Abstract
Objective:
To conduct a systematic review of the efficacy of single-agent bortezomib vs. single-agent thalidomide in patients with relapsed/refractory multiple.
Methods:
Publications in English from 1966 to June 2005 (MEDLINE, EMBASE, Cochrane library), publication reference lists, Janssen-Cilag data-on-file and abstracts from recent multiple myeloma conferences were reviewed. Prospective studies containing at least a single arm of either treatment group with n ≥ 30 were included. Studies adding dexamethasone for non-responders were excluded. Statistical pooling was performed for response rate and overall survival.
Results:
One bortezomib study (n = 333, NEJM 2005, 352; 2487–98) and 15 thalidomide (n = 1007) studies met these criteria and were included. Patient baseline characteristics including age, gender, IgG:IgA, disease duration and beta-2 microglobulin were well matched except that 48% of bortezomib patients had received prior thalidomide. Response rate, defined as serum M-protein reduction ≥ 50%, was 53% for patients receiving bortezomib vs. 32% for thalidomide (P < 0.001, n = 10 studies). Response rate determined by European Group for Blood and Marrow Transplantation (EBMT) criteria was 41% for patients receiving bortezomib vs. 22% for thalidomide (P < 0.001, n = 4 studies).
Conclusion:
Bortezomib was associated with a significantly higher response rate and complete remission rate using both M-protein and EBMT criteria.
Keywords: bortezomib, thalidomide, refractory, relapsed, multiple myeloma
Multiple myeloma remains an incurable disease despite intensive therapy, including high-dose melphalan and autologous stem cell transplantation (1). Novel treatments include thalidomide and its derivative lenalidomide and the proteasome inhibitor bortezomib (1–3).
Bortezomib has been shown to induce apoptosis by inhibiting activation of nuclear factor kappa B (4). However bortezomib has also been shown to inhibit angiogenesis and down-regulate the expression of cell adhesion molecules (5–7). Furthermore, bortezomib may help to overcome tumour resistance to corticosteroids or conventional cytotoxic agents, by inhibiting DNA repair mechanisms (8). The principal mechanism underlying the anti-tumour activity of thalidomide is uncertain, however it may be related to its anti-angiogenic and immunomodulatory activity, via modulation of TNF alpha, interleukin 10, and interleukin 2 and other cytokines (9).
The addition of corticosteroids such as dexamethasone has been shown to improve the response rate with bortezomib (10–12). The addition of corticosteroids also improves the response rate associated with thalidomide (13–15). However, the added toxicities associated with the addition of corticosteroids are not insubstantial, the most serious of which is venous thromboembolism with one study reporting a rate of 15% for thalidomide + dexamethasone vs. 4% for thalidomide alone (16). Thus, there remains a role for single-agent bortezomib and single-agent thalidomide, especially in patients intolerant to corticosteroids or where the administration of prophylactic anticoagulants is problematic. Moreover, a comparison of single-agent bortezomib and single-agent thalidomide can give an insight into the comparative biological impact of these two agents.
There have been two recent systematic reviews on the efficacy of single-agent thalidomide in patients with relapsed or refractory multiple myeloma (17, 18). The present study extends these findings by comparing the efficacy of thalidomide monotherapy with bortezomib monotherapy in patients with relapsed or refractory multiple myeloma.
Methods
Searching strategy
The published English-language literature from 1966 to June 2005 (MEDLINE, EMBASE, Cochrane library), publication reference lists, Janssen-Cilag Pty Ltd data-on-file and abstracts from recent multiple myeloma conferences were reviewed. Search terms included multiple myeloma, thalidomide and bortezomib.
Inclusion and exclusion criteria
Studies were included in this analysis if they were prospective, were of patients with relapsed or refractory multiple myeloma, used single-agent bortezomib or thalidomide and had at least 30 patients in each treatment arm. Studies were excluded if the treatment added dexamethasone for non-responders or was short term (< 3 months) or used fixed doses. The short term and fixed dose regimens do not correspond to usual clinical practice and were expected to show poor efficacy for thalidomide. Only bortezomib or thalidomide monotherapy studies were included in the analysis because of a lack of combination bortezomib and dexamethasone studies in the relapsed/refractory setting.
