Abstract
Three proteins secreted by Listeria monocytogenes facilitate escape from macrophage vacuoles: the cholesterol-dependent cytolysin listeriolysin O (LLO), a phosphoinositide-specific phospholipase C (PI-PLC) and a broad-range phospholipase C (PC-PLC). LLO and PI-PLC can activate several members of the protein kinase C (PKC) family during infection. PKCε is a novel PKC that contributes to macrophage activation, defence against bacterial infection, and phagocytosis; however, a role for PKCε in Lm infections has not been described. To study PKCε dynamics, PKCε-YFP chimeras were visualized in macrophages during Lm infection. PKCε-YFP was recruited to forming vacuoles during macrophage phagocytosis of Lm and again later to fully formed Lm vacuoles. The PKCε-YFP localization to the fully formed Lm vacuole was LLO-dependent but independent of PI-PLC or PC-PLC. PKCε-YFP recruitment often followed LLO perforation of the membrane, as indicated by localization of PKCε-YFP to Lm vacuoles after they released small fluorescent dyes into the cytoplasm. PKCε-YFP recruitment to vesicles also followed phagocytosis of LLO-containing liposomes or osmotic lysis of endocytic vesicles, indicating that vacuole perforation by LLO was the chief cause of the PKCε response. These studies implicate PKCε in a cellular mechanism for recognizing damaged membranous organelles, including the disrupted vacuoles created when Lm escapes into cytoplasm.
Introduction
Macrophages are essential for clearing Listeria monocytogenes (Lm) infections in mice (Mackaness, 1962; Adams and Hamilton, 1984; Kiderlen et al., 1984; Pamer, 2004). Lm enters macrophages by phagocytosis and then escapes from phagosomal vacuoles into the cytosol, where it can replicate and invade neighbouring cells. Listeria secretes a cholesterol-dependent cytolysin (CDC), listeriolysin O (LLO), which is necessary for escape from the phagosome into the cytosol (Portnoy et al., 1988; Cossart et al., 1989; Gedde et al., 2000). Lm also secretes two phospholipases C which have minor roles in escape (Smith et al., 1995): a phosphatidylinositol-specific phospholipase C (PI-PLC) and a broad-range phospholipase C (PC-PLC).
In addition to their involvement in bacterial escape, LLO and the bacterial phospholipases C (PLCs) induce signalling from the phagosome (Goldfine and Wadsworth, 2002). CDCs, including LLO, induce secretion of TNFα and IL-6 and activate macrophages by inducing iNOS expression (Park et al., 2004). In addition, Goldfine and colleagues identified a LLO-mediated activation of host PLC and phospholipase D following Lm infection of macrophages (Goldfine et al., 2000).
Protein kinases C (PKCs) are phospholipid-dependent, serine/threonine protein kinases whose 11 isozymes are placed into subfamilies based upon their cofactor requirements for activation (Newton, 2001). Conventional PKCs [α, β(I and II), γ] are activated by calcium (Ca2+), diacylglycerol (DAG), and phosphatidylserine (PS) or anionic phospholipids. Novel PKCs (δ, ε, η, θ) are activated by DAG and PS. Atypical PKCs (ζ, ι/λ) are activated by PS. LLO and PI-PLC activate recruitment of PKCδ to plasma membranes and PKC βII to early endosomes (Wadsworth and Goldfine, 2002). Inhibition of PKC βII increased Lm phagocytosis and decreased escape from vacuoles (Wadsworth and Goldfine, 2002), indicating that Lm exploits host PKC βII activity during infection.
Protein kinases C regulate a variety of cellular processes including cytoskeleton rearrangements and immune cell signalling (Tan and Parker, 2003). Signalling through the Fc-receptor induces PLC-mediated hydrolysis of phosphatidylinositol 4,5 bisphosphate, generating inositol-1,4,5 trisphosphate, which increases [Ca+2]i, and DAG, second messengers that activate conventional PKCs. PKCε is recruited to IgG-opsonized particles in forming phagosomes and is necessary for FcγR-mediated phagocytosis in macrophages (Larsen et al., 2000; 2002). PKCε has been implicated in innate immunity through its role in macrophage activation (Castrillo et al., 2001). PKCε also upregulates the expression of iNOS and subsequent NO production (Diaz-Guerra et al., 1996). Many bacteria (Listeria, Bacillus and Staphylococcus species) also secrete phospholipases, including PI-PLC and PC-PLC, which can produce DAG and potentially recruit host PLC for subsequent signal transduction.
Upon phosphorylation at three residues in the catalytic kinase domain, PKCs become mature; a prerequisite for binding to second messengers and activation (Keranen et al., 1995; Cenni et al., 2002). Mature but unactivated PKCs are localized in the cytosol; upon activation they translocate to the membrane (Newton, 2001). Novel PKCs translocate to the plasma membrane in response to DAG formation (Stahelin et al., 2005). Once bound to DAG, novel PKCs bind to anionic phospholipids which allows the pseudosubstrate domain to be released and PKC to phosphorylate its substrate. Inside cells, compartmentalization in space or time targets the different PKC isoforms to different signalling pathways. Targeting and substrate specificity for each PKC isoform depends upon subcellular and tissue localization (Akita, 2002).
