Abstract
Aims
To compare the efficacy and safety of either continuing or discontinuing rosiglitazone + metformin fixed-dose combination when starting insulin therapy in people with Type 2 diabetes inadequately controlled on oral therapy.
Methods
In this 24-week double-blind study, 324 individuals with Type 2 diabetes inadequately controlled on maximum dose rosiglitazone + metformin therapy were randomly assigned to twice-daily premix insulin therapy (target pre-breakfast and pre-evening meal glucose ≤ 6.5 mmol/l) in addition to either rosiglitazone + metformin (8/2000 mg) or placebo.
Results
Insulin dose at week 24 was significantly lower with rosiglitazone + metformin (33.5 ± 1.5 U/day, mean ± se) compared with placebo [59.0 ± 3.0 U/day; model-adjusted difference −26.6 (95% CI −37.7, −15,5) U/day, P < 0.001]. Despite this, there was greater improvement in glycaemic control [HbA1c rosiglitazone + metformin vs. placebo 6.8 ± 0.1 vs. 7.5 ± 0.1%; difference −0.7 (−0.8, −0.5)%, P < 0.001] and more individuals achieved glycaemic targets (HbA1c < 7.0% 70 vs. 34%, P < 0.001). The proportion of individuals reporting at least one hypoglycaemic event during the last 12 weeks of treatment was similar in the two groups (rosiglitazone + metformin vs. placebo 25 vs. 27%). People receiving rosiglitazone + metformin in addition to insulin reported greater treatment satisfaction than those receiving insulin alone. Both treatment regimens were well tolerated but more participants had oedema [12 (7%) vs. 4 (3%)] and there was more weight gain [3.7 vs. 2.6 kg; difference 1.1 (0.2, 2.1) kg, P = 0.02] with rosiglitazone + metformin.
Conclusions
Addition of insulin to rosiglitazone + metformin enabled more people to reach glycaemic targets with less insulin, and was generally well tolerated.
Keywords: insulin, metformin, randomized controlled trial, thiazolidinediones, Type 2 diabetes mellitus
Introduction
As Type 2 diabetes advances, progressive islet B-cell dysfunction leads to a deterioration in glycaemic control and most people eventually require insulin treatment, often at high doses because of insulin insensitivity [1]. One approach to optimizing the effectiveness of insulin is to continue oral glucose-lowering drugs (OGLDs) when starting insulin, rather than changing to insulin alone. Studies suggest that this reduces the insulin dose requirement by approximately 20% in combination with one oral agent, and approximately 40% with both a sulphonylurea and metformin, while achieving similar glycaemic control [2,3].
In a 1-year study of previously insulin-naive people, combination metformin and bedtime insulin gave greater improvement in glycaemic control with less hypoglycaemia than bedtime insulin with sulphonylurea, sulphonylurea plus metformin, or morning insulin [4]. Body weight was unchanged with insulin and metformin, but increased in the other groups. People on maximum-tolerated doses of OGLDs who continued metformin with insulin used less insulin, had less weight gain, and achieved better glycaemic control compared with insulin plus placebo [5,6]. Hence, guidelines recommend continuing metformin (and other OGLDs) when starting insulin [7,8].
People with poor glucose control on metformin alone can benefit from addition of a thiazolidinedione [9,10] and, in studies with rosiglitazone added to metformin, homeostasis model assessment (HOMA) estimates of insulin sensitivity and islet B-cell dysfunction also improved [11,12]. This suggests the actions of the two drugs are complementary [13–16]. In addition, because gastrointestinal side-effects with metformin are dose related [17], a lower incidence can be achieved by combination with rosiglitazone through reduction of metformin dose [12]. There is also reduced weight gain compared with thiazolidinedione monotherapy [10,18].
Adding rosiglitazone to insulin therapy was found to reduce glycated haemoglobin (HbA1c) by 1.2% (P < 0.001), along with a 12% reduction of insulin dosage, but with increased oedema and weight gain [19,20]. As the use of exogenous insulin is also associated with fluid retention, the tolerability of rosiglitazone and insulin in combination warrants further investigation.
