Abstract
Aims and methods:
In this double-blind, double-dummy, randomised, parallel group, multicentre study, the efficacy of dosing and re-dosing of a fixed combination of indomethacin, prochlorperazine and caffeine (Indoprocaf) was compared with encapsulated sumatriptan in the acute treatment of two migraine attacks. Additionally, in the group taking Indoprocaf, two different oral formulations were tested: effervescent tablets and encapsulated coated tablets.
Results:
Of 297 patients randomised (150 assigned to Indoprocaf and 147 to sumatriptan), 281 were included in the intention-to-treat efficacy analysis. The initial dosing of Indoprocaf and sumatriptan was similarly effective with pain-free rates higher than 30% (95% CI of odds-ratio: 0.57–1.28) and headache relief rates of about 60% (95% CI of odds-ratio: 0.82–1.84) with both the drugs. The efficacy of re-dosing of Indoprocaf as rescue medication was more effective than that of sumatriptan with pain-free values of 47% vs. 27% in the total attacks with a statistically significant difference in the first migraine attack in favour of Indoprocaf. The efficacy of re-dosing to treat a recurrence/relapse was very high without differences between the drugs (pain-free: 60% with Indoprocaf and 50% with sumatriptan in the total attacks). Indoprocaf and sumatriptan were well-tolerated.
Conclusion:
The study demonstrated that the efficacy of the initial dosing of Indoprocaf was not higher than that of sumatriptan, but that the strategy to use the lowest effective dose as soon as the headache occurred, followed by a second dose if the headache has not relieved or to treat a relapse, was very effective, especially with Indoprocaf.
What's new
Indoprocaf and sumatriptan are the most used drugs for the treatment of migraine in Italy and comparison studies between the oral forms of the two drugs had never been carried out before. In addition, the results of the study showed that the strategy to use the lowest effective dose as soon as the headache occurred, followed by a second dose if the headache had not relieved or to treat a relapse, was very effective.
Introduction
A fixed combination of indomethacin, prochlorperazine and caffeine (hereinafter Indoprocaf) is the most commonly used drug in Italy for the acute treatment of migraine and tension-type headache since more than 30 years. Indomethacin is a non-steroidal anti-inflammatory drug (NSAID), structurally related to serotonin and endowed with central analgesic and cranial vasoconstrictor properties (1,2). Indomethacin was described to block nitric oxide-induced dilation of dural meningeal vessels (3). In the rat, indomethacin administration markedly reduces nitroglycerin-induced Fos expression in several areas of pain control system, including nucleus trigeminalis caudalis (4). Moreover, several evidences indicate a central analgesic activity of this substance in both human and experimental studies (5,6). The efficacy of indomethacin in abolishing peripheral and central sensitisation in animal models was recently published (7,8). Indomethacin is the treatment of choice for chronic paroxysmal hemicrania and hemicrania continua (9) and showed to be effective in the acute treatment of migraine and in different types of primary headache (10–12). Prochlorperazine is a phenothiazine antiemetic, endowed with central cholinergic analgesic properties (13). Intramuscular or intravenous prochlorperazine is considered as adjunct first-line therapy for migraine attacks in emergency departments or offices, while rectal prochlorperazine is suggested to be used as associated treatment for migraine attacks with nausea and vomiting (14). Prochlorperazine has proved to be clinically effective in the acute treatment of migraine, also as monotherapy at an oral dosage of 3 mg (15). Caffeine is a methylxanthine used in several analgesic preparations because of its central cholinergic analgesic properties (16). The analysis of 30 clinical studies involving more than 10,000 patients showed that, if caffeine is combined with an analgesic, the dose of the analgesic required to obtain the same pain relief is reduced by 40% (17). A review of the benefit-risk of caffeine as an analgesic adjuvant concluded that adding caffeine to analgesics increases the number of patients who become free of headache (rate ratio, 1.36; CI, 1.17–1.58) (18).
The pharmacological and clinical profile of the fixed combination of indomethacin, prochlorperazine and caffeine (Indoprocaf) has recently been studied. The three active ingredients of Indoprocaf reverted hyperalgesia in in vivo models of migraine at doses 10 times lower than the corresponding analgesic ones (19). In these models Indoprocaf showed a significantly higher efficacy compared to the three single active principles (19). Indoprocaf, but not sumatriptan, was able to abolish the peripheral sensitisation induced by kainic acid and the central sensitisation induced by N–methyl–D–aspartate (NMDA), in in vivo models of hyperalgesia (7). This study suggested that, while triptans seem to be able to abort migraine attacks only if given before the establishment of cutaneous allodynia and central sensitisation (20), Indoprocaf should be able to abort migraine attacks independently from the time of administration, being able to abolish an already established peripheral and central sensitisation (7).
From a clinical point of view, in a multicentre, randomised, cross-over clinical trial, Indoprocaf suppositories showed to be significantly more effective than sumatriptan 25 mg suppositories in the acute treatment of migraine attacks with a good tolerability profile (21). In particular, the study showed that more attacks were pain-free at 2 h postdose (primary end-point) on Indoprocaf than on sumatriptan (49% vs. 34%; p < 0.01). The superiority of Indoprocaf to sumatriptan in this trial was also confirmed by other important secondary end-points, as time to pain-free, alleviation of nausea, sustained pain-free and consistency across and within patients. In a double-blind, multicentre, randomised, parallel group study, Indoprocaf, compared with nimesulide, showed to be very effective and well tolerated in the treatment of episodic tension-type headache (22).
Sumatriptan, an effective drug for the acute treatment of migraine with and without aura and cluster headache, is the first of the selective 5-HT1B/1D agonists (known as triptans) being discovered, and is widely considered to be the gold standard in migraine therapy (23). Indoprocaf and sumatriptan are the most used drugs for the treatment of migraine in Italy and comparison studies between the oral forms of the two drugs have never been carried out. This study, performed according to the second edition of the Guidelines for Controlled Trials of Drugs in Migraine (24), was designed to compare the efficacy and safety of Indoprocaf effervescent (a recently developed formulation of Indoprocaf) and encapsulated coated tablets with encapsulated sumatriptan 50-mg tablets in the acute treatment of migraine, using the percentage of pain-free attacks at 2 h after dosing in two migraine attacks in total, as primary efficacy end-point.
