reported LIC values a correction factor of 3·33 was used to convert the wet weight to dry weight values (Brittenham et al, 1982); for the adjusted values a correction factor of 6·66 was used (Olivieri & Brittenham, 1997).
Average daily doses are reported for the 1-year period of drug administration. For deferoxamine the reported doses are normalised to those administered with a 5-d treatment regimen.
Patients in the two lower LIC groups (LIC ≤ 3 and >3–7 mg Fe/g dw) who were randomised to treatment with deferoxamine were allowed to continue on their current deferoxamine dosage, even if this was higher than recommended in this table.
Safety and tolerability
Adverse events, irrespective of the relationship to study medication, which occurred in more than 10% of patients receiving either treatment, are shown in Table III. As arbitrarily defined by an increased frequency of at least 5% indicating a potential relationship to drug administration, adverse events observed more commonly with deferasirox included abdominal pain, nausea, vomiting, diarrhoea, back pain and skin rash. Those observed more commonly in patients receiving deferoxamine included cough, nasopharyngitis and viral infection. A similar incidence of several adverse events, such as headache and upper respiratory tract infection, was observed with both drugs, indicating a possible relationship to the underlying disease or to the administration of both drugs.
Table III.
Percentage of adverse events reported with greater than 10% frequency in either arm.
| Adverse events, % | Deferasirox (n = 132) | Deferoxamine (n = 63) | All patients (n = 195) |
|---|---|---|---|
| Sickle cell anaemia with crisis | 33·3 | 31·7 | 32·8 |
| Headache | 28·8 | 33·3 | 30·3 |
| Abdominal pain | 28·0 | 14·3 | 23·6 |
| Nausea | 22·7 | 11·1 | 19·0 |
| Pyrexia | 21·2 | 17·5 | 20·0 |
| Vomiting | 21·2 | 15·9 | 19·5 |
| Diarrhoea | 19·7 | 4·8 | 14·9 |
| Back pain | 18·2 | 5·9 | 17·4 |
| Upper respiratory tract infection | 18·2 | 19·0 | 18·5 |
| Arthralgia | 15·2 | 14·3 | 14·9 |
| Pain in extremity | 14·4 | 12·7 | 13·8 |
| Pharyngolaryngeal pain | 14·4 | 9·5 | 12·8 |
| Cough | 13·6 | 20·6 | 15·9 |
| Nasopharyngitis | 13·6 | 20·6 | 15·9 |
| Rash | 13·6 | 4·8 | 10·8 |
| Constipation | 9·8 | 14·3 | 11·3 |
| Chest pain | 9·1 | 12·7 | 10·3 |
| Viral infection | 4·5 | 11·1 | 6·7 |
The gastrointestinal adverse events that patients experienced with deferasirox were generally transient in nature and lasted about 1 week maximum. The number of patients receiving deferasirox and deferoxamine that reported serious adverse events was similar (46·2% and 42·9% respectively) and the most common serious adverse event in both groups was sickle cell anaemia with crisis (33·3% and 31·7% respectively).
Haematological parameters were similar in the deferasirox and deferoxamine groups. Mild, stable increases in serum creatinine were observed in 36·4% and 22·2% of patients receiving deferasirox and deferoxamine respectively. Change in the mean ± SD (range) creatinine from baseline to end-of-study was 6·30 ± 9·00 μmol/l (−8·90 to 39·80 μmol/l) with deferasirox and 3·06 ± 9·43 μmol/l (−17·80 to 22·10 μmol/l) with deferoxamine. The number of patients with serum creatinine values that also exceeded the ULN was similar in the two groups (2·3% and 3·2% respectively). Approximately 30% of patients in both the deferasirox and deferoxamine groups had urinary protein to creatinine ratios of >0·2 mg/mg at baseline, and this percentage did not change measurably in either group over the course of the study.
Two consecutive alanine aminotransferase (ALT) levels >5× ULN were observed in five patients (3·8%) treated with deferasirox and in none treated with deferoxamine. Three of these individuals treated with deferasirox had at least one ALT level >5× ULN during the screening period. The elevations were transient in four patients even with continued drug administration. In one patient, a persistently elevated ALT led to interruption of deferasirox therapy. After normalisation of the ALT level the individual was rechallenged with deferasirox and the elevation in ALT recurred. Treatment was permanently discontinued and the ALT returned to normal.