Castrillo et al. demonstrated that PKCε is necessary for lipopolysaccharide-induced macrophage activation and defence against infection by Escherichia coli and Staphylococcus aureus (Castrillo et al., 2001). Macrophages from PKCε –/– mice showed reduced ability to produce nitric oxide, TNF-α, and IL-1β in response to lipopolysaccharide and IFN-γ (Castrillo et al., 2001). The Toll-like receptor 4 (TLR4) adapter molecule TRAM was recently identified as a specific substrate for PKCε (McGettrick et al., 2006). Phosphorylation of TRAM by PKCε is necessary for TRAM-mediated TLR signalling (McGettrick et al., 2006). These observations indicate roles for PKCε in TLR signalling and the early signalling events necessary for macrophage activation (Aksoy et al., 2004; McGettrick et al., 2006). There is no evidence thus far that PKCε is recruited to membranes during bacterial entry.
To examine the role of PKCε in macrophage responses to infection, we expressed in macrophages a fluorescent chimera of PKCε, PKCε-YFP, and analysed its intracellular dynamics in macrophages during Lm infection. We identified a LLO- and PLC-independent accumulation of PKCε upon Lm entry into macrophages, as well as a later, LLO-dependent concentration of PKCε on vacuoles following perforation of the Lm vacuole membrane. This later PKCε recruitment could also be elicited by liposomes containing purified LLO or by osmotic lysis of endosomes, indicating a role for PKCε in the detection of damaged membrane organelles in macrophages.
Results
Protein kinase C ε is recruited to Lm-associated membranes in macrophages
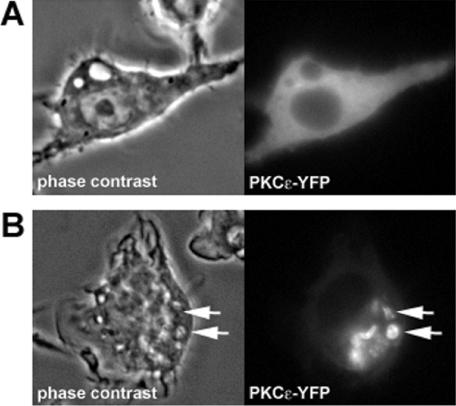
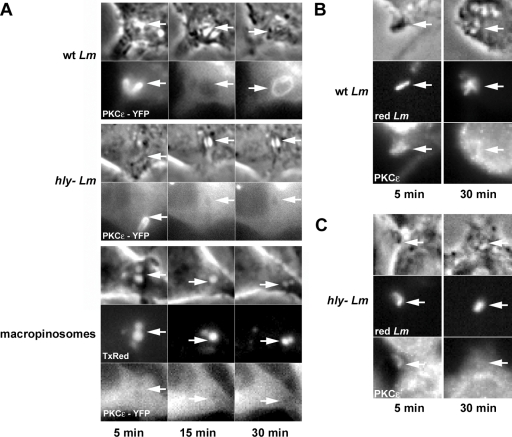
In resting cells, PKCε-YFP was mostly distributed uniformly throughout the macrophage cytoplasm, with minor perinuclear localization (Fig. 1A). After wild-type Lm infection of macrophages, PKCε-YFP robustly localized to membranes associated with the bacteria (Fig. 1B). We asked if Lm recruitment of PKCε to vacuolar membranes is affected by LLO. RAW 264.7 macrophages expressing PKCε-YFP were infected with wild-type Lm or hly (LLO-deficient) Lm, and localization of PKCε-YFP was analysed by time-lapse microscopy of live cells. Shortly after Lm entry, PKCε was recruited to both wild-type and hly Lm-associated membranes (Fig. 2A; 5 min). Time-lapse movies showed that the membranes that recruited PKCε-YFP during entry were close to the bacteria, consistent with a role for PKCε in Lm phagocytosis (Fig. 2A; Time 5). PKCε accumulation at Lm-containing phagosomes was biphasic, occurring during phagosome formation (5 min) and again around the fully formed Lm vacuoles. PKCε translocation was specific for Lm vacuoles; it did not translocate to macropinosomes loaded with only Texas Red dextran (TRDx; 10 000 MW dextran) (Fig. 2A). This second recruitment of PKCε-YFP was dependent upon the presence of LLO, as PKCε-YFP localized to wild-type Lm vacuoles but not hly Lm vacuoles (Fig. 2A, Time 30). Labelling of endogenous PKCε by immunofluorescence showed similar localization patterns: wild-type (Fig. 2B) and hly (Fig. 2C) Lm recruited PKCε upon entry (5 min), but at 30 min only vacuoles with wild-type Lm contained PKCε.
Fig. 1.
PKCε is recruited to Lm-associated membranes in macrophages. Macrophages expressing PKCε-YFP were either (A) uninfected or (B) infected with wild-type Lm. Representative phase-contrast and YFP images are shown. Arrows point to wild-type Lm and the recruitment of PKCε-YFP to associated membranes.
Fig. 2.
Wild-type Lm recruits PKCε to the phagosome. A. Macrophages expressing PKCε-YFP were either infected with wild-type Lm or hly Lm, or were labelled by endocytosis of Texas Red dextran (TxRed). Phase-contrast (upper panel), YFP and TRDx images were collected at 5 min (left image), 15 min (centre image) and 30 min (right image) after addition of Lm or dye. Arrows point to the vacuole. B and C. Distributions of endogenous PKCε. RAW macrophages were infected with either wild-type (B) or hly (C) Lm (SNARF-labelled; red) and PKCε was visualized by immunofluorescence and stained with Alexa Fluor 488-labelled secondary antibody.