Methods
Patients
In this multicentre study, conducted between December 2002 and June 2004, male or female outpatients (18–65 years) who met International Headache Society (IHS) criteria for migraine with or without aura (25) were enrolled by specialist physicians of Headache Centres throughout Italy. Patients were eligible for inclusion if they had a history of migraine of at least 1 year duration, an age at onset of migraine < 50 years and had experienced from one to six attacks per month during the month of screening. At least moderate headache severity most of the times and periods between attacks free from headache were also required. Patients were excluded if they used drugs for migraine prophylaxis or ergot derivatives during the month of screening, or if they had a history or current evidence of drugs (analgesics, ergot derivatives, opiates or major tranquillisers) or alcohol abuse according to IHS criteria (25). Other exclusion criteria were serious illness (including psychiatric diseases) or contraindications to Indoprocaf or to sumatriptan, and pregnancy or lactation. Patients were also excluded if they were known to be non-responders to Indoprocaf or to sumatriptan. Moreover, at baseline patients should have a normal 12-lead electrocardiogram (ECG), no clinically abnormal laboratory tests including haematology and blood chemistry and a negative pregnancy test.
The protocol and informed consent form were approved by independent Ethics Committee at each clinical centre. A description of the study risks and benefits was provided to all participants, who gave written informed consent prior to entry in the study. The study was conducted according to International Conference on Harmonisation (ICH) Good Clinical Practices, to the Guidelines for Controlled Trials of Drugs in Migraine (24) and to the Note for Guidance on Clinical Investigation of Medicinal Products for the Treatment of Migraine (26).
Study design and treatments
This study was designed as a double-blind, double-dummy, randomised, parallel group, multicentre trial. After 4 weeks of screening, patients were randomised to Indoprocaf-effervescent tablets or Indoprocaf-coated tablets or sumatriptan 50 mg tablets, for the treatment of two consecutive migraine attacks of moderate or severe intensity, separated by at least 48 h. The randomisation was 1 : 1 : 2 (Indoprocaf-effervescent tablets:Indoprocaf-coated tablets:sumatriptan 50 mg tablets) with a blocked randomisation by centre. The randomisation list was provided from a computer-generated code list. Patients were supplied with two doses of study medication for each of two attacks to be treated. The first dose had to be taken as soon as possible when the headache of moderate severity occurred. The second dose had to be taken either as rescue medication, when the severity of headache was still moderate (score 2) or severe (score 3) at 2 h after dosing, or to treat the relapse of headache, that is if the severity of headache was 0 (no headache) or 1 (mild headache) at 2 h and the headache returned within 48 h of initial dosing. In the Indoprocaf-effervescent tablets group, each dose of study medication consisted of one effervescent tablet containing indomethacin 25 mg, prochlorperazine 2 mg and caffeine 75 mg and one placebo capsule; in the Indoprocaf-coated tablets group, each dose of study medication consisted of one encapsulated tablet containing indomethacin 25 mg, prochlorperazine 2 mg and caffeine 75 mg and one placebo effervescent tablet; in the sumatriptan 50 mg tablets group, each dose of study medication consisted of one encapsulated tablet containing sumatriptan 50 mg and one placebo effervescent tablet.
Patients should not take any other drug during the first 2 h after initial dosing. For the patients who took a second dose of study medication, the use of any other drug to treat the headache was allowed only 2 h after the intake of the second dose of study medication. Ergot derivatives and opiates could not be used as a rescue medication. During the migraine attack, patients were not permitted to take coffee or beverages containing caffeine. Patients were scheduled to be seen after the treatment of two attacks and not later than 8 weeks after the randomisation. During this period ergot derivatives and migraine prophylactic drugs were not allowed.
At baseline visit, the investigators completed the MIDAS (Migraine Disability Assessment) questionnaire (27). During the screening and the study periods patients were given a diary card. During migraine attack, patients rated their headache severity in the diary using a four-grade scale (0, no headache; 1, mild headache, allowing normal activities; 2, moderate headache, disturbing normal activities; 3, severe headache, disabling activities, requiring bed-rest) just before the drug intake and at 0.5, 1, 1.5, 2, 3, 4 and 5 h after first dosing. At the same time-points, patients recorded the presence of associated symptoms (nausea, vomiting, photophobia, phonophobia and osmophobia). Patients also reported if they used the second dose as rescue medication or to treat the relapse of headache within 48 h and the severity of headache 0.5, 1, 1.5 and 2 h after the second dose intake.
At the final visit, unused tablets and capsules and empty boxes were returned and counted to assess adherence.
End-points
Pain-free, that is the percentage of attacks with no headache (0 on the severity of headache scale) at 2 h after dosing without use of rescue medication, in two migraine attacks in total was the primary end-point to compare Indoprocaf tablets and sumatriptan 50 mg tablets.
Secondary efficacy parameters were:
Pain-free in the first and in the second migraine attack analysed separately;
Headache relief, that is the percentage of attacks with mild or no headache (the severity of headache scale to 1 or 0) at 2 h, without use of rescue medication, in the first, in the second and in the total two migraine attacks;
Cumulative pain-free, that is the cumulative percentage of attacks pain-free at the different observation times without use of rescue medication;
Intra-individual consistency, that is the percentage of patients pain-free (or headache relieved) at 2 h in two of two migraine attacks;
Associated symptoms (nausea, vomiting, photophobia, phonophobia and osmophobia), that is the percentage of attacks with each associated symptom at 2 h;
Percentage of attacks free of any associated symptoms at 2 h;
Rescue medication, that is the percentage of attacks that needed the second dose of study drug as rescue medication between 2 and 48 h of initial dosing;
Second dose efficacy as rescue medication, that is the percentage of attacks with pain-free (or headache relief) 2 h after the use of a second dose of the study drug as rescue medication;
Total pain-free rate, that is the total percentage of attacks pain-free at 5 h without use of rescue medication, and 2 h after the second dose of study drug as rescue medication;
Recurrence, that is the percentage of attacks with headache relief at 2 h and worsening of headache within 24 h of initial dosing;
Sustained response, that is the percentage of attacks with headache relief at 2 h, no use of rescue medication and no recurrence within 24 h;
Relapse, that is the percentage of attacks with pain-free at 2 h and worsening of headache within 48 h of initial dosing;
Sustained pain-free, that is the percentage of attacks with pain-free at 2 h, no use of rescue medication and no relapse within 48 h;
Use of second dose to treat a recurrence or a relapse, that is the percentage of attacks that needed the second dose of study drug to treat a recurrence or a relapse;
Second dose efficacy to treat a recurrence or a relapse, that is the percentage of recurrences or relapses with pain-free (or headache relief) 2 h after the use of a second dose of the study drug.
As subanalysis, a comparison between Indoprocaf-coated and -effervescent tablets was performed on the following parameters: pain-free at 2 h, headache relief at 2 h, cumulative pain-free, rescue medication, second dose efficacy as rescue medication, total pain-free rate and second dose efficacy to treat a recurrence or a relapse.
