Abstract
The importance of treating hepatitis C virus (HCV)-associated morbidities in a growing population of patients coinfected with human immunodeficiency virus (HIV) has increased since the introduction of highly active antiretroviral therapy. As a result, investigative attention is turning to HCV-related liver disease and treatment-associated issues in coinfection. HIV/HCV-coinfected patients have higher HCV RNA loads and show more rapid progression of fibrosis than do monoinfected patients. Combination therapy with pegylated interferon plus ribavirin (RBV) is the standard of care for HCV in coinfected patients. Therapy slows fibrosis progression, but toxicity prevents identification of the most effective RBV dose. Coinfected patients have about a threefold greater risk of antiretroviral therapy-associated hepatotoxicity than patients with HIV only. Other challenges include anaemia, mitochondrial toxicity, drug–drug interactions and leucopenia. Thus, chronic hepatitis C should be treated in HIV/HCV-coinfected patients, but steps must be taken to prevent and treat potential toxicities. The first European Consensus Conference on the Treatment of Chronic Hepatitis B and C in HIV Co-infected Patients was held March 2005 in Paris to address these issues. This article reviews the peer-reviewed literature and expert opinion published from 1990 to 2005, and compares results with presentations and recommendations from the Consensus Conference to best present current issues in coinfection.
Keywords: coinfection, hepatitis C, human immunodeficiency virus, treatment
Introduction
Approximately 30% of patients infected with the human immunodeficiency virus (HIV) in the USA and Europe are also infected with the hepatitis C virus (HCV) [1–3]. The high prevalence of HIV/HCV coinfection is not unexpected because both viruses are transmitted by the same routes, although not with the same efficiency. As a result, the prevalence of HIV/HCV coinfection varies across subpopulations of HIV-infected patients; for example, persons acquiring HIV through exposure to contaminated blood (such as injection drug users or haemophiliacs) are more likely to have HCV coinfection than those acquiring HIV through sex [4].
Since the introduction of highly effective antiretroviral therapy (HAART) in the mid-1990s, liver disease has emerged as an important cause of morbidity and mortality in HIV-infected patients. As HIV-related mortality has declined, hepatitis C-related liver disease has become a leading cause of hospitalization and death in this population [5]. Chronic hepatitis C infection is now the most common indication for liver transplantation in the USA [6], and some estimates suggest that the number of deaths from HCV in the USA and Europe will triple over the next 10 years to surpass the number of deaths from AIDS.
Hepatologists and infectious disease specialists are increasingly turning their attention to HIV/HCV coinfection and its treatment. An international community of hepatologists, infectious disease specialists and others convened the first European Consensus Conference on the Treatment of Hepatitis B and C in HIV Co-infected Patients March 1 to 2, 2005, in Paris, France. This paper reviews the therapeutic issues affecting this coinfected population of patients and presents some of the recommendations from the consensus conference.
Natural history of hiv/hcv coinfection
Both HIV, a retrovirus, and HCV, a flavivirus, are single-stranded RNA viruses that produce chronic infection. Both viruses are also able to evade host immune responses, in part because of a high mutation rate resulting from rapid replication and a lack of ‘proofreading’ capabilities. The estimated daily virion production rate is 1010 in patients infected with HIV and 1012 in patients infected with HCV [7,8]. Both HIV and HCV exist in humans as a collection of closely related clones (e.g. quasispecies) that may evolve differently in such compartments as the liver, plasma and peripheral blood mononuclear cells (PBMNCs) [9,10]. In patients infected with HIV, antiviral drug therapy exerts selection pressure that may lead to the emergence of drug-resistant strains in the absence of adequate viral suppression, although specific anti-HCV drugs (e.g. HCV protease and polymerase inhibitors) are not yet commercially available, drug resistance to HCV protease inhibitors has been observed in early in vivo studies [11].
The presence of both HIV and HCV infection may complicate the natural history of both viruses and their treatment (Table 1) [12–18]. For example, coinfected patients have higher HCV viral loads than patients infected with HCV alone. In addition, HIV infection and related immunosuppression in patients with hepatitis C may be associated with more rapid progression of liver disease to cirrhosis, end-stage liver disease and death. In some studies, HIV/HCV coinfection was associated with a more rapid progression to AIDS and death [12]. HCV infection and related liver disease may be associated with an increased risk of antiretroviral therapy (ART)-induced hepatotoxicity, complicating the efforts to treat HIV disease [19].
Table 1.
Liver-associated morbidity and mortality in hepatitis C virus-positive patients coinfected with human immunodeficiency virus
| Author, year | n | Liver-related outcomes | Other |
|---|---|---|---|
| Darby et al. 1997 [12] | 4865 | Coinfection associated with a 5.1% increase in deaths from liver cancer and liver disease in all age groups | Haemophiliacs exposed to blood products with a high risk of HCV (1969–1985) |
| Soriano et al. 1999 [13] | 1670* | 143/1670 (8.6%) of hospital admissions of HIV-positive patients (93/143 HCV-positive) had clinical evidence of decompensated liver disease 4.8% of deaths over 4.5 years from chronic liver disease | Intravenous drug users |
| Rosenthal et al. 2003 [14] | 25 178 | 14.3% of deaths of coinfected patients in 2001 from end-stage liver disease 95% of coinfected patients who died of end-stage liver disease had chronic hepatitis C 25% of coinfected patients died of hepatocellular carcinoma | Compared with similar study in 1995, deaths from end-stage liver disease increased 5.1% (P < 0.01) High prevalence of alcohol consumption appears to contribute |
| Graham et al. 2001 [15] | Meta-analysis (eight studies) | Decompensated liver disease in coinfected patients: RR 6.14 (95% CI: 2.86–13.20) Histologically diagnosed cirrhosis in coinfected patients: RR 2.07 (95% CI: 1.40–3.07) | Combined RR of end-stage liver disease or cirrhosis: 2.92 (95% CI: 1.70–5.01) |
| Di Martino et al. 2001 [16] | 80 | Coinfection associated with: ↑ Knodell score (P = 0.011) ↑ Necroinflammatory scores (P = 0.023) | ↓ Response to IFN (P = 0.009) ↑ HCV RNA (P = 0.012) Fibrosis and progression to cirrhosis related to CD4+ cell count Age and alcohol associated with risk of cirrhosis and mortality |
| Benhamou et al. 1999 [17] | 122 | Compared with HIV monoinfection in 122 patients, coinfection associated with: 13% increase in extensive liver fibrosis (METAVIR 2–4; P < 0.05) Median fibrosis progression rate increased by 0.047 fibrosis units/yr (P < 0.0001) 24% increase in moderate or severe activity (P < 0.001) | Factors associated with higher liver fibrosis rates: Alcohol consumption (>50 g/day; P = 0.002) Age at HCV infection (<25 years; P < 0.001) Severe immunosuppression (CD4+ cell count ≤200/μL) |
| Benhamou et al. 2001 [18] | 182 | Multivariate analysis showed 4 independent predictors of progression to cirrhosis in coinfected patients: Absence of PI therapy: RR 4.74 (95% CI: 1.34–16.67) Heavy alcohol consumption (>50 g/day): RR 4.71 (95% CI: 1.92–11.57) Low CD4+ cell count (<200/μL): RR 2.74 (95% CI: 1.17–6.41) Age at HCV infection (≥20 years): RR 2.37 (95% CI: 1.04–5.38) | PI therapy might slow progression to cirrhosis |
CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferon; PI, protease inhibitor; RR, risk relative.
Hospital admissions.
Liver damage in HIV/HCV-coinfected patients has a number of potential causes including pre-existing liver disease (e.g. hepatitis B, D), alcohol abuse, opportunistic infections (Mycobacterium avium complex), nonalcoholic steatohepatitis, hepatotoxicity related to drugs used to treat HIV and its complications, and AIDS cholangiopathy in patients with CD4+ cell counts <100/μL. Since the introduction of HAART, liver-related morbidity and mortality has increased markedly in HIV-infected patients, particularly those coinfected with hepatitis C. The rate of liver-related complications increased from 5.4 to 26.7 admissions per 100 patient-years in HIV/HCV-coinfected patients treated at a large urban hospital between 1995 and 2000 (P < 0.001) [20]. In addition, liver disease was the second leading cause of death (0.23 cases per 100 person-years) behind HIV/AIDS (0.59 cases per 100 person-years) and ahead of cardiac disease (0.14 cases per 100person-years) among 23 441 patients enrolled in the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study [21].
Influence of hcv on hiv disease
The mechanisms by which HCV potentially affects HIV disease are not known; however, some experts speculate that HIV disease may be accelerated by HCV-related immune activation and subsequent impairments in immune recovery after effective ART. In a study of 3111 Swiss patients receiving HAART, Greub et al. [22] observed that HCV in HIV-infected patients was independently associated with an increased risk of progression to AIDS and AIDS-related death. The authors suggest that this finding is related to impaired CD4+ cell recovery in HCV-seropositive patients receiving potent ART. However, since HIV-monoinfected persons differ substantially from those with HIV/HCV coinfection with respect to many important social and medical characteristics (e.g. drug and alcohol use, psychiatric disease), the researchers may have been unable to adjust for all potential confounding factors that might be associated with poor outcomes in coinfected persons [22]. Others have suggested that HCV genotype might affect outcome in coinfected patients [23].