Lm PLCs do not affect PKCε recruitment to vacuolar membranes
As PKCε associates with membranes via binding to DAG as well as other lipids, we tested the hypothesis that PI-PLC or PC-PLC from Lm generates lipids for PKCε docking to Lm vacuoles. Specifically, we quantified the percentage of Lm vacuoles that recruited PKCε-YFP after infection with wild-type Lm or Lm mutants deficient in LLO (hly-), PI-PLC (plcA-), PC-PLC (plcB-) or combinations thereof (hly plcA-plcB-, plcA-plcB-). At 5 min after infection, similar percentages were observed for wild-type (37%), hly (42%), plcA- (45%), plcB- (47%), plcA-plcB- (47%), and hly plcA-plcB- (42%) Lm phagosomes, indicating that LLO and PLCs were not necessary for PKCε recruitment during bacterial entry (Fig. 3A).
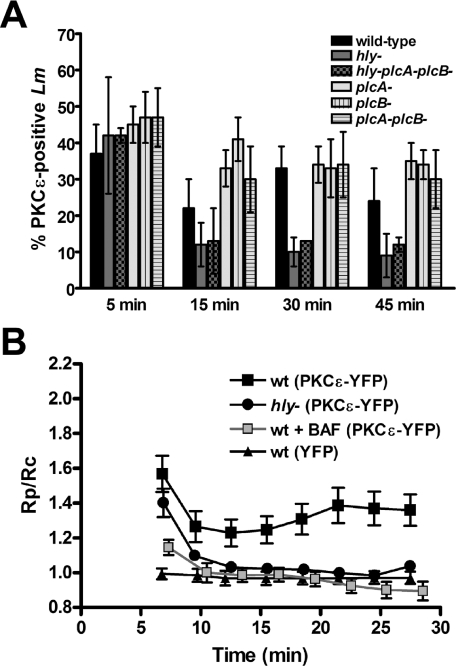
Fig. 3.
Conditions affecting PKCε recruitment to Lm vacuoles. A. Quantitative analysis of the timing of PKCε localization to Lm. Macrophages were infected for 3 min with the indicated strains of bacteria, then were washed and fixed at 5, 15, 30 and 45 min after infection. Bacteria were labelled with DAPI and scored for colocalization with PKCε-YFP. Fifty bacteria were counted in triplicate in three different experiments. B. Macrophages were transfected with plasmids for PKCε-YFP and CFP (or YFP and CFP for the negative control; n = 10). Transfected macrophages, untreated or treated with 500 nM bafilomycin A1 (BAF; n = 10), were infected with either wild-type (n = 18) or hly Lm (n = 11) for 3 min. Time-lapse phase-contrast, CFP and YFP images were taken of Lm phagosomes every 30 s for 30 min, and ratio images were prepared (YFP/CFP). The ratio of YFP/CFP in the phagosome (Rp) was divided by the ratio of YFP/CFP in the entire cell (Rc) to measure recruitment of PKCε-YFP to the phagosome (Rp/Rc).
At later time points, PKCε translocated more frequently to phagosomes containing LLO-expressing Lm (Fig. 3A; Time 15, 30 and 45 min). Approximately 25–35% of LLO-expressing Lm (wild-type, plcA-, plcB-, plcA-plcB-) recruited PKCε-YFP 15–45 min after infection, whereas only 10% of LLO– Lm (hly and hly plcA-plcB-) recruited PKCε-YFP at these later times. This indicated that the bacterial PLCs were not producing the DAG that recruited PKCε to the vacuolar membrane. Interestingly, at 15 min, the measurements for plcB-, plcA-, plcA-plcB-, and hly Lm were significantly different from that of wild-type Lm (P < 0.05). This suggests the PLCs may modulate the PKCε response.
Protein kinase C ε recruitment to the Lm vacuole membrane is dependent upon LLO and vacuolar acidification
We quantified the levels of PKCε translocated to LLO+ and LLO– Lm vacuoles (Fig. 3B). LLO– Lm vacuoles were not completely devoid of PKCε recruitment, indicating that PKCε was recruited to Lm vacuoles at low levels but that LLO somehow enhanced that recruitment. Macrophages coexpressing PKCε-YFP and CFP were infected with wild-type or hly Lm. Phase-contrast, YFP and CFP images were taken of phagosomes at regular intervals. Ratio images (YFP/CFP) were calculated, and the ratio of YFP/CFP of the phagosome (Rp) was divided by the ratio of YFP/CFP in the whole cell (Rc). Rp/Rc values greater than 1.0 indicated YFP chimera recruitment to vacuoles (Fig. 3B; triangles) (Henry et al., 2004). Both wild-type and hly Lm showed similar high amounts of PKCε-YFP recruited upon entry (Time 5), with Rp/Rc values of 1.6 and 1.4 respectively. The second recruitment to the wild-type Lm phagosome (Rp/Rc values of ∼1.3–1.4) was maximal at 22 min and was significantly higher than seen on hly Lm phagosomes (Rp/Rc values ∼1.05) (P < 0.01).