While that study evaluated the addition of rosiglitazone to insulin [19], the effect of adding insulin to rosiglitazone, which would be the commoner clinical scenario, has not been studied. Many individuals are treated with a combination of OGLDs before insulin is started; therefore, the continuation or discontinuation of combination therapy warrants investigation [8]. Theoretically, individuals taking optimized doses of rosiglitazone and metformin combination may require less insulin to achieve target glycaemic control compared with insulin alone or insulin plus either oral agent. Additionally, weight gain might be controlled by the metformin component, but hypoglycaemia might increase or decrease. The purpose of the current study was therefore to compare the efficacy and safety of either continuing or discontinuing rosiglitazone + metformin fixed-dose combination (rosi + met) when starting insulin in people with Type 2 diabetes inadequately controlled on maximal dose rosi + met.
Research design and methods
Study population
Men and women aged 18–70 years with Type 2 diabetes (defined according to World Health Organization criteria [21]) were considered for this 24-week, multicentre, randomized, double-blind, parallel group study. Participants were recruited from 73 centres in five European countries. The study was conducted in accordance with Good Clinical Practice guidelines and the 1996 version of the Declaration of Helsinki. Written informed consent was obtained from all participants. The study protocol and informed consent were approved by an ethics committee or institutional review board according to local requirements.
Participants had body mass index ≥ 25.0 kg/m2, HbA1c 7.1–10.0% at screening (visit 0; week −10), and were receiving ≥ 1500 mg metformin alone or in combination with other OGLDs at constant doses for ≥ 8 weeks prior to entry. Exclusion criteria included use of insulin in the 3 months before screening; a history of acidosis; ongoing oedema requiring pharmacological treatment; unstable or severe angina or any class of congestive heart failure; or myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass grafting or stroke within 3 months. Other exclusion criteria included blood pressure > 160/90 mmHg while on anti-hypertensive treatment, anaemia (Hb < 11.0 g/dl in men and < 10.0 g/dl in women), renal dysfunction (serum creatinine > 135 µmol/l in men and > 110 µmol/l in women), hepatic disease, and fasting serum C-peptide ≤ 0.50 nmol/l.
Study design
Eligible participants were enrolled into an 8-week, single-blind, run-in phase. During this time, prior OGLD treatment was changed to rosi + met 8 + 2000 mg (4 + 1000 mg twice daily). At the end of the run-in period, eligible participants with a clinic fasting plasma glucose (FPG) ≥ 7.0 mmol/l 2 weeks previously (n = 324) were randomized to either rosi + met 8 + 2000 mg plus insulin (n = 163) or matched placebo plus insulin (n = 161) double-blind for 24 weeks. Randomization was remote and concealed from centres, was gender stratified, and used a block randomization method. The combination of a thiazolidinedione with insulin remains investigational at the time of writing and is not a licensed indication in some countries.
The safety population included all randomized individuals who received at least one dose of double-blind study medication (rosi + met n = 162; placebo n = 160), and the intent-to-treat (ITT) population included all those who had at least one valid on-therapy observation for an efficacy variable (rosi + met n = 161; placebo n = 158). The baseline clinical characteristics of the groups were well matched (Table 1).
Table 1.
Clinical characteristics at randomization of the people with Type 2 diabetes studied
| Rosiglitazone + metformin and insulin | Placebo and insulin | |
|---|---|---|
| Safety population (n) | 162 | 160 |
| Age (years) | 57.2 ± 8.6 | 56.9 ± 9.1 |
| Sex [M/F, n (%)] | 84/78 (52/48) | 85/75 (53/47) |
| Race [white/other, n (%)] | 158/4 (98/2) | 158/2 (99/1) |
| Body weight (kg) | 88.9 ± 15.3 | 92.1 ± 16.8 |
| Body mass index (kg/m2) | 31.8 ± 4.7 | 32.5 ± 5.2 |
| Duration from diagnosis (years) | 9.2 ± 6.1 | 8.5 ± 6.0 |
| Prior glucose-lowering drug use [one/two daily at entry, n (%)] | 33/129 (20/80) | 31/129 (19/81) |
Mean ± sd, or n (%).
Baseline blood glucose control data are given in Table 2.
All participants were started on 24 U/day of locally sourced premixed insulin (12 U before breakfast and 12 U before the evening meal), the ‘baseline’ dose. Following training provided by the investigator or a trained designee, participants were asked to make self-monitored blood glucose (SMBG) measurements using a blood glucose meter calibrated to whole blood, and to record the results on diary cards. On beginning insulin they were requested to test four times daily to drive insulin dose adjustment according to protocol-specific algorithms, every 3–5 days until a target pre-breakfast and pre-evening meal blood glucose level of ≤ 6.5 mmol/l was achieved. After the target was reached, tests were requested to be carried out twice daily three times weekly, but four times daily for 3–5 days prior to the next clinic visit. If an SMBG level ≤ 4.0 mmol/l was recorded that could not be explained, the insulin dose was to be decreased by 10%.
