Abstract
The largest central synapse in adult Drosophila is a mixed electro-chemical synapse whose gap junctions require the product of the shaking-B (shak-B) gene. Shak-B2 mutant flies lack gap junctions at this synapse, which is between the giant fibre (GF) and the tergotrochanteral motor neuron (TTMn), but it still exhibits a long latency response upon GF stimulation. We have targeted the expression of the light chain of tetanus toxin to the GF, to block chemical transmission, in shak-B2 flies. The long latency response in the tergotrochanteral muscle (TTM) was abolished indicating that the chemical component of the synapse mediates this response. Attenuation of GAL4-mediated labelling by a cha-GAL80 transgene, reveals the GF to be cholinergic. We have used a temperature-sensitive allele of the choline acetyltransferase gene (chats2) to block cholinergic synapses in adult flies and this also abolished the long latency response in shak-B2 flies. Taken together the data provide evidence that both components of this mixed synapse are functional and that the chemical neurotransmitter between the GF and the TTMn is acetylcholine. Our findings show that the two components of this synapse can be separated to allow further studies into the mechanisms by which mixed synapses are built and function.
Keywords: giant fibre, innexins, neuron, neurotransmitter, tetanus toxin
Introduction
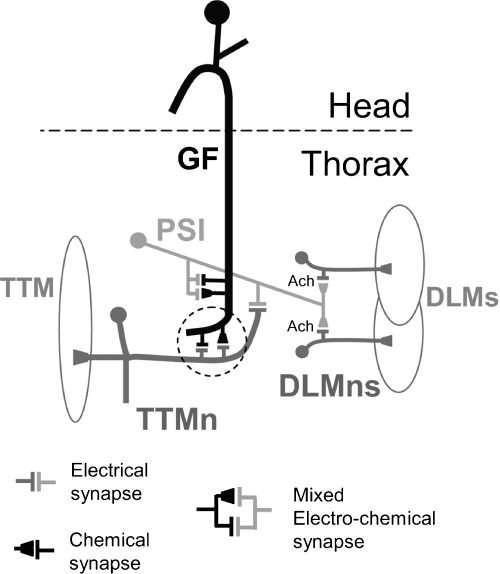
Mixed electro-chemical synapses are found in both vertebrate and invertebrate nervous systems including fish (Lin & Faber, 1988; Korn & Faber, 2005), crustaceans (Edwards et al., 1999), and insects (Blagburn et al., 1999).Their bi-partite nature has traditionally made them difficult to study as the two components are not easily separable. The giant fibre system (GFS) of Drosophila melanogaster is a simple neural circuit that mediates an escape response in adult flies and contains mixed electro-chemical synapses (reviewed in Allen et al., 2006). The two large giant fibres (GFs) (Fig. 1) relay information from the brain to the thoracic ganglia where they make electro-chemical synapses with the tergotrochanteral motor neuron (TTMn), which drives the leg extensor muscle and the peripherally synapsing interneuron (PSI; King & Wyman, 1980; Blagburn et al., 1999), which drives the dorsal longitudinal motor neurons (DLMns; King & Wyman, 1980; Gorczyca & Hall, 1984).
Fig. 1.
Schematic representing the known synaptic connections of the GFS. For simplicity only one side of the bilateral circuit is shown. The GF makes mixed electrochemical synapses with the PSI and with the TTMn in the thoracic ganglia. The GF-TTMn synapse is circled with a dotted line. The PSI synapses with the DLMns via cholinergic chemical synapses. The PSI synapses with five DLMns, but only two are indicated for clarity. PSI and TTMn are also electrically coupled. Adapted from Allen et al. (2006).
The shaking-B2 (shak-B2) mutation was originally generated during an adult EMS behavioural screen (Homyk et al., 1980) and independently, Passover alleles at the same locus were isolated in a mutagenic screen for flies that failed to escape to a light-off stimulus (Thomas & Wyman, 1984; Baird et al., 1990). The mutants show a very specific electrophysiological phenotype upon GF stimulation; a long latency and labile response is seen in tergotrochanteral muscle (TTM) and no responses are elicited in the dorsal longitudinal muscles (DLMs; Thomas & Wyman, 1984; Baird et al., 1990). The long latency response in TTM was originally thought to be due either, to a separate pathway from the brain to the thorax that was uncovered once the GF-TTMn synapse was rendered nonfunctional, or to a defect in the GF-TTMn synapse (Thomas & Wyman, 1984; Baird et al., 1990). Electrophysiological tests on flies in which neurite outgrowth of the GF was blocked showed that the only pathway from the brain to TTMn is via the GF (Allen et al., 2000). This suggested that a defect in the GF-TTMn synapse was the cause of the long latency seen in TTM. Moreover, it was shown that shak-B2 encodes a gap junction protein and the mutant flies have no functional gap junctions between the GF and TTMn (Phelan et al., 1996; Sun & Wyman, 1996; Phelan et al., 1998). EM work has revealed the existence of T-bars and synaptic vesicles, indicative of chemical transmission, in the presynaptic bends of the GF and at the GF-PSI contact points (Blagburn et al., 1999). This body of evidence led to the hypothesis that the chemical component of the synapse is responsible for the long latency TTM response in shak-B2 flies.
We have used these shak-B2 mutants, in combination with misexpression of a toxin, to test the function of the chemical component of this mixed synapse. We provide evidence that the GF is cholinergic by using the expression of GAL80 to block GAL4-mediated labelling of the neuron and have used a temperature sensitive allele that affects acetylcholine (ACh) production to demonstrate the nature of chemical transmission at the synapse.
