Abstract
Previous studies have shown a blunted ventilatory response to hypercapnia in mdx mice older than 7 months. We test the hypothesis that in the mdx mice ventilatory response changes with age, concomitantly with the increased functional impairment of the respiratory muscles. We thus studied the ventilatory response to CO2 in 5 and 16 month-old mdx and C57BL10 mice (n = 8 for each group). Respiratory rate (RR), tidal volume (VT), and minute ventilation (VE) were measured, using whole-body plethysmography, during air breathing and in response to hypercapnia (3, 5 and 8% CO2). The ventilatory protocol was completed by histological analysis of the diaphragm and intercostals muscles. During air breathing, the 16 month-old mdx mice showed higher RR and, during hypercapnia (at 8% CO2 breathing), significantly lower RR (226 ± 26 vs. 270 ± 21 breaths/min) and VE (1.81 ± 0.35 vs. 3.96 ± 0.59 ml min−1 g−1)(P < 0.001) in comparison to C57BL10 controls. On the other hand, 5 month-old C57BL10 and mdx mice did not present any difference in their ventilatory response to air breathing and to hypercapnia. In conclusion, this study shows similar ventilation during air breathing and in response to hypercapnia in the 5 month-old mdx and control mice, in spite of significant pathological structural changes in the respiratory muscles of the mdx mice. However in the 16 month-old mdx mice we observed altered ventilation under air and blunted ventilation response to hypercapnia compared to age-matched control mice. Ventilatory response to hypercapnia thus changes with age in mdx mice, in line with the increased histological damage of their respiratory muscles.
Keywords: Age, Duchenne muscular dystrophy, Hypercapnia mdx mouse, Ventilatory response
Introduction
Duchenne muscular dystrophy (DMD) is the most common fatal X-linked recessive disorder in humans, affecting approximately 1 in 3,500 live males. The disease is caused by mutations in the dystrophin gene (Mugneret et al. 1988), which encodes a large (427 kDa) cytoskeletal protein that is normally found at the sarcolemma, on the cytoplasmic side of the muscle cell membrane. These mutations lead to progressive weakness of all skeletal muscles, including the diaphragm and the other respiratory muscles, as assessed by maximal respiratory pressure and endurance measurements (Hahn et al. 1997; Matecki et al. 2001). The ventilatory insufficiency, caused by the dysfunction of the respiratory muscles, is, thus, a central problem in the management of DMD patients. Over the last decade, three main therapeutic approaches have been proposed to correct or to compensate for the lack of dystrophin and they have been extensively described in different reviews (Chakkalakal et al. 2005; Radley et al. 2007; Mouly et al. 2005; Skuk and Tremblay 2000). The first one, i.e., the molecular therapy, involves the delivery of the dystrophin gene to dystrophic fibers through the use of plasmid (Wolff et al. 1992) or viral vectors (Amalfitano and Parks 2002) or the correction of the mutant dystrophin via exon skipping (Goyenvalle et al. 2004). The second one is based on the delivery of muscle precursor cells to the dystrophic muscle (Mendell et al. 1995) or on the systemic delivery of stem cells with myogenic potential (Gussoni et al. 1999). The third one is a pharmacological approach based on the up-regulation of other proteins, which are endogenously expressed in dystrophic muscles and could, thus, compensate for the lack of dystrophin (Chakkalakal et al. 2004; Voisin et al. 2005). In parallel, other authors have proposed complementary therapies to try to decrease the degree of muscle wasting in DMD patients, such as exercise training (Matecki et al. 2001; Wanke et al. 1994), low-frequency electrical stimulation (Zupan 1992)or administration of different drugs (Fenichel et al. 1991, 1997; Politano et al. 2003; Tarnopolsky et al. 2004).
Before using in DMD patients, the efficacy of all these therapies should be tested on a murine model of DMD such as the mdx mouse, which lacks dystrophin due to a nonsense point mutation in exon 23 of the Dystrophin gene (Sicinski et al. 1989). Although, the majority of skeletal muscles of the mdx mouse show little fibrosis or functional alteration until late in life (Louboutin et al. 1993; Pastoret and Sebille 1995), their diaphragm presents major fibrosis and myofiber loss from an early age, as well as a greatly impaired contractile function (Petrof et al. 1993; Stedman et al. 1991). There is, thus, a much greater phenotypic resemblance between the human DMD muscles and the diaphragm of the mdx mice as compared to any other mdx muscle (Stedman et al. 1991). For this reason, mdx diaphragm function should be a main target to appreciate the efficiency of the different therapies on the DMD muscles’ functions.
Currently to appreciate the effects of different therapies, the major way to evaluate the two main parameters of the diaphragm contractile properties, i.e., force and endurance, is by ex vivo assessment of their maximal isometric twitch force, maximal isometric tetanic force and resistance to fatigue (Petrof et al. 1993; Matecki et al. 2005). It has also been shown that ventilatory function in vivo could be assessed by measuring the ventilatory parameters at rest, and in response to hypercapnia. Indeed, the in vivo ventilatory function depends on the neural control and drive, as well as on the blood flow regulation and muscle force production. However our knowledge about the in vivo respiratory physiology, even in well-known mouse models as the mdx mice, is very restricted. Dupont-Versteegden et al (Dupont-Versteegden et al. 1994; Dupont-Versteegden 1996)have reported decreased minute ventilation (VE) at rest in mdx mice, when compared to C57BL10 controls. Furthermore, Gosselin et al. 2003 showed in mdx mice older than 7 months of age, lower VE than C57BL10 controls and, specifically, a blunted ventilatory response to hypercapnia.