Participants were educated by a qualified nutrition professional on following a diet designed to maintain their current weight. Individuals were discontinued from the study (see numbers below) if they experienced unacceptable hypoglycaemia, exacerbation of ongoing oedema during the run-in, new oedema prior to randomization or lack of efficacy defined by increased FPG or symptomatic hyperglycaemia deemed by the investigator to be a safety risk.
Study assessments
Eleven study visits were scheduled from screening (week −10) to study end, at which fasting blood samples were taken (before the morning insulin injection). Assays were performed at a central laboratory (Quest Diagnostics, Heston, UK) using standard assays. The primary efficacy end point was the change in HbA1c from baseline (week 0) to week 24. HbA1c was measured by high-performance liquid chromatography (HPLC) using a Diabetes Control and Complications Trial (DCCT)-harmonized assay. Secondary end points included change in office-sampled laboratory-measured FPG from baseline to week 24, the proportion of participants who achieved HbA1c and FPG targets, daily insulin dose, and hypoglycaemic events during the last 12 weeks of the study (in the expectation that insulin dose would be relatively stable by week 12). Participants were instructed to record symptomatic hypoglycaemia on diary cards with an SMBG result. Investigators were asked to confirm hypoglycaemia by careful history and the SMBG result. Compliance with insulin-dose algorithms was assessed at week 24 based on diary card records. This suggested compliance ‘all of the time’ or ‘most of the time’ by 124 (77%) participants in the rosi + met group and by 110 (69%) participants in the placebo group.
Changes in islet B-cell dysfunction were assessed through changes in proinsulin, C-peptide and proinsulin:C-peptide ratio. Health outcome variables were measured using Diabetes Treatment Satisfaction Questionnaire status (DTSQs) and DTSQ change (DTSQc) total scores. These are self-administered and validated instruments [22]. Safety variables included adverse events (AEs) recorded by the investigator and assessed as mild, moderate or severe.
Statistical analysis
The differences between treatment groups for mean change in HbA1c, FPG and DTSQs were assessed by an ancova model with terms for treatment, country, gender and baseline measurement. DTSQc was analysed similarly but with no baseline measurement. Analysis of treatment difference for total daily insulin dose was assessed using a repeated-measures analysis model with terms for treatment, country, gender, time (visit) and treatment-by-time (visit). C-peptide, proinsulin and proinsulin:C-peptide ratio data were analysed non-parametrically as change from baseline because of non-normal data distribution. Comparisons between treatment groups in the proportion of participants achieving HbA1c ≤ 6.5% and < 7.0%, and FPG ≤ 6.5 mmol/l were assessed using a logistic regression model with terms for treatment, gender and baseline measurement, with treatment comparison estimated as odds ratios.
Treatment difference for the total number of hypoglycaemic events in the last 12 weeks of the study was assessed based on Poisson regression model analysis with terms of treatment, gender and baseline measurement, accounting for duration of therapy. Safety measures were summarized by treatment group.
Results
Study population
Twenty-seven participants (8%) from the safety population withdrew during the double-blind phase. The primary reasons were participant decision [6 (4%) in each group] and AEs [rosi + met 4 (2%); placebo 4 (3%)]. Withdrawals of note included in the rosi + met group two as a result of oedema and one as a result of abdominal discomfort, and in the placebo group one as a result of weight gain. One person on rosi + met and two on placebo were withdrawn by investigators for lack of efficacy, and three on rosi + met and one on placebo for protocol violation.