Materials and methods
Drosophila stocks
All stocks were cultured at 25 °C on standard medium unless stated otherwise. The P[GAL4] line c17 expresses in the GF and other neurons in the brain and optic lobes as well as sensory neurons. However, it does not express in the TTMn or any other identified neurons within the GFS (Allen et al., 1999; Trimarchi et al., 1999). The P[GAL4] line A307 expresses in the GF and weakly in the TTMn and some DLMns as well as some other neurons in the CNS (Phelan et al., 1996; Allen et al., 1998). The UAS-IMPTNT and UAS-TNT(G) lines are described in Sweeney et al. (1995) and Cha3.3kb-GAL80 has been described previously (Kitamoto, 2002). The shaking-B2 (shak-B2) mutation is an EMS-induced allele from a behavioural screen performed by Homyk et al. (1980). It acts as a functional null for the shak-B (neural) and shak-B (neural +16) gene products (Krishnan et al., 1993; Krishnan et al., 1995; Zhang et al., 1999). The chats2 allele used is that originally described by Greenspan et al. (1980) and causes an arginine to histidine change at amino acid 397 in the resulting protein (Wang et al., 1999). The CyO and MKRS balancer chromosomes are described in Lindsley & Zimm (1992).
Electrophysiology of flies expressing the tetanus toxin light chain
Flies were anaesthetized by cooling on ice and waxed onto a small podium, ventral side down, with the wings held outwards and secured in the wax. Tungsten electrodes were pushed through the eyes and into the brain for stimulation and a tungsten ground wire placed into the abdomen. A pulse of 40–60 V for 0.03 ms from a Grass S48 stimulator (Astro-Medical, West Warwick, USA) via a stimulus isolation unit was given to activate the GFs in the brain and recordings were made from the TTM and a contralateral DLM muscle with glass microelectrodes (resistance 40–60 MΩ). These were filled with 3M KCl, or saline and placed into the muscles through the cuticle. Responses were amplified using Getting 5 A amplifiers (Getting Instruments, San Diego, USA) and data digitized using an analogue-digital Digidata 1320 and Axoscope 9.0 software (MDS Inc, Toronto, Canada). For response latency recordings five single stimuli were given to each individual tested with a 5-s rest period between each stimulus. This usually enabled sufficient time for the weak GF-TTMn synapse of shak-B2 mutant flies to recover. In a few cases, where five responses were not initially obtained, more stimuli were given. To obtain data for synaptic following at two frequencies, trains of ten stimuli, at either 250 Hz or 100 Hz, were given with a 5-s rest period between each train.
For the thoracic stimulation, to activate the motor neurons directly, the stimulating electrodes were moved from the brain and carefully placed through the cuticle at the anterior end of the thorax and down into the fused thoracic ganglia in the ventral part of the thorax.
Electrophysiology of flies containing chats2
All chats2 flies were reared at the permissive temperature of 18 °C to allow them to develop to adulthood. Flies were collected on the day they eclosed and either kept at 18 °C for 48 h prior to testing or were moved to an incubating water bath and kept at 28 °C for 48 h prior to testing. Following this flies were prepared for electrophysiology as described above and tested within 10 min of being removed from the 18 °C or 25 °C environment. Each individual was given five stimuli at 1 Hz in the brain and recordings made. At this stimulation rate wild-type flies will show responses in TTM and DLM to every stimulus and shak-B2 mutants will show no responses in DLM and intermittent responses in TTM (Thomas & Wyman, 1984; Baird et al., 1990; this study). The stimulating electrodes were then moved as described above and thoracic stimulation of five stimuli at 1 Hz was given to the same individual.
CNS histochemistry
Adult nervous systems were dissected in 0.1 m PBS plus 0.1% Triton X-100, fixed briefly in 1% gluteraldehyde and stained for β-galactosidase activity as previously described (Jacobs et al., 2000). Images were taken on a Leica DMR microscope and figures assembled using Adobe Photoshop.