All adverse events were recorded in patient diaries and were assessed by the investigators for intensity, seriousness and relationship to study medication. Laboratory parameters (haematology, glucose, creatinine, alkaline phosphatase, total bilirubine, ALT, AST, gamma-GT, urea and total protein) were measured at screening and final visits by the laboratory of each centre.
Statistical analysis
On the basis of the results obtained in a previous study (21), the required sample size was estimated to be 264 patients (132 with Indoprocaf – 66 with Indoprocaf-effervescent tablets and 66 with Indoprocaf-coated tablets – and 132 with sumatriptan 50 mg tablets), giving the study a power of at least 0.80 to detect a difference of 15% in pain-free at 2 h between patients receiving Indoprocaf and those receiving sumatriptan, assuming a one-tailed test with a 0.05 significance level. Assuming that 10% of patients did not complete the study, it was estimated that approximately 300 patients would need to be enrolled.
The efficacy analysis was based on an ‘intention-to-treat’ (ITT) approach that included all randomised patients who received at least one dose of study medication (one effervescent tablet plus one capsule) and had available data for the primary efficacy parameter (pain-free at 2 h) for at least one migraine attack. Moreover, a ‘per-protocol’ (PP) sample, including all patients who had efficacy data on both migraine attacks of moderate intensity, separated by at least 48 h and without major violation of inclusion/exclusion criteria which might impact the efficacy of study medication, was analysed.
Pain-free, headache relief, cumulative pain-free, associated symptoms, use and efficacy of second dose as rescue medication, total pain-free rate, recurrences, sustained response, relapses, sustained pain-free, use and efficacy of second dose to treat a recurrence/relapse were analysed using χ2-test for each of the two attacks separately. In addition, the statistical analysis including the total migraine attacks was based on a categorial linear model for repeated measures including term for treatment, using the SAS procedure CATMOD. Odd ratios and corresponding two-sided 95% CI, derived from the CATMOD procedure, were given for treatment comparisons. The intra-individual consistency was analysed using χ2-test.
No alpha adjustment for multiple testing was applied because there was only a primary end-point (the pain-free at 2 h in two migraine attacks in total). All the other efficacy variables were considered as secondary end-points.
Missing values of severity of pain were replaced by carrying forward the preceding value.
Differences resulting in a p-value ≤ 0.05 were considered to be statistically significant. All randomised patients who received at least one dose of study medication (one effervescent tablet and/or one capsule) and for whom safety data were available after start of study medication were included in the safety sample.
All data analysis was carried out according to a pre-established analysis plan. The evaluation of the quality and completeness of the data, identification of important protocol deviations and handling of problem cases were performed regularly and finally decided before locking and unblinding the database. All study personnel and participants were blinded to treatment assignment for the duration of the study. Only the study statistician and the data manager saw unblinded data, but none had any contact with study participants.
Results
Study population
In total, 320 patients were assessed for eligibility and 297 were randomised across 14 centres in Italy. The recruitment started on December 5, 2002 and the trial was completed on June 15, 2004. The efficacy analyses were based on the ITT and PP samples. The results from the ITT and the PP analysis were similar and only the results based on the ITT sample will be shown.
During the study period, 15 patients did not take any study medication and were excluded from the safety sample, which therefore consisted of 282 patients, 143 treated with Indoprocaf (72 with coated tablets and 71 with effervescent tablets) and 139 with sumatriptan 50 mg tablets (Figure 1). One patient treated with sumatriptan without available diary data was not included in the ITT sample, which therefore consisted of 281 patients. In total, 276 migraine attacks were treated with Indoprocaf (142 with coated tablets and 134 with effervescent tablets) and 264 with sumatriptan. The demographic and baseline characteristics of the ITT sample were not different between the two treatment groups (Table 1). Overall, 78% of the patients were female and 22% were male. The mean age ± SD was 35 ± 9.8 years. In 92% of the patients migraine without aura was diagnosed: the remaining patients had a diagnosis of migraine with aura or with and without aura. During the screening period, all the patients experienced migraine attacks: NSAIDs, triptans, Indoprocaf and sumatriptan were used, respectively, in 38%, 37%, 10% and 8% of cases to treat the screening attacks. Seventy-seven per cent of the ITT sample reported MIDAS grade III (moderate disability) or IV (severe disability), without difference between Indoprocaf and sumatriptan. A higher percentage of patients treated with Indoprocaf-coated tablets compared with effervescent tablets reported MIDAS grade III or IV (85% vs. 70% respectively). At 0 h, patients in the ITT sample reported headache of moderate intensity in 47% of the attacks and severe headache in 51%; moreover, in 72% of the attacks, the study medication was taken within 60 min from the onset of headache, without difference between Indoprocaf and sumatriptan.
Figure 1.
Profile of subject disposition during the course of the study and inclusion in the analysis data sets: safety population (SP), intention-to-treat sample (ITT), per-protocol sample (PP)*. *Four patients treated with Indoprocaf, three with TBS (adverse event = 2 and withdrew consent = 1) and one with EFFE (adverse event), and three patients treated with sumatriptan (adverse event = 1, withdrew consent = 1 and lost to follow-up = 1) discontinued at any time during the study
Table 1.
Demographic and baseline characteristics of the patients (intention-to-treat sample)
| Indoprocaf tbs (n = 72) | Indoprocaf effe (n = 71) | Indoprocaf total (n = 143) | Sumatriptan (n = 138) | Total (n = 281) | |
|---|---|---|---|---|---|
| Sex (n, %) | |||||
| Male | 10 (14) | 20 (28) | 30 (21) | 31 (22) | 61 (22) |
| Female | 62 (86) | 51 (72) | 113 (79) | 107 (78) | 220 (78) |
| Age (years) | |||||
| Mean | 35 | 34 | 34 | 36 | 35 |
| Range | 20–60 | 19–58 | 19–60 | 18–64 | 18–64 |
| Migraine diagnosis (n, %) | |||||
| Migraine without aura | 67 (93) | 64 (90) | 131 (92) | 129 (93) | 260 (92) |
| Migraine with aura | 1 (1) | 5 (7) | 6 (4) | 4 (3) | 10 (4) |
| Migraine with and without aura | 4 (6) | 2 (3) | 6 (4) | 5 (4) | 11 (4) |
| MIDAS grade (n, %) | |||||
| I | 3 (4) | 11 (16) | 14 (10) | 14 (10) | 28 (10) |
| II | 8 (11) | 10 (14) | 18 (13) | 18 (13) | 36 (13) |
| III | 25 (35) | 23 (32) | 48 (33) | 48 (35) | 96 (34) |
| IV | 36 (50) | 27 (38) | 63 (44) | 58 (42) | 121 (43) |
Indoprocaf indicates indomethacin, prochlorperazine and caffeine; tbs, coated tablets; effe, effervescent tablets.
