Abstract
Porphyromonas gingivalis possesses two distinct fimbriae. The long (FimA) fimbriae have been extensively studied. Expression of the fimA gene is tightly controlled by a two-component system (FimS/FimR) through a cascade regulation. The short (Mfa1) fimbriae are less understood. The authors have recently demonstrated that both fimbriae are required for formation of P. gingivalis biofilms. Here, the novel finding that FimR, a member of the two-component regulatory system, is a transcriptional activator of the mfa1 gene is promoted. Unlike the regulatory mechanism of FimA by FimR, this regulation of the mfa1 gene is accomplished by FimR directly binding to the promoter region of mfa1.
Keywords: gene regulation, Porphyromonas gingivalis, two-component system
Introduction
Porphyromonas gingivalis is a gram-negative bacterium, which is considered to be a major periodontal pathogen (Socransky & Haffajee, 2005). It is also a pathogen that may be involved in coronary heart disease and preterm births (Boggess et al., 2005; Brodala et al., 2005; Chou et al., 2005). The ability of P. gingivalis to initiate a periodontal infection is mainly dependent on the expression of fimbriae (Malek et al., 1994). Two distinct fimbriae are found on the surface of the organism (Dickinson et al., 1988; Hamada et al., 1996). The long (major) filamentous structure is comprised of a FimA subunit protein encoded by the fimA gene. The short (minor) fimbriae are made up of a 67 kDa protein (Mfa1). Both fimbriae appear to be involved in bacterial pathogenicity (Amano et al., 2004).
The function of the FimA protein and regulation of fimA expression have been extensively studied. The FimA protein is required for P. gingivalis colonization on salivary coated surfaces, and the early colonization of dental plaque (Malek et al., 1994; Levesque et al., 2003; Maeda et al., 2004). A P. gingivalis fimA mutant shows impaired invasion capability of epithelial cells compared with wild-type strain, suggesting the involvement of FimA in the bacterial interaction with surface receptor(s) on gingival cells (Weinberg et al., 1997). Earlier studies by the authors showed that FimA expression was modulated by environmental cues, including temperature and hemin concentration, and by the presence of Streptococcus cristatus, an early colonizer of dental plaque (Xie et al., 1997, 2000b). FimR, a response regulator of the fimS/fimR two-component system was identified, and FimA expression was found to be dramatically reduced in fimR mutants (Hayashi et al., 2000). Investigation of the mechanism of regulation of fimA by FimR indicates that FimR does not bind directly to the fimA promoter, but rather binds to the promoter region of the first gene (pg2130) in the fimA cluster, suggesting that PG2130 is the FimR target gene, which in turn regulates expression of other genes in the fimA cluster, including the fimA gene (Nishikawa et al., 2004).
The short fimbriae (Mfa1) also contribute to P. gingivalis colonization. Coadhesion and biofilm development between P. gingivalis and Streptococcus gordonii require the interaction of Mfa1 with streptococcal protein SspB (Park et al., 2005). The authors have recently reported that the short fimbriae are required for P. gingivalis cell–cell aggregation, an essential step in microcolony formation (Lin et al., 2006). A mutant with a deficiency in minor fimbriae binds to a saliva-coated surface but does not form microcolonies as the wild-type strain does. Mfa1 expression appears to fluctuate under various growth conditions (Masuda et al., 2006). In a nutrient-limited medium, expression of FimA and Mfa1 are inhibited in P. gingivalis, whereas such differences are not found in gingipain expression. A recent study has shown that expression of mfa1 is repressed in the presence of some common oral plaque bacteria such as S. gordonii, Streptococcus sanguinis and Streptococcus mitis (Park et al., 2006). However, very little is known about regulatory mechanisms of mfa1 expression.
In this study, it is demonstrated that FimR is a positive regulator of Mfa1 expression. Evidence is provided that unlike FimR-dependent fimA expression, FimR regulates mfa1 expression by directly binding to the promoter region of mfa1.
Materials and methods
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Porphyromonas gingivalis 33 277 and its derivatives were grown from frozen stocks in trypticase soy broth (TSB) or on TSB blood agar plates, supplemented with yeast extract (1 mg mL−1), hemin (5 μg mL−1) and menadione (1 μg mL−1), at 37°C in an anaerobic chamber (85% N2, 10% H2, 5% CO2). Escherichia coli DH5α was the host for plasmids, and grown in Luria–Bertani (LB) broth at 37°C. Antibiotics were used, when appropriate, at the following concentrations: gentamicin (100 μg mL−1), erythromycin (5 μg mL−1), ampicillin (50 μg mL−1), kanamycin (50 μg mL−1) and tetracycline (10 μg mL−1).