As muscle force production decreases with time, the age of the mdx mice at the beginning of a therapeutic trial should be considered as a fundamental parameter in the evaluation of the effect of any treatment in relation to the muscle changes. Thus, to optimize the in vivo assessment of the function of their respiratory muscles, it should be important to evaluate how their ventilatory function changes with age. Indeed, many studies (Louboutin et al. 1993; Petrof et al. 1993; Stedman et al. 1991; Lefaucheur et al. 1995) have reported that the extent of damage of the diaphragm in mdx mice is different according to their age. Indeed, the diaphragm of mdx mice undergoes with age progressive degeneration and fibrosis. Besides, older mdx mice present more necrosis and decreased contractile properties compared to young adult ones. We thus make hypothesis that the increased damage of the respiratory muscles could induce changes in ventilation during air breathing and in response to hypercapnia in older compared to younger mdx mice. The aim of our study was thus to compare the ventilation during air breathing and the ventilatory response to hypercapnia in 5 month-and 16 month-old C57BL10 and mdx mice. These tests were complemented by the histological analysis of the diaphragm and intercostal muscles.
Materials and methods
Animals
Breeding pairs of C57BL10 mice were from our own production and mdx mice were purchased from A. Sebille (France). The respiratory test was performed on 5 month-old C57BL10 controls (26.6 g ± 1.3) and mdx mice (26.7 g ± 3.0) (n = 8 for each group) and on 16 month-old C57BL10 controls (31.3 g ± 1.4) and mdx mice (33.5 g ± 1.3) (n = 8 for each group). There were 29 males and three females. All mice were kept in their cages and housed at room temperature. They had access to food and water ad libitum. The diurnal cycle was regulated by natural lighting. All animal procedures were performed in accordance with institutional guidelines.
Histology
The diaphragm of each mouse was partitioned and rolled up. An intercostal section, which comprised the ribs and the attached intercostals muscles (internal and external), was dissected from three 5 month-old and three 16 month-old mice for each group (i.e., controls and mdx mice). The samples were quickly snap-frozen in isopentane, cooled in liquid nitrogen and then stored at −80C. Only the right portion of the diaphragm and the right intercostal section were used for the histological analysis.
Samples were cut into 10 lm thick transverse sections with a cryostat at −20C. Five unfixed cryostat sections from the middle part of the frozen muscle sample were incubated with Haematoxylin (Sigma). After washing with distilled water, sections were incubated with Eosin (Sigma). After a final wash, slides were mounted with Mowiol and observed under a Nikon Optiphot-2 microscope. Image analysis was performed using the ImageTool IT3 software package. Cross-sections of muscle fibers, containing on average 200 fibers, were analyzed in three steps:
(a) estimate of the fiber size, (b) calculation of the percentage of muscle fibers containing centralized nuclei and (c) determination of the muscular area as a percentage of the total area. The parameter used for the determination of the fiber size was the minimal “Feret’s diameter” (i.e., the minimal distance of parallel tangents at opposing borders of the muscle fiber) according to recently published data (Briguet et al. 2004). The variance coefficient (VC) of the “Feret’s diameter” was defined as follows: VC = (standard deviation of the “Feret’s diameter “/mean muscle fiber size) . 1000. The muscular area (%) was evaluated by measuring the muscular fiber area compared to the total area.
Measurements
Ventilatory response was measured in unrestrained animals by barometric plethysmography. The whole-body plethysmograph (450 ml) (Buxco, Troy, NY) (Fig. 1), which has been previously adapted to be used with mice, is based on the Drorbaugh and Fenn’s principle (Drorbaugh and Fenn 1955; Hamelmann et al. 1997). This is a double-chamber plethysmograph which allows the use a barometric analysis technique that compares the pressure difference between the animal chamber and a reference chamber. This technique has been previously described (Hamelmann et al. 1997). Plethysmography of unrestrained animals allowed us to perform pulmonary measurements on conscious, freely-moving rodents without invasive surgery and anesthesia, thereby avoiding side effects and modifications of the observed values. Moreover, in order to avoid restraining the freely-moving animals, body temperature was assumed to be stable at 37C (Tankersley et al. 1993). The box was calibrated by injecting 150 ll of air into the chamber with a syringe. For long-term measurements in a plethysmograph with air or CO2, a Bias Flow Regulator (BUXCO) should be used to provide a quiet, constant, and smooth flow through the animal chamber to prevent an excessive rise in CO2 concentration due to re-breathing. In our chamber configuration, which included a pneumotachograph that generated a flow signal during respiration, the Bias Flow Regulator was set to push air or a CO2 mixture in and out of the box with a flow rate of 700 ml/min. A thermo-hygrometer (Testo 605-H1) was placed between the Bias Flow Regulator and the chamber to measure the temperature and the moisture percentage in the gas mixture (atmospheric and hypercapnic).
Fig. 1.
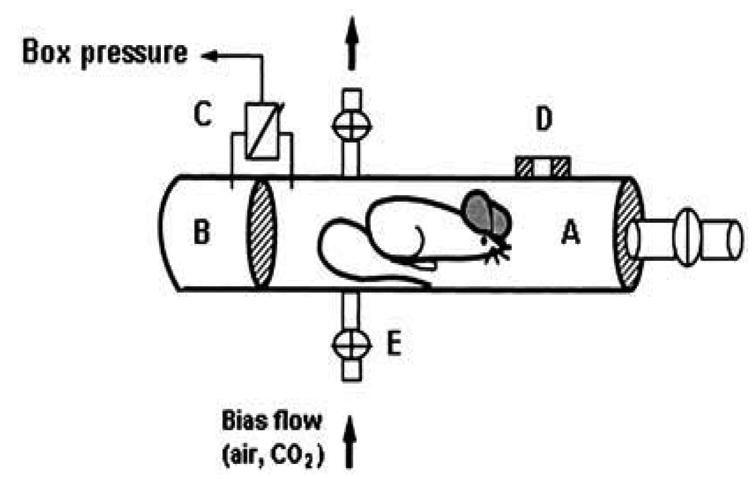
Schematic diagram of the whole-body plethysmograph. (A) Main chamber containing the mouse. (B) Reference chamber. (C) Pressure transducer connected to analyzer. (D) Pneumotachograph. (E) Inlet for bias flow with four-way stopcock
The respiratory stimuli were administered by replacing the airflow through the plethysmograph with three hypercapnic flows (3, 5 or 8% CO2; 21% O2; QSP N2). These hypercapnic mixtures were obtained commercially. A gas balloon was used to administer the gas mixture (air and hypercapnic). The animal was fully enclosed in the chamber, free from stress due to neck’s seals: it was free to move around within the enclosed space.