Of the 281 patients in the ITT sample, 41 patients were not included in the PP sample, mostly because only one migraine attack was treated or rescue medication had been taken within 2 h after dosing or the headache severity at 0 h was mild. The number of patients included in the PP sample was 240, 123 treated with Indoprocaf (63 with coated tablets and 60 with effervescent tablets) and 117 with sumatriptan.
Efficacy
Indoprocaf vs. sumatriptan
The pain-free rates in the total attacks at 2 h postdose were 34% with Indoprocaf and 37% with sumatriptan, without statistically significant differences between the drugs (95% CI of odds-ratio: 0.57–1.28) (Table 2).
Table 2.
Pain-free and headache relief with first dose, pain-free and headache relief with second dose as rescue medication, pain-free and headache relief with second dose to treat a recurrence/relapse (at 2 h postdose) and total pain-free rate with Indoprocaf and sumatriptan (intention-to-treat sample)*
| First attack | Second attack | Total attacks | ||||
|---|---|---|---|---|---|---|
| Indoprocaf | Sumatriptan | Indoprocaf | Sumatriptan | Indoprocaf | Sumatriptan | |
| Pain-free (first dose) | 45/143 (32) | 49/138 (36) | 48/133 (36) | 49/126 (39) | 93/276 (34) | 98/264 (37) |
| Headache relief (first dose) | 82/143 (57) | 79/138 (57) | 88/133 (66) | 70/126 (56) | 170/276 (62) | 149/264 (56) |
| Pain-free (second doseas rescue medication) | 21/46 (46)† | 10/43 (23) | 12/25 (48) | 13/42 (31) | 33/71 (47) | 23/85 (27) |
| Headache relief (second doseas rescue medication) | 28/46 (61)† | 17/43 (40) | 18/25 (72) | 21/42 (50) | 46/71 (65) | 38/85 (45) |
| Pain-free (second doseto treat a recurrence/relapse) | 13/26 (50) | 11/23 (48) | 14/19 (74) | 10/19 (53) | 27/45 (60) | 21/42 (50) |
| Headache relief (second doseto treat a recurrence/relapse) | 19/26 (73) | 19/23 (83) | 16/19 (84) | 16/19 (84) | 35/45 (78) | 35/42 (83) |
| Total pain-free rate‡ | 112/143 (78)† | 90/138 (65) | 105/133 (79) | 92/126 (73) | 217/276 (79)† | 182/264 (69) |
Values are number of attacks complying with the parameter/total no. of attacks (percentage). Indoprocaf indicates indomethacin, prochlorperazine and caffeine.
p < 0.05 vs. sumatriptan (χ2-test).
Total pain-free rate is the total percentage of attacks pain-free at 5 h without use of rescue medication and 2 h after the second dose of study drug as rescue medication.
Headache relief rates in the total attacks at 2 h postdose were 62% with Indoprocaf and 56% with sumatriptan, without a statistically significant difference between the two drugs (95% CI of odds-ratio: 0.82–1.84) (Table 2).
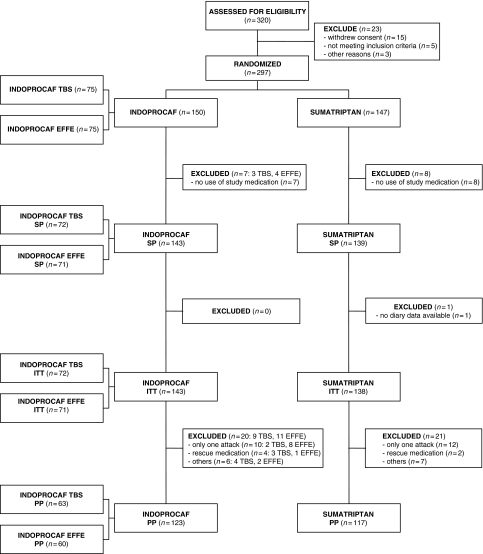
Concerning the cumulative pain-free rates, the pain-free efficacy of Indoprocaf and sumatriptan started at 0.5 h, but became relevant (about 15% of attacks) at 1.5 h postdose. At 3 h, about 50% of the attacks was pain-free both with Indoprocaf and with sumatriptan and then, the cumulative percentage of pain-free attacks, without use of rescue medication, continued to increase with both the drugs, reaching about 60–70% of pain-free attacks at 5 h (Figure 2).
Figure 2.
Cumulative pain-free rates without use of rescue medication in the total attacks (A) with Indoprocaf or sumatriptan and (B) with Indoprocaf-coated tablets (tbs) or Indoprocaf-effervescent tablets (effe) (intention-to-treat sample)
The percentage of patients reporting pain-free or headache relief in two of two migraine attacks (intra-individual consistency) was comparable between Indoprocaf (17% and 42%) and sumatriptan (20% and 42%), without statistically significant differences between drugs.
No statistically significant difference was found between Indoprocaf and sumatriptan in relieving each of the five associated symptoms at 2 h postdose. At baseline (0 h), nausea, photophobia and phonophobia were reported in 60–80%, osmophobia in 35–40% and vomiting in about 10% of the attacks. At 2 h postdose, the percentage of attacks with each associated symptom was more than halved: about 30% of nausea, photophobia and phonophobia, 15% of osmophobia and 5% of vomiting (Table 3). Both Indoprocaf and sumatriptan induced a high percentage of attacks free of any associated symptoms at 2 h postdose (higher than 50% in both cases) (Table 3).
Table 3.