Table 1.
Strains and plasmids used in this study
| Strains and plasmid | Relevant characteristics* | Source or reference |
|---|---|---|
| Strains | ||
| P. gingivalis | ||
| 33277 | Type strain from ATCC | Lab collection |
| FAT | P. gingivalis mutant with the fimA gene inactivated by insertion – the tetracycline the tetA(Q)gene, Tetr | Lin et al. (2006) |
| MFAE | P. gingivalis mutant with the mfa1 gene inactivated by insertion – a ermF–ermAM cassette, Emr | Lin et al. (2006) |
| FRE | P. gingivalis mutant with the fimR gene inactivated by insertion – a ermF–ermAM cassette, Emr | This study |
| E. coli | ||
| DH5α | F− ϕ80dlacZΔ(lacZYA–argF)U169 endA1 supE44 recA1 relA1 | BRL |
| Plasmids | Lee et al. (1996) | |
| pVA3000 | Suicide vector containing an ermF–ermAM cassette | Lee et al. (1996) |
| pBSK1.2-5 | pUC19 containing a tetA(Q)2 gene | Lepine et al. (1996) |
| PCRII-TOPO | Linearized plasmid with single 3′ dT residues, Kmr Amr | Invitrogen |
| pFR | PCRII-TOPO plasmid carrying a fimR gene | This study |
| pFRE | pFR plasmid an ermF–ermAM cassette inserted in the fimR gene | This study |
Kmr, Tetr, Emr, Amr, resistance to kanamycin, tetracyline, erythromycin, ampicillin.
Construction of the fimR− mutant
An insertional fimR mutant was constructed by allelic replacement. Briefly, the fimR gene was amplified by PCR using primers fimRF and fimRR (Table 2) and cloned into pCRII-TOPO (Invitrogen, Carlsbad, CA) to give rise to a pFR plasmid (Table 1). A 2.1-kb ermF–ermAM cassette (Fletcher et al., 1995) was amplified using plasmid pVA3000 as a template and ErmF and ErmR as primers, which introduced NdeI sites at both ends of the PCR product. The ermF–ermAM cassette was then inserted into the fimR gene cloned in plasmid pFR. The resulting plasmids pFRE were linearized with XhoI and introduced into P. gingivalis 33277-by electroporation. Electroporation was carried out by a modification of the procedure of Fletcher et al. (1995). Porphyromonas gingivalis 33277 competent cells were obtained by suspending early-log-phase cells in electroporation buffer (10% glycerol, 1.0 mM MgCl2). The cells were incubated with the linearized plasmid and were pulsed with a Bio-Rad gene pulser (Hercules, CA) at 2.5 kV. The cells were then immediately added to the TSB, and incubated anaerobically for 16 h. The fimR− mutants (FRE) resulting from a double-cross-over recombination were selected on Trypticase soy agar plates containing erythromycin (5 μg mL−1). The insertional mutation was confirmed by PCR analysis.
Table 2.
Oligonucleotide primers
| Gene | Primer name | Primer sequences (5′–3′) | Application |
|---|---|---|---|
| fimR | rfimR-F | ATGATTAGTATCGTACTC | The full-length fimR ORF amplification |
| rfimR-R | CTATTGCCAATCCACTAA | ||
| fimRF | TAGGCTTTTGCCAGATTGGA | Construction of fimR mutation | |
| fimRR | CCAAATCGGGAATTTAGCTC | ||
| ermF-ermAM | ErmF | CACCGTCATATGCGATAGCTTCCGCTATTGCT | ermF-ermAM cassette amplification |
| ErmR | GGAACTCATATGTCCCCGAAGCTGTCAGTAGT | ||
| fimA | fimAF | CGGAACGAATAACCCAGAGA | Real-time PCR |
| fimAR | CTGACCAACGAGAACCCACT | ||
| fimAProm-F | CGACGCTATATGCAAGACAA | The biotin-labeled promoter region | |
| fimAProm-R | Bio-TGTAACGGGTTCTGCCTCGT | ||
| Mfa1 | MfaProm-F | CTCTCGCGAGGGTCAATATC | The biotin-labeled promoter region |
| MfaProm-R | Bio-CGTCTTACCGGCTTCCCTAT | ||
| 67KD121F | CAGATGGGTTGTTGCTCA | The biotin-labeled coding region | |
| 67KD121R | Bio-ATAGAAAGTGCTGCTGGTAG | ||
| MfaF | CAGATGGGTTGTTGCTCA | Real-time PCR | |
| MfaR | GAAAGTGCTGCTGGTAG | ||
| MfaTSR1 | CTCGTTATCACATATCCGAACC | Identification of transcriptional start site | |
| MfaTSR2 | GAAGCAAAGCCCAATGAGAG | ||
| MfaTSR3 | CCGCTCGACTCACGAGACTA | ||
| MfaTSR4 | CACGACATAGAGTGTTCAGA | ||
| MfaTSR5 | CGTCTTGCCGACAGCAGAAT | ||
| MfaTSF1 | AGCCGGTAAGACGTAGCTGA | ||
| MfaTSF2 | ACGTAGAAGACAGCAGAATA | ||
| MfaTSF3 | TCTCTCGCGAGGGTCAATA | ||
| 16S | 16sRNA-F | TGGGTTTAAAGGGTGCGTAG | Real-time PCR |