Several other studies suggest that HCV coinfection does not affect HIV disease progression or response to ART. Sulkowski et al. [24] failed to identify an independent relationship between HCV infection and HIV disease in several cohorts after adjusting for potential confounders such as ART and its effectiveness. In a 6-year cohort study of 1995 coinfected patients, these investigators found no increase in the rate of progression to an AIDS-defining condition [1.03; 95% confidence interval (CI): 0.86–1.23] or death (1.05; 95% CI: 0.85–1.30) [24]. In addition, after adjusting for HIV treatment, HCV infection was not independently associated with death in the subsets of patients with CD4+ counts of 50 to 200 cells/μL. Furthermore, mortality did not differ between HIV-infected and HIV/HCV-coinfected patients receiving effective HAART, and no differences were found in increases in CD4+ cell count or CD4+ percentage during administration of HAART [25]. These data suggest that there are no major differences in HIV-related mortality between patients infected with both HIV and HCV and patients infected with HIV alone receiving ART.
Influence of hiv on hcv disease
HIV/HCV coinfection has been consistently associated with progression of HCV disease [17,26]. Because HAART has decreased HIV-specific mortality, HCV has become a major problem in coinfected patients.
Accelerated fibrosis progression
Compared with those without HIV, coinfected patients have higher HCV RNA levels and more rapid progression of hepatic fibrosis [16]. Factors associated with progressive fibrosis included advanced immunodeficiency (CD4+ cell counts <200/μL), higher daily alcohol consumption rate and older age at HCV infection. Benhamou et al. [17] compared the natural history of HCV disease in 244 matched patients divided equally into those with and those without HIV coinfection. The prevalences of significant liver fibrosis (METAVIR fibrosis scores 2–4) and moderate or severe inflammatory activity were higher in HIV-infected patients (60% and 54%, respectively) than in the non-HIV-infected patients (47% and 30%, respectively; P < 0.05 and P < 0.001). The investigators used fibrosis stage (METAVIR scoring system) and estimated duration of HCV infection to determine a fibrosis progression rate. The median fibrosis progression rates in coinfected and control patients were 0.153 fibrosis U/year (95% CI: 0.117–0.181) and 0.106 fibrosis U/year (95% CI: 0.084–0.125), respectively (P < 0.001 for the difference of 0.047 fibrosis units between group medians).
Preliminary evidence suggests that effective ART may slow the fibrosis progression rate in coinfected patients [27]. However, other studies show that ART can be associated with increased liver enzyme levels and hepatic steatosis in some patients, particularly those with hepatitis C. Further research is needed to determine the long-term effect of ART on the progression of liver disease in coinfected patients.
Progression to cirrhosis and hepatocellular carcinoma
Compared with HCV infection alone, HIV/HCV coinfection is associated with an increased risk of cirrhosis, end-stage liver disease and hepatocellular carcinoma. Graham et al. [15] conducted a meta-analysis of eight studies in coinfected patients to evaluate the risk of progression to decompensated liver disease and histologically established cirrhosis. The pooled, adjusted relative risk (RR) of developing histologically confirmed cirrhosis was 2.07 (95% CI: 1.40–3.07), and the RR of developing decompensated liver disease was 6.14 (95% CI: 2.86–13.20). Other investigators reported that coinfected patients who went on to develop hepatocellular carcinoma were younger (42.2 vs 68.9 years; P < 0.001) and had a shorter duration of disease (17.8 vs 28.1 years; P < 0.05) at the time of diagnosis than HIV-negative patients [28].
Hepatitis C viremia
Human immunodeficiency virus-mediated immune suppression appears to facilitate HCV replication, impair immune-mediated HCV clearance or both. Studies demonstrate that coinfected patients have significantly higher serum HCV titres than do patients infected with HCV alone [29,30]. This association is independent of HCV genotype [31] and, in some studies, is linked to lower CD4+ cell counts as well, suggesting a relationship between viremia and cellular and innate immunity. Whereas HCV is predominantly hepatotrophic, hepatitis C may also replicate in PBMCs [32]. In an in vitro study of PBMCs from healthy donors, HIV infection increased the susceptibility of macrophages to HCV infection [33]. In fact, HIV coinfection appeared to be required to establish HCV replication in this cell line. Interestingly, immune reconstitution following effective ART has been associated with transient increases in the levels of hepatitis C viremia followed by a return to pre-ART levels. However, suppression of hepatitis C viremia following effective ART has not been consistently observed, suggesting additional factors may be responsible for the association of HIV and HCV load [34].
Risk of ART-associated hepatotoxicity
Whereas ART should not be withheld from coinfected patients requiring HIV treatment, chronic hepatitis C is associated with an increased risk of drug-induced hepatotoxicity (Table 2) [35,36]. In a study of HIV-infected patients receiving HAART, HCV infection was associated with a 2.46 increased RR (95% CI: 1.43–4.24) for liver enzyme elevations (5 × upper limit of normal) and an absolute increase of at least 100 U/L [37]. In a study of other HAART-associated risk factors, the following were also associated with increased RRs for liver toxicity: alcohol abuse (RR 5.87; 95% CI: 1.49–23.15; P = 0.01), HCV coinfection (RR 3.99; 95% CI: 1.32–12.10; P = 0.01) and older age (RR 1.11; 95% CI: 1.04–1.18; P = 0.001) [38]. Across several studies, the risk of liver injury appears to be particularly great with the use of nevirapine and full-dose ritonavir; however, the administration of low-dose ritonavir (≤200 mg/day) has not been associated with a greater risk of hepatotoxicity. The mechanisms underlying the association of HCV and hepatotoxicity have not been fully described, but in some patients liver enzyme elevations may be a manifestation of immune reconstitution that follows ART. After immune recovery, CD4+ cell counts rise and the ability of immunocytes to identify and lyse HCV-infected hepatocytes may be increased [39]. However, there is little direct evidence of this phenomenon in vivo and alternative mechanisms of liver injury probably contribute.
Table 2.
Incidence and relative risk of severe hepatotoxicity associated with highly active antiretroviral therapy*
| Antiretroviral drug regimen | n | Cases | Person- time (100 person- months) | Incidence (cases/ persons exposed) (95% CI) | Incidence (cases/100 person-months) (95% CI) | Relative risk (95% CI) |
|---|---|---|---|---|---|---|
| Dual nucleoside analogue | 87 | 5 | 246 | 5.7 (1.2–12.9) | 2.0 (0.7–4.7) | 1.0 |
| Protease inhibitor (all) | 112 | 26 | 795 | 12.3 (8.2–17.5) | 3.3 (2.1–4.8) | 2.2 (0.9–5.4) |
| Ritonavir (single protease inhibitor) | 22 | 6 | 96 | 27.3 (10.7–50.2) | 6.3 (2.3–21.6) | 4.8 (1.6–14.1) |
| Ritonavir plus saquinavir | 28 | 9 | 79 | 32.1 (15.9–52.4) | 11.4 (5.2–21.6) | 5.6 (2.1–15.3) |
| Saquinavir† | 17 | 1 | 98 | 5.9 (0.15–28.7) | 1.0 (0.7–4.8) | 1.0 (0.1–8.2) |
| Indinavir | 117 | 8 | 520 | 6.8 (3.0–13.1) | 1.5 (0.7–3.0) | 1.2 (0.4–3.5) |
| Nelfinavir | 51 | 3 | 153 | 5.9 (1.2–16.2) | 2.0 (0.4–5.7) | 1.0 (0.3–4.1) |
| Total | 298 | 31 | 1041 | 10.4 (7.2–14.4) | 3.1 (2.1–4.3) | NA |
Reprinted with permission from Sulkowski et al. [35].
CI, confidence interval; NA, not applicable; PEG-IFN, pegylated interferon; RBV, ribavirin.
Because use of individual drugs was studied, some overlap occurred during the study period; thus, the individual numbers of patients and cases and the person-time for specific protease inhibitor categories do not equal the ‘Total.’
Saquinavir hard gelatin capsule formulation without concurrent ritonavir prescription. The case occurring in a patient receiving saquinavir alone (i.e. not in combination with ritonavir) is also counted in the indinavir category because the patient was taking both drugs at the time of the toxicity.