Addition of bafilomycin A1 to macrophages increases the pH of endocytic compartments by inhibition of the proton ATPase, and consequently inhibits LLO pore-forming activity (Beauregard et al., 1997; Shaughnessy et al., 2006). We asked if inhibiting LLO activity with bafilomycin A1 would affect PKCε recruitment to the Lm vacuole. Treatment of macrophages with bafilomycin A1 before and during infection reduced wild-type Lm activation of PKCε to baseline levels (Fig. 3B). This indicated that LLO pore-forming activity was necessary for the late PKCε recruitment to the Lm vacuole.
Protein kinase C ε is recruited to perforated Lm vacuoles
We previously showed by measuring the sequential release of small [Lucifer Yellow (LY); 522 MW] and large (TRDx; 10 000 average MW) fluorescent probes from Lm vacuoles that LLO-expressing (LLO+) Lm perforate vacuolar membranes (Shaughnessy et al., 2006). In the previous study, 50% of LLO+ Lm perforated the vacuole, which was characterized by the selective release of LY from the vacuole or the sequential release of LY then TRDx (Shaughnessy et al., 2006). The other 50% of LLO+ Lm vacuoles and 100% of LLO– Lm vacuoles did not perforate the vacuole (Shaughnessy et al., 2006). We define perforation of the Lm vacuole as the differential release of LY and TRDx; either by loss of LY only or by sequential loss of LY and then TRDx.
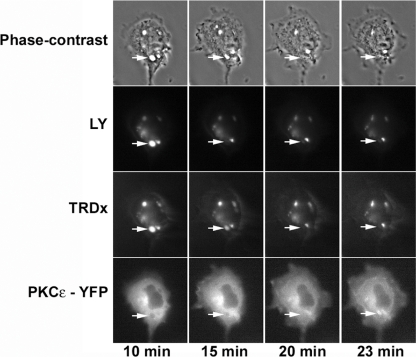
We adapted this method to ask if vacuoles recruiting PKCε were first perforated by LLO. Macrophages expressing PKCε-YFP were infected with wild-type Lm in the presence of LY and TRDx. The fluorescence of LY, TRDx, and PKCε-YFP was then imaged in the Lm vacuoles over time. Time-lapse fluorescence revealed that PKCε-YFP translocation to wild-type Lm vacuoles occurred after perforation of the vacuole. That is, the sequential loss of fluorescence of LY and TRDx from the phagosome was apparent 10–15 min after Lm addition whereas translocation of PKCε was not detected until approximately 23 min (Fig. 4). As in previous studies (Shaughnessy et al., 2006), half of wild-type Lm vacuoles (6 of 11 recorded events) showed perforation. Of the six phagosomes that perforated, five later recruited PKCε-YFP (Fig. 4). Of the five events in which Lm did not lose LY and TRDx, only one showed PKCε-YFP translocation to the vacuole. No vacuoles perforated after recruitment of PKCε-YFP. A Pearson's chi-squared test applied to these results indicated that the correlation between perforation and PKCε recruitment was significant (P = 0.0356). These results indicated that LLO pore-forming activity is necessary for accumulation of PKCε.
Fig. 4.
PKCε localizes after perforation of the Lm phagosome. PKCε-YFP-expressing macrophages were infected with wild-type Lm in the presence of LY and TRDx. Time-lapse phase-contrast and fluorescence images of YFP, LY and TRDx in Lm phagosomes were taken at regular intervals. Shown are representative images taken at 10, 15, 20 and 23 min after infection. Arrow points to a Lm phagosome that loses LY and TRDx then acquires PKCε-YFP.
Protein kinase C ε is recruited by vacuolar LLO
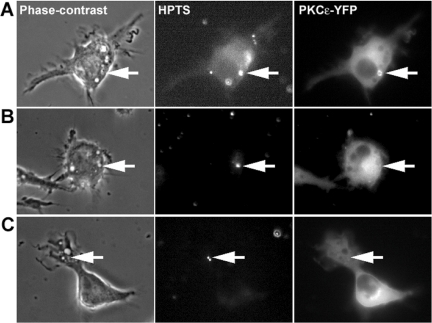
We next asked if LLO activity in the vacuole was sufficient to recruit PKCε. Macrophages were allowed to phagocytose pH-sensitive liposomes containing LLO. These liposomes (phosphatidylethanolamine–cholesterylhemisuccinate) become unstable at pH < 6.0 and release their contents, allowing delivery of encapsulated molecules, including LLO, into the endocytic compartment (Lee et al., 1996). Macrophages expressing PKCε-YFP were fed pH-sensitive liposomes in which LLO was co-encapsulated with a small fluorescent dye, 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS). Delivery of LLO (Fig. 5A), but not heat-inactivated LLO (hiLLO) (Fig. 5B) or HPTS alone (Fig. 5C), into the endocytic compartment caused PKCε-YFP translocation to the phagosomes. LLO and HPTS liposomes recruited PKCε-YFP to 5% of the vacuoles, whereas liposomes containing HPTS alone or hiLLO plus HPTS never recruited PKCε-YFP (50 vacuoles were counted for three separate experiments). This indicated that endosomal LLO was sufficient to recruit PKCε to compartments and suggests that PKCε recruitment signals vacuole perforation.
Fig. 5.