| 16sRNA-R | CAATCGGAGTTCCTCGTGAT | ||
| rgpA | rgpAF | CAACCAGTCTTGGGCTTCTC | Real-time PCR |
| rgpAR | CCACCATAGCAAACATACCG |
RNA isolation and qPCR
Porphyromonas gingivalis strains were grown anaerobically on Trypticase soy agar plates at 37°C for 48 h. Porphyromonas gingivalis cells were collected and mixed in Trizol Reagent (Invitrogen). The RNA in the supernatant was then purified using an RNeasy mini spin column (Qiagen, Valencia, CA). To minimize contamination with genomic DNA, RNA samples were digested on the column with RNase-free DNase. The total RNA was tested using an Agilent 2100 Bioanalyzer to insure the quality of the samples. Gene expression was measured using the QuantiTect SYBR Green reverse transcriptase polymerase chain reaction (RT-PCR) Kit (Qiagen) and the iCycler iQ real-time detection system (Bio-Rad Laboratories, Inc.) according to the manufacturer's instructions. The primers for the fimA gene were fimAF and fimAR, and for the mfa1 gene were mfa1F and mfa1R. Expression of the 16S rRNA gene and rgpA (a gene encoding for arginine-gingipain) were tested as a control to normalize samples for variations in sample volume loading. Amplification reactions consisted of a reverse transcription cycle at 50°C for 30 min, an initial activation at 95°C for 10 min, and 40 cycles of 94°C for 15 s, 60°C for 30 s and 72°C for 30 s. For quantitative analysis of gene expression, fivefold serial dilutions of total RNA, from 0.4 to 250 ng, were used as the template in each 50-μL reaction to generate a standard curve. Data were collected only from the reactions in which correlation coefficients of standard curves were ≥0.99 and where the melting curves showed a single peak. Values represent the mean±SD of duplicate samples obtained from three independent experiments.
Western blot analysis
Porphyromonas gingivalis strains were grown on TSB blood agar plates for 48 h. The surface proteins were collected by sonication and centrifugation as described previously (Xie et al., 2004). Protein concentrations of the samples were determined using a Bio-Rad protein assay. The soluble proteins (5 μg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), along with prestained MW standards (Bio-Rad) and were transferred to nitrocellulose membranes (Gibco BRL) with Mini Transblot Electrophoretic transfer cell (Bio-Rad Laboratories) at 100 V for 1 h. The membrane was treated with 30 mL of blocking solution [3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) containing 0.1% Tween-20, pH 7.4] for 1 h and incubated for 1 h with a polyclonal anti-FimA or anti-Mfa1 antibody diluted 1 : 1000 in PBS containing 0.1% Tween-20, pH 7.4. The membrane was then rinsed twice and washed three times for 15 min each with 0.1% Tween-20 in PBS. The membrane was incubated with antirabbit horseradish peroxidase-conjugated secondary antibodies for 1 h and rinsed and washed as described above. Antigen–antibody reactivity was visualized by enhanced chemiluminescence (GE Healthcare Bio-Sciences Corp, Piscataway, NJ).
5′ RACE analysis of Mfa1 transcripts
The transcriptional start site of mfa1 was determined using a FirstChoice RLM-RACE Kit (Ambion, Austin, TX). Briefly, a 45 base 5′ RNA adapter oligonucleotide was ligated to the 5′ end of the total RNA (10 μg) using T4 RNA ligase. Reverse transcription (RT) of cDNA was performed using M-MLV reverse transcriptase with primer MfaTSR2 of mfa1. Nested PCR was performed by first using 5′ RACE outer primer and mfa1-specific reverse primer MfaTSR2 to amplify 5′ adapter-linked cDNA molecules of mfa1. Inner PCR was then conducted with 5′ RACE inner primer and MfaTSR1, and with the PCR product generated from the outer primers as templates. Five microliters of each PCR reaction was analyzed by 1.5% agarose gel electrophoresis. The PCR fragments of the inner PCR product were extracted and cloned into a pCRII-TOPO vector (Invitrogen) and sequenced using ABI capillary sequencer (Perkin-Elmer).