Results
Blocking chemical synaptic transmission with tetanus toxin
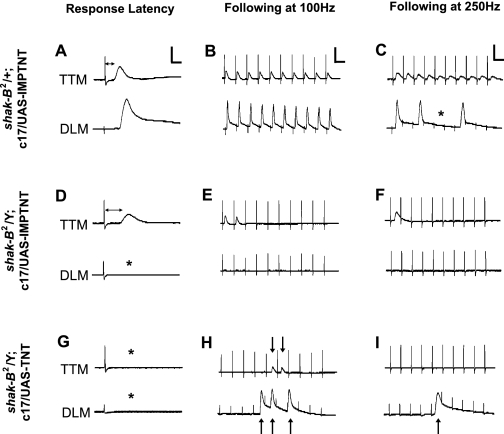
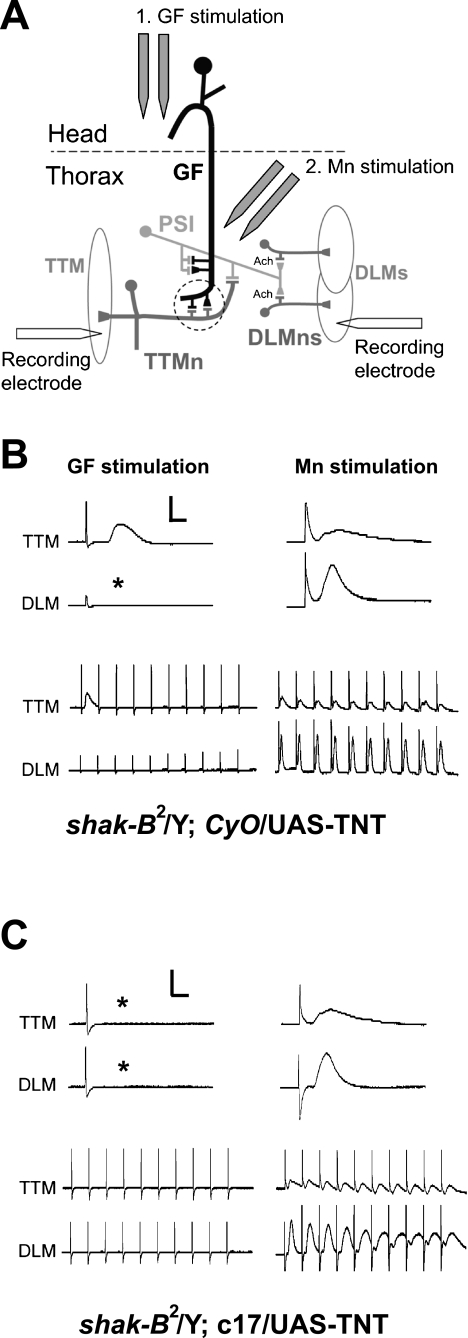
Typically, shak-B2 mutant flies show a long latency response in TTM that is very labile. This may be due to the chemical component of the GF-TTMn synapse that develops, in the absence of gap junctions. To test this hypothesis we used the GAL4-UAS system to selectively block chemical transmission from the GF to the TTMn. We targeted the expression of either an active form of the light chain of the tetanus toxin (TNT), or an inactive form of the toxin (IMPTNT), to the GF using the GF-specific P[GAL4] line c17 and UAS transgenes encoding the two forms of the toxin (Sweeney et al., 1995; Allen et al., 1999; Trimarchi et al., 1999). Hemizygous shak-B2 males that expressed TNT in their GFs gave no responses in TTM or DLM upon stimulation (Table 1; Fig. 2G–I). This suggests that the chemical component of the GF-TTMn synapse is responsible for the long latency response in TTM. Interestingly, these flies showed an increase in spontaneous activity in both TTM and DLM (see following traces, Fig. 2H and I). Other genotypes served as controls. Control female flies, either heterozygous for shak-B2 alone or heterozygous for shak-B2 and expressing IMPTNT, showed wild-type electrophysiological recordings upon activation of the GFs (Table 1; Fig. 2A–C). Females, heterozygous for shak-B2, expressing TNT in their GFs also exhibited wild-type responses (Table 1); presumably because the gap junctions between the GF and TTMn are sufficient for normal connectivity. Finally, shak-B2 hemizygous males, and shak-B2 hemizygous males expressing IMPTNT, showed the previously characterized mutant responses of a long latency and poor following to trains of stimuli for TTM and no responses in the DLMs (Baird et al., 1990; Table 1; Fig. 2D–F). To confirm that any long latency to TTM was due to a defective GF-TTMn synapse, the stimulating electrodes were placed into the thoracic ganglia of two of the shak-B2 hemizygous males, five of the shak-B2 c17/UAS-IMPTNT males and five of the shak-B2 CyO/UAS-TNT males tested, to activate the motor neurons directly. This by-passes the GF and always resulted in short latency responses in both TTM and DLM, even if the fly had given no responses on GF stimulation (Fig. 3B and data not shown). To ensure that expression of the active tetanus light chain toxin was not affecting the neuromuscular junctions (NMJs) of either TTM or DLM, thoracic ganglia stimulation was also performed. Of the seven shak-B2 c17/UAS-TNT males, thoracic ganglia stimulation was performed on six and these all showed responses in both TTM and DLM (Fig. 3C and data not shown).
Table 1.
Synaptic function in shak-B2 mutant flies expressing the tetanus light chain
| TTM | DLM | ||||||
|---|---|---|---|---|---|---|---|
| Genotype | n | Latency (ms) ± SEM | Following at 100 Hz ± SEM | Following at 250 Hz ± SEM | Latency (ms) ± SEM | Following at 100 Hz ± SEM | Following at 250 Hz ± SEM |
| shak-B2/+ | 6 | 0.85 ± 0.03 | 100 ± 0.0% | 100 ± 0.0% | 1.43 ± 0.07 | 84 ± 10.7% | 27.6 ± 7.2% |
| shak-B2/Y | 7a | 1.62 ± 0.17** | 17.5 ± 5.5% | 10.5 ± 0.5% | No responses | No responses† | No responses |
| shak-B2/+; c17/IMPTNT | 7 | 0.87 ± 0.02 | 100 ± 0.0% | 88 ± 9.2% | 1.44 ± 0.08 | 82.9 ± 12.1% | 33.1 ± 8.2% |
| shak-B2/Y; c17/IMPTNT | 6b | 1.27 ± 0.1* | 13.5 ± 1.7% | 10.5 ± 0.5% | No responses | No responses | No responses |
| shak-B2/+; c17/TNT | 12 | 0.92 ± 0.02 | 97.7 ± 1.3% | 78.7 ± 7.6% | 1.49 ± 0.04 | 92.7 ± 5.0% | 43.5 ± 8.6% |
| shak-B2/Y; c17/TNT | 7 | No responses | No responses | No responses | No responses | No responses† | No responses† |
| shak-B2/+; CyO/TNT | 7 | 0.86 ± 0.03 | 100 ± 0.0% | 86.3 ± 6.4% | 1.38 ± 0.06 | 91.1 ± 8.9% | 40.6 ± 9.4% |
| shak-B2/Y; CyO/TNT | 7c | 1.36 ± 0.09** | 18 ± 7.3% | 12 ± 2.5% | No responses | No responses | No responses |
3/7
2/6
2/7 flies gave no responses in both TTM and DLM. TTM averages are from those that did respond.