Effects of haart in coinfected patients
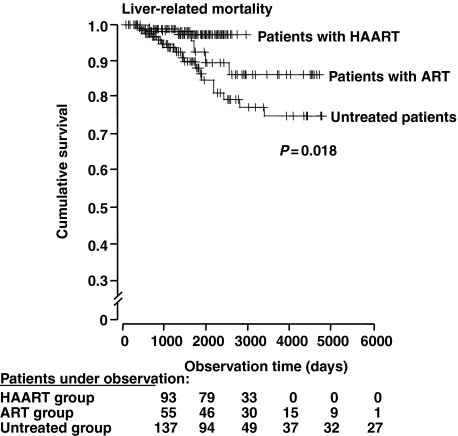
Overall, HAART slows the progression of hepatic fibrosis in coinfected patients. Qurishi et al. [40] reported a Kaplan–Meier analysis of liver-related mortality and confirmed that ART significantly improves survival in coinfected patients (P = 0.02). Regression analysis identified the following factors as being significantly associated with liver-related survival (Fig. 1): HAART [odds ratio (OR) 0.12 (95% CI: 0.02–0.56)], antiretroviral treatment [OR 0.28 (95% CI: 0.10–0.78)], CD4+ T-cell count [OR 0.75 (95% CI: 0.64–0.87) per 0.05 × 109 cells/L], serum cholinesterase [OR 0.96 (95% CI: 0.94–0.99) per 100 U/L], and older age [OR 1.07 (95% CI: 1.03–1.11) per year] [41].
Fig. 1.
Kaplan–Meier analysis of liver-related mortality. To calculate liver-related mortality, deaths because of nonhepatic causes were censored. Vertical marks indicate censored patients. ART, antiretroviral therapy; HAART, highly active antiretroviral therapy. (Reprinted with permission from Qurishi et al. [40]).
A study by Marine-Barjoan et al. [42] supported these results and showed that coinfected patients treated with HAART had slower progression of hepatic fibrosis and that fibrosis was more likely to develop when HAART therapy was delayed. Thus, although ART may be associated with hepatotoxicity in some HCV-coinfected patients, emerging evidence suggests that control of HIV disease confers some histologic and clinical benefit in HCV-infected patients.
Treating hcv infection in coinfected patients
The goal of therapy for chronic hepatitis C is viral eradication or sustained virologic response (SVR), an outcome associated with improved histologic results and decreased risk of progression to cirrhosis, end-stage liver disease and hepatocellular carcinoma.
Screening
Because the prevalence of coinfection is high, all HIV-infected patients should always be screened for HCV using a third-generation enzyme-linked immunoblot assay [43]. Because HIV and HCV share risk factors, HCV-infected patients should also be screened for HIV. Persons found to be positive for HCV antibodies should also be tested for serum HCV RNA, as the detection of viremia indicates active disease. Patients with advanced HIV disease (CD4+ cell count <100/μL) and those with acute HCV infection may not have detectable HCV antibodies. Accordingly, HCV RNA testing should also be done if HCV is clinically suspected after a negative HCV antibody result. Coinfected patients should also be screened for protective immunity against hepatitis B and A infection and, in the absence of past infection, vaccinated.
The decision to perform a liver biopsy should be made on a patient-by-patient basis. Liver histology indicates the grade and stage of HCV-related liver disease and may also provide information about comorbidities such as alcoholic liver disease, nonalcoholic steatohepatitis and mitochondrial toxicity. However, histologic staging may be less important in patients in whom HCV treatment is likely to be effective as treatment may be beneficial regardless of histologic stage (e.g. genotypes 2, 3 and/or genotype 1 with low viral load). Persons with cirrhosis should be screened on a semi-annual basis for hepatoma with serum alpha-fetoprotein and liver imaging studies [e.g. ultrasound, computed tomography (CT), magnetic resonance imaging]. Upper endoscopy may also be indicated in cirrhotic patients to evaluate and manage potential esophageal varices.
Because of the limitations, expense and risks of biopsy, noninvasive markers of liver disease are highly desirable. Unfortunately, alanine aminotransferase levels do not correlate well with the histologic severity of liver disease, and normal levels do not exclude the presence of significant hepatic fibrosis. As a result, investigators have studied a number of noninvasive approaches to evaluation of the stage of liver disease in patients with chronic hepatitis C. Transient elastography (FibroScan®; EchoSens, Paris, France) measures liver stiffness as a rapid and reproducible surrogate of hepatic fibrosis [44]. A panel of biochemical markers has been used to estimate the degree of hepatic fibrosis and activity (HCV FIBROSURE™ Laboratory Corporation of America, Research Triangle Park, NC, USA). At a cut-off value of 0.31 and 0.36, the two tests had negative predictive values 91% for excluding cirrhosis and 85% for excluding significant necrosis [45]. A retrospective analysis of 832 patients from the AIDS Pegasys Ribavirin International Coinfection Trial (APRICOT) identified platelet count, age, and alanine and aspartate aminotransferase levels as significantly different, varying with stage of fibrosis: F0–1, F2–3 and F4–6 [46]. Investigators used an equation that included these variables to create an index (FIB-4). With a cut-off outside of 1.45–3.25, the FIB-4 index correctly classified 87% of patients in the APRICOT subset according to stage of fibrosis. Use of the FIB-4 would have resulted in a 71% decrease in biopsies for staging. Although this evolving evidence suggests that such laboratory tests can replace liver biopsy in the work-up of coinfected patients, significant fibrosis can still be missed or misdiagnosed [47].
Identifying candidates for therapy
Published treatment guidelines recommend the provision of HCV treatment to HIV-infected patients for whom the potential benefits of therapy outweigh the potential risk of treatment-related toxicity. SVR rates are relatively high (∼60%) in persons with HCV genotype 2 or 3 and with HCV genotype 1 and relatively low levels of hepatitis C viremia (HCV RNA < 800 000 IU/mL). Conversely, SVR rates are significantly lower (∼18%) in persons with HCV genotype 1 and relatively high levels of viremia.
In addition to viral parameters, host factors also predict HCV treatment response. For example, host genetic factors may play a role particularly with respect to significantly lower SVR rates (50%) observed among African-American than among white American patients with hepatitis C [48]. Interestingly, in several large studies, HIV disease (e.g. low CD4 cell count, high HIV RNA level) and its treatment were not associated with HCV treatment response. Conversely, hepatitis C disease stage may affect response rates. In most studies, SVR is more common among those with no or minimal hepatic fibrosis compared with those with bridging fibrosis or cirrhosis. However, HCV may be eradicated in patients with advanced fibrosis, and the impetus to treat such patients before hepatic decompensation is strong. Interferon (IFN)-based therapy is contraindicated in patients with decompensated liver disease (Child–Pugh stage B or C).
The decision to treat hepatitis C must take into account comorbid conditions that limit life expectancy or increase the risks associated with HCV therapy; for example, HIV disease should be stable with or without ART. Because IFN can exacerbate pre-existing mental illness, persons with underlying psychiatric disease, such as depression or bipolar disorder, should be evaluated before HCV treatment is initiated. Similarly, although HCV treatment is not contraindicated in persons actively using illicit drugs or alcohol, substance abuse is associated with high rates of treatment nonadherence and may compromise treatment outcomes.
Therapeutic options for hcv in hiv-infected patients
Candidates for HCV therapy should be patients in whom the potential benefits of treatment exceed the potential risks. HIV disease status is a major consideration is this risk:benefit assessment. For patients with relatively high CD4+ cell counts (>350/μL) for whom ART may be deferred, HCV treatment may be considered. Conversely, patients with low CD4+ cell counts (<200/μL) with untreated HIV infection should not receive HCV therapy until HIV infection is effectively treated.
Recommended HCV therapy in coinfected patients
Published guidelines for anti-HCV therapy [49–51] indicate that the standard of care in coinfected patients is pegylated IFN alfa (PEG-IFN) plus ribavirin (RBV) (Table 3) [52–58]. Efficacy and safety outcomes after treatment of HIV/HCV coinfection were published recently by investigators from APRICOT and from the Randomized Controlled Trial of Pegylated-Interferon alfa-2b plus Ribavirin vs Interferon alfa-2b plus Ribavirin for the Initial Treatment of Chronic Hepatitis C in HIV Co-Infected Patients (RIBAVIC) [57,59]. Both trials compared standard IFN alfa-2a or alfa-2b and the pegylated formulations of these IFNs for 48 weeks independent of HCV genotype [57,59]. All patients also received RBV 800 mg/day; although lower than routinely given to noncoinfected patients, this dosage was selected because of concerns about RBV-associated anaemia and possible drug–drug interactions between RBV and nucleoside reverse transcriptase inhibitors.
Table 3.
Summary of trials of PEG-IFN plus RBV in coinfected patients
| Author, year | Genotype: treatment duration (week) | Type of PEG-IFN | RBV dose (mg/day) | n | Virologic response at end of treatment, n (%) | SVR,*n (%) | Withdrawals owing to adverse events, n (%) |
|---|---|---|---|---|---|---|---|
| Perez-Olmeda et al. 2003 [53] | Genotype 1: 48 Genotypes 2, 3: 24 | alfa-2b | 800 | 68 | 27 (40) | 19 (28) | 10 (15) |
| Voigt et al. 2003 [54] | Genotype 1: 48 Genotypes 2, 3: 24 | alfa-2b | 800 | 72 | 33 (46) | 19 (26) | 12 (17) |
| Carrat et al. 2004 [57] | All genotypes: 8 | alfa-2b | 800 | 205 | NA | 55 (27) | 77 (38) |
| Chung et al. 2004 [58] | All genotypes: 48 | alfa-2a | 600† | 66 | 27 (41) | 18 (27) | 8 (12) |
| Torriani et al. 2004 [56] | All genotypes: 48 | alfa-2a | 800 | 289 | NA | 115 (40) | NA (12) |
| Moreno et al. 2004 [55] | All genotypes: 48 | alfa-2b | 800 | 35 | 14 (40) | 11 (31) | 6 (17) |
Reprinted with permission from Rockstroh [52].