FimR cloning and expression in E. coli
DNA fragments of the fimR were amplified by PCR with primers 5′-ATGATTAGTATCGTACTC and 5′-CTATTGCCAATCCACTAA, which produced a 684 bp PCR product. The PCR products were then cloned into pCRII-TOPO. Recombinant FimR (rFimR) was expressed in E. coli using a pET protein expression system (Novagen, San Diego, CA). The FimR DNA fragment was subcloned into the pET-30b down stream of a histidine tag. The recombinant FimR was expressed in E. coli BL21 (DE3) cells carrying the pET-30b/FimR plasmid in the presence of IPTG and kanamycin. His-tagged rFimR was purified with Ni2+-charged His-bind resin. The His-tag on the recombinant protein was cleaved with enterokinase and removed by His-bind resin. Enterokinase was then removed using Ekapture agarose.
Electrophoretic mobility shift assay (EMSA)
EMSAs were performed using the LightShift Chemiluminescent EMSA Kit (PIERCE, Rockford, IL) according to the manufacturer's instructions. Biotin-labeled DNA fragments were generated using 5′ biotin-incorporated primers (Invitrogen). For phosphorylation, rfimR was incubated with binding buffer containing 50 mM acetylphosphate lithium potassium salt (Sigma, Saint Louis, MO) at room temperature for 30 min. Binding of rFimR to DNA was carried out in a 20-μL reaction mixture containing 20 fmol biotin-labeled DNA, 10 mM Tris, pH 7.5, 50 mM KCl, 1 mM dithiothreitol, 10 ng μL−1 poly (dI–dC), 2% glycerol, 0.05% NP-40, 2 mM MgCl with various amounts of purified rFimR protein (10, 20 and 40 pmol μL−1) at room temperature for 30 min. Samples were then loaded and run into a 5% nondenaturing polyacrylamide gel in 0.5 × TBE buffer. The electrophoresis was carried out for 2 h at a constant 100 V at 4°C. The DNA and protein complexes were then transferred to a positively charged nylon membrane (380 mA, 30 min). The biotin end-labeled DNA was detected using the streptavidin–horseradish peroxidase conjugate and the chemiluminescent substrate. Each EMSA experiment was repeated at least three times.
Results
Role of FimR in mfa1 expression
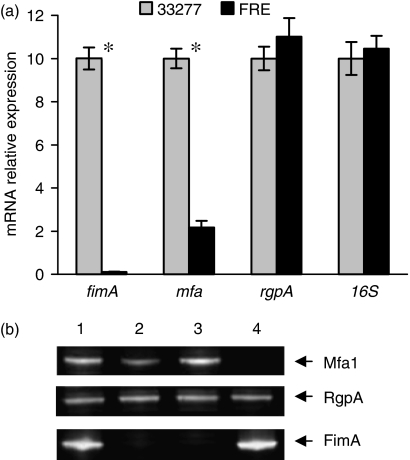
The fimA gene is the only gene known to be tightly controlled by the FimS/FimR system. It was postulated that the expression of other genes may also be controlled by this two component regulatory system. To investigate effects of FimR on expression of the mfa1 gene, an insertional fimR mutant was constructed by allelic replacement. Expression of fimA and mfa1 in the fimR− mutant was determined using real-time PCR analysis. Statistically significant differences of the level of gene expression in 33277 and the fimR− mutant were calculated by a Student's t-test. As shown in Fig. 1a, expression of the fimA gene was abolished in the fimR− mutant strain FRE. This result is consistent with previous observations (Hayashi et al., 2000; Nishikawa et al., 2004). The striking finding is that expression of the mfa1 gene was also repressed threefold in the fimR− mutant, although not to the degree observed with the fimA expression. However, the fimR− mutation had no effect on expression of rgpA, a gene encoding the arginine-specific protease, or the P. gingivalis 16S RNA gene. This analysis suggests the FimS/FimR system is required for expression of both major and minor fimbriae.
Fig. 1.
Expression of fimbrial genes in Porphyromonas gingivalis. (a) Fold changes in gene transcription in wild type strain 33277 and the fimR− mutant were measured by real-time PCR. Primers used for each gene are shown in Table 2. Results shown are means and SDs from triplicate experiments. Fold differences were calculated using the relative comparison method. Significant differences (P<0.001 by t test) are labeled with asterisks. (b) Western blot analyses show the expression of Mfa1, RgpA, and FimA in wild type 33 277 (lane 1), the fimR− mutant FRE (lane 2), the fimA− mutant FAT (lane 3), and the mfa1− mutant MFAE (lane 4).