Insulin doses
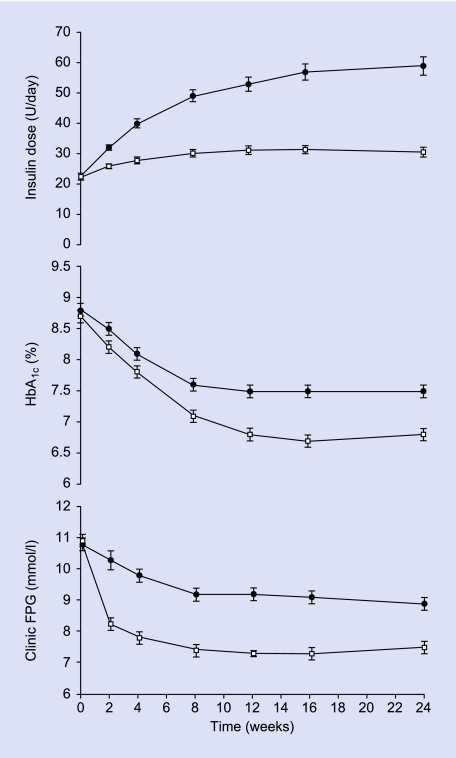
Daily insulin dose in the two groups was diverging by week 2 (Fig. 1) and was significantly lower in the rosi + met group at every visit from week 2, with treatment differences increasing with time. Most dose adjustment occurred in the first 4 weeks in both groups (Fig. 1). At week 24, there was a model-adjusted mean difference of −26.6 (95% CI −37.7, −15.5) U/day, P < 0.001 (Table 2).
FIGURE 1.
Time courses for daily insulin dose, HbA1c and clinic FPG for people with Type 2 diabetes starting insulin while continuing rosiglitazone + metformin (□) or transferring to placebo (•). Data are mean ± se for ITT populations with LOCF, except for dose which is without LOCF. For statistical significance see Results and Table 2.
Table 2.
Changes in efficacy and health perception measures from baseline to week 24 when starting insulin and continuing or discontinuing rosiglitazone + metformin fixed-dose combination therapy
| Rosiglitazone + metformin and insulin | Placebo and insulin | Treatment comparison as difference or odds ratio | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 24 | Baseline | Week 24 | p | ||
| Insulin dose (U/day) | 21.7 ± 0.4* | 33.5 ± 1.5 | 23.1 ± 0.4* | 59.0 ± 3.0 | −26.6 (−37.7, −15.5) | < 0.001 |
| HbA1c (%) | 8.7 ± 0.1 | 6.8 ± 0.1 | 8.8 ± 0.1 | 7.5 ± 0.1 | −0.7 (−0.8, −0.5) | < 0.001 |
| Clinic FPG (mmol/l) | 10.9 ± 0.2 | 7.5 ± 0.2 | 10.8 ± 0.2 | 8.9 ± 0.2 | −1.4 (−1.9, −0.9) | < 0.001 |
| Target achievers [n (%)]: | ||||||
| HbA1c ≤ 6.5% | 74 (46) | 29 (19) | 4.1 (2.4, 6.9) | < 0.001 | ||
| HbA1c < 7.0% | 113 (70) | 53 (34) | 5.0 (3.0, 8.1) | < 0.001 | ||
| Clinic FPG ≤ 6.5 mmol/l | 58 (36) | 25 (16) | 3.0 (1.8, 5.2) | < 0.001 | ||
| Mean daily SMBG† (mmol/l) | 10.0 ± 0.2 | 6.8 ± 0.1 | 10.0 ± 0.2 | 8.2 ± 0.2 | ||
| Hypoglycaemia events (n) | 166 | 130 | 1.1 (0.6, 2.1) | NS | ||
| ≥ 1 event [n (%)]† | 40 (25) | 43 (27) | ||||
| ≥ 6 events [n (%)]† | 9 (5) | 5 (3) | ||||
| Body weight (kg) | 89.6 ± 1.3 | 93.2 ± 1.3 | 91.2 ± 1.3 | 93.7 ± 1.3 | 1.1 (0.2, 2.1) | 0.021 |
| C-peptide (nmol/l)‡ | 0.75 (0.58, 0.97) | 0.66 (0.41, 0.89) | 0.77 (0.59, 1.01) | 0.70 (0.45, 0.97) | −0.10 (−0.27, 0.07) vs. −0.10 (−0.25, 0.12) | NS |
| Proinsulin (pmol/l)‡ | 6.4 (3.8, 9.7) | 4.8 (2.6, 10.3) | 6.8 (4.3, 10.1) | 6.6 (4.0, 14.2) | −0.8 (−4.3, 2.5) vs. 1.0 (−1.8, 4.6) | < 0.001 |
| Proinsulin:C-peptide‡ (pmol/nmol) | 8.1 (5.0, 12.7) | 7.6 (4.5, 14.0) | 9.1 (6.2, 13.2) | 10.7 (7.6, 17.6) | −0.3 (−3.7, 3.2) vs. 2.0 (−0.8, 6.6) | < 0.001 |
| DTSQs score | 28.5 ± 0.6 | 29.2 ± 0.5 | 27.7 ± 0.6 | 27.4 ± 0.6 | 1.5 (0.2, 2.7) | 0.024 |
| DTSQc score | — | 11.5 ± 0.8 | — | 9.6 ± 0.7 | 1.9 (0.4, 3.4) | 0.014 |
FPG, clinic/laboratory fasting plasma glucose; NS, non-significant; SMBG, self-monitored blood glucose.