Attacks with associated symptoms at baseline and 2 h postdose and attacks free of any associated symptoms at 2 h postdose (intention-to-treat sample)*
| First attack | Second attack | Total attacks | ||||
|---|---|---|---|---|---|---|
| Indoprocaf (n = 143) | Sumatriptan (n = 138) | Indoprocaf (n = 133) | Sumatriptan (n = 126) | Indoprocaf (n = 276) | Sumatriptan (n = 264) | |
| Nausea | ||||||
| Baseline | 86 (60) | 79 (57) | 75 (56) | 80 (64) | 161 (58) | 159 (60) |
| 2 h | 47 (33) | 35 (25) | 32 (24) | 31 (25) | 79 (29) | 66 (25) |
| Vomiting | ||||||
| Baseline | 12 (8) | 15 (11) | 17 (13) | 16 (13) | 29 (11) | 31 (12) |
| 2 h | 4 (3) | 7 (5) | 8 (6) | 6 (5) | 12 (4) | 13 (5) |
| Photophobia | ||||||
| Baseline | 100 (70) | 108 (78) | 91 (68) | 92 (73) | 191 (69) | 200 (76) |
| 2 h | 46 (32) | 49 (36) | 38 (29) | 42 (33) | 84 (30) | 91 (35) |
| Phonophobia | ||||||
| Baseline | 94 (66) | 104 (75) | 91 (68) | 94 (75) | 185 (67) | 198 (75) |
| 2 h | 44 (31) | 45 (33) | 38 (29) | 36 (29) | 82 (30) | 81 (31) |
| Osmophobia | ||||||
| Baseline | 54 (38) | 46 (33) | 53 (40) | 48 (38) | 107 (39) | 94 (36) |
| 2 h | 23 (16) | 22 (16) | 20 (15) | 18 (14) | 43 (16) | 40 (15) |
| Attacks free of associated symptoms | ||||||
| 2 h | 70 (49) | 72 (52) | 73 (55) | 68 (54) | 143 (52) | 140 (53) |
Values are number (percentage) of attacks. Indoprocaf indicates indomethacin, prochlorperazine and caffeine.
No statistically significant difference was found between Indoprocaf and sumatriptan in the percentage of attacks needing the second dose as rescue medication (26% vs. 32%) (Table 4). Considering the patients who used a second dose as rescue medication after 2 h, a higher global pain-free rate was shown for Indoprocaf (47%) vs. sumatriptan (27%), with a statistically significant higher percentage of attacks pain-free (46% vs. 23%, p < 0.05) at 2 h postdose with Indoprocaf compared with sumatriptan in the first migraine attack, as well as a higher global headache relief rate (65% with Indoprocaf compared with 45% with sumatriptan) with a statistically significant higher percentage of attacks headache-relieved (61% vs. 40%, p < 0.05) at 2 h postdose (Table 2).
Table 4.
Recurrences, sustained response, relapses, sustained pain-free and use of second dose (intention-to-treat sample)*
| First attack | Second attack | Total attacks | ||||
|---|---|---|---|---|---|---|
| Indoprocaf | Sumatriptan | Indoprocaf | Sumatriptan | Indoprocaf | Sumatriptan | |
| Recurrences (24 h) | 16/82 (20) | 19/79 (24) | 16/88 (18) | 16/70 (23) | 32/170 (19) | 35/149 (24) |
| Sustained response | 64/143 (45) | 60/138 (44) | 72/133 (54) | 54/126 (43) | 136/276 (49) | 114/264 (43) |
| Relapses (48 h) | 22/45 (49) | 20/49 (41) | 16/48 (33) | 13/49 (27) | 38/93 (41) | 33/98 (34) |
| Sustained pain-free | 23/143 (16) | 29/138 (21) | 32/133 (24) | 35/126 (28) | 55/276 (20) | 64/264 (24) |
| Use of second dose asrescue medication (2–48 h) | 46/143 (32) | 43/138 (31) | 25/133 (19) | 42/126 (33) | 71/276 (26) | 85/264 (32) |
| Use of second doseto treat a recurrence/relapse | 26/143 (18) | 23/138 (17) | 19/133 (14) | 19/126 (15) | 45/276 (16) | 42/264 (16) |
Values are number of attacks complying with the parameter/total no. of attacks (percentage). Indoprocaf indicates indomethacin, prochlorperazine and caffeine.
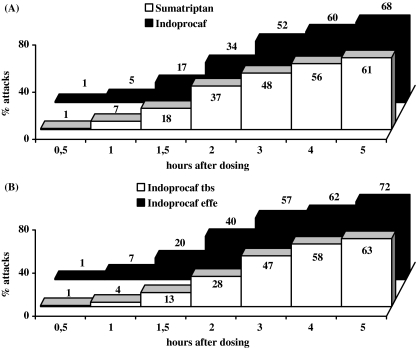
For the secondary end-point total pain-free rate, a statistically significant difference (p < 0.05) was found in favour of Indoprocaf compared with sumatriptan in the first (78% vs. 65%) and the total attacks (79% vs. 69%) in the percentage of attacks pain-free at 5 h after the first dose of study drug (without use of rescue medication) or 2 h after the use of the second dose as rescue medication (Table 2; Figure 3).
Figure 3.
Total pain-free rate: percentage of attacks pain-free at 5 h after first dose of Indoprocaf or sumatriptan, or after second dose of study drug used as rescue medication (χ2-test; intention-to-treat sample)
No statistically significant difference was found between Indoprocaf and sumatriptan in the percentage of recurrences within 24 h (19% vs. 24%), relapses within 48 h (41% vs. 34%), sustained response (49% vs. 43%) or sustained pain-free (20% vs. 24%) (Table 4).
No statistically significant difference was found between Indoprocaf and sumatriptan in the percentage of attacks needing the second dose to treat a recurrence or a relapse (16% in both cases in total attacks) (Table 4). The pain-free rate at 2 h after the second dose used to treat a recurrence or a relapse was very high with both the drugs (60% vs. 50%) (Table 2).
Summarising the results obtained treating 540 attacks with Indoprocaf or sumatriptan, a higher percentage of pain-free attacks was reported with Indoprocaf than with sumatriptan (76% vs. 66% of attacks with pain-free at 2 h or pain-free at 5 h or pain-free with second dose as rescue medication or pain-free with second dose to treat a relapse) (Figure 4).
Figure 4.
Summary of results obtained treating the total attacks with Indoprocaf (n = 276 attacks) or sumatriptan (n = 264 attacks) (intention-to-treat sample)
Indoprocaf-coated tablets vs. Indoprocaf-effervescent tablets
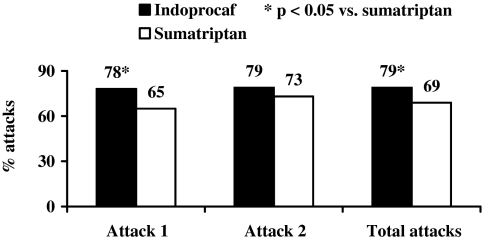
A statistically significant difference was found in favour of Indoprocaf-effervescent tablets in the pain-free rate (41% vs. 22%) and in the headache relief rate (66% vs. 49%) at 2 h postdose in the first attack (Table 5; Figure 5). The cumulative pain-free rates of Indoprocaf-coated tablets were lower than those of Indoprocaf-effervescent tablets at all the times, and lower to those of sumatriptan within 2 h, but similar within 3 and 5 h postdose (Figure 2). No statistically significant differences between Indoprocaf-effervescent and coated tablets were found in the efficacy of the re-dosing (Table 5). A statistically significant difference (p < 0.05) in favour of the effervescent tablets was found in the total pain-free rate in the total attacks (84% vs. 73%) (Table 5 and Figure 5). The total pain-free rate of Indoprocaf-coated tablets was lower than that of effervescent tablets, but higher than that of sumatriptan (Table 5).