Data extraction
Two people independently extracted the data for the thalidomide studies and they resolved any discrepancies by joint review of the source literature. The data extracted included summary statistics on the pretreatment characteristics of patients in the studies, as well as efficacy measures. The primary efficacy measure was response to treatment. This was measured either as a serum M-protein reduction of at least 50% or using the European Group for Blood and Marrow Transplantation (EBMT) criteria (19). Secondary outcome was complete response (CR) rate.
Data analysis
The data were analysed on an intent-to-treat basis in which all patients randomised to the particular treatment were included. The outcome variables were proportions that were analysed on an intent-to-treat basis using logistic regression for fixed effects models and the method of derSimonian and Laird for random effects models (20). Where possible, the heterogeneity between studies has been explored and taken into account when assessing the estimated difference in outcome between treatment with bortezomib and thalidomide. The random effects estimates were numerically close or identical to the estimates obtained using the fixed effects models, and therefore only the fixed effects estimates are reported.
Results
Included studies
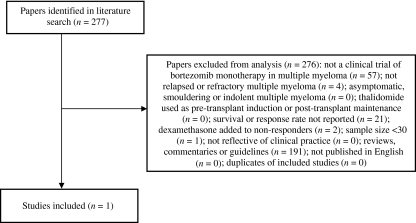
Only one study (the APEX study) was identified where patients were treated with bortezomib monotherapy (21) (Fig. 1). Data from the SUMMIT trial (10) were excluded because patients who experienced progressive disease or did not respond adequately were treated with bortezomib plus adjunctive dexamethasone. Thus the APEX trial was the only bortezomib trial suitable for inclusion in the systematic review. Where possible, the data analysed in this report are taken from the final study report addendum (dated 3 August 2005) in which 2-yr follow-up data were available for the bortezomib arm. In the APEX trial, 333 patients were randomised to treatment with bortezomib monotherapy.
Figure 1.
Flow chart for inclusion and exclusion of bortezomib studies.
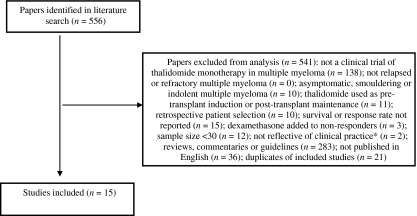
Fifteen studies of thalidomide for the treatment of relapsed or refractory multiple myeloma were consistent with the inclusion/exclusion criteria (Fig. 2). The number of patients in each trial ranged from 30 to 169 (Table 1).
Figure 2.
Flow chart for inclusion and exclusion of thalidomide studies. *Short-term studies (< 3 months) or studies using fixed doses regimens were excluded as these do not correspond to usual clinical practice and were expected to show poor efficacy.
Table 1.
Trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma
| Reference | Patients treated |
|---|---|
| Barlogie B et al. (25) | 169 |
| Grosbois BB et al. (26) | 120 |
| Neben K et al. (27) | 83 |
| Yakoub-Agha I et al. (22) | 83 |
| Mileshkin L et al. (28) | 75 |
| Schey SA et al. (29) | 69 |
| Tosi P et al. (30) | 65 |
| Waage A et al. (31) | 65 |
| Hus M et al. (32) | 53 |
| Alexanian RW et al. (33) | 45 |
| Hattori Y et al. (34) | 44 |
| Cibeira MR et al. (35) | 42 |
| Offidani M et al. (36) | 32 |
| Kumar S et al. (37) | 32 |
| Richardson P et al. (38) | 30 |
Patient characteristics
The patients treated with bortezomib in the APEX trial were similar to those treated in the thalidomide studies in terms of baseline characteristics (Table 2). However, in the APEX trial, 48% of patients had prior treatment with thalidomide, while no patients in the thalidomide studies had prior treatment with bortezomib.
Table 2.