Mean ± se, n (%), median (IQ range), or treatment difference from baseline to week 24 (95% CI).
ITT population with LOCF (insulin dose without LOCF), or safety population for hypoglycaemia.
Starting insulin dose taken as baseline.
Observational data without statistical comparison.
Hormonal data are analysed non-parametrically, and given as median (25–75%ile), with change from baseline rather than treatment difference.
Glycaemic control
The group continuing rosi + met achieved significantly greater improvement in HbA1c between baseline and week 24 compared with the placebo group [difference −0.7 (−0.8, −0.5)%, P < 0.001; Fig. 1, Table 2]. A significantly greater proportion of people receiving rosi + met achieved treatment targets of HbA1c ≤ 6.5 and < 7.0% compared with those in the placebo group (P < 0.001; Table 2); 46% in the rosi + met group achieved the stricter target.
The fall in clinic FPG was early and rapid in the group continuing rosi + met (Fig. 1). At week 24, there was a greater reduction in clinic FPG in the rosi + met group compared with the placebo group, with a difference between the two groups of −1.4 (−1.9, −0.9) mmol/l, P < 0.001 (Table 2). A significantly greater proportion of individuals who received rosi + met achieved the clinic FPG target ≤ 6.5 mmol/l (P = 0.001; Table 2).
SMBG data were collected to assist in insulin dose titration and were not compared statistically. In the rosi + met group, mean daily SMBG measurement fell from 10.0 ± 0.2 mmol/l at baseline to 6.8 ± 0.1 mmol/l at week 24. The mean SMBG reduced from 10.0 ± 0.2 mmol/l to 8.2 ± 0.2 mmol/l in the placebo group (Table 2).
Hypoglycaemia and body weight
During the last 12 weeks of the study, the number of participants who reported biochemically confirmed hypoglycaemia with and without symptoms was similar between groups: rosi + met vs. placebo 33 vs. 32 and 9 vs. 13, respectively. The number of participants who reported symptomatic hypoglycaemia which was not biochemically confirmed was also similar with rosi + met and placebo (5 vs. 5 individuals). The number of participants reporting at least one hypoglycaemic event of any kind during the last 12 weeks of the study was very similar in the two groups: 40 (25%) in the rosi + met group and 43 (27%) in the placebo group. Although the total number of reported events was higher with rosi + met (166 vs. 130 events), the event rate was not statistically significantly different (Table 2). No severe events or events leading to withdrawal from study medication occurred.
Body weight increased in both groups, with a significantly greater increase in the rosi + met group [mean difference 1.1 (0.2, 2.1) kg (P = 0.021) Table 2].
Islet B-cell function and dysfunction
Despite lower FPG levels with rosi + met (above), serum C-peptide levels were similar in the two groups at 24 weeks, with identical change from baseline (Table 2). Proinsulin levels, while similar at baseline, fell only in the rosi + met group, and were notably lower at 24 weeks, with a significantly different change in levels from baseline compared with the placebo group (P < 0.001, Table 2). Consistent with these data, the proinsulin:C-peptide ratio change from baseline was also different in the two groups (P < 0.001; Table 2).
Health perception measures
Measures of health perception suggested a greater level of treatment satisfaction in participants who received rosi + met compared with placebo. From baseline to week 24, the DTSQs score increased in the rosi + met group and decreased with placebo [difference 1.5 (0.2, 2.7) points, P = 0.024; Table 2]. The DTSQc total score, a measure of improvement in satisfaction, was significantly higher in the rosi + met group than in the placebo group at week 24 [difference 1.9 (0.4, 3.4) points, P = 0.014; Table 2].
Safety and tolerability
Both treatment regimens were generally well tolerated. A similar proportion of participants had on-therapy AEs in the two treatment groups: 60 people (38%) in the rosi + met group and 69 people (43%) with placebo. Six people (4%) in the rosi + met group and eight people (5%) in the placebo group had on-therapy non-fatal serious AEs. All were reported by the investigator as apparently unrelated to study medication. One death occurred before treatment with study drugs, and one on rosi + met as a result of circulatory arrest.