Paediatric growth across the various age groups of children assessed by growth velocity was similar with deferasirox and deferoxamine. The change in growth velocity in patients <6, 6 to <12 and 12–16 years was 7·1 ± 1·7, 6·0 ± 3·2 and 4·6 ± 4·0 cm/year, respectively, for those treated with deferasirox versus 6·1 ± 1·9, 4·8 ± 2·5 and 3·4 ± 3·8 cm/year, respectively, for those treated with deferoxamine. In addition, there were no differences in sexual maturity between patients receiving deferasirox and deferoxamine as assessed by Tanner stage (Tanner, 1978).
Efficacy
Iron intake was calculated from the total amount of red blood cells transfused as previously described (Cappellini et al, 2006). For exchange transfusions, the mg amount of iron removed was calculated based on the volume of packed red blood cells removed (1 ml red blood cells removed equals 1·08 mg iron). The mean and median amount of iron administered in the form of blood transfusions during the study was nearly identical in patients receiving deferasirox and deferoxamine (0·22 mg/kg/d and 0·23 mg/kg/d respectively). The majority of patients (58·5%) received simple transfusions during the course of the study. The remaining patients received either exchange transfusions alone (18·5%), a combination of simple and exchange transfusions (21·0%), or no transfusions (2·1%).
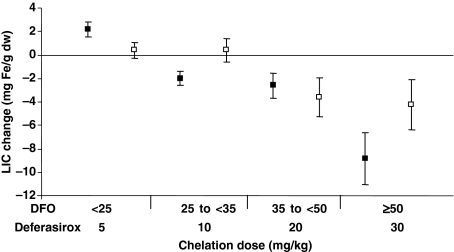
Administration of deferasirox resulted in a statistically significant reduction in LIC for the overall population, adjusted for transfusion category, of −3·0 ± 6·2 mg Fe/g dw (P < 0·001). This was similar to the reduction observed with deferoxamine for the overall population, adjusted for transfusion category, of −2·8 ± 10·4 mg Fe/g dw (P = 0·022). Statistically significant reductions in LIC at the level of P < 0·05 were observed with treatment at deferasirox doses of 10–30 mg/kg and at a deferoxamine dose of 35 to <50 mg/kg (Fig 1). Similar reductions in LIC occurred across all age groups of patients enroled into the trial.
Fig 1.
Adjusted change (mean ± SD) in liver iron concentration (LIC) according to assigned treatment category for deferasirox (solid squares) and deferoxamine (DFO, open squares). Statistically significant reductions in LIC were observed with deferasirox in the dose groups treated with 10 (n = 60), 20 (n = 45) and 30 (n = 14) mg/kg with P-values of 0·001, 0·014, and <0·001 respectively.
Comparing patients who were categorised as only receiving simple transfusions to those only receiving exchange transfusions, the reductions in LIC observed with deferasirox were −1·6 ± 5·78 mg Fe/g dw (n = 62) and −6·6 ± 5·60 mg Fe/g dw (n = 22) respectively. The corresponding reductions in patients receiving deferoxamine were −1·4 ± 3·12 mg Fe/g dw (n = 35) and −1·4 ± 3·90 (n = 10) respectively.
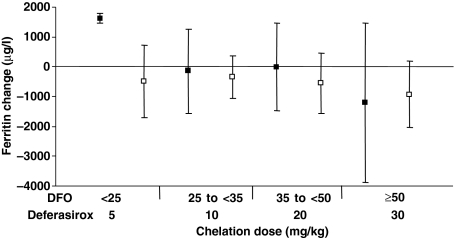
Changes in serum ferritin between baseline and end-of-study were dose dependent and generally paralleled the changes in LIC observed (Fig 2). However, there was notable intra-patient variability in this parameter during the study. In the overall patient populations treated with deferasirox and deferoxamine, the changes observed were −183 ± 1651 μg/l and −558 ± 951 μg/l respectively.
Fig 2.
Change (mean ± SD) in serum ferritin according to assigned baseline liver iron concentration (LIC) category for deferasirox (solid squares) and deferoxamine (DFO, open squares).