PKCε localizes to perforated compartments. PKCε-YFP-expressing macrophages were infected with liposomes containing (A) HPTS and LLO, (B) HPTS and hiLLO, or (C) HPTS. Shown are representative phase-contrast and fluorescence HPTS and YFP images with arrows pointing to endosomes containing liposomes. PKCε-YFP was only recruited to vacuoles containing functional LLO.
Protein kinase C ε is recruited to osmotically lysed endosomes
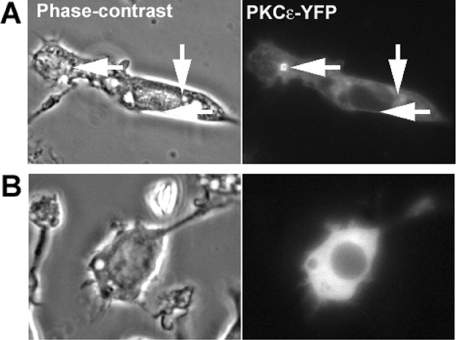
The results to this point are consistent with two models: (i) LLO is required for PKCε recruitment or (ii) LLO is not required and other signals that induce membrane damage will serve equally for the same task. To distinguish between these possibilities, we determined the effect of osmotic lysis of endosomes on PKCε translocation. Endosomes in RAW macrophages expressing PKCε-YFP were ruptured by osmotic lysis, a commonly used method to deliver antigens from endosomes into the cytosol (Okada and Rechsteiner, 1982; Moore et al., 1988). Briefly, macrophages were allowed to endocytose hypertonic medium, and then hypotonic medium was added, causing endosome rupture and release of their contents into cytoplasm. Finally an isotonic solution was added to allow macrophage recovery. When this procedure was performed with macrophages expressing PKCε-YFP, multiple vacuoles recruited PKCε-YFP to their membranes (Fig. 6A). Omission of 10% polyethylene glycol 1000 from the hypertonic medium does not allow cytoplasmic release of macromolecules (Okada and Rechsteiner, 1982; Moore et al., 1988). In macrophages exposed to this control condition, PKCε-YFP no longer translocated to endocytic membranes (Fig. 6B). Moreover, isotonic medium and hypotonic medium alone also did not cause PKCε-YFP to translocate to endocytic membranes (data not shown). This indicates that PKCε recruitment to Lm vacuoles is part of a cytoplasmic signalling mechanism that recognizes damaged organelles.
Fig. 6.
PKCε localizes to osmotically lysed endosomes. Representative phase-contrast and YFP images of PKCε-YFP-expressing macrophages exposed to either (A) hypertonic media containing polyethylene glycol 1000, which lyses endosomes osmotically, or (B) hypertonic media with no polyethylene glycol 1000, which does not lyse endosomes.
Discussion
This work demonstrates that PKCε localizes twice to Lm-associated membranes during Lm infection of macrophages. PKCε localization during Lm entry is independent of LLO and the bacterial PLCs. Later, PKCε localizes to the Lm-containing vacuole after perforation by LLO. This indicates a role for PKCε in recognizing damaged Lm vacuoles.
A role for PKCε in Lm pathogenesis
Protein kinase C ε localized to Lm upon entry into macrophages, independently of LLO or the bacterial PLCs. This localization is likely related to that described for FcγR-mediated phagocytosis of IgG-opsonized particles (Diaz-Guerra et al., 1996; Larsen et al., 2000; 2002). Inhibition of PKCε slowed the rate of FcγR-mediated phagocytosis and decreased NO production (Larsen et al., 2000; 2002). NO production is important for the clearance of Lm in activated macrophages. It is possible that PKCε recruitment during Lm entry signals the induction of NO production near the vacuole.
The activity of LLO inside Lm vacuoles stimulated a second localization of PKCε. The recruitment of PKCε to wild-type Lm vacuoles followed LLO-mediated vacuole perforation and required LLO activity. This LLO-dependent recruitment of PKCε-YFP suggests either that Lm exploits host cell signalling mechanisms or that the host uses PKCε as a means to signal for the presence of a damaged vacuole. The low level of PKCε recruitment to vacuoles without LLO may indicate the normal level of PKCε that is recruited to phagosomes which is necessary for defence against bacterial infection.
Our hypothesis at the outset of these studies was that Lm PLCs recruit PKCε to the vacuolar membrane. Both host cells and Lm produce PLCs that hydrolyse phospholipids, releasing DAG on membranes. Therefore, both the host and Lm are equipped to activate PKCε. It was previously reported that Lm PLCs activate host PKCs (Goldfine and Wadsworth, 2002; Wadsworth and Goldfine, 2002). However, as we did not see a decrease in PKCε recruitment relative to wild-type Lm after infection with Lm mutants plcA-, plcB- or the double mutant plcA-plcB-, we conclude that they have no role in activating PKCε. These results therefore implicate host PLCs for PKCε activation (Goldfine et al., 2000). DAG, localized in macrophages expressing the DAG-binding domain from PKCδ (C1δ-GFP), appeared on Lm-associated membranes both during entry and later on the vacuole membrane (data not shown).
Cholesterol-dependent cytolysins could form pores that specifically translocate proteins into the host cell. This is supported by studies of another CDC, streptolysin O, which secretes an effector molecule through its pore into the host cell (Madden et al., 2001). Likewise, it was hypothesized that LLO is a specific translocator for the Lm-secreted proteins, PI-PLC and PC-PLC (Goldfine and Wadsworth, 2002). Our studies indicate no role for the bacterial PLCs in the activation of PKCε, despite the fact that PKCε recruitment occurs after membranes have been permeabilized by LLO.