NA, not available; PEG-IFN, pegylated interferon; RBV, ribavirin; SVR, sustained virologic response.
Negative for HCV RNA 24 weeks after end of treatment.
Increased to 1000 mg/day at 12 weeks.
Pegylated-IFN plus RBV was significantly more effective than standard IFN plus RBV in both APRICOT and RIBAVIC [57,59]. The APRICOT investigators reported the highest SVR rate achieved in coinfected patients treated with PEG-IFN rather than standard IFN plus RBV: an overall SVR rate of 40%vs 12% (genotype 1, 29%vs 7%; genotypes 2, 3, 62%vs 20%, respectively). In patients with cirrhosis, a group that is difficult to treat, the SVR rate was 30%. Although RIBAVIC also demonstrated superiority of PEG-IFN plus RBV, the improvement in outcomes was less than anticipated. The overall SVR in RIBAVIC was 27%vs 20% (genotype 1 or 4, 17%vs 6%; genotype 2, 3, or 5, 44%vs 43%).
Several factors potentially account for the lower SVR rates in RIBAVIC: [1] early treatment discontinuations and [2] proportion of patients with bridging fibrosis or cirrhosis [57,59]. Early treatment discontinuations were more common in RIBAVIC than in APRICOT (37%vs 15%) and were predominantly related to patients’ decisions to stop therapy or not to return for further treatment to avoid serious adverse events (16%vs 13%, respectively). In addition, more patients in RIBAVIC had bridging fibrosis or cirrhosis than in APRICOT (40%vs 16%), representing a population with lower expected HCV treatment efficacy and tolerability. The high incidence of adverse events in the two trials is a reminder that coinfected patients with advanced liver disease should be monitored particularly closely for drug-related toxicities. In addition, coinfected patients receiving combination therapy for HCV should not be also receiving didanosine, a drug associated with significant mitochondrial toxicity. Didanosine toxicity is believed to result from increased phosphorylation of the drug because of inosine monophosphate dehydrogenase (IMPDH) inhibition by RBV, leading to an increased effect on mitochondrial DNA polymerase gamma [60].
Findings by Laguno et al. [61] support the results of APRICOT, although Laguno et al. had fewer enrollees and their analysis grouped genotypes 1 and 4. In this randomized, single-center, open-label trial, investigators compared the efficacy and safety of 48 weeks of standard IFN (3 × 106 U TIW) and PEG-IFN (100–150 μg/week) plus RBV (800–1200 mg/day) in 95 coinfected patients. Patients with HCV genotypes 2 or 3 and baseline HCV RNA levels <800 000 IU/mL received only 24 weeks of treatment. Intent-to-treat analysis showed that SVR rates were significantly higher among patients treated with PEG-IFN plus RBV than among patients treated with standard IFN (44%vs 21%; P = 0.017). The SVR rate was 38% among patients with genotype 1 or 4 treated with PEG-IFN but only 7% among patients treated with standard IFN (P = 0.007). Differences in SVR rates were not significant in patients with HCV genotypes 2 or 3 (53%vs 47%; P = 0.730). The CD4+ cell count but not its percentage dropped in both treatment groups, and HIV RNA viral load did not change from baseline. The superiority of PEG-IFN plus RBV over standard IFN was also shown in a similar multicenter, randomized trial conducted by Chung et al. [62], who demonstrated that treatment may be beneficial even in the absence of an SVR: 35% of 66 patients who did not achieve an SVR had improved liver histology.
Side effects were common in the APRICOT, RIBAVIC and Laguno et al. [57,59,61] studies; treatment was discontinued for adverse events in 12%, 17% and 17% of patients, respectively, treated with PEG-IFN plus RBV. Hepatic decompensation, an outcome not anticipated in HCV monotherapy trials, was identified in substantial numbers of patients in APRICOT and RIBAVIC but not in Laguno et al. [63]. All episodes developed in cirrhotic patients. Other risk factors associated with this event were hyperbilirubinemia; elevated alkaline phosphatase and decreased haemoglobin (Hb) or platelet concentrations; and treatment with didanosine. There was no correlation with HCV RNA, histologic activity, CD4+ cell counts, or combination therapy with either PEG-IFN or standard IFN.
In the coinfected patient, PEG-IFN is administered at either 180 μg (PEG-IFN alfa-2a) or 1.5 μg/kg (PEG-IFN alfa-2b). At this time, the dose of RBV for the treatment of HCV in the HIV-coinfected patient is not well defined. Although most clinical trials in this population studied fixed doses of RBV (800 mg/day), data from studies of HIV-seronegative patients indicate that higher doses of RBV (1000–1200 mg/day) are more effective than lower doses (800 mg/day) in persons infected with HCV genotype 1 [64–66]. Logistic regression analysis of the results of the study by Manns et al. [64] indicated that RBV dose (in mg/kg) was a significant predictor of SVR. Therefore, although clinical trials in coinfected patients generally administer RBV at 800 mg/day, dosages of 1000 to 1200 mg/day are recommended for patients with HCV genotype 1 [43]. This recommendation is supported by findings from Laguno et al. [61], who reported that patients were treated with weight-based RBV dosages (800–1200 mg/day) without safety concerns. More importantly, in a prospective, randomized, controlled trial in HIV-seronegative patients, Hadziyannis et al. [67] reported that both the dose of RBV and total duration of combination therapy should be individualized according to HCV genotype. Patients with HCV genotype 1 require treatment with a standard dose of RBV for 48 weeks. However, those with HCV genotype 2 or 3 infection appear to be adequately treated with combination therapy with the low dose of RBV (800 mg/day) for only 24 weeks.
Unresolved issues in HCV treatment
Unresolved issues include the duration of HCV therapy, optimal RBV dose for HCV genotype 1 and role of newer agents. In clinical trials in coinfected patients, the duration of therapy for all HCV genotypes was 48 weeks. Currently, the standard of care for patients infected with HCV is 48 weeks for genotype 1 and 24 weeks for genotype 2 or 3. Preliminary studies suggest that all patients be treated for 48 weeks. Treatment for 24 weeks in HIV-infected patients with HCV genotype 2 or 3 infection is associated with a high rate of relapse at the end of therapy [61]. Recent data from HCV treatment in HIV seronegative patients indicate that the duration of HCV treatment should be determined by the viral response kinetics of the individual patient rather than a standard duration of therapy for all patients. For example, PEG-IFN plus RBV for 24 weeks was as effective as 48 weeks among HCV genotype 1-infected patients who achieved an undetectable HCV RNA level after 4 weeks of treatment [68,69]. Conversely, the relapse rate was >50% among HCV genotype 1-infected patients who achieved an undetectable HCV RNA level for the first time after 24 weeks of therapy, suggesting longer treatment may be needed [70]. Further research is needed to clarify the appropriate duration of HCV treatment in coinfected patients as well as the role of individual viral kinetics in determining the appropriate treatment course.
Studies in monoinfected patients demonstrated that RBV significantly increases SVR rates [71,72]. Studies in coinfected patients generally used low-dose RBV (800 mg/day). The Spanish Pegasys Plus Ribavirin for HCV Treatment in HIV/HCV Coinfection (PRESCO) trial looked at efficacy and safety of PEG-IFN alfa-2a plus RBV for the treatment of chronic hepatitis C in HIV-coinfected patients [73]. This trial enrolled 582 coinfected patients to receive PEG-IFN alfa plus weight-based dosages of RBV (1000/1200 mg/day). The duration of treatment was extended beyond that of APRICOT and RIBAVIC (genotypes 1, 4: 12 or 18 months; genotypes 2, 3: 6 or 12 months). The end-of-treatment response in PRESCO was 50%—12% higher than that reported in APRICOT — which suggests that higher doses of RBV may be beneficial [59]. Furthermore, preliminary analysis also suggests that higher doses of RBV were not associated with an excessive risk of anaemia and/or mitochondrial toxicity.
A number of newer therapeutic agents in development include cellular IMPDH or IMPDH inhibitors, viral key enzyme inhibitors (i.e. protease, helicase and polymerase inhibitors), internal ribosomal entry site inhibitors, small and expressed interfering RNAs, ribozymes and several new IFNs (i.e. IFN alfa-2b fused with albumin, consensus IFN and IFN-γ) [74,75]. Several RBV-like molecules also in development have the potential to improve the outcomes compared with standard RBV.