Occasionally PSPs were recorded but were spontaneous muscle contractions and not responses to the stimuli.
P < 0.001
P < 0.005 in a Student's unpaired t-test compared to shak-B2/+ females.
Fig. 2.
Expression of tetanus toxin in the GF abolishes the TTM response in shak-B2 mutants. Responses in the TTM and a DLM are shown when individual flies were given a single brain stimulus or ten brain stimuli at either 100 or 250 Hz. (A–C) responses in a shak-B2/+ c17/UAS-IMPTNT control fly show wild-type latencies and following frequencies at 100 and 250 Hz including the DLM not following 1:1 at 250 Hz (*) due to the failure of the PSI-DLMns synapses (Tanouye & Wyman, 1980). (D–F) responses in a shak-B2/Y c17/UAS-IMPTNT fly showing no output to DLM and a long latency response and poor following in TTM at both frequencies. (G–I) responses in a shak-B2/Y c17/UAS-TNT fly show no responses in either TTM or DLM upon stimulation but increased spontaneous activity (marked with arrows). Vertical scale bars, 50 mV for all traces; horizontal, 1 ms for response latencies, 10 ms for following at 100 Hz, and 4 ms for following at 250 Hz.
Fig. 3.
NMJ function is unaffected by the shak-B2 mutation or expression of tetanus toxin using the c17 line. (A) Schematic showing the positions of the stimulating and recording electrodes for either GF or motorneuron stimulation. (B) Responses in TTM and DLM to a single stimulus, or ten stimuli at 250 Hz, in the brain (GF stimulation) or the thorax (Mn stimulation) from a shak-B2/Y UAS-TNT/CyO fly. (C) Responses in TTM and DLM to a single stimulus, or ten stimuli at 250 Hz, in the brain (GF stimulation) or the thorax (Mn stimulation) from a shak-B2/Y c17/UAS-TNT fly. In (B) and (C) Mn stimulation always resulted in a muscle response to every stimulus.
Statistical analysis of the results (see legend to Table 1) suggests that the chemical component of the synapse is responsible for the long latency response seen in TTM and this is blocked by expression of tetanus toxin. As tetanus toxin blocks chemical transmission generally the results do not provide any information concerning the transmitter.
Identifying the GF as a cholinergic neuron
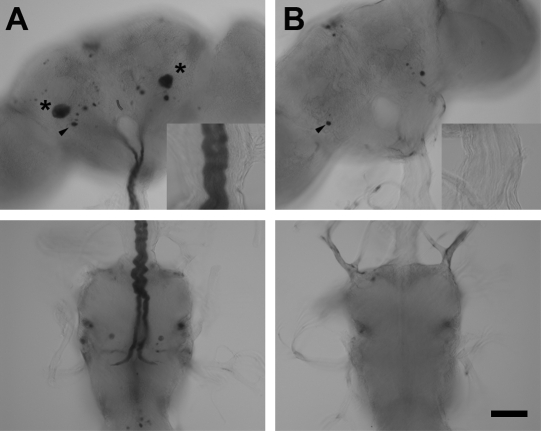
The major excitatory neurotransmitter in the Drosophila CNS is ACh (Lee & O'Dowd, 1999). Previous studies, using antibodies against choline acetyltransferase (ChAT) or generating a cha-GAL4 line, have shown extensive expression in the adult CNS but not identified the GF as cholinergic (Gorczyca & Hall, 1987; Yasuyama et al., 1996; Salvaterra & Kitamoto, 2001). We examined the CNS from cha-GAL4 flies expressing GFP carefully, but were not able to identify the GF as a cholinergic neuron unequivocally as the large domains of expression made such identification problematic (data not shown). To determine whether the GF is cholinergic we utilized a cha3.3kb-GAL80 line (Kitamoto, 2002) and reasoned that if the GF was cholinergic, expression of GAL80 protein in the neuron would inhibit GAL4-mediated expression of a UAS-reporter. When flies were generated containing the GAL4 line A307 that expresses in the GFs, a UAS-lacZ reporter transgene and cha3.3kb-GAL80, the reporter could not be detected in the GFs in any of the preparations (n = 15), even with excessive staining, but could in other GAL4-expressing neurons (Fig. 4). This was also the case for a second GF-expressing GAL4 line, c17 (data not shown). Controls showed 100% of the GFs examined to stain (n = 9). This indicates that the GF is a cholinergic neuron.
Fig. 4.
The GF is a cholinergic neuron. Dissected adult nervous systems stained for LacZ. (A) UAS-lacZ; A307 control preparation showing distinct staining in the GFs (*) and a few other cells in the brain and ventral nerve cord including a cell that lies just ventral to each GF (arrowhead). Inset is a higher power view of a cervical connective through which the labelled GFs can be easily identified. (B) UAS-lacZ; A307; cha3.3kb-GAL80 preparation. Note the lack of staining in the GFs but the presence of staining in the small ventral cell (arrowhead) that is in the position to be a cell body of a giant commissural interneuron. Inset higher power view of a cervical connective shows the GFs to be present but unlabelled. Scale bar, 50 µm; 25 µm for insets.