Discussion
Sickle cell disease results in acute complications and progressive multi-organ failure. Transfusion therapy reduces the number of erythrocytes containing sickle haemoglobin and reduces the vascular complications associated with the disease. In the STOP trial, which recruited asymptomatic children at risk for cerebral infarction, transfusions resulted in a 90% reduction in the rate of first stroke (Adams et al, 1998). In addition to preventing primary and secondary stroke in children, preventative transfusion programmes decrease the frequency of total hospitalisations, vasoocclusive events, acute chest syndrome and growth failure (Vichinsky et al, 2005). Chronic transfusions have been recommended in order to decrease the morbidity of end-organ failure associated with heart, pulmonary and renal disease in children and adults (National Institutes of Health, 2002).
Despite the significant benefits that such transfusions may bring, they result in iron overload that is clearly associated with morbidity and mortality in β-thalassaemia (Gabutti & Piga, 1996; Schrier & Angelucci, 2005). Evidence is also accumulating regarding the deleterious effects of iron overload in patients with sickle cell disease. This transfusion-related complication is estimated to affect a sizable number of sickle cell patients (Hagar & Vichinsky, 2000). As indicated by the baseline LIC and serum ferritin levels of patients enroled into this clinical trial, patients with sickle cell disease receiving blood transfusions may have markedly elevated iron burdens. These levels are similar to those commonly encountered in β-thalassaemia major and may place them at risk for serious complications.
This comparative study demonstrated that deferasirox was reasonably well tolerated in patients with sickle cell disease who have relatively normal renal and hepatic function. There was no excess in sickle cell crises or infections when compared with deferoxamine. Although the absolute magnitude of the reduction may be under- or overestimated because biosusceptometry using SQUID, rather than liver biopsy, was utilised in order to determine the change in LIC, similar reductions in LIC were observed with deferasirox and deferoxamine in the overall populations and across all age groups. As would be expected, greater reductions in LIC were observed with deferasirox in patients receiving exchange transfusions. A similar trend was not observed with deferoxamine, possibly reflecting the relatively small sample size. Changes in serum ferritin were somewhat more variable than those observed in LIC, as would be expected for this parameter in patients with sickle cell disease (Brittenham et al, 1993).
The most notable adverse events reported were transient gastrointestinal symptoms and skin rash. The gastrointestinal symptoms observed might be, at least in part, related to the presence of lactose in the formulation of deferasirox. Tablets contain lactose in an approximately 1:1 ratio by weight to the active drug substance deferasirox. Mild, stable increases in serum creatinine were observed in a greater percentage of patients receiving deferasirox than those receiving deferoxamine, although the percentage of patients with serum creatinine values above the normal range was similar in both groups. A dose reduction algorithm for increased serum creatinine resulted in stabilisation or normalisation of this parameter in all patients. Reversible increases in ALT were seen in a relatively small percentage of patients, and were not associated with changes in alkaline phosphatase or bilirubin levels. Such elevations in ALT may be associated with hepatic iron toxicity itself (Jensen et al, 2003). However, at least one patient enroled in this trial did appear to have elevations in ALT that were caused by deferasirox administration. This conclusion was based on the isolated recurrence of elevated aminotransferase upon rechallenge with deferasirox. Drug-induced hepatitis was not confirmed because the ALT values returned to normal within a short enough timeframe, so a liver biopsy was not performed.
Although these data indicate that deferasirox was well tolerated in individuals with sickle cell disease who had normal baseline serum creatinine levels, the safety of deferasirox in sickle cell patients with abnormal serum creatinine is unknown. Further studies will be required in this regard. In addition, whereas iron chelation may be required for many years or even life-long, the duration of this study was only 1 year. Therefore, long term follow-up of patients receiving continued treatment is required, and this is currently ongoing in an extension of the present study.
Compliance with the administration of parenteral deferoxamine chelation therapy has proved challenging to all groups of patients with transfusional iron overload (Cappellini et al, 2006). This is particularly true for patients with sickle cell disease (Treadwell et al, 2005). Treatment adherence to therapy in the present study was mandated by the study protocol and was similar in both treatment groups. However, in routine clinical practice, compliance with a once-daily, oral regimen would be expected to be superior to compliance with a parenteral regimen. Such improved compliance with therapy should lead to a reduction in morbidity and mortality from iron overload.