Assessing response
Early virologic response (EVR) assessed after 12 weeks of therapy is an important indicator of virologic failure. The failure to achieve an undetectable HCV RNA level or reductions in HCV RNA ≥2 log10 has a negative predictive value of 98–100% for treatment failure [76]. Therefore, HCV treatment should be discontinued if an adequate EVR is not achieved at 12 weeks. Patients should be made aware of the importance of strict adherence to dose and schedule during the first 3 months of combination therapy to increase the probability of achieving an EVR. Similar to HCV RNA levels during treatment in monoinfected patients, levels in coinfected patients at 24 weeks should determine further therapy among those with detectable HCV RNA after 12 weeks of therapy: treatment should be discontinued in those with detectable HCV RNA after 24 weeks and continued in those without. Some experts recommend the continuation of therapy in patients with marked hepatic fibrosis or cirrhosis and virologic failure as a means to prevent liver disease progression. However, the benefits of this approach are currently unclear and require additional evidence before being incorporated into practice.
Other treatment-related issues
CD4+ cell threshold for treatment
Published guidelines do not provide a consensus CD4+ threshold for treating hepatitis C in HIV/HCV-coinfected patients. Whereas the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America do not specify a threshold, an International Expert Opinion Panel and the Centers for Disease Control recommend thresholds of 200 and 500 cells/μL, respectively [49–51]. In the major randomized clinical trials of treatments for coinfected patients [i.e. by RIBAVIC, APRICOT and AIDS Clinical Trial Group (ACTG) investigators], baseline CD4+ cell count had no effect on SVR rates; however, eligible patients in these studies were required to have baseline counts ≥200/μL with the exception of APRICOT, in which persons with CD4+ counts 100–200/μL and HIV RNA levels >5000 copies/mL were enrolled.
Effect of IFN on CD4+ cell count
Soriano et al. [77] reported marked decreases in the absolute CD4+ cell counts in HIV-infected patients with chronic hepatitis C treated with IFN. A similar observation was made in the APRICOT, RIBAVIC and ACTG studies, in which combination PEG-IFN plus RBV significantly lowered the absolute CD4+ cell counts in coinfected patients [62]. However, in each study, the CD4 cell percentage, representing the proportion of the total lymphocyte count, was increased and the absolute CD4+ cell count returned to baseline within 24 weeks after treatment was stopped. Furthermore, in APRICOT, PEG-IFN was associated with an approximate 0.7 log10 reduction in HIV RNA, confirming a modest antiretroviral effect of PEG-IFN in some patients. In addition, HIV-related opportunistic infections were rarely observed in the published studies. Thus, combination PEG-IFN and RBV does not appear to be detrimental to HIV disease.
Treatment of relapsed patients
By definition, a relapsed patient has undetectable HCV RNA at the end of treatment and emerging viremia after treatment is stopped. At present, no treatment strategies address relapse in coinfected patients. However, possible strategies to produce an SVR in this population include extending the duration of therapy, administering higher RBV doses, or deferring treatment until other classes of HCV therapy are introduced. An effort should be made to determine the previously administered dose and duration of treatment in patients considered to have relapsed. If the relapsing patients received suboptimal doses, retreatment with PEG-IFN plus RBV at recommended doses and duration of therapy may produce SVR. Alternatively, if the relapsed patient had dose reductions because of anaemia, retreatment and concomitant recombinant human erythropoietin (rHuEPO) to maintain RBV dose might be considered.
Treatment of nonresponders
Human immunodeficiency virus/HCV-coinfected patients are deemed nonresponders if they adhered to therapy and did not achieve an EVR at week 12 (<2 log10) or 24 (undetectable HCV RNA). Management strategies for these patients have not been studied but may depend on fibrosis stage. In patients with minimal fibrosis, treatment might be deferred until newer classes of anti-HCV drugs become available. There is evidence from the ACTG 5071 trial that therapy can improve histologic measures, irrespective of viral response [62]. Therefore, patients with marked fibrosis might be retreated with high-dose combination therapy or treated with maintenance PEG-IFN. However, the absence of data precludes consensus recommendation regarding the appropriate management of such patients.
Liver transplantation
Liver transplantation is the primary treatment option for eligible coinfected patients with Child–Pugh stage B or C liver disease [43]. HAART therapy has significantly improved short- and mid-term outcomes in HIV-infected patients undergoing liver transplantation. In a study by Ragni et al. [78], cumulative survival among 24 HIV-positive HAART recipients was similar to that among age- and race-comparable HIV-negative recipients (P = 0.37, by log-rank test). At 12, 24 and 36 months after orthotopic liver transplantation, respective survival rates were 87.1%, 72.8% and 72.8% among HIV-positive patients and 86.6%, 81.6% and 77.9% among HIV-negative patients. However, HCV infection was associated with compromised patient survival (P = 0.02). The major issue in this HCV-coinfected subpopulation is reinfection of the graft — an outcome that may lead to rapid development of cirrhosis in the graft [52]. The best approach at this time appears to be PEG-IFN plus RBV combination therapy for the first 3 months after transplantation. However, coinfected liver transplantation patients present special drug treatment issues because of drug–drug interactions and the decreased glomerular filtration rate associated with transplantation.
Other therapeutic considerations in coinfected patients
The majority of adverse events seen in coinfected patients receiving ART and IFN/RBV combination therapy are those typically seen in monoinfected patients receiving either therapy. Therefore, this part of the discussion is devoted to only those events of particular concern in the coinfected population. To achieve the SVR rates reported in clinical trials, patients with hepatitis C must receive IFN and RBV in recommended doses. However, the adverse effects of medications are known to result in dose modifications that can compromise virologic outcomes.
Anaemia in the coinfected population
Anaemia is common in HIV-infected patients because of ‘anaemia of chronic disease,’ blood loss and drug effects.
RBV-related anaemia
Patients with HIV/HCV coinfection present with anaemia and are more susceptible to its development [79]. Thus, it is essential to consider the effects of a treatment on Hb concentration. The most important side effect of RBV is a dose-dependent haemolytic anaemia [80]. Hb concentrations return to normal within 4–8 weeks after RBV is stopped. RBV-related anaemia is secondary to accumulation of phosphorylated derivatives of the drug within the erythrocyte, competition with high-energy phosphate stores, oxidative stress and extravascular haemolysis. Despite the use of combination therapy that includes low-dose RBV (800 mg/day), 12% and 17% of coinfected patients discontinue treatment because of adverse events, primarily haematologic events [57,59]. In one study, anaemia necessitated dose reductions in 16% of 288 coinfected patients treated with combination therapy for chronic hepatitis C and in 26% of those whose antiretroviral regimen included zidovudine [59]. In 3.8% of patients, Hb dropped to below 8 g/dL, despite the use of low doses of RBV.
Despite the risk of anaemia, coinfected patients are treated with HCV combination therapy because of its demonstrated ability to improve SVR rates. One approach to managing anaemia is to reduce the dose of RBV or discontinue the drug. However, virologic outcomes are compromised when the RBV dose is decreased to 600 mg/day for Hb concentrations below 10 g/dL and when RBV is discontinued for Hb concentrations below 8.5 g/dL. As a result, alternative strategies and drugs are being evaluated.
Effect of zidovudine on RBV-associated anaemia
Anaemia because of combination therapy with PEG-IFN plus RBV may be more problematic in HIV-infected patients, particularly in those receiving concomitant medication such as zidovudine. In a randomized trial in 107 patients, Brau et al. [81] reported greater Hb declines during the first 16 weeks (−3.64 g/dL) in patients receiving combination therapy with RBV 800 mg/day plus zidovudine than in patients who did not receive zidovudine (−2.08 g/dL). In addition, patients treated with RBV in combination therapy who also were receiving zidovudine had more anaemia-related RBV dose reductions than those not receiving zidovudine (60%vs 16%) [82].
Recombinant human erythropoietin
Recombinant human erythropoietin is used successfully to treat patients with RBV-associated anaemia and does not adversely affect HCV clearance. In addition to maintaining the RBV dose, rHuEPO may increase adherence and improve health-related quality of life [83]. Sulkowski et al. [84] conducted a 16-week, open-label, randomized, parallel-group, multicenter study in 66 anaemic (Hb ≤ 12 g/dL) coinfected patients receiving IFN/RBV for at least 16 weeks. Patients were randomized 1:1 to receive epoetin alfa 40 000 U s.c. once weekly or standard of care (no rHuEPO). The rHuEPO dose was increased by 20 000 U if Hb had not returned to pretreatment baseline concentrations after 4 weeks. Mean baseline Hb was 11.1 ± 0.3 g/dL in the rHuEPO group and did not differ significantly from that in the standard of care group (P = 0.33). Treatment corrected the anaemia in all patients, including those receiving zidovudine. The mean increases in Hb concentrations from baseline to week 16 in the rHuEPO and control groups were 2.6 ± 0.3 and 0.2 ± 0.3 g/dL, respectively (P < 0.001). Treatment with the recombinant haematopoietic growth factor allowed significantly more patients to receive RBV doses above 10.6 mg/kg/day (67%vs 45%; P = 0.09). Health-related quality-of-life scores were greater and fatigue decreased significantly in the rHuEPO group compared with the standard of care group. The drug was well tolerated, and most adverse events were mild to moderate in severity. These findings are supported by studies reporting the benefits of rHuEPO in HCV-monoinfected patients receiving combination therapy [85].