Protocol
Two plethysmographs, with the same temperature and moisture conditions, were used simultaneously with control and mdx mice placed in one or the other in a randomized way, to ensure the conditions of homogeneity between the two groups. Each plethysmograph was calibrated before every set of experiments. Each mouse was weighed and placed in the main chamber for a period of familiarization that lasted for at least 30 min, but was continued until the animal was quiet and motionless (mean familiarization time: 80 ± 14 min for the 5 month-old and 65 ± 27 min for the 16 month-old mice).
Each animal was first submitted to air breathing for 15 min; during this time we measured and recorded the baseline ventilatory parameters for 5 min. Then, each mouse was exposed to three levels of hypercapnia in normoxia with the following sequence, 3, 5 and 8% CO2 that were separated by periods of air breathing until ventilation returned to the baseline values. For each test, there was a 5-min familiarization period followed by the registration of the ventilatory parameters (5 min). The total duration of each recording session was of approximately 2 h 30 min. After the last test, the mice were removed from the plethysmograph, weighed and replaced in their cage. Each animal was tested at a given time, either in the morning or in the afternoon. In each group, the same numbers of animals were tested in the morning and in the afternoon. The animals were observed during the experiments, and the periods of high locomotor activity were noted.
Dry air and respiratory stimuli were administered by gas balloon. During all experiments, the gas from the cage was continuously sampled and analyzed using an O2 analyzer (Beckman OM11, France) and a CO2 analyzer (Cosma rubies 3000, France) to ensure constant gas level; the temperature and moisture percentage in the gas mixture were measured using a thermo-hygrometer.
Analysis of results and statistics
Valid data were selected on the basis of the visual inspection of the locomotor activity during the experiments. Indeed, freely-moving mice normally exhibit an exploratory behavior accompanied by sniffing, grooming and rearing. These activities alter the breathing pattern especially at rest, and this could affect the accuracy of ventilatory measurements. The ventilatory parameters of each test were thus registered during air breathing and in hypercapnic conditions by selecting data samples that were free from behavioral artifacts (Tankersley et al. 1993, 1997).
We measured the tidal volume (VT) and respiratory rate (RR) manually. The VE was calculated as a product of RR and VT. We then calculated the averages for all these parameters. VE and VT were normalized according to body weight.
We also registered the breathing pattern during the first and last minute of the 8% hypercapnic test to compare them.
Data were analyzed by three-way (age . disease . gas mixture) repeated measure analysis of variance with post hoc (Scheffé) analysis. Weight data were analyzed by two-way (age . disease) analysis of variance with post hoc (Scheffé) analysis. Histological parameters were analyzed using the Student’s t-test. Values for all measurements were expressed as means ± standard error of the mean (SEM); P values for significance were set at 0.05. All analyses were carried out using the Statistica program, version 6.1.188.0 (StatSoft, Inc.).
Results
Animals
We did not find any difference in the weight of the C57BL10 and mdx mice at the same age (i.e., 26.6 ± 1.3 vs. 26.7 ± 3.0 g for the 5 month-old and 31.3 ± 1.4 vs. 33.5 ± 1.3 g for the 16 month-old mice), although, the older mice were significantly heavier than the younger ones in both groups (P < 0.001).
Histological analysis
As shown in Figure 2, diaphragm sections from both 5 (b) and 16 (c) month-old mdx mice showed clear signs of dystrophic changes with extensive necrosis, myofiber loss and connective tissue proliferation. When compared to those of 5 month-old control mice (a), the 5 month-old mdx mice presented a greater variation in the size of muscles fibers (P < 0.001) and increased number of central nuclei (P < 0.001) and a significant decrease in the muscular area percentage (P < 0.001) (Table 1). An analysis of diaphragm sections from 16 month-old mdx mice revealed a lower number of central myonuclei (P < 0.01), less uniform fiber diameters (P < 0.001) and a decreased muscular area (P < 0.05), when compared to those of 5 month-old mdx mice. In Figure 3, sections from intercostal muscles of 5 (a) and 16 (b) month-old mdx mice are shown. Their analysis revealed that, in 16 month-old mdx mice, these muscles had a similar percentage of muscle fibers with centralized nuclei (i.e., 24% vs. 20%), a higher variance coefficient (i.e., 430 vs. 332), which indicates an increase in size variations of muscle fibers and a decreased muscular area (70% vs. 94%), when compared to the intercostal muscles of 5 month-old mdx animals.
Fig. 2.