Table 5.
Pain-free and headache relief with first dose, pain-free and headache relief with second dose as rescue medication, pain-free and headache relief with second dose to treat a recurrence/relapse (at 2 h postdose) and total pain-free rate with Indoprocaf-coated tablets and -effervescent tablets (intention-to-treat sample)*
| First attack | Second attack | Total attacks | ||||
|---|---|---|---|---|---|---|
| Indoprocaf tbs | Indoprocaf effe | Indoprocaf tbs | Indoprocaf effe | Indoprocaf tbs | Indoprocaf effe | |
| Pain-free (first dose) | 16/72 (22) | 29/71 (41)† | 23/70 (33) | 25/63 (40) | 39/142 (28) | 54/134 (40) |
| Headache relief (first dose) | 35/72 (49) | 47/71 (66)† | 45/70 (64) | 43/63 (68) | 80/142 (56) | 90/134 (67) |
| Pain-free (second doseas rescue medication) | 11/27 (41) | 10/19 (53) | 6/16 (38) | 6/9 (67) | 17/43 (40) | 16/28 (57) |
| Headache relief (second doseas rescue medication) | 14/27 (52) | 14/19 (74) | 10/16 (63) | 8/9 (89) | 24/43 (56) | 22/28 (79) |
| Pain-free (second doseto treat a recurrence/relapse) | 4/8 (50) | 9/18 (50) | 8/9 (89) | 6/10 (60) | 12/17 (71) | 15/28 (54) |
| Headache relief (second doseto treat a recurrence/relapse) | 7/8 (88) | 12/18 (67) | 9/9 (100) | 7/10 (70) | 16/17 (94) | 19/28 (68) |
| Total pain-free rate‡ | 52/72 (72) | 60/71 (85) | 52/70 (74) | 53/63 (84) | 104/142 (73) | 113/134 (84)† |
Values are number of attacks complying with the parameter/total no. of attacks (percentage). Indoprocaf indicates indomethacin, prochlorperazine and caffeine; tbs indicates coated tablets; effe indicates effervescent tablets.
p < 0.05 vs. Indoprocaf tbs.
Total pain-free rate is the total percentage of attacks pain-free at 5 h without use of rescue medication and 2 h after the second dose of study drug as rescue medication.
Figure 5.
(A) Percentage of attacks pain-free at 2 h postdose with Indoprocaf-coated tablets (tbs) or Indoprocaf-effervescent tablets (effe) without use of rescue medication; (B) total pain-free rate: percentage of attacks pain-free at 5 h after first dose of Indoprocaf-coated tablets (tbs) or Indoprocaf-effervescent tablets (effe) or after second dose of study drug used as rescue medication (χ2-test; intention-to-treat sample)
Safety
Indoprocaf and sumatriptan were regarded as safe and well-tolerated treatments for the acute attacks of migraine. A total of 31 patients (22%) in the Indoprocaf group and 25 patients (18%) in the sumatriptan group reported at least one treatment-emergent adverse event (Table 6). Twenty-three patients treated with Indoprocaf (16%) and 14 patients treated with sumatriptan (10%) reported at least one related treatment-emergent adverse event. No serious adverse events were reported with Indoprocaf, while only one serious adverse event (severe headache) was reported with sumatriptan. Three patients in the Indoprocaf group (one for malaise, one for malaise and loss of consciousness and one for vertigo) and one patient in the sumatriptan group (for headache) dropped out because of adverse events (Table 6). A similar number of treatment-emergent adverse events was found after one (36 events) or two doses (39 events) of Indoprocaf; on the contrary, the number of treatment-emergent adverse events was three times higher with two doses (31 events) of sumatriptan in comparison to one dose (nine events). The most frequent non-serious related adverse events were reported in the nervous system or gastrointestinal system with both the drugs, with vertigo (4.9% of safety sample) for Indoprocaf and somnolence (2.2% of safety sample) for sumatriptan as the most commonly reported events. No differences were detected in the safety profile between Indoprocaf-coated tablets and -effervescent tablets. There was no clinically significant mean change in laboratory and/or blood pressure values from baseline to the final study assessment.
Table 6.
Summary of adverse events (safety sample)*
| Indoprocaf (n = 143) | Sumatriptan (n = 139) | |
|---|---|---|
| Patients reporting at least one TEAE (n, %) | 31 (22) | 25 (18) |
| Patients reporting at least one related TEAE (n, %) | 23 (16) | 14 (10) |
| Serious adverse events (n) | – | 1 |
| Patients dropped-out as a result of adverse events (n) | 3 | 1 |
Indoprocaf indicates indomethacin, prochlorperazine and caffeine; TEAE indicates treatment-emergent adverse events.
Discussion
The aim of this double-blind, double-dummy, randomised, parallel group, multicentre study was to compare the efficacy and safety of Indoprocaf tablets with sumatriptan 50 mg tablets in the acute treatment of migraine. As subanalysis, a comparison between Indoprocaf-coated and -effervescent tablets on the most important efficacy parameters was also performed.
In this study patients with migraine with or without aura according to the IHS criteria (25) were enrolled. The patient population analysed had a more severe baseline intensity of migraine (47% of moderate and 51% of severe migraine) compared to that commonly included in triptan studies (65% of moderate and 35% of severe migraine) (28,29) and 77% of patients reported a baseline moderate or severe disability (MIDAS grade III and IV) (27).
Differently from the most part of the studies with triptans, where headache relief was used as primary efficacy end-point (23), and according to the most recent guidelines (24,26), in this study pain-free at 2 h postdose was used as primary efficacy end-point. Moreover, all the secondary efficacy parameters were analysed according to these recent international guidelines (24,26).
Moreover, in this study it was planned to analyse two migraine attacks treated with the same drug, differently from the most part of the studies with triptans, where only one attack was studied (23). A repeated intake of the study medication for multiple attacks is recommended, because it is expected to increase the discriminative power of a trial if outcome is averaged across multiple attacks for each patient and it is used to evaluate consistency of response (24).
In this study, it was chosen to compare the lowest effective oral doses of Indoprocaf and sumatriptan for the initial dosing. However, it was given the possibility to take a second dose as rescue medication after 2 h or to treat a recurrence/relapse within 48 h, to evaluate the efficacy of re-dosing of the drugs in the same attack.