To determine production of long (major) and short (minor) fimbriae in the fimR− mutant, western blotting was performed with a polyclonal anti-FimA or anti-Mfa1 antibody to compare fimbrial production in wild-type strain (33277), the fimR− mutant (FRE), the fimA− mutant (FAT) and the mfa1− mutant (MFAE). Density of protein bands was determined by UVP Bioimaging System (UVP, CA). This analysis revealed that the expression of the fimA and mfa1 genes was consistent at the mRNA level and protein level (Fig. 1b). FimA protein was not detectable in the fimR− mutant, while a 50% lower level of Mfa1 protein was found in the fimR− mutant compared with that in wild-type strain 33277. Similarly, there was no apparent change in RgpA production in the fimR− mutant, which was detected by anti-RgpA serum.
Identification of the transcriptional start site of the mfa1 gene
To identify the promoter region of mfa1, the transcriptional start site was first determined. The RACE experiment was first conducted with mfa1-specific reverse primers MfaTSR1 located at 135 bp up-stream of the potential start codon and MfaTSR2 located at 37 bp downstream of the potential start codon (Fig. 2a). The transcriptional start site (the A) of mfa1 was at 434 bp upstream from the potential start codon (Fig. 2b). To verify the result, the RACE experiment was repeated with mfa1-specific reverse primers MfaTSR3 and MfaTSR4 located at 237 bp upstream of the potential start codon. The same transcriptional start site was identified.
Fig. 2.
Determination of the transcriptional start site of mfa1. (a) Four different mfa1 sense-strand primers (lanes 2–5, primers MfaTSR2, MfaTSR1, MfaTSR4, MfaTSR3, respectively) were used with 5′ RLM-RACE primers to determine the approximate transcriptional start for mfa1. The PCR products were visualized on a 1.5% agarose TAE gel containing ethidium bromide. Molecular weight standards (indicated in base pairs) are in lane 1. (b) DNA sequence of the mfa1 promoter region. The transcriptional start site A (+1) and the potential start codon ATG are bolded. The primers used for RLM-RACE and RT-PCR are underlined. (c) RT-PCR analysis. Lane 1, 1 kb ladder marker; lane 2 is RT-PCR with MfaTSF3 (from −139 to −121) and MfaTSR5 (from +805 to +824); lane 3 is RT-PCR with MfaTSF2 (from −66 to −47) and MfaTSR5 (from +805 to +824); lane 4 is RT-PCR MfaTSF1 (from +6 to +25) and MfaTSR5 (from +805 to +824).
To confirm the result of RACE, reverse-transcriptional PCR using three sets of primers was performed. As shown in Fig. 2c, the PCR product was generated only with primers MfaTSF1 (corresponding to +6 to +25) and MfaTSR5 (+805 to +824). There was no PCR product generated from RT-PCR using the primers (MfaTSF3, from −139 to −121 or MfaTSF2, from −66 to −47) which correspond to the DNA sequences upstream from the transcriptional start site. This transcriptional start site is 390 bp upstream of the site previously reported (Park et al., 2006). It is likely that mfa1 gene possesses two functional promoters, which are also detected in the fimA gene of P. gingivalis (Xie & Lamont, 1999; Nishikawa et al., 2004).
Binding of FimR to the promoter region of mfa1
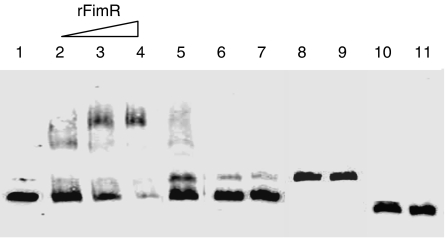
The previous study has shown that the mechanism of FimR activation of the fimA gene involves a regulatory cascade (Nishikawa et al., 2004). It was postulated that different mechanisms might be involved in FimR-mediated mfa1 expression, since expression regulation of mfa1 by FimR was not controlled as tightly as observed for fimA expression. One possibility is that FimR modulates mfa1 expression by directly binding to the promoter region of mfa1. To test this hypothesis, electrophoretic mobility shift assays were performed. The mfa1 promoter (positioned from +18 to −138), fimA promoter (positioned from −22 to−190) (Xie & Lamont, 1999) and mfa1 coding DNA (positioned from +1253 to +1373) were generated by PCR with the 5′ biotin-labeled primers (Table 1). The recombinant FimR (rFimR) was expressed in pET expression system and purified from E. coli. The rHGP44 protein, a binding domain of P. gingivalis gingipains (Xie et al., 2006), expressed in the same system and purified by the same procedures as rFimR was used as a control. Cold competitor chase experiments with a 100-fold excess of unlabeled DNA probe as a specific competitor were also used to demonstrate the specificity of rFimR binding. As shown in Fig. 3, the DNA fragment of the mfa1 promoter region was shifted in the presence of the rFimR. Retarded mfa1 promoter-rFimR complex was detected with as little as 10 pmol μL−1 rFimR (Fig. 3). As the concentration of rFimR increased, the retarded protein–DNA complex became evident, with complete loss of the mfa1 promoter DNA. The unlabeled mfa1 promoter fragments effectively competed with the labeled fragment, suggesting a