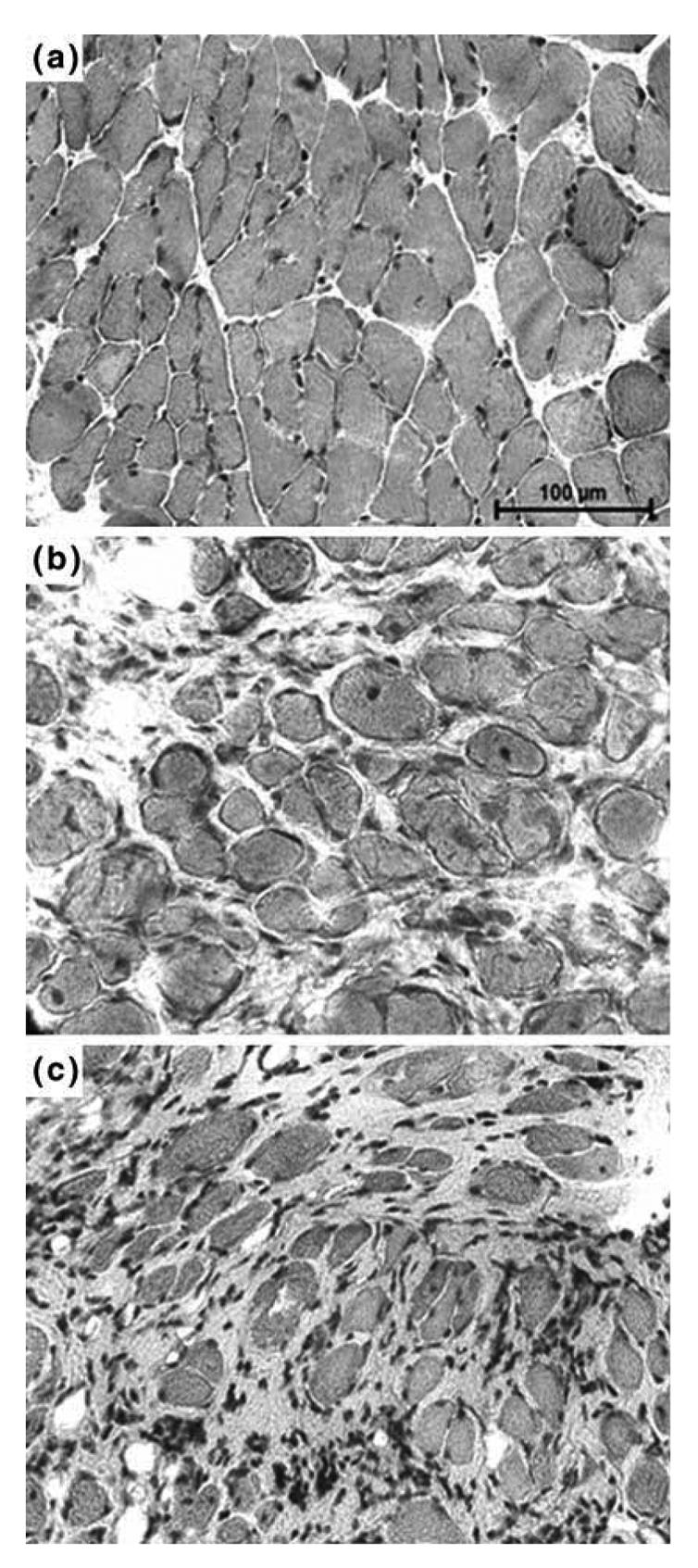
Histology of 5 month-old C57 and mdx mice and 16 month-old C57 mice. Cryostat sections of (a) 5 month-old C57 mice diaphragm, (b) 5 month-old mdx mice diaphragm and (c) 16 month-old mdx mice diaphragm were subjected to Haematoxylin and eosin staining
Table 1.
Histological analysis of diaphragms in mdx and C57 mice
| mdx mice | |||
|---|---|---|---|
| Diaphragm C57BL10 mice | 5 month-old | 5 month-old | 16 month-old |
| % Centralized nuclei (a) | 0.25 ± 0.5 | 20.6 ± 2.5*** | 14.1±0.4:II |
| 0.0–1 | 18.9–24.3 | 12.9–16.3 | |
| Variance coefficient “Feret’s diameter” (b) | 204.9 ± 6.6 | 393.6 ±15.2*** | 1000±64.2III |
| 170–270 | 322.2–589.8 | 793.4–1174.8 | |
| % Muscular area (c) | 96.2 ±1.3 | 48.5 ± 0.7*** | 32.6 ± 3.0I |
| 94.5–97.7 | 47.8–49.1 | 24.2–37.5 | |
Cryostat sections of diaphragms from 5 and 16 month-old mdx and 5 month-old C57 mice were subjected to quantitative analysis by histomorphometry. (a) Percentage of muscle fibers with centralized nuclei, (b) Variance coefficient (VC) of the minimal “Feret’s diameter” and (c) percentages of muscular area were compared in diaphragms. Values are means ± SEM; n = 8 mice/group. Comparison of the histological parameters between C57 vs. mdx 5 month-old mice:
P < 0.001; and between 5 and 16 month-old mdx mice: P < 0.05; P < 0.01; P < 0.001
Fig. 3.
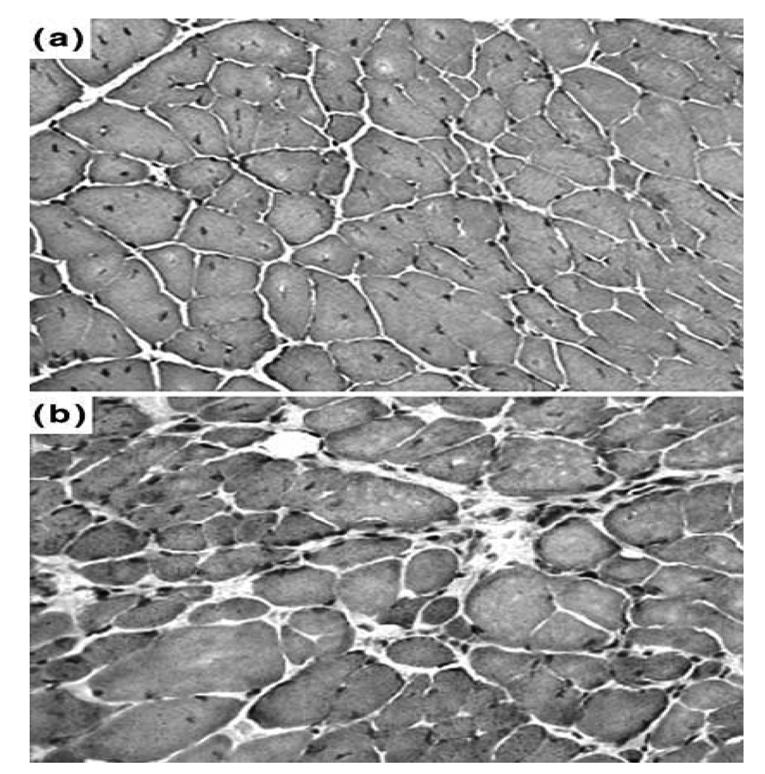
Histology of 5 and 16 month-old mdx mice. Cryostat sections of (A) 5 and (B) 16 month-old mdx mice intercostal muscles were subjected to Haematoxylin and eosin staining
Ventilatory parameters during air breathing and in response to hypercapnia
Room air breathing
There was no difference in the RR (135 ± 15 vs. 131 ± 19 breaths/min), VT (6.5 ± 1.7 vs. 5.8 ± 0.8 llg−1) and VE (0.86 ± 0.23 vs. 0.75 ± 0.08 ml min−1g−1)of 5 month-old mdx and 5 month-old C57BL10 mice (Fig. 4). However, when we compared the 16 month-old animals, the RR was significantly higher in mdx mice than in controls (151 ± 17 vs. 111 ± 11 breaths/min) (P < 0.01) and the VT tended to be weaker in the mdx group even though this difference was not statistically significant (5.1 ± 0.9 vs. 6.9 ±1.7 llg−1). VE was, thus, not significantly different between the two groups (0.76 ±0.12 vs. 0.75 ± 0.14 ml min−1g−1) (Fig. 5).