Recombinant human erythropoietin is uncommonly associated with some adverse effects: thrombosis, hypertension and pure red blood cell aplasia [86–88]. However, no serious rHuEPO-related events occurred in the study by Sulkowski et al. [84]. Instead, all adverse events occurred in the control group and consisted of one case each of chest pain, myocardial infarction and psychosis. Notably, no thrombotic events or pure red blood cell aplasia were reported. Use of the recombinant haematopoietic growth factor requires regular Hb monitoring. Unfortunately, clinical studies have yet to establish the effect of rHuEPO on SVR, the potential cost–benefit of this treatment, and the beneficial effects of prophylactic rHuEPO on quality of life in patients with hepatitis C.
Alternatives to RBV
New alternatives to RBV may reduce the risk of anaemia and the need for rHuEPO in the coinfected population without compromising virologic outcomes. Taribavirin, a liver-targeting prodrug of RBV, is now in phase 3 clinical trials [89]. A synthetic guanosine analogue, taribavirin, is converted to RBV by hepatic adenosine deaminase and thus preferentially targets the liver and shows significantly less accumulation within erythrocytes [89]. Because lower levels of red blood cell RBV phosphates compete with high-energy phosphate stores, taribavirin produces less compromise of cellular energetics than RBV. The pharmacology of the drug suggests its ability to prevent RBV-associated anaemia in coinfected patients.
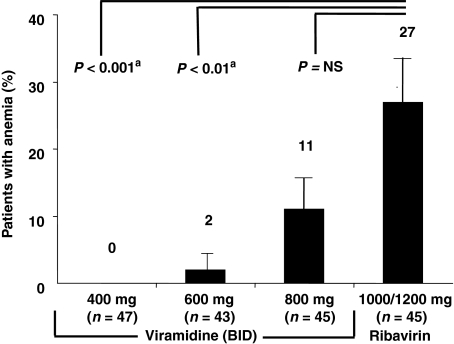
Early studies demonstrated the antiviral potential of taribavirin. A phase 2 randomized, active-controlled, multicenter study compared taribavirin and RBV in combination with PEG-IFN alfa-2a in 180 treatment-naïve patients with chronic hepatitis C. At the end of treatment, there were no significant differences between groups in the proportion of patients with undetectable HCV RNA levels (range: 55–63%), regardless of HCV genotype [90]. Importantly, significantly fewer patients treated with taribavirin developed anaemia compared with those treated with RBV [4%vs 27%; P < 0.001 (Fig. 2)] [91]. In fact, anaemia (Hb < 10 g/dL) did not occur in the viramidine 400 mg b.i.d. group and occurred in only one patient in the 600 mg b.i.d. group, the dose selected for phase 3 trials [90]. In contrast, 11% and 27% of the taribavirin 800 mg b.i.d. and RBV 1000/1200 mg/day groups, respectively, became anaemic.
Fig. 2.
Anaemia in hepatitis C virus-infected patients treated with ribavirin or taribavirin. aAdjusted for multiple comparisons. [Reprinted with permission from Gish (91)].
Currently, taribavirin 600 mg b.i.d. is being compared with RBV 1000/1200 mg/day in 2 phase 3 trials: VISER1 and VISER2 (VIramidine's Taribavirin Safety and Efficacy vs Ribavirin). These studies have completed enrollment are expected to determine the comparative efficacy of taribavirin vs RBV and to evaluate the drug's red blood cell-sparing properties. If the drug is at least as effective as RBV in combination therapy and is found to preserve Hb concentrations, it may eliminate the issue of RBV-related anaemia in coinfected patients and prevent the need for dose modifications or rHuEPO therapy.
Mitochondrial toxicity
Nucleoside analogue reverse transcriptase inhibitors used to treat HIV in coinfected patients can cause mitochondrial toxicity, which is identified by elevated levels of lactate or pancreatic enzymes. This risk is increased in coinfected patients also receiving drugs with similar mechanisms of action. In the APRICOT and RIBAVIC studies, mitochondrial toxicity was identified in 3% and 5% of patients, respectively. In a prospective analysis of 113 coinfected patients, Laguno et al. [92] identified evidence of this disorder in 12% of patients treated with combination therapy for HCV plus HAART, although most patients were asymptomatic.
Administering didanosine to patients treated with combination therapy increases the risk of mitochondrial toxicity [93]. Ribavirin monophosphate inhibits IMPDH, the primary phosphate donor to didanosine [94]. This inhibition increases the intracellular concentrations of didanosine triphosphate and the occurrence of resultant toxicities such as lactic acidosis. Therefore, all coinfected patients receiving combination therapy and ART should be monitored for lipid, lactic acid and amylase levels. If lactate levels are above 5 mmol/L, treatment should be stopped immediately and supportive treatment supplied as needed [95]. Patients with lactate elevations of 2.1–5 mmol/L can continue with a non-nucleoside reverse transcriptase inhibitor or a regimen without the causative agent.
Drug–drug interactions
Anti-HCV drugs can produce adverse outcomes in coinfected patients receiving ART. For example, RBV and pyrimidine antiretroviral agents such as zidovudine exert antagonistic effects on HIV replication in vitro [96]. This effect appears to be secondary to RBV-induced inhibition of the phosphorylation of azidothymidine. However, the agent also enhances the inhibitory effects of purine 2′,3′-dideoxynucleosides on replication of HIV in vitro [97]. Thus, the net inhibitory and facilitatory activities suggest the effect might not be clinically important — an observation supported by clinical trial results [59,62,98]. The package insert for didanosine warns about potential complications when didanosine is administered with RBV. The interaction can produce mitochondrial toxicity with lactic acidosis, myopathy, neuropathy and cardiomyopathy, all of which are presumably secondary to RBV-dependent increased phosphorylation of didanosine and mitochondrial DNA polymerase (polymerase-γ) damage [81,99]. In addition, a preliminary report of 62 coinfected patients raises the issue that treatment with protease inhibitors coadministered with IFN/RBV combination therapy may decrease the SVR [100]. In the study, patients receiving HAART with or without a PI had SVR rates of 11.1% and 44.4%, respectively. Clearly, this issue requires study in a larger trial.
Leucopenia
In the pivotal trials of combination therapy for patients with chronic hepatitis C, neutropenia resulted in dose reductions of PEG-IFN alfa-2a and alfa-2b in 24% and 18% of patients, respectively [64,65]. After IFN therapy, both neutrophil and lymphocyte counts decline within 2 weeks and stabilize thereafter [101]. The reduction in granulocytes is more severe when PEG-IFN is given. Soza et al. [102] assessed combination therapy and neutropenia in 119 patients with chronic hepatitis C. During treatment, neutrophil counts decreased by an average of 34%. Documented or suspected bacterial infections developed in 22 patients (18%), but in no patient with neutropenia. Some physicians manage neutropenia with IFN dose reduction; however, absolute neutrophil counts that trigger this response are variable. Preliminary studies show filgrastim (recombinant human granulocyte-colony-stimulating factor) significantly improves neutrophil counts and may be a useful adjuvant is some settings [101].
Conclusions
Hepatitis C virus coinfection is common in HIV-positive patients in the USA and Europe. Because HIV infection can accelerate progression of HCV-related liver disease, treatment of chronic hepatitis C is generally recommended. Either virus can alter the outcomes associated with the other. At this time, up to 40% of coinfected patients can achieve SVR with combination PEG-IFN plus RBV therapy. However, the ability to achieve SVR depends on adhering to the recommended doses of both drugs; thus, steps should be taken to prevent and treat potentially dose-limiting complications, such as hepatotoxicity, mitochondrial toxicity, anaemia, fatigue, depression and neutropenia. New and investigational agents have the potential to improve the outcomes and should be studied in coinfected patients as well as more traditional HCV-infected subgroups.
Acknowledgments
Both MSS and YB received grant/research support from Akros Pharma Inc, Human Genome Sciences, Idenix Pharmaceuticals Inc, Roche Pharmaceuticals, Schering-Plough Corporation and Valeant Pharmaceuticals International. In addition, MSS received support from Vertex Pharmaceuticals Incorporated and has received grant support from the National Institute on Drug Abuse (DA-16065, DA-13806) and YB received support from Gilead Sciences.