When combined with appropriate laboratory monitoring, the availability of deferasirox as a once-daily, oral option for safe and effective chelation therapy has the potential to prevent complications of iron overload. In paediatric and adult patients with sickle cell disease this should consequently help prevent serious complications, such as stroke and organ failure, by facilitating the appropriate use of blood transfusions.
Appendix
Appendix I.
Participating centres and investigators.
| Canada | Olivieri N, Toronto General Hospital, Toronto. |
| France | Bachir D, Hôpital Henri Mondor, Créteil; Bernaudin F, Centre Hospitalier Intercommunal, Créteil; de Montalembert M, Hôpital Necker, Paris. |
| Italy | Cappellini MD, Ospedale Maggiorre-IRCCS, Milan; Cianciulli P, Ospedale Galliera, Rome; Forni GL, Centro della Microcitemia, Ospedale Galliera, Genoa; Lombardo T, Ospedale S Bambino, Catania; Magnano C, Az. Osp. Di Rilievo Nazionale e di Alta, Cantania. |
| UK | Okpala I, St. Thomas Hospital, London; Porter J, University College Hospital, London. |
| USA | Adewoye A, Boston Medical Center, Boston; Bellevue R, New York Methodist Hospital, Brooklyn, NY; Benjamin L, Montefiore Hospital, Bronx, NY; Cataland S, Ohio State University, Columbus, OH; Clowney B, Santee Hematology/Oncology, Sumter, SC; Coates T, Children's Hospital Los Angeles, CA; Cruz J, Wake Forest University, Winston-Salem, NC; Eckman J, Emory University School of Medicine, Atlanta, GA; Frankel L, Scott & White Memorial Hospital, Temple; Freiberg A, Penn State Milton S Hershey Medical Center, Hershey, PA; Gardner R, Children's Hospital, New Orleans, LA; Giardina P, Weill Medical College of Cornell University, New York, NY; Gonzalez F, Liberty Hematology Oncology Center, Columbia, SC; Hassell K, University of Colorado Health Sciences Center, Denver, CO; Heeney M, Children's Hospital of Boston, Boston, MA; Kelly P, Children's Hospital Medical Center, Cincinnati, OH; Krishnamurthi L, Children's Hospital of Pittsburgh, Pittsburgh, PA; Kutlar A, Medical College of Georgia, Augusta, GA; Kwiatkowski J, Children's Hospital of Philadelphia, Philadelphia, PA; Labotka R, University of Illinois at Chicago, Chicago, IL; Lane P, Emory University School of Medicine, Atlanta, GA; Mathias L, Loma Linda University Medical Center, Loma Linda, CA; Mueller, BU, Texas Children's Hospital, Houston, TX; Nuss R, University of Colorado Health Science Center, Denver; Onyekwere O, Howard University, Washington, DC; Owen W, Children's Hospital of the King's Daughter, Norfolk, VA; Prasannan L, Scott and White Memorial Hospital and Clinics, Temple, TX; Rice L, The Methodist Hospital, Houston, TX; Scher C, Tulane University, New Orleans, LA; Swerdlow P, Wayne State University School of Medicine, Detroit, MI; Tebbi C, Tampa Children's Hospital at St. Joseph's, Tampa, FL; Thompson A, Children's Memorial Hospital, Chicago, IL; Vichinsky E, Children's Hospital and Research Center at Oakland, Oakland, CA; Wilson F, University of South Alabama, Mobile, AL; Wise R, Palmetto Health, Columbia, SC; Yasin Z, University of Cincinnati, Cincinnati OH. |
References
- Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, Abboud M, Gallagher D, Kutlar A, Nichols FT, Bonds DR, Brambilla D. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. New England Journal of Medicine. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Seminars in Hematology. 2001;38(Suppl. 1):30–36. doi: 10.1016/s0037-1963(01)90058-7. [DOI] [PubMed] [Google Scholar]
- Brittenham GM, Farrel DE, Harris JW, Feldman ES, Danish EH, Muir WA, Tripp JH, Bellon EM. Magnetic susceptibility measurement of human iron stores. New England Journal of Medicine. 1982;307:1671–1675. doi: 10.1056/NEJM198212303072703. [DOI] [PubMed] [Google Scholar]