Baseline characteristic in the bortezomib study (APEX) and the thalidomide studies
| Thalidomide | ||||
|---|---|---|---|---|
| Baseline patient characteristic | Bortezomib | Median | Range | No. of studies |
| Median age, years | 62 | 63 | 56–69 | 15 |
| Gender, %male | 56 | 56 | 44–73 | 14 |
| IgG:IgA | 60:23 | 70:16 | – | 7 |
| β2 microglobulin (mg/L) | 3.7 | 3.5 | 2.9–4.6 | 6 |
| Disease duration, months | 42 | 44 | 31–55 | 9 |
Response rate
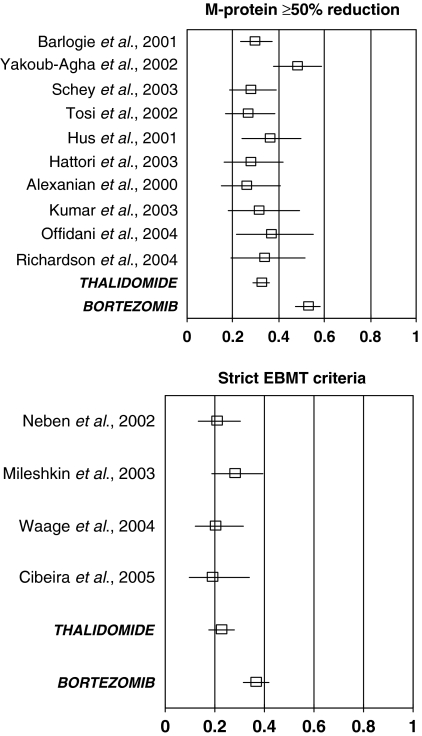
The primary outcome for most studies was response rate. The best response was available from 14 of the 15 thalidomide studies. However, response to treatment was not measured consistently between studies. To the extent possible from the often-limited description of the response rate criteria, studies with comparable definitions were compared using either of two criteria: one based primarily on reduction in serum M-protein and the other using the EBMT criteria (19). Fortunately, the bortezomib trial was able to furnish both estimates.
M-protein response rate
The first criterion was based on reduction in serum M-protein by at least 50% from baseline (i.e., those patients who had a CR or a partial response (PR). For patients with a low baseline serum M-protein, at least a 90% reduction in urine M-protein was usually also specified.
Different studies used different criteria for the baseline determination and the degree of confirmation (e.g., number of repeat measurements and their timing) for a potential responder. Although the definition of M-protein response varied between studies, the reported response rates showed little variation between the 10 thalidomide studies that report this outcome (Fig. 3). The variation in response rates between the thalidomide studies was not statistically significant (χ2 = 13.5, 9 d.f., P = 0.14) and the mean response rate was 32% (95% CI: 29%, 36%).
Figure 3.
Response rates for relapsed or refractory multiple myeloma patients treated with either thalidomide or bortezomib. Response rate was defined using the EBMT criteria or as a confirmed reduction of at least 50% in serum M-protein and by at least 90% for urine M-protein for patients with low baseline serum M-protein.
Most of the variation in response rate between the thalidomide studies reflects the high reported response rate in one study (χ2 = 10.7, 1 d.f., P = 0.002) (22). The description of the study methods used by Yakoub-Agha does not explicitly state that confirmation of the reduction in M-protein was required when assessing a PR (serum M-protein reduction by at least 50% from baseline).
The M-protein response rate with bortezomib treatment was 53% (95% CI: 47%, 58%). This is higher than was observed in each of the 10 thalidomide studies and is statistically significantly higher than the mean response rate for thalidomide (χ2 = 37,1 d.f., P < 0.0001).
Within the APEX trial, the response rate assessed using M-protein was similar for patients with no prior exposure to thalidomide (55%, 95/172) or with prior exposure to thalidomide (50%, 80/161; χ2 = 1.0, 1 d.f., P = 0.3). When the comparison between the 10 thalidomide studies and the APEX trial was restricted to patients without prior exposure to thalidomide, bortezomib was still associated with a statistically significantly higher response rate (χ2 = 30, 1 d.f., P < 0.0001).
EBMT response rate
The second response endpoint, reported in four of the thalidomide studies and in the APEX study, was from the EBMT criteria. This definition modifies the M-protein response to take into account additional clinically relevant information and results in fewer patients being classified as having responded.
The variation in EBMT response rates between the four thalidomide studies was not statistically significant (χ2 = 2.0, 3 d.f., P = 0.6; Fig. 3) and the mean response rate was 22% (95% CI: 18%, 28%). The EBMT response rate with bortezomib treatment was 41% (95% CI: 35%, 46%). This is higher than was observed in each of the four thalidomide studies and is statistically significantly higher than the mean EMBT response rate for thalidomide (χ2 = 23.0, 1 d.f., P < 0.0001).