Reducing ACh using the chats2 mutant allele
To test whether chemical synaptic transmission from GF to TTMn is cholinergic, we took advantage of a temperature sensitive allele of the Drosophila cha gene, which encodes choline acetyltransferase (ChAT), a major enzyme in ACh synthesis. We recorded responses to GF-activating stimuli in flies in which we had used a temperature sensitive allele of cha to reduce the amount of ACh within the CNS. The chats2 mutants are viable at 18 °C, but they die at the restrictive temperature of 30 °C due to severely reduced ChAT activity (Salvaterra & McCaman, 1985; Takagawa & Salvaterra, 1996). The protein produced from this allele is thermolabile, but the cha mRNA levels are also reduced in homozygous mutants after 48 h at 30 °C, which further reduces ChAT activity (Wang et al., 1999). Twenty-eight degrees C is considered a semipermissive temperature and adults shifted from 18 °C to 28 °C become paralysed but will move their legs if agitated. Females heterozygous for shak-B2 but homozygous for the chats2 allele and shifted to 28 °C exhibited normal responses to GF stimulation in TTM but no responses in DLM due to failure of the PSI-DLMns synapses (Table 2, Fig. 5B). This is consistent with the study of Gorczyca & Hall (1984) in which they used temperature-sensitive alleles of cha to determine that these peripheral synapses were cholinergic. chats2 females that were not temperature-shifted showed normal responses in both TTM and DLM (Table 2). This shows that there is sufficient ChAT activity in these flies for normal PSI-DLMns transmission even though protein levels are known to be somewhat reduced at the permissive temperature (Takagawa & Salvaterra, 1996). Females heterozygous for shak-B2 and chats2 that were reared at 18 °C, or shifted to 28 °C for 48 h, all showed normal responses upon GF stimulation (Table 2, Fig. 5A) indicating that the shift in temperature did not adversely affect synaptic function.
Table 2.
Responses of shak-B2 and chats2 flies at 18 °C and 28 °C
| TTM ± SEM | DLM ± SEM | |||||
|---|---|---|---|---|---|---|
| Genotype | n | Temperature | GF stimulation | Mn stimulation | GF stimulation | Mn stimulation |
| shak-B2/+; chats2/MKRS | 6 | 28 °C | 100 ± 0.0% | 100 ± 0.0% | 100 ± 0.0% | 100 ± 0.0% |
| shak-B2/+; chats2/MKRS | 6 | 18 °C | 100 ± 0.0% | 100 ± 0.0% | 100 ± 0.0% | 100 ± 0.0% |
| shak-B2/+; chats2/chats2 | 6 | 28 °C | 100 ± 0.0% | 100 ± 0.0% | No responses | 100 ± 0.0% |
| shak-B2/+; chats2/chats2 | 6 | 18 °C | 100 ± 0.0% | 100 ± 0.0% | 100 ± 0.0% | 100 ± 0.0% |
| shak-B2/Y; chats2/chats2 | 6 | 28 °C | No responses | 100 ± 0.0% | No responses | 100 ± 0.0% |
| shak-B2/Y; chats2/chats2 | 6a | 18 °C | 27 ± 13.2% | 100 ± 0.0% | No responses | 100 ± 0.0% |
| shak-B2/Y; chats2/MKRS | 6b | 28 °C | 67 ± 16.0% | 100 ± 0.0% | No responses | 100 ± 0.0% |
| shak-B2/Y; chats2/MKRS | 6c | 18 °C | 77 ± 16.7% | 100 ± 0.0% | No responses | 100 ± 0.0% |
3/6
1/6
1/6 flies gave no responses in both TTM and DLM.
The percentage responses are calculated from all (6×5) stimuli given. At 28 °C, shak-B2/Y; chats2/chats2 flies gave significantly fewer responses than all other genotypes (Kruskal–Wallis anova, H = 14.94, d.f. = 3, P < 0.01). At 18 °C, shak-B2/Y; chats2/chats2 flies gave some responses but significantly fewer than all other genotypes (Kruskal–Wallis anova, H = 11.68, d.f. = 3, P < 0.01).
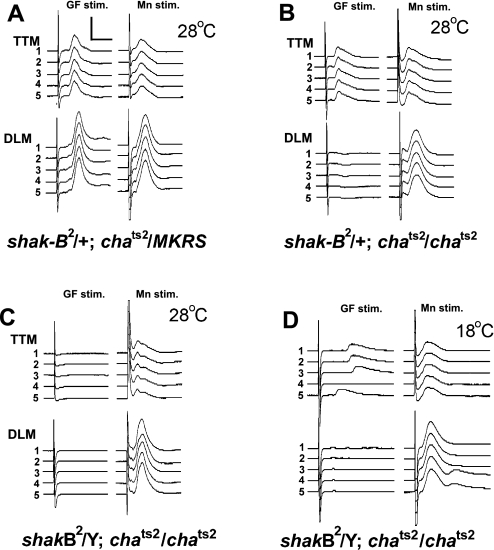
Fig. 5.
The response in the TTM is blocked in shak-B2; chats2 double-mutants at the restrictive temperature. Traces from the TTM and DLM of individual flies given five stimuli (1–5) at 1 Hz. (A) shak-B2/+ chats2/MKRS control female showing WT responses upon GF stimulation (GF stim) at 28 °C. (B) shak-B2/+ chats2/chats2 female showing a normal response in TTM and a loss of the DLM response at 28 °C. (C) shak-B2/Y; chats2/chats2 male showing no responses in DLM and a loss of responses in TTM at 28 °C. (D) shak-B2/Y; chats2/chats2 male showing no responses in DLM but responses in TTM at 18 °C. In all cases, individuals showed responses in both muscles upon thoracic stimulation (Mn stim.).Vertical scale bar, 50 mV; horizontal scale bar, 2 ms.