Fig. 4.
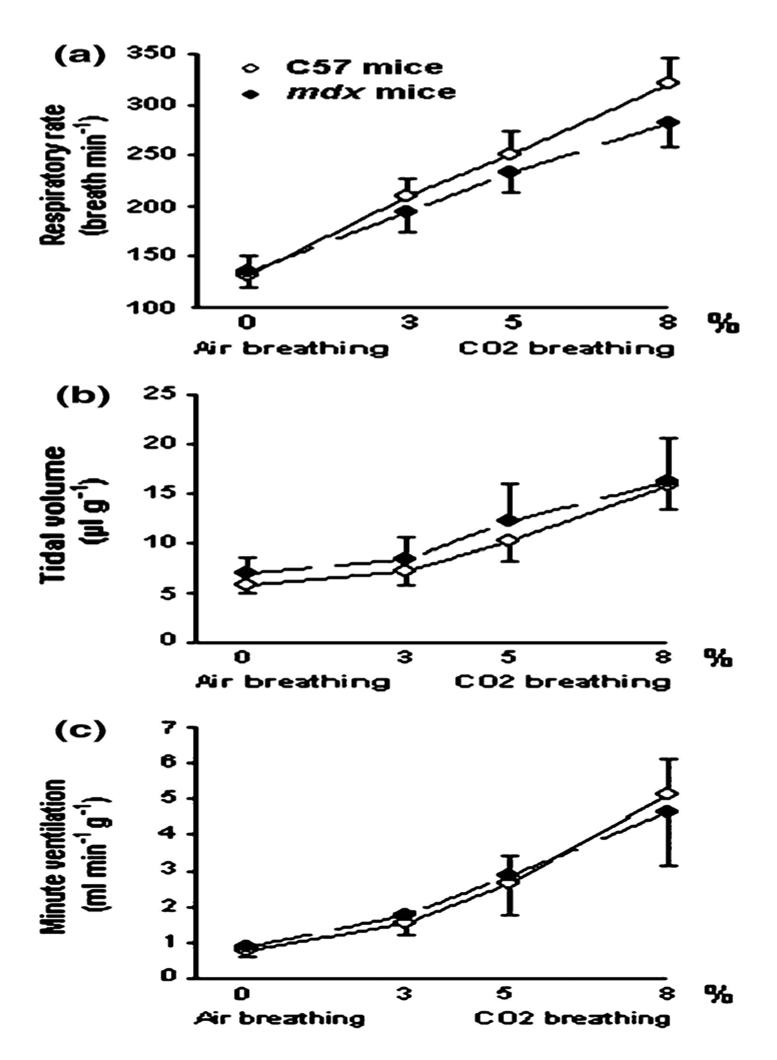
Ventilatory parameters in response to air and 3, 5 and 8% CO2 breathing in 5 month-old C57 and mdx mice. Significant difference between young adult C57 and young adult mdx mice: NS: non significant
Fig 5.