Regarding the primary efficacy parameter, there was no statistically significant difference between Indoprocaf and sumatriptan in the percentage of pain-free attacks (respectively, 34% and 37% of total attacks). This pain-free rate at 2 h confirms the widely published mean absolute pain-free response of 30% with oral sumatriptan (23). Therefore, the encapsulation of sumatriptan did not influence the efficacy of the drug, as evidenced also in a recently published meta-analysis comparing the time-course of response up to 4 h of encapsulated and commercial sumatriptan (30).
No significant differences were found for headache relief between Indoprocaf and sumatriptan (62% vs. 56% respectively). Headache relief response rates of about 60% are commonly reported with the triptans (23).
Analysing the cumulative percentage of attacks becoming pain-free without use of rescue medication, it was observed that the pain-free response of both the drugs started at 30 min, but became relevant (more than 15%) starting from 1.5 h postdose. At 3 h, 50% of the attacks was pain-free both with Indoprocaf and with sumatriptan and then, the cumulative percentage of pain-free attacks, without use of rescue medication, continued to increase with both the drugs, reaching 60–70% of pain-free attacks at 5 h postdose.
The percentage of attacks with each associated symptom at baseline was similar in all the treatment groups and confirms those reported in literature (60–70% of nausea, photophobia and phonophobia; 10–20% of vomiting and 30–40% of osmophobia) (28). Both drugs showed a similar efficacy in relieving the associated symptoms: at 2 h postdose, the percentage of attacks with each associated symptom was more than halved and more than 50% of the attacks were free of any associated symptoms.
No statistical difference was found between Indoprocaf and sumatriptan in the percentage of attacks with use of the second dose of study drug as rescue medication, about 30%, that is similar to the percentage reported in the literature with the triptans (28).
For both drugs the average percentages of recurrences (22%) or relapses (38%) and of sustained response (46%) or sustained pain-free (22%) obtained in this study confirm those reported in the literature with the triptans (23).
The pain-free rate of oral Indoprocaf (34%) was lower than that obtained in an open, cross-over trial (21) with rectal Indoprocaf (49%) vs. rectal sumatriptan (34%). Rectal Indoprocaf has almost the same quantitative composition of the oral tablets (both the formulations contain indomethacin 25 mg and caffeine 75 mg; prochlorperazine 2 mg and 4 mg are contained, respectively, in the rectal and in the oral formulation); rectal sumatriptan contains 25 mg of active ingredient, that is the halved dosage of the oral dose used in this study. Comparing the results of these studies, it could be suggested that for the oral administration the double quantitative of active ingredients is required compared to the rectal one, probably because of the well known delayed absorption during migraine attacks (23). Therefore, considering the very low dosage of Indoprocaf used in this study, the results obtained are particularly valuable (especially with the effervescent tablets). Moreover, the rectal formulations of both Indoprocaf and sumatriptan showed to induce a lower percentage of relapse (respectively, 8% and 12%) than the oral forms (41% and 34%) (21).
The second dose of study medication, taken as rescue medication when the severity of headache was still at a score of 2 or 3 at 2 h after dosing, showed to be very effective, especially with Indoprocaf with a statistically significant difference compared with sumatriptan in the first attack (46% vs. 23% of pain-free attacks and 61% vs. 40% of headache-relieved attacks after 2 h).
Considering the total percentage of attacks pain-free at 5 h with the initial dose of study drug (without use of rescue medication) or at 2 h after the second dose of study drug, a statistically significant difference was obtained in favour of Indoprocaf compared with sumatriptan (79% vs. 69%). This difference is mainly because of the higher pain-free efficacy both of the first dose of Indoprocaf from 3 to 5 h and of the second dose compared with sumatriptan.
It has to be considered that to our knowledge this is the first study where the effect of a second dose of the same drug as rescue medication in the acute treatment of migraine attacks was investigated.
The efficacy of the second dose of study medication was confirmed when the second dose was taken to treat a recurrence or a relapse, that is when the headache relieved at 2 h and the headache returned within 48 h of initial dosing with an average high pain-free rate (55%).
Considering the total number of attacks treated with the study drugs, only 24% of attacks with Indoprocaf and 34% with sumatriptan were not treated effectively in the 48 h of observation time.
Moreover, a higher pain-free rate at 2 h was found for Indoprocaf-effervescent tablets, with a statistical difference vs. Indoprocaf-coated tablets only in the first attack (41% vs. 22%). A statistical difference between the two formulations was found also in the total pain-free rate, because also the second dose of Indoprocaf-effervescent tablets as rescue medication was more effective than that of coated tablets. The possible reasons for this difference between Indoprocaf-effervescent and -coated tablets could be a delayed oral absorption of coated tablets in patients with migraine attacks, the encapsulation of coated tablets or the fact that a higher percentage of patients treated with the coated tablets compared with effervescent tablets reported MIDAS grade III or IV (85% vs. 70% respectively). However, although Indoprocaf-coated tablets were less effective than the effervescent tablets, they were at least as effective as sumatriptan for the majority of the secondary end-points.
Indoprocaf and sumatriptan showed a similar safety profile, with a percentage of patients reporting at least one treatment emergent adverse event, which was similar or lower to that reported in the literature with sumatriptan 50 mg (28,31). In the open, cross-over trial comparing the rectal formulations of Indoprocaf and sumatriptan, both the drugs were particularly well tolerated with only 9% of patients reporting at least one adverse event (21).
This study, conducted according to the most recent guidelines (24,26), demonstrated that the efficacy of the initial dosing of Indoprocaf was not higher than that of sumatriptan, but that the strategy to use the lowest effective dose as soon as the headache occurred, followed by a second dose if the headache has not relieved or to treat a relapse, was very effective, especially with Indoprocaf.
Acknowledgments
The authors wish to acknowledge other investigators from Italy who participated in this study: Grazia Sances (University Centre for Adaptive Disorders and Headache, IRCCS ‘C. Mondino’ Institute of Neurology Foundation, Pavia, Italy); Gianluca Bruti (Pain Centre, Emergency Department, ‘La Sapienza’ University, Rome); Francesco De Cesaris (Headache Centre, Department of Internal Medicine, University of Florence); Maria Pia Prudenzano (Headache Centre, Neurologic Clinic I, University of Bari); Sergio De Filippis, Gabriella Coloprisco (Regional Referral Headache Centre, Department of Medical Sciences, II School of Medicine, ‘La Sapienza’ University, Rome); Lidia Savi (Headache Centre, Neurologic Clinic II, University of Torino); Cristiana Rossi, Andrea Alberti, Francesca Coppola, Antonio Baldi (Headache Centre, Neurologic Clinic, Department of Medical and Surgical Specialties and Public Health, University of Perugia); Graziella Di Meo (Headache Centre, University of Chieti); Ferdinando Maggioni, Filippo Dainese (Headache Centre, Department of Neurosciences, University of Padova); Paolo Liberini (Department of Neurology, Spedali Civili, Brescia); Giorgio Bono (Department of Neurology, Ospedale di Circolo e Fondazione Macchi, Varese); Filippo Brighina, Antonina Aloisio (Institute of Neuropsychiatry, University of Palermo); Giovanni Regesta, Marco Fonzari (Department of Neurology, San Martino Hospital, Genova).