Twelve participants (7%) in the rosi + met group had oedema, compared with four (3%) in the placebo group; all were judged mild or moderate, but two cases in the rosi + met group led to withdrawal from the study. In those participants who developed oedema, mean weight gain was 4.0 ± 1.7 kg in the placebo group and 5.0 ± 0.8 kg in the rosi + met group. There were no cases of heart failure in either treatment group, but one occurrence of angina pectoris (judged severe) on rosi + met, associated with weight loss (−7.1 kg).
Conclusions
In this randomized controlled study, the addition of insulin to rosi + met significantly improved glycaemic control and increased the proportion of individuals achieving glycaemic targets when compared with insulin alone. Importantly, these changes were seen despite markedly lower insulin doses in the group continuing on rosi + met, and with no difference in the proportion of participants reporting hypoglycaemia during the last 12 weeks of the study (the period for which the insulin dose was stable). This improved blood glucose control with lower insulin doses was reflected in improved treatment satisfaction measures, known to primarily reflect changes in perceived control [23]. A comparison between the insulin-sparing effect of rosi + met in this study and previous studies of OGLDs in combination with insulin suggests rosi + met may have an additional insulin-sparing effect over that seen with metformin plus insulin [2,3].
The increased incidence of oedema in participants continuing on rosi + met compared with insulin plus placebo (7 vs. 3%) was lower than in a previous study of insulin plus rosiglitazone [19], although still measurable, and with no cases of heart failure. The lower rate of oedema in this study may reflect the gradual up titration of insulin therapy. The lower dose of insulin required with rosi + met may also have helped, as oedema associated with thiazolidinedione treatment is greater when thiazolidinediones are used in combination with insulin [20].
The significantly greater mean weight gain seen with rosi + met therapy in this study is consistent with a systematic review reporting that weight gain with thiazolidinediones in combination with insulin is consistently associated with improved glycaemic control [2]. The weight difference from the placebo group is consistent with the difference in blood glucose control [4]. The gain of 3.7 kg in the present study was less than the 5.3 kg previously observed when rosiglitazone 8 mg/day was used with insulin [19], suggesting that metformin may have a mitigating effect on the weight change, in line with previous reports of metformin added to insulin [2,3] and of metformin plus rosiglitazone [10,18].
Mechanistically, the lower insulin dose required in the rosi + met group supports the rationale for improving insulin sensitivity, as indicated by the combination of lower insulin requirements and lower HbA1c. Reducing the insulin dose may offer health benefits in terms of reducing the risk of hypoglycaemia [24] and hyperinsulinaemia [25], along with other putative benefits such as reducing the hunger stimulus and the frequency of injections, together with some cost offset. The lack of change in fasting serum C-peptide despite lower glucose levels implies improved islet B-cell function, but the present study cannot discriminate whether this is a direct effect or is secondary to the improvement in blood glucose and metabolic control. However, the fall in proinsulin and proinsulin:C-peptide ratio, not seen with the smaller improvement of glucose control in the placebo group, implies improved processing of proinsulin to insulin with continuation of rosi + met when starting insulin. More stable blood glucose control has been known for decades to be related to preservation of endogenous insulin secretion, and this may be another reason why the insulin sensitizers did not result in increased hypoglycaemia in this study. It is yet to be determined whether these changes have long-term implications for preservation of endogenous insulin secretion.
This study provides further support for current guidelines that recommend continuation of OGLD therapy while insulin is introduced [7,8]. This study is also in agreement with recent studies suggesting that titration of insulin to achieve glycaemic targets allows improved glycaemic control while minimizing the risk of hypoglycaemia [26–28]. In the current study, almost half of the group randomized to rosi + met was able to achieve HbA1c ≤ 6.5%, the target in the latest evidence-based guidelines such as those of the International Diabetes Federation [8,29,30], despite starting levels (mean 8.7%) well above usual recommendations for starting insulin. In contrast, less than one-third of those randomized to insulin plus placebo achieved this target.
There are several limitations that must be considered when making comparisons with other studies. The regimen used for insulin dose adjustment was less aggressive than in some recent studies [4,19], which may account for the higher HbA1c values obtained with insulin monotherapy here compared with studies that have used alternative insulin regimens [4]. In addition, it is important to note that, while the aim of this study was to examine continuation or discontinuation of rosi + met when initiating insulin, current guidelines advise the continuation of metformin when transferring patients to insulin [7,8].