specific interaction between rFimA and the mfa1 promoter. To investigate the role of phosphorylation of FimR in its binding to the mfa1 promoter region, EMSA experiments were also performed with the phosphorylated rFimR. No significant difference was detected in the level of DNA binding between the phosphorylated rFimR and unphosphorylated rFimR (data not shown). In agreement with a previous report (Nishikawa et al., 2004), rFimR did not bind to the fimA promoter region, suggesting that regulation of fimA expression by FimR is through a different mechanism. Moreover, incubation of rHGP44 with mfa1 promoter fragment did not retard the DNA movement in polyacrylamide gel. There was also no DNA shift detected when rFimR was incubated with the coding region of mfa1. These data clearly show that FimR protein can bind specifically to the mfa1 promoter region, acting as an activator of mfa1 transcription. EMSA experiments were also performed to examine whether the rFimR binds to the other promoter region identified by Park et al. (2006). The biotin-labeled DNA fragment corresponding to this promoter region did not shift in the presence of the rFimA protein (data not shown), suggesting that only the promoter identified here is involved for mfa1 expression mediated by FimR.
Fig. 3.
Nucleic acid binding properties of FimR. Electrophoretic mobility shift assays were performed using the biotin-labeled DNA probes. Lane 1, the biotin-labeled mfa1 promoter region (156 bp) alone; lane 2–4, mobility of the biotin-labeled mfa1 promoter region was noted in the presence of increasing amounts of rFimR protein (10, 20, and 40 pmol μL−1, respectively), as indicated on the top; lane 5, the biotin-labeled mfa1 promoter region, rfimR 40 pmol μL−1, and 100-fold excess of unlabeled mfa1 probe; lane 6 is the biotin-labeled mfa1 promoter region alone; lane 7 is the biotin-labeled mfa1 promoter region, and rHGP44 40 pmol μL−1; lane 8 is the biotin-labeled fimA probe (176 bp) alone; lane 9 is the biotin-labeled fimA probe (176 bp), and rFimR 40 pmol μL−1; lane 10 is the biotin-labeled mfa1 coding region (121 bp) alone; lane 11 is the biotin-labeled mfa1 coding region (121 bp) and rFimR 40 pmol μL−1.
Discussion
The two-component regulatory system is a major mechanism of signal transduction and is widespread in bacteria. Six putative two-component regulatory systems were detected by surveying the P. gingivalis W83 genome database for homologues of the two-component sensor histidine kinase (Hasegawa et al., 2003). Although most target genes of P. gingivalis two-component systems are unknown, the role of the FimS/FimR in expression of the fimA gene is well defined. Expression of minor fimbriae (mfa1) in a fimR mutanthas been investigated. A comparison of the transcriptional level of the mfa1 in P. gingivalis wild-type strain and in the fimR mutant indicates that the FimS/FimR system is a positive regulator for the mfa1 gene, although the system controls two fimbrial genes at different levels. It is hypothesized that the FimS/FimR system regulates expression of each fimbrial gene through a unique mechanism. The previous study suggested that regulation of fimA expression by FimR is through a regulation cascade involving interaction of FimR and the promoter region of the first gene in the fimA cluster (Nishikawa et al., 2004). Here it is demonstrated that FimR binds directly to the promoter region of the mfa1 gene, suggesting a direct role of FimR in activation of mfa1 expression. It has also been reported previously that the transcriptional activity of fimA was reduced in the fimA mutant, indicating multiple levels of control of fimA expression in P. gingivalis (Xie et al., 2000a). This may explain the much tighter control of fimA expression by FimR. However, the possibility cannot be excluded that other regulatory elements are also involved in expression of the mfa1 gene.
A two-component regulatory system typically contains a membrane-bound histidine kinase sensor and a cytosolic response regulator. Phosphorylation, mediated by histidine kinase at a specific aspartate residue, activates DNA-binding activity of the response regulator and initiates the corresponding cellular response. However, no apparent difference was found in DNA-binding affinity between rFimRs with or without acetyl phosphate treatment. Observation suggests that different mechanisms may be involved in P. gingivalis FimR activation. Activation of a regulatory protein not corresponding to phosphorylation was also observed in Streptococcus mutans (Biswas & Biswas, 2006). Phosphorylation of CovR, a global response regulator, had no effect on its DNA-binding affinity. The fact that FimR was not activated by phosphorylation may also be due to the short lifetime of the phosphorylated state, which has been observed in other bacteria (Stock et al., 2000).