Funding
This study was supported by funding from Solvay Pharma S.p.A.
References
- 1.Sicuteri F. The holistic hypothesis of migraine. Ann Ital Med Int. 1997;12(Suppl. 1):1S–32S. [Google Scholar]
- 2.Hu XH. Central mechanism of indomethacin analgesia. Eur J Pharmacol. 1994;263:53–7. doi: 10.1016/0014-2999(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 3.Akerman S. The effect of anti-migraine compounds on nitric oxide-induced dilation of dural meningeal vessels. Eur J Pharmacol. 2002;452:223–8. doi: 10.1016/s0014-2999(02)02307-5. [DOI] [PubMed] [Google Scholar]
- 4.Tassorelli C. The effects on the central nervous system of nitroglycerin - Putative mechanisms and mediators. Prog Neurobiol. 1999;57:607–24. doi: 10.1016/s0301-0082(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 5.Jurna I. Central effect of the non-steroid anti-inflammatory agents, indomethacin, ibuprofen, and diclofenac, determined in C fibre-evoked activity in single neurones of the rat thalamus. Pain. 1990;41:71–80. doi: 10.1016/0304-3959(90)91111-U. [DOI] [PubMed] [Google Scholar]
- 6.Guieu R. Analgesic effect of indomethacin shown using the nociceptive flexion reflex in humans. Ann Rheum Dis. 1992;51:391–3. doi: 10.1136/ard.51.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghelardini C. Indomethacin, alone and combined with prochlorperazine and caffeine, but not sumatriptan, abolishes peripheral and central sensitization in in vivo models of migraine. J Pain. 2004;5:413–9. doi: 10.1016/j.jpain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Jakubowski M, et al. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005;45:850–61. doi: 10.1111/j.1526-4610.2005.05153.x. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders – 2nd edition. Cephalalgia. 2004;24(Suppl. 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Sicuteri F. Termination of migraine headache by a new anti-inflammatory vasoconstrictor agent. Clin Pharmacol Ther. 1965;6:336–44. doi: 10.1002/cpt196563336. [DOI] [PubMed] [Google Scholar]
- 11.Pradalier A. Migraine and non-steroidal anti-inflammatory agents. Pathol Biol. 1992;40:397–405. [PubMed] [Google Scholar]
- 12.Dodick DW. Indomethacin-responsive headache syndromes. Curr Pain Headache Rep. 2004;8:19–26. doi: 10.1007/s11916-004-0036-6. [DOI] [PubMed] [Google Scholar]
- 13.Ghelardini C, et al. Prochlorperazine induces central antinociception mediated by the muscarinic system. Pharmacol Res. 2004;50:351–8. doi: 10.1016/j.phrs.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 14.US Headache Consortium. Evidence-based Guidelines for Migraine Headache in the Primary Care Setting: Pharmacological Management of Acute Attacks. http://www.aan.com/ (accessed 23 May 2007)
- 15.Sharma S, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42:896–902. doi: 10.1046/j.1526-4610.2002.02210.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghelardini C. Caffeine induces central cholinergic analgesia. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:590–5. doi: 10.1007/pl00005094. [DOI] [PubMed] [Google Scholar]
- 17.Laska EM, et al. Caffeine as an analgesic adjuvant. JAMA. 1984;251:1711–8. [PubMed] [Google Scholar]
- 18.Zhang WY. A benefit-risk assessment of caffeine as an analgesic adjuvant. Drug Saf. 2001;24:1127–42. doi: 10.2165/00002018-200124150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Galeotti N. Indomethacin, caffeine and prochlorperazine alone and combined revert hyperalgesia in ‘‘in vivo’’ models of migraine. Pharmacol Res. 2002;46:245–50. doi: 10.1016/s1043-6618(02)00126-3. [DOI] [PubMed] [Google Scholar]
- 20.Burstein R. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 21.Di Monda V, et al. Efficacy of a fixed combination of indomethacin, prochlorperazine, and caffeine versus sumatriptan in the acute treatment of multiple migraine attacks: a multicenter, randomized, crossover trial. Headache. 2003;43:835–44. doi: 10.1046/j.1526-4610.2003.03161.x. [DOI] [PubMed] [Google Scholar]
- 22.Cerbo R, et al. Efficacy of a fixed combination of indomethacin, prochlorperazine, and caffeine in the treatment of episodic tension-type headache: a double-blind, randomized, nimesulide-controlled, parallel group, multicentre trial. Eur J Neurol. 2005;12:759–67. doi: 10.1111/j.1468-1331.2005.01056.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari MD. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–58. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 24.International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000. pp. 765–86. [DOI] [PubMed]
- 25.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(Suppl. 7):1–96. [PubMed] [Google Scholar]
- 26.Note for Guidance on Clinical Investigation of Medicinal Products for the Treatment of Migraine. CPMP/EWP/788/01 Final, December.
- 27.Stewart WF, et al. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia. 1999;19:107–14. doi: 10.1046/j.1468-2982.1999.019002107.x. [DOI] [PubMed] [Google Scholar]
- 28.Spierings ELH, et al. Oral almotriptan vs oral sumatriptan in the abortive treatment of migraine. A double-blind, randomized, parallel-group, optimum-dose comparison. Arch Neurol. 2001;58:944–50. doi: 10.1001/archneur.58.6.944. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein J, et al. Crossover comparison of rizatriptan 5 mg and 10 mg versus sumatriptan 25 mg and 50 mg in migraine. Headache. 1998;38:737–47. doi: 10.1046/j.1526-4610.1998.3810737.x. [DOI] [PubMed] [Google Scholar]
- 30.Mandema JW. Therapeutic benefit of eletriptan compared to sumatriptan for the acute relief of migraine pain – results of a model-based meta-analysis that accounts for encapsulation. Cephalalgia. 2005;25:715–25. doi: 10.1111/j.1468-2982.2004.00939.x. [DOI] [PubMed] [Google Scholar]
- 31.Tfelt-Hansen P. Triptans in migraine. A comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;60:1259–87. doi: 10.2165/00003495-200060060-00003. [DOI] [PubMed] [Google Scholar]