The present results appear to be generally applicable as a result of the inclusion of a typical Type 2 diabetes starter population in line with treatment recommendations, although excluding those with a history of cardiac failure [7,8]. A future investigation could consider a comparator group treated with insulin added to metformin monotherapy; meanwhile, however, the results appear better than those achieved in other recent studies of insulin initiation in which OGLDs were continued [26,28,31], although inter-study comparisons must be made with caution.
In conclusion, the addition of insulin to rosi + met as a fixed-dose combination proved effective, well tolerated, and enabled more individuals to reach glycaemic targets, with a lower insulin dose requirement than for insulin monotherapy.
Competing interests
PDH and CJB have received funding from GlaxoSmithKline at Newcastle University and Aston University, respectively, for activities related to rosiglitazone and other products. The other authors are employees of GlaxoSmithKline, the manufacturer of rosiglitazone and the combination product (Avandamet) tested in this paper. GlaxoSmithKline funded the study as part of the development programme for Avandamet.
Acknowledgments
The authors thank all the people with diabetes and investigators (Appendix) who participated in this study. The authors gratefully acknowledge the editorial assistance of Carol Mason in the development of this manuscript.
Glossary
Abbreviations
- AE
adverse event
- DTSQ
Diabetes Treatment Satisfaction Questionnaire
- FPG
fasting plasma glucose
- HbA1c
glycated haemoglobin
- ITT
intent-to-treat
- OGLD
oral glucose-lowering drug
- rosi + met
rosiglitazone + metformin fixed-dose combination
- SMBG
self-monitored blood glucose
Appendix
Study investigators
Austria: Claudia Francesconi; France: Jean-Raymond Attalli, Chantal Bully, Jean Caron, Bernard Charbonnel, Guillaume Charpentier, Alain Duplan, Philippe Giraud, Didier Gouet, Jean-Louis Grenier, Anne-Marie Leguerrier, Olivier Ziegler; Germany: Siegfried Adam, Hasan Alawi, Helmut Anderten, Elke Austenat, Gudrun Banse, Udo Biermann, Marion Braun, Klaus Busch, Richard Daikeler, Joachim Dehmel, Werner Dohmen, Frank Franzmann, Horst Frick, Klaus Funke, Bernd Glückermann, Claus-Michael Grimm, Josef Grosskopf, Thomas Hampel, Markolf Hanefeld, Agnes Himpel-Bönninghoff, Eduard Hülsmann, Wolfgang Jung, Monika Kiper, Christiane Klein, Dieter Klein, Uwe Kleinecke-Pohl, Christine Kosch, Karl-Heinz Krause, Dieter Langguth, Peter Lauer, Sigrun Lukas, Stephan Maxeiner, Andrea Molle, Gerhard Orlovius, Thomas Segiet, Bernhard Stähr, Jorg Von Hübbenet, Jurgen Wachter, Peter Weisweiler, Ulrich Wendisch, Knut Wippermann; Italy: Carlo Coscelli, Valter Donadon, Fausto Santeusanio, Ennio Scaldaferri, Giorgio Viviani; Spain: Javier Ampudia, Juan Jose Beitia, Juan Jose Cornejo Sanz, Guillem Cuatrecasas Cambra, Luis De Teresa, Jose Ramon Domínguez, Luis Escobar, Gabriel Giménez, Monica Marazuela, Ramon Villar Garcia, Ramon Gomis, Angel Luis Marco Mur, Teresa Muros De Fuentes, Eduardo Pena Gonzalez, Jose Sabán.
References
- 1.Klein R. The medical management of hyperglycemia over a 10-year period in people with diabetes. Diabetes Care. 1996;19:744–750. doi: 10.2337/diacare.19.7.744. [DOI] [PubMed] [Google Scholar]
- 2.Yki-Jarvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care. 2001;24:758–767. doi: 10.2337/diacare.24.4.758. [DOI] [PubMed] [Google Scholar]
- 3.Goudswaard AN. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2004. CD003418. [DOI] [PMC free article] [PubMed]
- 4.Yki-Järvinen H. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1999;130:389–396. doi: 10.7326/0003-4819-130-5-199903020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Chow CC. Comparison of insulin with or without continuation of oral hypoglycemic agents in the treatment of secondary failure in NIDDM patients. Diabetes Care. 1995;18:307–314. doi: 10.2337/diacare.18.3.307. [DOI] [PubMed] [Google Scholar]
- 6.Douek IF. Continuing metformin when starting insulin in patients with Type 2 diabetes: a double-blind randomized placebo-controlled trial. Diabet Med. 2005;22:634–640. doi: 10.1111/j.1464-5491.2005.01475.x. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Diabetes Association. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2003. pp. S1–S152. [DOI] [PubMed]
- 8.International Diabetes Federation Clinical Guidelines Taskforce. Global Guideline for Type 2 Diabetes. [2006-September-4]. Available from: http://www.idf.org/webdata/docs/IDF%20GGT2D.pdf.