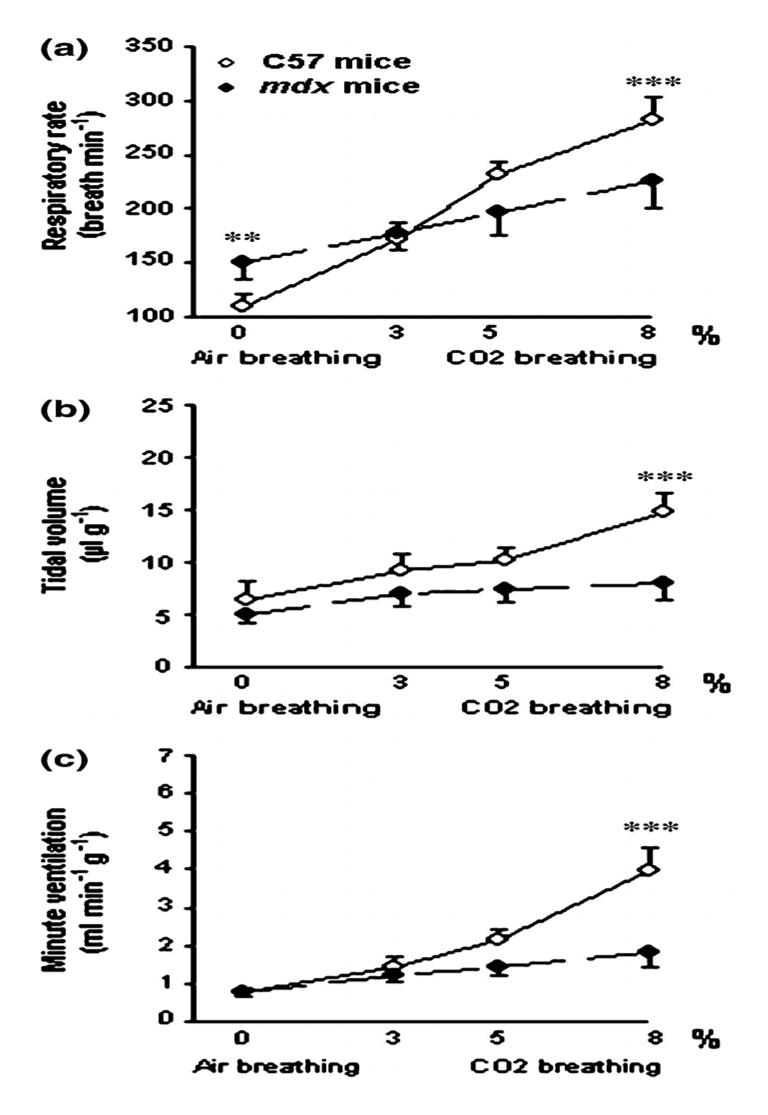
Ventilatory parameters in response to air and 3.5 and 8% CO2 breathing in 16 month-old C57 and mdx mice. Significant difference between old C57 and old mdx mice.**P<0.01; ***P <0.001.
Moreover, there were no significant differences in the ventilatory parameters of the 5 month and 16 month-old C57BL10 mice or of the 5 month and 16 month-old mdx animals (Figs. 4 and 5).
Adaptation to hypercapnia
As shown in Figure 4, in 5 month-old mice, no differences were observed in the ventilatory response to hypercapnia of both C57BL10 and mdx mice. Indeed, in both groups, the RR (a) increased significantly and progressively from its baseline value (i.e., during air breathing) upon stimulation with 3, 5 and 8% CO2 (P < 0.001). The VT (b) and VE (c) increased also during CO2 breathing, but this change was only significant in response to 8% CO2 (P < 0.001) compared to the values during air breathing.
On the contrary, in the 16 month-old mice, we observed tremendous differences in the ventilatory response to hypercapnia of the two groups (Fig. 5). No significant difference was reported for the breathing pattern between the first and the last minute of the 8% CO2 hypercapnic test. In the older mice, although the RR (a) was higher at 8% than at 3% CO2 or during air breathing (P < 0.001), the VT (b) and VE (c) did not change during CO2 breathing. Thus, when we compared the 16 month-old mdx and the 16 month-old C57BL10 mice at 8% CO2 breathing, as illustrated in Fig. 5, the RR (226 ± 26 vs. 270 ± 21 breaths/min) (a), VT (8.0 ± 1.8 vs. 14.9 ± 1.8 ll g−1) (b) and, therefore, the VE (1.81 ± 0.35 vs. 3.96 ± 0.59 ml min−1g−1) (c) were significantly lower in the mdx mice than in the controls (P < 0.001). In conclusion, the 16 month-old mdx mice presented a much blunted ventilatory response to hypercapnia compared to the same age controls.
Effect of age on the adaptation to hypercapnia in C57BL10 and mdx mice
Although, the 16 month-old C57BL10 mice showed a lower RR increase than the 5 month-old ones at 3% (171 ± 16 vs. 210 ± 16 breaths/min) (P < 0.05), 5% (215 ± 11 vs. 250 ± 23 breaths/min) (P < 0.05), and 8%CO2 (270 ± 21 vs. 315 ± 25 breaths/min) (P < 0.01), VT and VE did not change significantly.
In the mdx mice, at 5% CO2, both VT (7.4 ± 1.3 vs. 12.1 ± 3.8 μlg−1) and VE (1.45 ± 0.23 vs. 2.88 ± 1.10 ml min−1g−1) were lower in the older mice than in the younger ones (P < 0.001 and P < 0.01 respectively). At 8% CO2, the RR (226 ± 26 vs. 282 ± 23 breaths/min), VT (8.0 ± 1.8 vs. 16.2 ± 4.4 μlg−1) and VE (1.81 ± 0.35 vs. 4.64 ± 1.52 ml min−1g) were greatly reduced in the 16 month-old mice (P < 0.001) in comparison to the 5 month-old ones. The 16 month-old mdx mice thus presented a much blunted ventilatory response to CO2 as compared to the 5 month-old mdx mice.
Discussion
This study shows that, in spite of the presence of significant pathological structural changes in their respiratory muscles, 5 month-old mdx mice have similar ventilation during air breathing and in response to hypercapnia than same-age control animals. On the contrary, 16 month-old mdx mice present an altered ventilatory response, including a blunted response to hypercapnia, a finding that can be explained by the increased and widespread damaged of the respiratory muscles in the older mdx mice.
Since the life expectancy of mdx mice in standard conditions, as it applies also to our mice, is greatly reduced in comparison to wild type animal, we have chosen to use 16 month-old animals to make up our older group of mdx mice. This choice is supported by Lefaucheur et al. (1995) who observed that mdx mice die before 24 months of age. Lynch et al. (2001) have reported a longer life span for their mdx mice, but these animals were pathogen free. Finally Pastoret and Sebille (Pastoret and Sebille 1995) define mdx mice as old, when they are between 65 and 104 week-old.
Ventilatory parameters during air breathing
We find that 5 month-old C57BL10 and mdx mice do not show differences in their breathing pattern during air inhalation. This suggest that the respiratory muscle dysfunction, which is already present in these young mdx mice, is not enough to induce a change in their breathing pattern at this age.