Glossary
Abbreviations:
- ACTG
AIDS Clinical Trial Group
- AIDS
acquired immune deficiency syndrome
- APRICOT
AIDS Pegasys Ribavirin International Coinfection Trial
- ART
antiretroviral therapy
- CT
computed tomography
- EVR
early virologic response
- HAART
highly effective antiretroviral therapy
- Hb
haemoglobin
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IFN
interferon
- IMPDH
inosine monophosphate dehydrogenase
- IRES
internal ribosomal entry site
- PBMC
peripheral blood mononuclear cell
- PEG-IFN
pegylated interferon
- PRESCO
Pegasys Plus Ribavirin for HCV Treatment in HIV/HCV Coinfection
- RBV
ribavirin
- rHuEPO
recombinant human erythropoietin
- RIBAVIC
Randomized Controlled Trial of Pegylated-Interferon alfa-2b plus Ribavirin vs Interferon alfa-2b plus Ribavirin for the Initial Treatment of Chronic Hepatitis C in HIV Co-Infected Patients
- SVR
sustained virologic response
References
- 1.Staples CT., Jr Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29:150–154. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 2.Benfield T. Proceedings of the 12th World AIDS Conference. Geneva, Switzerland: 1998. Hepatitis C in the EuroSIDA cohort of European HIV-infected patients: prevalence and prognostic value [abstract 22261] June 28–July 3. [Google Scholar]
- 3.Denis F. Seroprevalence of HBV, HCV and HDV hepatitis markers in 500 patients infected with the human immunodeficiency virus. Pathol Biol (Paris) 1997;45:701–708. [PubMed] [Google Scholar]
- 4.Sherman KE. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 5.Bica I, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 6.Wong JB. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho DD. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 8.Neumann AU, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 9.Maggi F, et al. Differences in hepatitis C virus quasispecies composition between liver, peripheral blood mononuclear cells and plasma. J Gen Virol. 1997;78(Pt 7):1521–1525. doi: 10.1099/0022-1317-78-7-1521. [DOI] [PubMed] [Google Scholar]
- 10.Venturi G, et al. Antiretroviral resistance mutations in human immunodeficiency virus type 1 reverse transcriptase and protease from paired cerebrospinal fluid and plasma samples. J Infect Dis. 2000;181:740–745. doi: 10.1086/315249. [DOI] [PubMed] [Google Scholar]
- 11.Reesink HW, et al. Final results of a phase 1B, multiple-dose study of VX-950, a hepatitis C virus protease inhibitor [abstract 96] Hepatology. 2005;42(Suppl. 1):234A–235A. [Google Scholar]
- 12.Darby SC, et al. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. UK Haemophilia Centre Directors’ Organisation. Lancet. 1997;350:1425–1431. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- 13.Soriano V. Impact of chronic liver disease due to hepatitis viruses as cause of hospital admission and death in HIV-infected drug users. Eur J Epidemiol. 1999;15:1–4. doi: 10.1023/a:1007506617734. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal E, et al. Mortality due to hepatitis C-related liver disease in HIV-infected patients in France (Mortavic 2001 study) AIDS. 2003;17:1803–1809. doi: 10.1097/00002030-200308150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Graham CS, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 16.Di Martino V, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology. 2001;34:1193–1199. doi: 10.1053/jhep.2001.29201. [DOI] [PubMed] [Google Scholar]
- 17.Benhamou Y, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 18.Benhamou Y, et al. Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology. 2001;34:283–287. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 19.Sulkowski MS. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl. 1):S77–S84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 20.Gebo KA. Hospitalization rates differ by hepatitis C status in an urban HIV cohort. J Acquir Immune Defic Syndr. 2003;34:165–173. doi: 10.1097/00126334-200310010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Weber R, et al. Proceedings of the 12th Conference on Retroviruses and Opportunistic Infections. Boston, MA: 2005. HIV and non-HIV-related deaths and their relationship to immunodeficiency: the D:A:D Study [abstract 595] February 22–25 Available at: http://www.retroconference.org/2005/cd/Abstracts/24898.htm. [Google Scholar]
- 22.Greub G, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 23.Yoo TW. Effect of hepatitis C virus (HCV) genotype on HCV and HIV-1 disease. J Infect Dis. 2005;191:4–10. doi: 10.1086/426513. [DOI] [PubMed] [Google Scholar]
- 24.Sulkowski MS. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 25.Rockstroh JK, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 26.Romeo R, et al. Hepatitis C is more severe in drug users with human immunodeficiency virus infection. J Viral Hepat. 2000;7:297–301. doi: 10.1046/j.1365-2893.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 27.Brau N, et al. Control of HIV viral load through highly active antiretroviral therapy (HAART) slows down liver fibrosis progression in HIV/HCV-coinfection and makes it the same as in HCV monoinfection [abstract 91] J Hepatol. 2004;40(Suppl 1):33. [Google Scholar]
- 28.Garcia-Samaniego J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:179–183. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- 29.Sherman KE, et al. Quantitative evaluation of hepatitis C virus RNA in patients with concurrent human immunodeficiency virus infections. J Clin Microbiol. 1993;31:2679–2682. doi: 10.1128/jcm.31.10.2679-2682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daar ES, et al. Relation between HIV-1 and hepatitis C viral load in patients with hemophilia. J Acquir Immune Defic Syndr. 2001;26:466–472. doi: 10.1097/00126334-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 31.Cribier B, et al. HIV increases hepatitis C viraemia irrespective of the hepatitis C virus genotype. Res Virol. 1997;148:267–271. doi: 10.1016/s0923-2516(97)88363-x. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima N. Characterization of long-term cultures of hepatitis C virus. J Virol. 1996;70:3325–3329. doi: 10.1128/jvi.70.5.3325-3329.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laskus T, et al. Human immunodeficiency virus facilitates infection/replication of hepatitis C virus in native human macrophages. Blood. 2004;103:3854–3859. doi: 10.1182/blood-2003-08-2923. [DOI] [PubMed] [Google Scholar]
- 34.Babik JM. Impact of highly active antiretroviral therapy and immunologic status on hepatitis C virus quasispecies diversity in human immunodeficiency virus/hepatitis C virus-coinfected patients. J Virol. 2003;77:1940–1950. doi: 10.1128/JVI.77.3.1940-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulkowski MS. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 36.Sulkowski MS. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35:182–189. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 37.den Brinker M, et al. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. 2000;14:2895–2902. doi: 10.1097/00002030-200012220-00011. [DOI] [PubMed] [Google Scholar]
- 38.Nunez M. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:426–431. doi: 10.1097/00126334-200108150-00002. [DOI] [PubMed] [Google Scholar]
- 39.John M. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease. AIDS. 1998;12:2289–2293. doi: 10.1097/00002030-199817000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Qurishi N, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 41.Bräu N, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Marine-Barjoan E, et al. Impact of antiretroviral treatment on progression of hepatic fibrosis in HIV/hepatitis C virus co-infected patients. AIDS. 2004;18:2163–2170. doi: 10.1097/00002030-200411050-00008. [DOI] [PubMed] [Google Scholar]
- 43.Alberti A, et al. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol. 2006;55:403–408. doi: 10.1016/j.jhep.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Foucher J, et al. Diagnosis of cirrhosis by transient elastography (Fibroscan®): a prospective study. Gut. 2005;???????:???????. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poynard T, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8. doi: 10.1186/1476-5926-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterling R, et al. Proceedings of the 12th Conference on Retroviruses and Opportunistic Infections. Boston, MA: 2005. Can routine non-invasive tests predict liver histology in HIV/HCV co-infection? Analysis of patients entering the AIDS PEGASYS Ribavirin International Co-infection Trial (APRICOT) [abstract 120] February 22–25 Available at: http://www.retroconference.org/2005/cd/Abstracts/25102.htm. [Google Scholar]
- 47.Thuluvath PJ. Noninvasive markers of fibrosis for longitudinal assessment of fibrosis in chronic liver disease: are they ready for prime time. Am J Gastroenterol. 2005;100:1981–1983. doi: 10.1111/j.1572-0241.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 48.Muir AJ. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 49.Soriano V, et al. Care of patients with chronic hepatitis C and HIV co-infection: recommendations from the HIV-HCV International Panel. AIDS. 2002;16:813–828. doi: 10.1097/00002030-200204120-00001. [DOI] [PubMed] [Google Scholar]
- 50.Strader DB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 51.Benson CA. Treating opportunistic infections among HIV-exposed and infected children: recommendations from CDC, the National Institutes of Health, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2004;53:1–112. [PubMed] [Google Scholar]
- 52.Rockstroh JK. HIV and hepatitis C virus co-infection. Lancet Infect Dis. 2004;4:437–444. doi: 10.1016/S1473-3099(04)01059-X. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Olmeda M, et al. Pegylated IFN-alpha2b plus ribavirin as therapy for chronic hepatitis C in HIV-infected patients. AIDS. 2003;17:1023–1028. doi: 10.1097/00002030-200305020-00011. [DOI] [PubMed] [Google Scholar]