Within the APEX trial, the EBMT response rate was higher for patients with no prior exposure to thalidomide (44%, 76/172) compared with patients with prior exposure to thalidomide (28%, 45/161; χ2 = 9.6, 1 d.f., P = 0.002; data from APEX study data files with last date of follow-up for response of 14 December 2003). When the comparison between the four thalidomide studies and the APEX trial was restricted to patients without prior exposure to thalidomide, the bortezomib-thalidomide difference was increased and was still statistically significant (χ2 = 23, 1 d.f., P < 0.0001).
One of the thalidomide studies (23) has been omitted from this analysis because the response rate reported was the best M-protein response within 60 d of starting treatment with thalidomide. Because some responses will have occurred after day 60, this measure is not comparable with those used in the other thalidomide studies. Indeed, the reported response rate in this study (17%, 20/120) was lower than the M-protein response rates reported in the other thalidomide studies (Fig. 3). Including the response rate reported from this study would have produced a biased, low, estimated response rate for treatment with thalidomide.
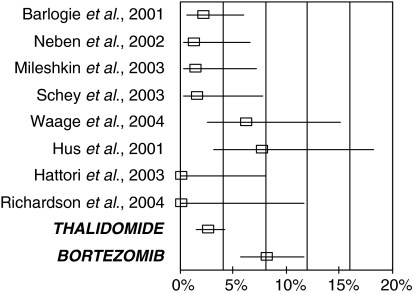
Complete response rate
Complete response rates were available for eight of the thalidomide studies and for the bortezomib (APEX) trial (Fig. 4). CR required confirmed absence of M-protein and usually also required fewer than 5% plasma cells in the bone marrow in a patient and no signs or symptoms of disease.
Figure 4.
Complete response rate in patients with refractory multiple myeloma treated with either thalidomide or bortezomib.
The variation in CR rates between the eight thalidomide studies was not statistically significant (χ2 = 11.9, 7 d.f., P = 0.1) and the mean CR rate was 2% (95% CI: 1%, 4%). The CR rate with bortezomib treatment was 8% (95% CI: 6%, 12%). This is higher than was observed in each of the eight thalidomide studies and is statistically significantly higher than the mean CR for thalidomide (χ2 = 15.6, 1 d.f., P = 0.0001).
Discussion
In this systematic review in patients with relapsed/refractory multiple myeloma, single-agent bortezomib was associated with a significantly higher response rate. In keeping with this, the response rate for bortezomib was also higher than in each of the individual thalidomide studies.
The response rate was statistically significantly higher in patients treated with bortezomib than patients treated with thalidomide regardless of the criteria used to assess response. When assessed using the simple definition of an M-protein reduction of at least 50%, the response rate observed for bortezomib was 53% compared with a mean response rate of 32% for thalidomide. In comparison, using the EBMT criteria the response rate for bortezomib was 41% compared with a mean response rate of 22% for thalidomide. These differences highlight the need to identify which criteria are used when making comparisons between therapeutic agents, as stricter criteria are likely to result in more conservative estimates. Hopefully, as the new response criteria recently proposed by Durie et al. are adapted more widely, such comparisons will be made easier (24).
The slight difference in response rates reported in the original APEX study and the present analysis are a result of different analytical approaches. The original APEX publication used a modified intent-to-treat analysis based on patients who received at least one dose of bortezomib and had measurable disease at baseline (n = 315). In contrast, in the present study all patients randomised to receive treatment were evaluated (n = 333). Patients with no baseline reading were considered to be non-responders in the present analysis.
The differences in CR rates between bortezomib and thalidomide are consistent with the overall response rates reported. The CR rate for bortezomib was 8% compared with a mean CR rate of 2% for thalidomide. Again, it should be noted that the CR rate for bortezomib was higher than in each of the individual thalidomide studies.