On the contrary, the 16 month-old mdx mice present a modification in their breathing pattern during air breathing, i.e., higher respiratory frequency, when compared to same age C57BL10 controls. This rapid shallow breathing could be interpreted as an adaptation of the ventilatory control due to the imbalance between the chest wall loading and the weakness of the respiratory muscles that increases over time.
Although our baseline VT in C57BL10 mice was very close to the value reported by Dupont-Versteegden et al. (1994), our respiratory frequency in all groups, during air breathing, was lower than the values observed by these Authors (Dupont-Versteegden 1996) and Gosselin et al. (2003). This difference could be due to our long familiarization period, the time needed for the mouse to be quiet and motionless.
Response to hypercapnia in the 5 month-old mdx mice
In our study, 5 month-old mdx mice show the same response to hypercapnia as the 5 month-old C57BL10 controls. Since Gosselin et al. (2003) have reported a blunted ventilatory response to hypercapnia in 7 and 10–12 month-old mdx mice, we think that the age-related changes in the ventilatory response to hypercapnia must take place between 5 and 7 months of age.
Our data demonstrate that, at 5 months of age, the functional impairment of the respiratory muscles is not sufficient to induce a blunted response to hypercapnia. This finding is unexpected, since the diaphragm of our 5 month-old mdx mice present dystrophic patterns that include variation in fiber size, central nuclei, necrosis and an important proliferation of the connective tissue accompanied by a very much reduced muscular area (Fig. 2b), which represents only 50% of the value observed in control mice. Furthermore, Petrof et al. (1993) have reported a 50% reduction of the maximum isometric tension in young mdx mice. Yet, in spite of these severe dystrophic manifestations, we found a normal ventilatory response to hypercapnia. We, thus, suggest that the hypercapnia-induced hyperventilation does not represent the maximal work that can be realized by the residual respiratory muscles and that an adequate ventilatory reserve exists in young mdx animals. A compensatory recruitment of the accessory respiratory muscle mass may occur in 5 month-old mdx mice (Stedman et al. 1991), thereby, partially explaining the normal response to CO2. Indeed, in our study we observe, as previously described by other Authors (Stedman et al. 1991; Laws and Hoey 2004) that these accessory muscles are relatively spared compared to the diaphragm in 5 month-old mdx mice.
As the response to hypercapnia reflects the respiratory muscle force output and, above all, the chemoreceptors’ sensitivity to CO2, it seems that the respiratory centers of the 5 month-old mdx mice present a normal sensitivity to CO2.
Response to hypercapnia in the 16 month-old mdx mice
On the other hand, the 16 month-old mdx group shows a blunted ventilatory response to hypercapnia. In fact, although the respiratory frequency increases slightly, the VT and VE do not change upon stimulation.
The blunted hypercapnic drive, which we observe in the 16 month-old mdx mice in comparison to the C57BL10 controls, seems to reflect mainly the impairment of the respiratory muscle force output. Indeed, previous studies by others (Petrof et al. 1993; Dupont-Versteegden and McCarter 1992; Lynch et al. 1997) and us (Matecki et al. 2005) have shown a progressive loss in the diaphragm’s force with age. Our results are in accordance with those by Gosselin et al. (2003) who found in 7 and 10–12 month-old mdx mice a blunted ventilatory response to hypercapnia. This altered response is in agreement with the modifications in the structure of respiratory muscles, which can be observed around this age. As shown in our study and by others (Louboutin et al. 1993; Stedman et al. 1991), the diaphragm of old mdx mice presents extensive necrosis, myofiber loss with consequent replacement by adipose tissue. Indeed, our old mdx mice have a decreased muscular area which represents only about 30% of the values we observe in control mice. Moreover, Lynch et al. (1997) reported that in old mdx mice the maximal isometric tension and the maximal diaphragmatic power were decreased to 48% and 31% of the same values for control mice. In addition, we demonstrate an increase in myofibers’ loss also in the intercostal muscles of our 16 month-old mdx mice. Considering that these muscles are relatively spared at 5 months of age, we hypothesize that, in older mice, the extent of the degenerative process could hinder their compensatory recruitment that has been implicated to explain the normal ventilation observed in younger mdx mice. Indeed, it is possible that, in young mdx mice, the ventilatory workload might have been transferred to the anterior scalene and the intercostal muscles to compensate for the already impaired diaphragm function, thus accelerating the degenerative process of these muscles (Stedman et al. 1991). For all these reasons we suggest that, in the 16 month-old mdx mice, the accessory respiratory muscles can not develop any longer a sufficient maximal tension to compensate the impairment of diaphragm.
Moreover, since the blunted ventilatory response to CO2 in the 16 month-old mdx is present from the onset of the hypercapnic stimulation, we hypothesize that weakness rather than fatigue is the cause of this limited response.
In summary, our results show that while the younger mdx mice respond to hypercapnia as well as the C57BL10 controls, the 16 month-old mdx mice have a very limited ventilatory response. The ventilatory response to hypercapnia, which represents the in vivo respiratory muscle force output, decreases with age concomitantly with the alterations in the function of the respiratory muscles. One of the consequences of these findings is that we cannot evaluate the in vivo effectiveness of a treatment for DMD patients by hypercapnic stimulation of 5 month-old or younger mdx mice. The knowledge, that, in mdx mice, the ventilation during air breathing and in response to CO2 changes according to age is, therefore, valuable for the assessment of future therapeutic trials for muscular dystrophy.
Acknowledgments
The authors thank Gérald Hugon for careful technical help with the immunochemistry techniques used in this work.