Hemizygous shak-B2 males that were also homozygous for chats2 and had been shifted to 28 °C gave no responses in DLM upon GF stimulation, as expected, but also gave no responses in TTM (Table 2, Fig. 5C). The chemical component of the GF-TTMn synapse is therefore not functional when ACh is reduced within the CNS. Of the six males of the same genotype, continually reared at 18 °C, three gave no responses and three gave characteristic long latency, intermittent, responses in TTM upon GF stimulation with a total of eight of 30 stimuli (27%) eliciting responses across the six preparations (Table 2, Fig. 5D). Function was decreased compared to controls (Table 2) indicating reduced ChaT activity of chats2 homozygotes at the permissive temperature. This is consistent with data reported by Salvaterra & McCaman (1985). Shak-B2; chats2/MKRS males also showed responses in TTM at 18 °C and 28 °C indicating that the temperature shift alone did not reduce synaptic function.
To confirm that the glutamatergic NMJs were unaffected by any reduction in ACh or change in temperature, we again used thoracic ganglia stimulation to activate TTMn and the DLMns directly. This resulted in responses in DLM and TTM irrespective of temperature or whether flies were homozygous for shak-B2 or chats2 (Mn stim, Fig. 5). Thus, the abolition of DLM responses in control flies, or TTM responses in shak-B2 flies, was due to failure of synapses within the CNS and not the NMJs.
Discussion
We have used shak-B2 mutant flies to investigate the chemical component of the mixed GF-TTMn synapse within the CNS of Drosophila. By blocking chemical transmission in shak-B2 mutant flies using tetanus toxin we can deduce that the chemical component is functional in the absence of gap junctions. We have shown elsewhere that the GF is the only pathway from the brain to the TTMn (Allen et al., 2000) and yet when we remove the gap junctions a residual, albeit less reliable, pathway exists. Simultaneous removal of the gap junctions and blockade of cholinergic synapses in shak-B2; chats2 double-mutants blocks the GF-TTMn synapse at the restrictive temperature. When GAL80 is expressed under the control of a fragment from the cha promoter it blocks GAL4-mediated expression of a reporter in GFs. These results indicate that the chemical component of the GF-TTMn synapse uses ACh as its neurotransmitter.
Although the GFS is the most studied adult neural circuit in Drosophila, there are several elements of this escape pathway's outputs that are poorly understood. For example, the GF also activates the tibial levator (TLM; Trimarchi & Schneiderman, 1993), the dorsal ventral flight muscles (DVMs; Tanouye & Wyman, 1980), and possibly wing elevators (Tanouye & King, 1983; Hammond & O'Shea, 2007) but the neurons involved in this are unknown. As our analysis involves stimulating the GF and recording outputs to TTM and a DLM, the formal possibility still exists that there is a second parallel, unidentified, polysynaptic pathway from the GF to the TTMn that is uncovered when gap junctions are removed from the GF in shak-B2 flies. This is unlikely, however, as several studies in which the GF-TTMn presynaptic terminal has been perturbed exhibit a range of longer response latencies, corresponding with the morphological abnormalities seen (Allen et al., 1999; Allen et al., 2000; Godenschwege et al., 2002a; Godenschwege et al., 2002b; Godenschwege et al., 2006). This is consistent with a monosynaptic connection being weakened rather than ‘switching’ to a polysynaptic pathway. In addition, shak-B2 flies sometimes give no responses in TTM upon GF stimulation. If a second pathway existed, it would have to also have to have elements sensitive to loss of gap junctions formed by Shak-B. Our interpretation therefore explains the data best. Confirmation of GF-TTMn being monosynaptic only will require intracellular recordings from TTMn.
Several neural circuits have been identified in Drosophila that use mixed electro-chemical synapses including the GFS (Blagburn et al., 1999), sensory afferents from the halteres to flight motorneurons (Trimarchi & Murphey, 1997) and auditory pathways in the Johnston's organ (Sivan-Loukianova & Eberl, 2005). The results for the haltere afferents-to-B1 nicely parallel our results for the GF-TTMn. Both the haltere afferents onto the B1 motorneuron (Trimarchi & Murphey, 1997) and the GF-TTMn synapse (this study) are reduced in efficacy in shak-B2 mutant animals and the residual response is blocked by cholinergic blockers. Thus both mixed synapses use ACh as the transmitter and both contain gap junctions that require Shak-B. Given the range of behavioural phenotypes altered in shak-B2 mutants, it is unlikely that these will be the only synapses in the CNS that have these properties.
Electron microscopy and cell biological approaches have shown that the GF-PSI synapse is also a mixed electro-chemical synapse. Unlike GF-TTMn, it appears that the chemical component of this synapse is unable to function on its own as no responses are seen in shak-B2 mutants (Thomas & Wyman, 1984; Baird et al., 1990; this study). Although not demonstrated here, the chemical component of the GF-PSI mixed synapse is likely cholinergic. A recent study has determined that the Dalpha7 subunit of the nicotinic acetylcholine receptor (nAChR) is needed for transmission from the PSI to the DLMns and inputs to the GFs (Fayyazuddin et al., 2006). From the expression data of Fayyazuddin et al. this subunit seems not to be present at the GF-PSI or GF-TTMn synapses, however, this is yet to be determined.