The transcriptional start site of the mfa1 gene located at 434 bp upstream of the putative start codon was detected, which is also 390 bp upstream of the site previously reported (Park et al., 2006). The transcriptional site revealed here is confirmed by RT-PCR analysis. Data of this study suggest that transcription of the mfa1 gene originated at a distal upstream transcriptional start site and read through the promoter region suggested by Park et al. (2006). Moreover, FimR appears to act on the promoter region identified here, suggesting that this promoter may make significant contributions toward mfa1 expression through the FimS/FimR system. Gene expression under the control of two promoters is not uncommon in bacteria. In E. coli, two promoters direct transcription of acs encoding, an acetate-scavenging enzyme required for fitness during periods of carbon starvation – the distal acsP1 and the proximal acsP2 (Beatty et al., 2003). It is suggested that each promoter may interact with different regulatory elements. Two promoter regions in the P. gingivalis fimA gene were also reported (Xie & Lamont, 1999; Nishikawa et al., 2004). A cascade regulation starting with FimR appears to act on the upstream promoter (Nishikawa et al., 2004). The observations that FimR binds only to the upstream promoter region of the mfa1 gene and that activity of the downstream promoter is inhibited by S. gordonii, S. sanguinis and S. mitis (Park et al., 2006) imply the complexity of regulation of mfa1 expression. It is possible that two promoters of mfa1 are regulated in response to different environmental signals. The hypothesis is currently under investigation.
In conclusion, P. gingivalis fimbriae play a predominant role in the attachment of the organism to a variety of oral surfaces (Lamont & Jenkinson, 2000; Amano et al., 2004), although other surface proteins, such as gingipains, may also be involved in the bacterial colonization (Tokuda et al., 1996; Chen et al., 2001). It has been recently reported by the authors that both major fimbriae and minor fimbriae contribute to the formation of P. gingivalis biofilm (Lin et al., 2006). Major fimbriae are required for initial attachment and the minor fimbriae appear to play an important role in microcolony formation by facilitating cell–cell interactions. The data presented here provide evidence that these two distinct fimbriae are under the control of a two-component regulatory system: FimS/FimR. Expression of major fimbriae (FimA) is extremely low in the fimR mutant, and minor fimbriae production in this mutant is inhibited by least 50%. Therefore, it is proposed that FimR can be an attractive target for inhibition of P. gingivalis colonization.
Acknowledgments
This work was supported by Public Health Service grant DE014699 from the National Institute of Dental and Craniofacial Research to H.X.
Statement
Re-use of this article is permitted in accordance with the Creative Commons Deed, Attribution 2.5, which does not permit commercial exploitation.
References
- Amano A, Nakagawa I, Okahashi N, Hamada N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J Periodontal Res. 2004;39:136–142. doi: 10.1111/j.1600-0765.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- Beatty CM, Browning DF, Busby SJ, Wolfe AJ. Cyclic AMP receptor protein-dependent activation of the Escherichia coli acsP2 promoter by a synergistic class III mechanism. J Bacteriol. 2003;185:5148–5157. doi: 10.1128/JB.185.17.5148-5157.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Biswas I. Regulation of the glucosyltransferase (gtfBC) operon by CovR in Streptococcus mutans. J Bacteriol. 2006;188:988–998. doi: 10.1128/JB.188.3.988-998.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005;192:554–557. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Brodala N, Merricks EP, Bellinger DA, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2005;25:1446–1451. doi: 10.1161/01.ATV.0000167525.69400.9c. [DOI] [PubMed] [Google Scholar]
- Chen T, Nakayama K, Belliveau L, Duncan MJ. Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect Immun. 2001;69:3048–3056. doi: 10.1128/IAI.69.5.3048-3056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HH, Yumoto H, Davey M, Takahashi Y, Miyamoto T, Gibson FC, III, Genco CA. Porphyromonas gingivalis fimbria-dependent activation of inflammatory genes in human aortic endothelial cells. Infect Immun. 2005;73:5367–5378. doi: 10.1128/IAI.73.9.5367-5378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DP, Kubiniec MA, Yoshimura F, Genco RJ. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988;170:1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher HM, Schenkein HA, Morgan RM, Bailey KA, Berry CR, Macrina FL. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada N, Sojar HT, Cho MI, Genco RJ. Isolation and characterization of a minor fimbria from Porphyromonas gingivalis. Infect Immun. 1996;64:4788–4794. doi: 10.1128/iai.64.11.4788-4794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Nishiyama S, Nishikawa K, Kadowaki T, Yamamoto K, Noguchi T, Yoshimura F. A novel type of two-component regulatory system affecting gingipains in Porphyromonas gingivalis. Microbiol Immunol. 2003;47:849–858. doi: 10.1111/j.1348-0421.2003.tb03451.x. [DOI] [PubMed] [Google Scholar]