Listeriolysin O: an activator of signalling events from the phagosome
The substrate of PKCε on the Lm vacuole is not yet known, so it is unclear what downstream molecules are involved. LLO and other CDCs have previously been shown to mediate host signalling pathways. CDCs activate TLR4 signalling events, including induction of TNFα, IL-6 and iNOS (Park et al., 2004). PKCε has also been implicated in TLR signalling (Aksoy et al., 2004; McGettrick et al., 2006). Recently, the TLR4 adaptor molecule TRAM was identified as a specific substrate for PKCε; phosphorylation of TRAM by PKCε was necessary for downstream TRAM-mediated TLR4 signalling (McGettrick et al., 2006). It is unknown whether TLR4 is involved in recognition of Lm; however, activation of MyD88 (another TLR4 adaptor molecule) is essential for the innate immune defence against Lm (Edelson and Unanue, 2002; Seki et al., 2002; Pamer, 2004). Therefore, it is possible that Lm is recognized by TLR4 and signals through TRAM and PKCε on the Lm vacuoles. Future studies should determine if PKCε recruitment to the Lm vacuole enhances signalling by TLRs or other pattern-recognition receptors.
The role of PKCε in cellular responses to membrane damage
It is unknown what happens to damaged compartments and how the host cell either repairs or recycles the membrane. Damaged organelles could be sealed and ready to use again or, if the membrane is beyond repair, the membrane may be sequestered and degraded by autophagy. PKCε signalling may be involved in the breakdown and recycling of damaged membrane.
The PKCε recruitment to Lm vacuoles may indicate a general mechanism for recognizing damaged organelles. What could be the consequences of recruiting PKCε to a damaged organelle? Many viruses and intracellular pathogens reach cytoplasm by disrupting vesicles that contain them after endocytosis by host cells. For Lm, inflammatory responses of infected macrophages and dendritic cells require LLO (Vazquez et al., 1995; Brzoza et al., 2004). Perhaps inflammatory or immune responses of host cells to invasive microbes are activated by PKCε binding to lipids or proteins which normally reside in the outer (luminal) leaflet of endocytic vesicle membranes, but which are exposed to cytoplasm when the integrity of the bilayer is compromised.
Experimental procedures
Reagents
The fluorophores Texas Red phalloidin, TRDx (MW = 10 kDa), LY, HPTS, and 4′,6-diamindino-2-phenylindole (DAPI) were obtained from Molecular Probes (Eugene, OR). Bafilomycin A1 was obtained from Calbiochem (La Jolla, CA).
Bacterial strains
The Lm wild-type strain DP-L10403, hly deletion strain DP-L2161, plcA- deletion strain DP-L1552, plcB- deletion strain DP-L1935, plcA-plcB- deletion strain DP-L1936, hly plcA-plcB- deletion strain DP-L2319 used in this study were gifts from Daniel Portnoy (University of California, Berkeley).
Bacterial preparation
Listeria monocytogenes were grown overnight at room temperature in Brain Heart Infusion broth. They were subcultured the next day and grown for ∼1 h to an OD600 of 0.500 at 37°C. Subcultured bacteria (1 ml) were washed three times in 1 ml Ringer's buffer (RB; 155 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 2 mM NaH2PO4, 10 mM Hepes and 10 mM glucose, pH 7.2) followed by centrifugation (4500 g). Where indicated, bacteria were pre-labelled with SNARF-1, carboxylic acid, acetate succinimidyl ester (Molecular Probes). Washed bacteria were labelled with 3 μl ml−1 SNARF-1 solution in DMSO for 15 min at 37°C with shaking, then washed four times with 1 ml RB before use.
Macrophage preparation
RAW 264.7 macrophages were obtained from ATCC (Manassas, VA) and grown in Advanced Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA), with 2% heat-inactivated FBS (Invitrogen), 100 unit ml−1 of penicillin/streptomycin mixture (Sigma Chemical, St Louis, MO), and l-glutamine, at 37°C with 5% CO2. Cells were plated the day before the experiment onto 25 mm coverslips, in 6 well plates, at 3 × 105 cells well−1. Macrophages were transfected with plasmids for fluorescent chimera expression using FuGENE 6 transfection reagent, according to the manufacturer's protocol (Roche Diagnostics, GmbH, Mannheim, Germany).
Fixed cell assay and immunofluorescence
Macrophages were infected with Lm (multiplicity of infection ∼1) for 3 min. Coverslips were washed with RB and incubated with DMEM, 10% FBS, and 25 μg ml−1 gentamicin. Coverslips were fixed with cytoskeletal fix (30 mM Hepes, 10 mM EGTA, 0.5 mM EDTA, 5 mM MgSO4, 33 mM potassium acetate, 5% polyethylene glycol 400, and 4% paraformaldehyde) at either 5, 15, 30 or 45 min after infection. Cells were rinsed with phosphate-buffered saline (PBS) and 2% goat serum, permeabilized with 0.3% Triton X-100 in PBS, and incubated for 15 min in PBS and 2% goat serum plus DAPI (2 μg ml−1 from a 100 μg ml−1 stock in water). For immunofluorescence staining, non-transfected macrophages were incubated with 1:50 dilution of mouse monoclonal anti-PKCε antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in 2% goat-serum overnight at 4°C and rinsed three times for 5 min with 2% goat-serum. A 1:1000 dilution of Alexa Fluor 488-labelled secondary antibody (Molecular Probes) in 2% goat-serum was incubated for 1 h at 37. Coverslips were mounted on glass slides containing Prolong Antifade Gold (Molecular Probes). For each coverslip, 50 Lm were scored for the presence of PKCε localization.