Of the 15 thalidomide studies, only six reported data on progression-free survival. These data appeared to be inconsistent, probably reflecting variation in the frequency and intensity of follow-up between studies. In one study (22), patients were reported to have low progression rates with death occurring sooner after progression than was apparent in other thalidomide studies, suggesting that the definition of progression may differ between the Yakoub-Agha and other studies. Treatment at relapse was not reported consistently across the studies and as this treatment could influence survival, we have chosen not to report overall survival data.
The results of this analysis are consistent with those obtained from recent systematic reviews of the efficacy of single-agent thalidomide in relapsed/refractory multiple myeloma. In the present study, the mean overall response rate for thalidomide using the most commonly reported criteria (M-protein reduction of at least 50%) was 32%. Two recent systematic reviews of single-agent thalidomide in patients with relapsed/refractory multiple myeloma reported overall response rates of approximately 28% (17, 18). This is remarkably consistent with the present analysis, considering that different inclusion and exclusion criteria were used in each of these reviews.
One of the limitations of this analysis is that there is inevitably more variability when combining results from different studies than if a randomised controlled trial comparing the two treatments was available. The value of combining data from multiple studies depends on the comparability of the studies, both in terms of the patients being treated and the methods of assessment being used. Patients receiving bortezomib were very similar to those receiving thalidomide in terms of key patient characteristics. Age, gender, IgG:IgA, disease duration, and beta-2 microglobulin were well matched between the two groups. One difference between the patient groups was that 48% of the bortezomib patients had previously received thalidomide. Despite the similarity in cardinal disease descriptors, the patients necessarily came from different populations, within different treatment infrastructures and practices. The magnitude of these possible differences is unknown.
A comparison of bortezomib in combination with dexamethasone compared with thalidomide in combination with dexamethasone is not yet possible. The addition of corticosteroids does improve the response rate associated with thalidomide (13–15). Similarly, the evidence to date suggests an additive or synergistic effect of bortezomib with dexamethasone in relapsed/refractory patients (12). The synergistic effect of bortezomib with dexamethasone has also been observed in patients who have not responded to bortezomib monotherapy (10, 11).
In conclusion, bortezomib was associated with a significantly higher response rate than thalidomide in patients with relapsed/refractory multiple myeloma. Comparisons of thalidomide and bortezomib in combination with other agents are warranted.
Acknowledgments
HMP has participated on advisory boards for Celgene and Janssen-Cilag and has received honoraria. DKS was an employee of Covance when the research was conducted. JH and DKS are currently employed by Janssen-Cilag. Dr Peter Tobin (Janssen-Cilag) provided assistance with the preparation of this manuscript. Janss en-Cilag provided funding for this research.
References
- 1.San-Miguel J. Perspective on the current use of bortezomib in multiple myeloma. Haematologica. 2006;91:871–2. [PubMed] [Google Scholar]
- 2.Singhal S, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–71. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos M. Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): results of a phase 3 study (MM-010) [abstract] Blood. 2005;106:6a–7a. [Google Scholar]
- 4.Adams J. Proteasome inhibition in cancer: development of PS-341. Semin Oncol. 2001;28:613–9. doi: 10.1016/s0093-7754(01)90034-x. [DOI] [PubMed] [Google Scholar]
- 5.Hideshima T. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–6. [PubMed] [Google Scholar]
- 6.Hideshima T, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–4. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 7.LeBlanc R, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 8.Mitsiades N, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 9.Ribas C. Advances in the treatment of multiple myeloma: the role of thalidomide. Leuk Lymphoma. 2003;44:291–8. doi: 10.1080/1042819021000035671. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma: final time-to-event results from the SUMMIT trial. Cancer. 2006;106:1316–9. doi: 10.1002/cncr.21740. [DOI] [PubMed] [Google Scholar]