- 54.Voigt E, et al. Proceedings of the 2nd International AIDS Society Conference on HIV Pathogenesis and Treatment. Paris, France: 2003. Factors related to outcome of treatment with pegylated interferon alpha 2b (IEG-IFN) plus ribavirin (RBV) in HCV/HIV-coinfected patients [abstract 976] July 13–16 Available at: http://www.iasociety.org/abstract/show.asp?abstract_id=10798. [Google Scholar]
- 55.Moreno L, et al. Pegylated interferon alpha2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. AIDS. 2004;18:67–73. doi: 10.1097/00002030-200401020-00008. [DOI] [PubMed] [Google Scholar]
- 56.Torriani FJ, et al. Proceedings of the 11th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: 2004. Final results of APRICOT: a randomized, partially blinded, international trial evaluating peginterferon-α-2a + ribavirin vs interferon-α-2a + ribavirin in the treatment of HCV in HIV/HCV co-infection [abstract 112] February 8–11. Available at: http://www.retroconference.org/2004/cd/Abstract/112.htm. [Google Scholar]
- 57.Carrat F, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 58.Chung R, et al. Proceedings of the 11th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: 2004. A randomized, controlled trial of PEG-interferon-alfa-2a plus ribavirin vs interferon-alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-co-infected persons: follow-up results of ACTG A5071 [abstract 110] February 8–11. Available at: http://www.retroconference.org/2004/cd/Abstract/110.htm. [Google Scholar]
- 59.Torriani FJ, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 60.Hoggard PG. Effects of drugs on 2′,3′-dideoxy-2′,3′-didehydrothymidine phosphorylation in vitro. Antimicrob Agents Chemother. 1997;41:1231–1236. doi: 10.1128/aac.41.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laguno M, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18:F27–F36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 62.Chung RT, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mauss S, et al. Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon-based therapy. AIDS. 2004;18:F21–F25. doi: 10.1097/00002030-200409030-00002. [DOI] [PubMed] [Google Scholar]
- 64.Manns MP, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 65.Fried MW, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 66.Tod M, et al. Pharmacokinetic/pharmacodynamic and time-to-event models of ribavirin-induced anaemia in chronic hepatitis C. Clin Pharmacokinet. 2005;44:417–428. doi: 10.2165/00003088-200544040-00006. [DOI] [PubMed] [Google Scholar]
- 67.Hadziyannis SJ, et al. Peginterferon-α2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 68.Zeuzem S, et al. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol. 2006;44:97–103. doi: 10.1016/j.jhep.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Mangia A, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609–2617. doi: 10.1056/NEJMoa042608. [DOI] [PubMed] [Google Scholar]
- 70.Ferenci P, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–433. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Poynard T, et al. Randomised trial of interferon α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 72.McHutchison JG, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 73.Nunez M, et al. Program and Abstracts of the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC: 2004. Efficacy and safety of peginterferon-a2a plus ribavirin (PegIFN + RBV) for the treatment of hepatitis C in HIV co-infected patients: the PRESCO Trial [abstract V-1148] October 30-November 2. [Google Scholar]
- 74.McHutchison JG. Future therapy of hepatitis C. Hepatology. 2002;36(Suppl. 1):S245–S252. doi: 10.1053/jhep.2002.36795. [DOI] [PubMed] [Google Scholar]
- 75.McHutchison JG. Future trends in managing hepatitis C. Gastroenterol Clin North Am. 2004;33(Suppl. 1):S51–S61. doi: 10.1016/j.gtc.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Soriano V, et al. Hepatitis C virus-RNA clearance in HIV-coinfected patients with chronic hepatitis C treated with pegylated interferon plus ribavirin. Antivir Ther. 2004;9:505–509. [PubMed] [Google Scholar]
- 77.Soriano V, et al. CD4+ T-lymphocytopenia in HIV-infected patients receiving interferon therapy for chronic hepatitis C. [letter] HIV-Hepatitis Spanish Study Group. AIDS. 1994;8:1621–1622. doi: 10.1097/00002030-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 78.Ragni MV, et al. Survival of human immunodeficiency virus-infected liver transplant recipients. J Infect Dis. 2003;188:1412–1420. doi: 10.1086/379254. [DOI] [PubMed] [Google Scholar]
- 79.Dieterich DT. Hematologic disorders associated with hepatitis C virus infection and their management. Clin Infect Dis. 2003;37:533–541. doi: 10.1086/376971. [DOI] [PubMed] [Google Scholar]
- 80.Sulkowski MS. Changes in haemoglobin during interferon alpha-2b plus ribavirin combination therapy for chronic hepatitis C virus infection. J Viral Hepat. 2004;11:243–250. doi: 10.1111/j.1365-2893.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 81.Brau N, et al. Treatment of chronic hepatitis C in HIV/HCV-coinfection with interferon alpha-2b + full-course vs. 16-week delayed ribavirin. Hepatology. 2004;39:989–998. doi: 10.1002/hep.20107. [DOI] [PubMed] [Google Scholar]
- 82.Alvarez D. Zidovudine use but not weight-based ribavirin dosing impacts anaemia during HCV treatment in HIV-infected persons. J Viral Hepat. 2006;13:683–689. doi: 10.1111/j.1365-2893.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 83.Sulkowski MS. Anemia in the treatment of hepatitis C virus infection. Clin Infect Dis. 2003;37(Suppl. 4):S315–S322. doi: 10.1086/376911. [DOI] [PubMed] [Google Scholar]
- 84.Sulkowski MS, et al. Epoetin alfa once weekly improves anemia in HIV/hepatitis C virus-coinfected patients treated with interferon/ribavirin: a randomized controlled trial. J Acquir Immune Defic Syndr. 2005;39:504–506. doi: 10.1097/01.qai.0000167158.90722.73. [DOI] [PubMed] [Google Scholar]
- 85.Afdhal NH, et al. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302–1311. doi: 10.1053/j.gastro.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 86.Tobu M, et al. Erythropoietin-induced thrombosis as a result of increased inflammation and thrombin activatable fibrinolytic inhibitor. Clin Appl Thromb Hemost. 2004;10:225–232. doi: 10.1177/107602960401000304. [DOI] [PubMed] [Google Scholar]
- 87.Bennett CL, et al. Pure red-cell aplasia and epoetin therapy. N Engl J Med. 2004;351:1403–1408. doi: 10.1056/NEJMoa040528. [DOI] [PubMed] [Google Scholar]
- 88.Nigg L. Prolonged course of pure red cell aplasia after erythropoietin therapy. Eur J Haematol. 2004;73:376–379. doi: 10.1111/j.1600-0609.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- 89.Lin C-C. Viramidine, a prodrug of ribavirin, shows better liver-targeting properties and safety profiles than ribavirin in animals. Antivir Chem Chemother. 2003;14:145–152. doi: 10.1177/095632020301400304. [DOI] [PubMed] [Google Scholar]
- 90.Gish R. Safety and efficacy of viramidine in combination with pegylated interferon alfa-2a for treatment of hepatitis C in therapy-naïve patients [abstract 479] J Hepatol. 2004;40(Suppl. 1):141–142. [Google Scholar]
- 91.Gish RG. Treating HCV with ribavirin analogues and ribavirin-like molecules. J Antimicrob Chemother. 2006;57:8–13. doi: 10.1093/jac/dki405. [DOI] [PubMed] [Google Scholar]
- 92.Laguno M, et al. Incidence and risk factors for mitochondrial toxicity in treated HIV/HCV-coinfected patients. Antivir Ther. 2005;10:423–429. [PubMed] [Google Scholar]
- 93.Lafeuillade A. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet. 2001;357:280–281. doi: 10.1016/S0140-6736(00)03618-7. [DOI] [PubMed] [Google Scholar]
- 94.Salmon-Ceron D. Mitochondrial toxic effects and ribavirin. Lancet. 2001;357:1803–1804. doi: 10.1016/S0140-6736(00)04921-7. [DOI] [PubMed] [Google Scholar]
- 95.Cengiz C. HIV and liver diseases: recent clinical advances. Clin Liver Dis. 2005;9:647–666. doi: 10.1016/j.cld.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 96.Vogt MW, et al. Ribavirin antagonizes the effect of azidothymidine on HIV replication. Science. 1987;235:1376–1379. doi: 10.1126/science.2435003. [DOI] [PubMed] [Google Scholar]
- 97.Baba M. Ribavirin antagonizes inhibitory effects of pyrimidine 2′,3′-dideoxynucleosides but enhances inhibitory effects of purine 2′,3′-dideoxynucleosides on replication of human immunodeficiency virus in vitro. Antimicrob Agents Chemother. 1987;31:1613–1617. doi: 10.1128/aac.31.10.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salmon-Ceron D, et al. Interferon-ribavirin in association with stavudine has no impact on plasma human immunodeficiency virus (HIV) type 1 level in patients coinfected with HIV and hepatitis C virus: a CORIST-ANRS HC1 trial. Clin Infect Dis. 2003;36:1295–1304. doi: 10.1086/374837. [DOI] [PubMed] [Google Scholar]
- 99.Ogedegbe AO. Antiretroviral-associated liver injury. Clin Liver Dis. 2003;7:475–499. doi: 10.1016/s1089-3261(03)00023-0. [DOI] [PubMed] [Google Scholar]
- 100.Poizot-Martin I, et al. Efficacy and tolerance of HCV treatment in HIV-HCV coinfected patients: the potential interaction of PI treatment. HIV Clin Trials. 2003;4:262–268. doi: 10.1310/50jb-vnbb-7hrg-7gur. [DOI] [PubMed] [Google Scholar]
- 101.Ong JP. Managing the hematologic side effects of antiviral therapy for chronic hepatitis C: anemia, neutropenia, and thrombocytopenia. Cleve Clin J Med. 2004;71(Suppl. 3):S17–S21. doi: 10.3949/ccjm.71.suppl_3.s17. [DOI] [PubMed] [Google Scholar]
- 102.Soza A, et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology. 2002;36:1273–1279. doi: 10.1053/jhep.2002.36502. [DOI] [PubMed] [Google Scholar]