- 9.Einhorn D. Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The Pioglitazone 027 Study Group. Clin Ther. 2000;22:1395–1409. doi: 10.1016/s0149-2918(00)83039-8. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca V. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. J Am Med Assoc. 2000;283:1695–1702. doi: 10.1001/jama.283.13.1695. [DOI] [PubMed] [Google Scholar]
- 11.Jones TA. Addition of rosiglitazone to metformin is most effective in obese, insulin-resistant patients with type 2 diabetes. Diabetes Obes Metab. 2003;5:163–170. doi: 10.1046/j.1463-1326.2003.00258.x. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CJ, et al. Rosiglitazone/metformin fixed-dose combination compared with uptitrated metformin alone in type 2 diabetes: a 24-week, randomized, double-blind, parallel-group study. Clin Ther. 2005;27:1548–1561. doi: 10.1016/j.clinthera.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Bailey CJ. Avandamet: combined metformin–rosiglitazone treatment for insulin resistance in type 2 diabetes. Int J Clin Pract. 2004;58:867–876. doi: 10.1111/j.1742-1241.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki Y, et al. Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in Type II diabetic patients. Diabetologia. 2001;44:2210–2219. doi: 10.1007/s001250100031. [DOI] [PubMed] [Google Scholar]
- 15.Stumvoll M. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 16.Bailey CJ. Treating insulin resistance in type 2 diabetes with metformin and thiazolidinediones. Diabetes Obes Metab. 2005;7:675–691. doi: 10.1111/j.1463-1326.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 17.Garber AJ. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose–response trial. Am J Med. 1997;103:491–497. doi: 10.1016/s0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 18.Rosak C. Rosiglitazone plus metformin is effective and well tolerated in clinical practice: results from large observational studies in people with type 2 diabetes. Int J Clin Pract. 2005;59:1131–1136. doi: 10.1111/j.1368-5031.2005.00652.x. [DOI] [PubMed] [Google Scholar]
- 19.Raskin P. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226–1232. doi: 10.2337/diacare.24.7.1226. [DOI] [PubMed] [Google Scholar]
- 20.Nesto RW, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27:256–263. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its ComplicationsPart IDiagnosis and Classification of Diabetes Mellitus. Geneva: WHO Department of Noncommunicable Disease Surveillance; 1999. [Google Scholar]
- 22.Bradley C. Diabetes Treatment Satisfaction Questionnaire (DTSQ) In: Bradley C, editor. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Management. Langhorne: Harwood Academic Publishers; 1994. [Google Scholar]
- 23.Rosenstock J. Patient satisfaction and glycemic control after 1 year with inhaled insulin (Exubera) in patients with type 1 or type 2 diabetes. Diabetes Care. 2004;27:1318–1323. doi: 10.2337/diacare.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 24.UK Prospective Diabetes Study (UKPDS) Group. United Kingdom Prospective Diabetes Study 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann Intern Med. 1998;128:165–175. doi: 10.7326/0003-4819-128-3-199802010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Despres JP, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 26.Riddle MC. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 27.Yki-Järvinen H. Treat To Target simply—the LANMET study. Diabetes. 2004;53(Suppl. 2):A519. [Google Scholar]
- 28.Raskin P, et al. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 29.European Diabetes Policy Group. A desktop guide to Type 2 diabetes. Diabet Med. 1999;16:716–730. [PubMed] [Google Scholar]
- 30.Lebovitz HE, et al. ACE/AACE consensus conference on the implementation of out patient management of diabetes mellitus: consensus conference reccommendations. Endocr Pract. 2006;12(Suppl. 1):6–12. doi: 10.4158/EP.12.S1.6. [DOI] [PubMed] [Google Scholar]
- 31.Janka HU. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005;28:254–259. doi: 10.2337/diacare.28.2.254. [DOI] [PubMed] [Google Scholar]