Ratiometric imaging
Macrophages expressing PKCε-YFP (or untagged YFP when indicated) and CFP were infected with Listeria for 3 min (or 0.5 mg ml−1 of TRDx when indicated), then excess bacteria were washed away with RB (30 times with 1 ml). Where indicated, 500 nM bafilomycin A1 (from a 100 μM stock in DMSO) was added to the macrophages for 1 h prior to infection and throughout the experiment.
Experiments used an inverted fluorescence microscope (Nikon TE300, Japan) equipped with transmitted light and a mercury arc lamp with epifluorescence illumination. To measure YFP and CFP fluorescence, two filter wheels (Lambda 10–2, Sutter Instruments, Novato, CA) held excitation filters (S500/20× and S436/10×, for YFP and CFP respectively, Chroma Technology Corporation, Rockingham, VT) and emission filters (S535/30m and S470/30m) with the dichroic mirror set (86002v1bs, Chroma Technology Corporation). A cooled CCD camera (Quantix Photometrics, Tucson, AZ) collected images and Metamorph software version 6.3 (Universal Imaging, West Chester, PA) controlled the equipment and image processing.
A ratio image was obtained by dividing each YFP image by the corresponding CFP image and multiplying by 1000. A binary mask was produced from the addition of the YFP and CFP images followed by application of a manual threshold. The binary and divided images were combined in a logical AND to produce ratio images that excluded non-cellular signals. A region was drawn around the phagosome in the ratio image and the average ratio of YFP/CFP in the phagosome was calculated. A second region drawn around the entire cell was used to measure the average fluorescence intensities of YFP and CFP over the entire cell. Relative ratios of YFP/CFP in the phagosome (Rp) were then divided by the YFP/CFP for the entire cell (Rc) to obtain a cell-normalized phagosome ratio (Rp/Rc).
Measurement of vacuole perforation by Lm
In a method adapted from previous work (Shaughnessy et al., 2006), macrophages expressing PKCε-YFP were infected with a 100 μl mixture of wild-type Lm, TRDx (0.5 mg ml−1) and LY (0.5 mg ml−1) for 3 min. After infection, cells were washed thoroughly with RB. Lm-infected macrophages were located by phase-contrast optics. Using the 86006 dichroic filter set (Chroma Technology Corporation), four images were taken every minute for 30 min: phase-contrast, LY (exc. 436 nm/em. 535 nm), TRDx (exc. 580 nm/em. 630 nm) and YFP (exc. 492 nm/em. 535). The sequential loss of fluorescence from LY and TR was recorded relative to the timing of PKCε-YFP localization.
Purification of LLO
Recombinant LLO was purified from E. coli strain BL21(DE3) transformed with the pET29b vector expressing LLO with a C-terminal six-histidine tag as previously described (Mandal and Lee, 2002). The protein yield was measured using the BCA assay (Pierce, Rockford, IL), and protein purity was analysed using SDS-PAGE. For some experiments, LLO was heat-inactivated at 70°C for 10 min. Haemolytic activity was measured using the sheep red blood cell-based haemolysis assay, as previously described (Mandal and Lee, 2002).
Preparation of LLO liposomes
LLO/HPTS, hiLLO/HPTS, or HPTS liposomes were prepared with phosphatidylethanolamine (Avanti, Alabaster, AL) and cholesterylhemisuccinate (Sigma) in a 2:1 molar ratio using the thin film method (Lee et al., 1996; Mandal and Lee, 2002). LLO and HPTS were encapsulated inside liposomes at 0.25 mg ml−1 and 35 mM HPTS, respectively, in 30 mM Tris buffer, 100 mM NaCl, at pH 8.5, under non-reducing conditions. Liposomes underwent repeated sonication and freeze-thaw cycles. Unencapsulated protein and HPTS were removed by purification on a Sepharose CL-4B column (Amersham Pharmacia, Uppsala, Sweden).
Osmotic lysis of pinosomes
Macrophage pinosomes were osmotically lysed according to previously published methods and adapted for this work (Okada and Rechsteiner, 1982; Moore et al., 1988). RAW macrophages expressing PKCε-YFP were exposed to hypertonic medium (0.5 M sucrose, 10% polyethylene glycol 1000, in RB) for 10 min (when stated, polyethylene glycol 1000 was not added). Coverslips were then washed five times and medium was replaced with hypotonic medium (60% RB and 40% water) for 3 min, then washed again and replaced with isotonic medium (RB). Phase-contrast and YFP images were taken after osmotic lysis.
Acknowledgments
The suggestions of Dr Michelle Lennartz are greatly appreciated. Supported by NIH Grants AI-035950 to J.A.S. and AI-047173 and AI-058080 to K.D.L.