- 11.Jagannath S, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–72. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 12.Kropff MH. Bortezomib in combination with dexamethasone for relapsed multiple myeloma. Leuk Res. 2005;29:587–90. doi: 10.1016/j.leukres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, et al. Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol. 2001;12:991–5. doi: 10.1023/a:1011132808904. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, et al. Efficacy of low-dose thalidomide and dexamethasone as first salvage regimen in multiple myeloma. Hematol J. 2004;5:318–24. doi: 10.1038/sj.thj.6200403. [DOI] [PubMed] [Google Scholar]
- 15.Anagnostopoulos A. Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol. 2003;121:768–71. doi: 10.1046/j.1365-2141.2003.04345.x. [DOI] [PubMed] [Google Scholar]
- 16.Weber D. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21:16–19. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 17.Glasmacher A. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584–93. doi: 10.1111/j.1365-2141.2005.05914.x. [DOI] [PubMed] [Google Scholar]
- 18.Prince HM. An analysis of clinical trials assessing the efficacy and safety of single-agent thalidomide in patients with relapsed or refractory multiple myeloma. Leuk Lymphoma. 2007;48:46–55. doi: 10.1080/10428190601001904. [DOI] [PubMed] [Google Scholar]
- 19.Blade J. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 20.derSimonian R. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Richardson PG, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 22.Yakoub-Agha I, et al. Thalidomide in patients with advanced multiple myeloma: a study of 83 patients–report of the Intergroupe Francophone du Myelome (IFM) Hematol J. 2002;3:185–92. doi: 10.1038/sj.thj.6200175. [DOI] [PubMed] [Google Scholar]
- 23.Grosbois B. Thalidomide in the treatment of advanced multiple myeloma. A prospective study of 120 patients. Blood. 2001;98:163a. [Google Scholar]
- 24.Durie BG, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 25.Barlogie B, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–4. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- 26.Grosbois B. Thalidomide (Thal) in the treatment of advanced multiple myeloma (MM). A prospective study of 120 patients. Blood. 2001;98:163a. [Google Scholar]
- 27.Neben K. Dose-dependent effect of thalidomide on overall survival in relapsed multiple myeloma. Clin Cancer Res. 2002;8:3377–82. [PubMed] [Google Scholar]
- 28.Mileshkin L, et al. Multicenter phase 2 trial of thalidomide in relapsed/refractory multiple myeloma: adverse prognostic impact of advanced age. Blood. 2003;102:69–77. doi: 10.1182/blood-2002-09-2846. [DOI] [PubMed] [Google Scholar]
- 29.Schey SA. An UK myeloma forum phase II study of thalidomide; long term follow-up and recommendations for treatment. Leuk Res. 2003;27:909–14. doi: 10.1016/s0145-2126(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 30.Tosi P, et al. Salvage therapy with thalidomide in patients with advanced relapsed/refractory multiple myeloma. Haematologica. 2002;87:408–14. [PubMed] [Google Scholar]
- 31.Waage A, et al. Early response predicts thalidomide efficiency in patients with advanced multiple myeloma. Br J Haematol. 2004;125:149–55. doi: 10.1111/j.1365-2141.2004.04879.x. [DOI] [PubMed] [Google Scholar]
- 32.Hus M. Thalidomide treatment of resistant or relapsed multiple myeloma patients. Haematologica. 2001;86:404–8. [PubMed] [Google Scholar]
- 33.Alexanian RWD. Thalidomide for resistant and relapsing myeloma. Semin Hematol. 2000;37:22–25. [Google Scholar]
- 34.Hattori Y. Thalidomide-induced severe neutropenia during treatment of multiple myeloma. Int J Hematol. 2004;79:283–8. doi: 10.1532/ijh97.03136. [DOI] [PubMed] [Google Scholar]
- 35.Cibeira MT. Sydney, Australia: Thalidomide in refractory and relapsed multiple myeloma: duration of response 10th International Workshop on Multiple Myeloma; p. 140. Haematologica. [Google Scholar]
- 36.Offidani M, et al. Common and rare side-effects of low-dose thalidomide in multiple myeloma: focus on the dose-minimizing peripheral neuropathy. Eur J Haematol. 2004;72:403–9. doi: 10.1111/j.1600-0609.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, et al. Response rate, durability of response, and survival after thalidomide therapy for relapsed multiple myeloma. Mayo Clin Proc. 2003;78:34–39. doi: 10.4065/78.1.34. [DOI] [PubMed] [Google Scholar]
- 38.Richardson P, et al. Thalidomide for patients with relapsed multiple myeloma after high-dose chemotherapy and stem cell transplantation: results of an open-label multicenter phase 2 study of efficacy, toxicity, and biological activity. Mayo Clin Proc. 2004;79:875–82. doi: 10.4065/79.7.875. [DOI] [PubMed] [Google Scholar]