It appears that by changing the properties of the GF during development the connectivity diagram was altered. We observed increased spontaneous activity in both TTM and DLM in shak-B2 mutant flies that were expressing TNT in the GFs throughout development. In contrast, we saw no spontaneous activity in shak-B2 chats2 males in which the GF-TTMn was blocked acutely in adults. Blocking chemical and electrical components of the GF may alter the homeostasis of TTMn and PSI early in development so that they receive greater input from other (thoracic) inputs. Blocking either of the components individually, or reduction of either component, has no noticeable effect. This suggests that chemical transmission from the GF to TTMn has a role during normal synaptic development. This dual role for the chemical and electrical components is not unprecedented as transient gap junction communication is needed for the correct development of chemical synapses in the optic lamina (Curtin et al., 2002). Indeed, activity is now seen as a vital aspect of neural cell development (Spitzer, 2006).
One developmental question that remains unanswered is whether the chemical component of the GF-TTMn synapse is stronger in shak-B2 flies than it is in wild type. It may make a stronger chemical synapse during development because there are no gap junctions present. The study of Blagburn et al. (1999) is inconclusive as to whether there are a greater number of chemical synaptic zones in shak-B2 flies compared to wild type. This hypothesis could be tested physiologically by recording from the motor neurons as performed by Fayyazuddin et al. (2006), or, genetically requiring either dominant negative expression or temperature sensitive mutations of shak-B to acutely block gap junctions.
Now that we have a better understanding of this mixed synapse and can dissect the two components genetically, we can analyse further the development and plasticity of the synapse. Studies of the role of activity can take advantage of these data to determine whether neural activity affects the development of the synapse. And studies of plasticity of the synapse can be combined with blockade of activity to assess the normal development of this synapse. Such analyses should shed light on the development and function of all mixed synapses.
Acknowledgments
Many thanks to Dr Richard Baines for supplying the chats2 line, Dr Scott Waddell for supplying the cha3.3kb-GAL80 line and to Dr Pauline Phelan and Kate VanHegan for helpful discussions during this work. This study was funded in part by grants from the Wellcome Trust (069710/Z/02/Z, to M.J.A.) and the NIH (NS15571, to R.K.M.).
Glossary
Abbreviations
- ACh
acetylcholine
- ChAT
choline acetyltransferase
- DLMns
dorsal longitudinal muscle motorneurons
- DLMs
dorsal longitudinal muscles
- GF
giant fibre
- GFS
giant fibre system
- NMJ
neuromuscular junction
- PSI
peripherally synapsing interneuron
- TNT
tetanus toxin light chain
- TTM
tergotrochanteral muscle
- TTMn
tergotrochanteral motorneuron.
References
- Allen MJ, Drummond JA, Moffat KG. Development of the giant fiber neuron of Drosophila melanogaster. JCompNeurol. 1998;397:519–531. doi: 10.1002/(sici)1096-9861(19980810)397:4<519::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Godenschwege TA, Tanouye MA, Phelan P. Making an escape: development and function of the Drosophila giant fibre system. SeminCellDevBiol. 2006;17:31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Shan X, Caruccio P, Froggett SJ, Moffat KG, Murphey RK. Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. JNeurosci. 1999;19:9374–9384. doi: 10.1523/JNEUROSCI.19-21-09374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MJ, Shan XL, Murphey RK. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. MolCellNeurosci. 2000;16:754–765. doi: 10.1006/mcne.2000.0903. [DOI] [PubMed] [Google Scholar]
- Baird DH, Schalet AP, Wyman RJ. The Passover locus in Drosophila melanogaster: complex complementation and different effects on the giant fiber neural pathway. Genetics. 1990;126:1045–1059. doi: 10.1093/genetics/126.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagburn JM, Alexopoulos H, Davies JA, Bacon JP. Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: a structural study. JCompNeurol. 1999;404:449–458. [PubMed] [Google Scholar]
- Curtin KD, Zhang Z, Wyman RJ. Gap junction proteins expressed during development are required for adult neural function in the Drosophila optic lamina. JNeurosci. 2002;22:7088–7096. doi: 10.1523/JNEUROSCI.22-16-07088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. TINS. 1999;22:153–161. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- Fayyazuddin A, Zaheer MA, Hiesinger PR, Bellen HJ. The nicotinic acetylcholine receptor Dalpha7 is required for an escape behavior in Drosophila. PLoS Biol. 2006;4:e63. doi: 10.1371/journal.pbio.0040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nature Neurosci. 2002a;5:1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. CurrBiol. 2006;16:12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Simpson JH, Shan X, Bashaw GJ, Goodman CS, Murphey RK. Ectopic expression in the giant fiber system of Drosophila reveals distinct roles for roundabout (Robo), Robo2, and Robo3 in dendritic guidance and synaptic connectivity. JNeurosci. 2002b;22:3117–3129. doi: 10.1523/JNEUROSCI.22-08-03117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca M, Hall JC. Identification of a cholinergic synapse in the giant fiber pathway of Drosophila using conditional mutations of acetylcholine synthesis. JNeurogenet. 1984;1:289–313. doi: 10.3109/01677068409107093. [DOI] [PubMed] [Google Scholar]