References
- Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Akita Y. Protein kinase C-epsilon (PKC-epsilon): its unique structure and function. J Biochem (Tokyo) 2002;132:847–852. doi: 10.1093/oxfordjournals.jbchem.a003296. [DOI] [PubMed] [Google Scholar]
- Aksoy E, Goldman M, Willems F. Protein kinase C epsilon: a new target to control inflammation and immune-mediated disorders. Int J Biochem Cell Biol. 2004;36:183–188. doi: 10.1016/s1357-2725(03)00210-3. [DOI] [PubMed] [Google Scholar]
- Beauregard KE, Lee K-D, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzoza KL, Rockel AB, Hiltbold EM. Cytoplasmic entry of Listeria monocytogenes enhances dendritic cell maturation and T cell differentiation and function. J Immunol. 2004;173:2641–2651. doi: 10.4049/jimmunol.173.4.2641. [DOI] [PubMed] [Google Scholar]
- Castrillo A, Pennington DJ, Otto F, Parker PJ, Owen MJ, Bosca L. Protein kinase Cε is required for macrophage activation and defense against bacterial infection. J Exp Med. 2001;194:1231–1242. doi: 10.1084/jem.194.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenni V, Doppler H, Sonnenburg ED, Maraldi N, Newton AC, Toker A. Regulation of novel protein kinase C epsilon by phosphorylation. Biochem J. 2002;363:537–545. doi: 10.1042/0264-6021:3630537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P, Vicente MF, Mengaud J, Baquero F, Perez-Diaz JC, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Guerra MJ, Bodelon OG, Velasco M, Whelan R, Parker PJ, Bosca L. Up-regulation of protein kinase C-epsilon promotes the expression of cytokine-inducible nitric oxide synthase in RAW 264.7 cells. J Biol Chem. 1996;271:32028–32033. doi: 10.1074/jbc.271.50.32028. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage Listericidal activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine H, Wadsworth SJ. Macrophage intracellular signaling induced by Listeria monocytogenes. Microbes Infect. 2002;4:1335–1343. doi: 10.1016/s1286-4579(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Goldfine H, Wadsworth SJ, Johnston NC. Activation of host phospholipases C and D in macrophages after infection with Listeria monocytogenes. Infect Immun. 2000;68:5735–5741. doi: 10.1128/iai.68.10.5735-5741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RM, Hoppe AD, Joshi NJ, Swanson JA. The uniformity of phagosome maturation in macrophages. J Cell Biol. 2004;164:185–194. doi: 10.1083/jcb.200307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- Kiderlen AF, Kaufmann SH, Lohmann-Matthes ML. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984;14:964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Larsen EC, DiGennaro JA, Saito N, Mehta S, Loegering DJ, Mazurkiewicz JE, Lennartz MR. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J Immunol. 2000;165:2809–2817. doi: 10.4049/jimmunol.165.5.2809. [DOI] [PubMed] [Google Scholar]
- Larsen EC, Ueyama T, Brannock PM, Shirai Y, Saito N, Larsson C, et al. A role for PKC-ε in FcγR-mediated phagocytosis by RAW 264.7 cells. J Cell Biol. 2002;159:939–944. doi: 10.1083/jcb.200205140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-D, Oh Y-K, Portnoy DA, Swanson JA. Delivery of macromolecules into cytosol using liposomes containing hemolysin from Listeria monocytogenes. J Biol Chem. 1996;271:7249–7252. [PubMed] [Google Scholar]
- McGettrick AF, Brint EK, Palsson-McDermott EM, Rowe DC, Golenbock DT, Gay NJ, et al. Trif-related adapter molecule is phosphorylated by PKCε during Toll-like receptor 4 signaling. Proc Natl Acad Sci USA. 2006;103:9196–9201. doi: 10.1073/pnas.0600462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- Madden JC, Ruiz N, Caparon M. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in Gram-positive bacteria. Cell. 2001;104:143–152. doi: 10.1016/s0092-8674(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Mandal M, Lee K-D. Cytosolic delivery of macromolecules in vivo utilizing listeriolysin O-mediated endosomolysis: enhanced cytotoxic T-lymphocyte induction and tumor protection. Biochim Biophys Acta. 2002;1563:7–17. doi: 10.1016/s0005-2736(02)00368-1. [DOI] [PubMed] [Google Scholar]
- Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Okada CY, Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982;29:33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Park JM, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200:1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. 2006;8:781–792. doi: 10.1111/j.1462-5822.2005.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahelin RV, Digman MA, Medkova M, Ananthanarayanan B, Melowic HR, Rafter JD, Cho W. Diacylglycerol-induced membrane targeting and activation of protein kinase Cepsilon: mechanistic differences between protein kinases Cdelta and Cepsilon. J Biol Chem. 2005;280:19784–19793. doi: 10.1074/jbc.M411285200. [DOI] [PubMed] [Google Scholar]
- Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003;376:545–552. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez MA, Sicher SC, Wright WJ, Proctor ML, Schmalzried SR, Stallworth KR, et al. Differential regulation of TNF-alpha production by listeriolysin-producing versus nonproducing strains of Listeria monocytogenes. J Leukoc Biol. 1995;58:556–562. doi: 10.1002/jlb.58.5.556. [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, Goldfine H. Mobilization of protein kinase C in macrophages induced by Listeria monocytogenes affects its internalization and escape from the phagosome. Infect Immun. 2002;70:4650–4660. doi: 10.1128/IAI.70.8.4650-4660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]