- Hayashi J, Nishikawa K, Hirano R, Noguchi T, Yoshimura F. Identification of a two-component signal transduction system involved in fimbriation of Porphyromonas gingivalis. Microbiol Immunol. 2000;44:279–282. doi: 10.1111/j.1348-0421.2000.tb02496.x. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immunol. 2000;15:341–349. doi: 10.1034/j.1399-302x.2000.150601.x. [DOI] [PubMed] [Google Scholar]
- Levesque C, Lamothe J, Frenette M. Coaggregation of Streptococcus salivarius with periodontopathogens: evidence for involvement of fimbriae in the interaction with Prevotella intermedia. Oral Microbiol Immunol. 2003;18:333–337. doi: 10.1034/j.1399-302x.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- Lin X, Wu J, Xie H. Porphyromonas gingivalis minor fimbriae are required for cell–cell interactions. Infect Immun. 2006;74:6011–6015. doi: 10.1128/IAI.00797-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Nagata H, Yamamoto Y, Tanaka M, Tanaka J, Minamino N, Shizukuishi S. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect Immun. 2004;72:1341–1348. doi: 10.1128/IAI.72.3.1341-1348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek R, Fisher JG, Caleca A, Stinson M, van Oss CJ, Lee JY, Cho MI, Genco RJ, Evans RT, Dyer DW. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J Bacteriol. 1994;176:1052–1059. doi: 10.1128/jb.176.4.1052-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Murakami Y, Noguchi T, Yoshimura F. Effects of various growth conditions in a chemostat on expression of virulence factors in Porphyromonas gingivalis. Appl Environ Microbiol. 2006;72:3458–3467. doi: 10.1128/AEM.72.5.3458-3467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Yoshimura F, Duncan MJ. A regulation cascade controls expression of Porphyromonas gingivalis fimbriae via the FimR response regulator. Mol Microbiol. 2004;54:546–560. doi: 10.1111/j.1365-2958.2004.04291.x. [DOI] [PubMed] [Google Scholar]
- Park Y, Simionato MR, Sekiya K, Murakami Y, James D, Chen W, Hackett M, Yoshimura F, Demuth DR, Lamont RJ. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect Immun. 2005;73:3983–3989. doi: 10.1128/IAI.73.7.3983-3989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, James CE, Yoshimura F, Lamont RJ. Expression of the short fimbriae of Porphyromonas gingivalis is regulated in oral bacterial consortia. FEMS Microbiol Lett. 2006;262:65–71. doi: 10.1111/j.1574-6968.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Tokuda M, Duncan M, Cho MI, Kuramitsu HK. Role of Porphyromonas gingivalis protease activity in colonization of oral surfaces. Infect Immun. 1996;64:4067–4073. doi: 10.1128/iai.64.10.4067-4073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Belton CM, Park Y, Lamont RJ. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Lamont RJ. Promoter architecture of the Porphyromonas gingivalis fimbrillin gene. Infect Immun. 1999;67:3227–3235. doi: 10.1128/iai.67.7.3227-3235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Cai S, Lamont RJ. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect Immun. 1997;65:2265–2271. doi: 10.1128/iai.65.6.2265-2271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Chung WO, Park Y, Lamont RJ. Regulation of the Porphyromonas gingivalis fimA (Fimbrillin) gene. Infect Immun. 2000a;68:6574–6579. doi: 10.1128/iai.68.12.6574-6579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Cook GS, Costerton JW, Bruce G, Rose TM, Lamont RJ. Intergeneric communication in dental plaque biofilms. J Bacteriol. 2000b;182:7067–7069. doi: 10.1128/jb.182.24.7067-7069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Kozlova N, Lamont RJ. Porphyromonas gingivalis genes involved in fimA regulation. Infect Immun. 2004;72:651–658. doi: 10.1128/IAI.72.2.651-658.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Belogortseva NI, Wu J, Lai WH, Chen CH. Inhibition of human immunodeficiency virus Type 1 entry by a binding domain of Porphyromonas gingivalis gingipain. Antimicrob Agents Chemother. 2006;50:3070–3074. doi: 10.1128/AAC.01578-05. [DOI] [PMC free article] [PubMed] [Google Scholar]