- Gorczyca MG, Hall JC. Immunohistochemical localization of choline acetyltransferase during development and in Chats mutants of Drosophila melanogaster. JNeurosci. 1987;7:1361–1369. doi: 10.1523/JNEUROSCI.07-05-01361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ, Finn JA, Jr, Hall JC. Acetylcholinesterase mutants in Drosophila and their effects on the structure and function of the central nervous system. JCompNeurol. 1980;189:741–774. doi: 10.1002/cne.901890409. [DOI] [PubMed] [Google Scholar]
- Hammond S, O'Shea M. Escape flight initiation in the fly. JCompPhysiolA. 2007;193:471–476. doi: 10.1007/s00359-006-0203-9. [DOI] [PubMed] [Google Scholar]
- Homyk T, Jr, Szidonya J, Suzuki DT. Behavioral mutants of Drosophila melanogaster. III. Isolation and mapping of mutations by direct visual observations of behavioral phenotypes. MolGenGenet. 1980;177:553–565. doi: 10.1007/BF00272663. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Todman MG, Allen MJ, Davies JA, Bacon JP. Synaptogenesis in the giant-fibre system of Drosophila: interaction of the giant fibre and its major motorneuronal target. Development. 2000;127:5203–5212. doi: 10.1242/dev.127.23.5203. [DOI] [PubMed] [Google Scholar]
- King DG, Wyman RJ. Anatomy of the giant fiber pathway in Drosophila 1. 3 thoracic components pathway. JNeurocytol. 1980;9:753–770. doi: 10.1007/BF01205017. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. ProcNatl AcadSciUSA. 2002;99:13232–13237. doi: 10.1073/pnas.202489099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Krishnan SN, Frei E, Schalet AP, Wyman RJ. Molecular basis of intracistronic complementation in the Passover locus of Drosophila. ProcNatl AcadSciUSA. 1995;92:2021–2025. doi: 10.1073/pnas.92.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan SN, Frei E, Swain GP, Wyman RJ. Passover: a gene required for synaptic connectivity in the giant fiber system of Drosophila. Cell. 1993;73:967–977. doi: 10.1016/0092-8674(93)90274-t. [DOI] [PubMed] [Google Scholar]
- Lee D, O'Dowd DK. Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons. JNeurosci. 1999;19:5311–5321. doi: 10.1523/JNEUROSCI.19-13-05311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Faber DS. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. I. Characteristics of electrotonic and chemical postsynaptic potentials. JNeurosci. 1988;8:1302–1312. doi: 10.1523/JNEUROSCI.08-04-01302.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The Genome of Drosophila Melanogaster. San Diego: Academic Press; 1992. [Google Scholar]
- Phelan P, Nakagawa M, Wilkin MB, Moffat KG, O'Kane CJ, Davies JA, Bacon JP. Mutations in shaking-B prevent electrical synapse formation in the Drosophila giant fiber system. JNeurosci. 1996;16:1101–1113. doi: 10.1523/JNEUROSCI.16-03-01101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P, Stebbings LA, Baines RA, Bacon JP, Davies JA, Ford C. Drosophila Shaking-B protein forms gap junctions in paired Xenopus oocytes. Nature. 1998;391:181–184. doi: 10.1038/34426. [DOI] [PubMed] [Google Scholar]
- Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with GAL4/UAS-GFP. Brain ResGene ExprPatterns. 2001;1:73–82. doi: 10.1016/s1567-133x(01)00011-4. [DOI] [PubMed] [Google Scholar]
- Salvaterra PM, McCaman RE. Choline acetyltransferase and acetylcholine levels in Drosophila melanogaster: a study using two temperature-sensitive mutants. JNeurosci. 1985;5:903–910. doi: 10.1523/JNEUROSCI.05-04-00903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan-Loukianova E, Eberl DF. Synaptic ultrastructure of Drosophila Johnston's organ axon terminals as revealed by an enhancer trap. JCompNeurol. 2005;491:46–55. doi: 10.1002/cne.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Sun YA, Wyman RJ. Passover eliminates gap junctional communication between neurons of the giant fiber system in Drosophila. JNeurobiol. 1996;30:340–348. doi: 10.1002/(SICI)1097-4695(199607)30:3<340::AID-NEU3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, Okane CJ. Targeted expression of tetanus toxin light-chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Takagawa K, Salvaterra P. Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. NeurosciRes. 1996;24:237–243. doi: 10.1016/0168-0102(95)00999-x. [DOI] [PubMed] [Google Scholar]
- Tanouye MA, King DG. Giant fiber activation of direct flight muscles in Drosophila. JExpBiol. 1983;105:241. –&. [Google Scholar]
- Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila. JNeurophysiol. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Wyman RJ. Mutations altering synaptic connectivity between identified neurons in Drosophila. JNeurosci. 1984;4:530–538. doi: 10.1523/JNEUROSCI.04-02-00530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JR, Jin P, Murphey RK. Controlling the motor neuron. IntRevNeurobiol. 1999;43:241–264. doi: 10.1016/s0074-7742(08)60548-6. [DOI] [PubMed] [Google Scholar]
- Trimarchi JR, Murphey RK. The shaking-B2 mutation disrupts electrical synapses in a flight circuit in adult Drosophila. JNeurosci. 1997;17:4700–4710. doi: 10.1523/JNEUROSCI.17-12-04700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JR, Schneiderman AM. Giant fiber activation of an intrinsic muscle in the mesothoracic leg of Drosophila melanogaster. JExpBiol. 1993;177:149–167. doi: 10.1242/jeb.177.1.149. [DOI] [PubMed] [Google Scholar]
- Wang W, Kitamoto T, Salvaterra PM. Drosophila choline acetyltransferase temperature-sensitive mutants. NeurochemRes. 1999;24:1081–1087. doi: 10.1023/a:1021021213625. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Kitamoto T, Salvaterra PM. Differential regulation of choline acetyltransferase expression in adult Drosophila melanogaster brain. JNeurobiol. 1996;30:205–218. doi: 10.1002/(SICI)1097-4695(199606)30:2<205::AID-NEU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Curtin KD, Sun YA, Wyman RJ. Nested transcripts of gap junction gene have distinct expression patterns. JNeurobiol. 1999;40:288–301. doi: 10.1002/(sici)1097-4695(19990905)40:3<288::aid-neu2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]