References
- Amalfitano A, Parks RJ. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr Gene Ther. 2002;2:111–133. doi: 10.2174/1566523024605618. [DOI] [PubMed] [Google Scholar]
- Briguet A, Courdier-Fruh I, Foster M, et al. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord. 2004;14:675–682. doi: 10.1016/j.nmd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Harrison MA, Carbonetto S, et al. Stimulation of calcineurin signaling attenuates the dystrophic pathology in mdx mice. Hum Mol Genet. 2004;13:379–388. doi: 10.1093/hmg/ddh037. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Thompson J, Parks RJ, et al. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. Faseb J. 2005;19:880–891. doi: 10.1096/fj.04-1956rev. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Dupont-Versteegden EE, McCarter RJ, Katz MS. Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol. 1994;77:1736–1741. doi: 10.1152/jappl.1994.77.4.1736. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE. Exercise and clenbuterol as strategies to decrease the progression of muscular dystrophy in mdx mice. J Appl Physiol. 1996;80:734–741. doi: 10.1152/jappl.1996.80.3.734. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, McCarter RJ. Differential expression of muscular dystrophy in diaphragm versus hindlimb muscles of mdx mice. Muscle Nerve. 1992;15:1105–1110. doi: 10.1002/mus.880151008. [DOI] [PubMed] [Google Scholar]
- Fenichel GM, Florence JM, Pestronk A, et al. Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology. 1991;41:1874–1877. doi: 10.1212/wnl.41.12.1874. [DOI] [PubMed] [Google Scholar]
- Fenichel G, Pestronk A, Florence J, et al. A beneficial effect of oxandrolone in the treatment of Duchenne muscular dystrophy: a pilot study. Neurology. 1997;48:1225–1226. doi: 10.1212/wnl.48.5.1225. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Barkley JE, Spencer MJ, et al. Ventilatory dysfunction in mdx mice: Impact of tumor necrosis factor-alpha deletion. Muscle Nerve. 2003;28:336–343. doi: 10.1002/mus.10431. [DOI] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Hahn A, Bach JR, Delaubier A, et al. Clinical implications of maximal respiratory pressure determinations for individuals with Duchenne muscular dystrophy. Arch Phys MedRehabil. 1997;78:1–6. doi: 10.1016/s0003-9993(97)90001-0. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda K, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- Laws N, Hoey A. Progression of kyphosis in mdx mice. J Appl Physiol. 2004;97:1970–1977. doi: 10.1152/japplphysiol.01357.2003. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Pastoret C, Sebille A. Phenotype of dystrophinopathy in old mdx mice. Anat Rec. 1995;242:70–76. doi: 10.1002/ar.1092420109. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Fichter-Gagnepain V, Thaon E, et al. Morphometric analysis of mdx diaphragm muscle fibres. Comparison with hindlimb muscles. Neuromuscul Disord. 1993;3:463–469. doi: 10.1016/0960-8966(93)90098-5. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Rafael JA, Hinkle RT, et al. Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am J Physiol. 1997;272:C2063–2068. doi: 10.1152/ajpcell.1997.272.6.C2063. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Hinkle RT, Chamberlain JS, et al. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol. 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matecki S, Topin N, Hayot M, et al. A standardized method for the evaluation of respiratory muscle endurance in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2001;11:171–177. doi: 10.1016/s0960-8966(00)00179-6. [DOI] [PubMed] [Google Scholar]
- Matecki S, Rivier F, Hugon G, et al. The effect of respiratory muscle training with CO2 breathing on cellular adaptation of mdx mouse diaphragm. Neuromuscul Disord. 2005;15:427–436. doi: 10.1016/j.nmd.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Amato AA, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Mouly V, Aamiri A, Perie S, et al. Myoblast transfer therapy: is there any light at the end of the tunnel? Acta Myol. 2005;24:128–133. [PubMed] [Google Scholar]
- Mugneret F, Sidaner I, Nivelon-Chevallier A, et al. [Translocation (X; Y) and genetic counseling] J Genet Hum. 1988;36:93–97. [PubMed] [Google Scholar]
- Pastoret C, Sebille A. mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci. 1995;129:97–105. doi: 10.1016/0022-510x(94)00276-t. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Stedman HH, Shrager JB, et al. Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol. 1993;265:C834–841. doi: 10.1152/ajpcell.1993.265.3.C834. [DOI] [PubMed] [Google Scholar]
- Politano L, Nigro G, Nigro V, et al. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 2003;22:15–21. [PubMed] [Google Scholar]
- Radley HG, De Luca A, Lynch GS, et al. Duchenne muscular dystrophy: focus on pharmaceutical and nutritional interventions. Int J Biochem Cell Biol. 2007;39:469–477. doi: 10.1016/j.biocel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Geng Y, Ryder-Cook AS, et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Skuk D, Tremblay JP. Progress in myoblast transplantation: a potential treatment of dystrophies. Microsc Res Tech. 2000;48:213–222. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<213::AID-JEMT9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Fitzgerald RS, Mitzner WA, et al. Hypercapnic ventilatory responses in mice differentially susceptible to acute ozone exposure. J Appl Physiol. 1993;75:2613–2619. doi: 10.1152/jappl.1993.75.6.2613. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Fitzgerald RS, Levitt RC, et al. Genetic control of differential baseline breathing pattern. J Appl Physiol. 1997;82:874–881. doi: 10.1152/jappl.1997.82.3.874. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky MA, Mahoney DJ, Vajsar J, et al. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology. 2004;62:1771–1777. doi: 10.1212/01.wnl.0000125178.18862.9d. [DOI] [PubMed] [Google Scholar]
- Voisin V, Sebrie C, Matecki S, et al. L-arginine improves dystrophic phenotype in mdx mice. Neurobiol Dis. 2005;20:123–130. doi: 10.1016/j.nbd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Wanke T, Toifl K, Merkle M, et al. Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest. 1994;105:475–482. doi: 10.1378/chest.105.2.475. [DOI] [PubMed] [Google Scholar]
- Wolff JA, Ludtke JJ, Acsadi G, et al. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- Zupan A. Long-term electrical stimulation of muscles in children with Duchenne and Becker muscular dystrophy. Muscle nerve. 1992;15:362–367. doi: 10.1002/mus.880150316. [DOI] [PubMed] [Google Scholar]