- Brittenham GM, Cohen AR, McLaren CE, Martin MB, Griffith PM, Nienhuis AW, Young NS, Allen CJ, Farrell DE, Harris JW. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. American Journal of Hematology. 1993;42:81–85. doi: 10.1002/ajh.2830420116. [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, Aydinok Y, Kattamis A, Kilinc Y, Porter J, Capra M, Galanello R, Fattoum S, Drelichman G, Magnano C, Verissimo M, Athanassiou-Metaxa M, Giardina P, Kourakli-Symeonidis A, Janka-Schaub G, Coates T, Vermylen C, Olivieri N, Thuret I, Opitz H, Ressayre-Dfaffer C, Marks P, Alberti D. A Phase III study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- Fischer R, Harmatz P, Nielsen P. Does liver biopsy overestimate liver iron concentration? Blood. 2006;108:1775–1776. doi: 10.1182/blood-2006-03-013482. [DOI] [PubMed] [Google Scholar]
- Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematologica. 1996;95:26–36. doi: 10.1159/000203853. [DOI] [PubMed] [Google Scholar]
- Hagar RW, Vichinsky EP. Major changes in sickle cell disease. Advances in Pediatrics. 2000;47:249–272. [PubMed] [Google Scholar]
- Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. The Journal of the American Medical Association. 1983;249:1743–1745. [PubMed] [Google Scholar]
- Jensen PD, Jensen FT, Christensen T, Nielsen JL, Ellegaard J. Relationship between hepatocellular injury and transfusional iron overload prior to and during iron chelation with desferrioxamine: a study in adult patients with acquired anemias. Blood. 2003;101:91–96. doi: 10.1182/blood-2002-06-1704. [DOI] [PubMed] [Google Scholar]
- Manci EA, Culberson DE, Yang YM, Gardner TM, Powell R, Haynes J, Jr, Shah AK, Mankad VN. Investigators of the Cooperative Study of sickle cell disease. Causes of death in sickle cell disease: an autopsy study. British Journal of Haematology. 2003;123:359–365. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- McLaren GD, Muir WA, Kellermeyer RW. Iron overload disorders: natural history, pathogenesis, diagnosis, and therapy. Critical Reviews in Clinical Laboratory Sciences. 1983;19:205–266. doi: 10.3109/10408368309165764. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Management and Therapy of Sickle Cell Disease. National Heart, Lung, and Blood Institute; 2002. URL http://www.nhibi.nih.gov(NIH Publication No. 96-2117:153–160) [Google Scholar]
- Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- Porter JB. Practical management of iron overload. British Journal of Haematology. 2001;115:239–252. doi: 10.1046/j.1365-2141.2001.03195.x. [DOI] [PubMed] [Google Scholar]
- Schrier SL, Angelucci E. New strategies in the treatment of thalassemias. Annual Review of Medicine. 2005;56:157–171. doi: 10.1146/annurev.med.56.082103.104718. [DOI] [PubMed] [Google Scholar]
- Styles LA, Vichinsky E. Effects of a long-term transfusion regimen on sickle cell-related illnesses. Journal of Pediatrics. 1994;125:909–911. doi: 10.1016/s0022-3476(05)82006-2. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Fetus into Man: Physical Growth from Conception to Maturity. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- The Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP2) Trial Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. New England Journal of Medicine. 2005;353:2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- Treadwell MJ, Law AW, Sung J, Hackney-Stephens E, Quirolo K, Murray E, Glendenning GA, Vichinsky E. Barriers to adherence of deferoxamine usage in sickle cell disease. Pediatric Blood and Cancer. 2005;44:500–507. doi: 10.1002/pbc.20290. [DOI] [PubMed] [Google Scholar]
- Vanorden HE, Hagemann TM. Deferasirox — an oral agent for chronic iron overload. Annals of Pharmacotherapy. 2006;40:1110–1117. doi: 10.1345/aph.1G566. [DOI] [PubMed] [Google Scholar]
- Vichinsky E, Butensky E, Fung E, Hudes M, Theil E, Ferrell L, Williams R, Louie L, Lee PD, Harmatz P. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. American Journal of Hematology. 2005;80:70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- Wanko SO, Telen MJ. Transfusion management in sickle cell disease. Hematology/Oncology Clinics of North America. 2005;19:803–826. doi: 10.1016/j.hoc.2005.07.002. [DOI] [PubMed] [Google Scholar]