Abstract
The use of dry powder inhalers (DPIs) to administer treatments for respiratory diseases has increased significantly in recent years. There is now a wide range of DPIs available that vary considerably in design, required operational techniques, output characteristics and drug delivery across a range of inhalation patterns. Different patient populations may find individual types of DPI easier to use correctly than others and selecting the right DPI for particular patient requirements will improve compliance with therapy. For example, some DPIs offer a greater resistance against inspirational flow rate than others which affects the total emitted dose and also fine particle mass of the aerosol released. An individual patient may therefore receive different amounts of drug when inhaling from different DPIs. Therefore, it is important that the prescriber is fully aware of the characteristics of the different types of DPI, so that he or she can prescribe the device that is most appropriate to an individual patient's needs. This review explores the characteristics of currently available DPIs and evaluates their efficacy and patient acceptability. The differences in output characteristics, ease of use and patient preferences between available devices is shown to affect treatment efficacy and patient compliance with therapy. Changing the DPI prescribed to a patient to a cheaper or generic device may therefore adversely affect disease control and thereby increase the cost of treatment.
Review Criteria
The information presented in this review has been sourced from published literature. In particular, the review has focussed on comparative studies that have measured in vitro aerosol output characteristics of different types of dry powder inhaler, and also clinical studies that have assessed ease of handling and patient preferences for different dry powder inhalers.
Message for the Clinic
Different types of dry powder inhaler have different output and handling characteristics. Therefore, it is important to select the best DPI for any given patient, based on aerosol delivery, ease of handling and patient preference because these factors directly affect treatment compliance and therefore disease control. Substituting a patient's preferred DPI for a cheaper, generic device may prove to be a false economy and deleteriously influence treatment efficacy and patient outcomes.
Introduction
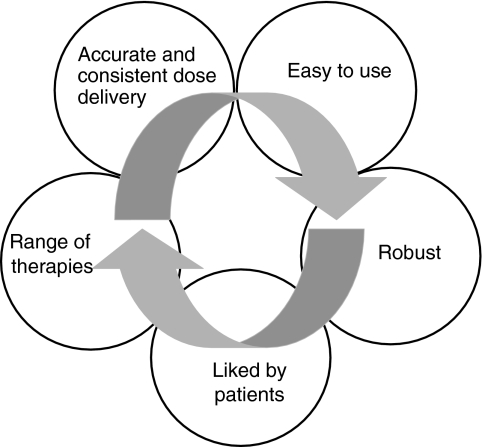
The benefits of inhaled therapy for the treatment of lung diseases have been recognised for many years. In comparison with oral or parenteral formulations, minute but therapeutic doses of drug are delivered topically into the airways causing local effects within the lung. Unwanted systemic effects are minimised as the medication acts with maximum pulmonary specificity together with a rapid onset and duration of action. Consequently, aerosol formulations of bronchodilators and corticosteroids are the mainstay of modern treatment for asthma and chronic obstructive pulmonary disease (COPD). Central to the success of inhaled treatment has been the availability of efficient aerosol delivery systems or ‘inhalers’. To provide consistent clinical control, an appropriate inhaler should satisfy the criteria that are described in Figure 1.
Figure 1.
Criteria for an ideal inhaler
The pressurised metered dose inhaler (pMDI) was first introduced 50 years ago for the delivery of bronchodilators (1). It was readily accepted by patients and soon formulated to contain other classes of asthma medications. It was particularly useful for the administration of corticosteroids which hitherto had been administered orally. Because of the large doses needed for oral administration, corticosteroid treatment for asthma was associated with an unacceptably high adverse event profile (2). As a result of the availability of inhaled formulations, with the introduction of beclometasone dipropionate in 1972, inhaled corticosteroids are now part of the cornerstone of asthma treatment. Virtually every class of inhaled drug is now formulated as a pMDI. This small and unobtrusive device remains the most commonly used inhalation device worldwide (3), with estimates of annual production in excess of 800 million units (4). A significant factor in its enduring popularity is its advantage of being cheap and relatively simple to manufacture on a large commercial scale and also the availability of a range of drugs that can be formulated for pMDIs.
In addition to pMDIs, dry powder inhalers (DPIs) have been available since 1967. The Spinhaler™ (Aventis) was first introduced for the delivery of sodium cromoglycate (5). It was developed because it was not possible for a pMDI to accommodate the large (20 mg) required dose of sodium cromoglycate for each administration. To deliver a dose of such magnitude, this aerosol delivery system comprised an inhaler which was supplied with separate capsules. Each gelatin capsule contained a single dose of drug, which was placed inside the inhaler before each use and the empty capsule was discarded. Presentation of the formulation in a capsule also provided protection from moisture ingress. This is essential to maintain good powder flow and ensure that the drug particles have the potential to be deposited in the lungs during normal patient use. The Spinhaler™ was regarded as inconvenient to use because of the number of steps required to administer each dose. Nevertheless, this type of system is still widely used especially when protection of the drug formulation from moisture is important, for example the recent introduction of tiotropium in the Handihaler™ (Boehringer-Ingelheim) device.
A DPI has some distinct advantages over pMDIs for the delivery of inhaled drugs to two particular groups of patients. Many children and elderly patients have difficulty using a pMDI correctly, because of the high velocity at which each dose is released and, therefore have problems following the inhalation technique recommended in the Patient Information Leaflet. Extensive training is required to achieve correct use of a pMDI. To deliver the drug effectively into the lung, the patient must actuate the pMDI as they start to inhale. This requires a high degree of ‘hand/lung’ co-ordination and failure to achieve this often results in reduced effectiveness of treatment and poor disease control (6,7). Because the Spinhaler™ required inspiratory effort to draw the medication from the device, drug was only released while the patient inhaled. Therefore, the issue of ‘hand-lung’ co-ordination was resolved. However, this problem has been substituted by another problem that affects all DPIs. To ensure that the dose emitted from a DPI contains drug particles that have the greatest potential to be delivered to the conducting airways, it is necessary for the patient to generate adequate inspiratory effort. The faster the inspiration rate through the DPI (and hence acceleration), the better is the quality of the emitted dose for lung deposition. This applies to all DPIs, but for some the effect is minimal, whereas other DPIs show significant flow-dependent dose emission, which may result in erratic dose emission and in turn compromise consistent disease control. The latter problem could be overcome by recommending adjustable maintenance dosing regimens to a patient's management plan.
Concurrent with the introduction of DPIs was a growing environmental concern that the chlorofluorocarbon propellants used in pMDIs were causing irreparable damage to the ozone layer in the atmosphere (8). The pharmaceutical industry was therefore committed to the development of non-chlorofluorocarbons (CFC) propellants for use in pMDIs and also DPIs that required no propellant at all. The reformulation to change the propellant used in pMDIs to those based on hydrofluoroalkanes, in place of CFC was not easy and some difficulties still remain. Consequently, other DPIs began to appear on the market. The first such DPIs were similar to the Spinhaler™, for example salbutamol (9) and beclometasone dipropionate (10) delivered via the Rotahaler™ (GlaxoSmithKline) and ipratropium bromide delivered by the Aerohaler™ (Boehringer-Ingelheim) (11). Dose emission from some of these DPIs was less than that from the corresponding pMDI and therefore the recommended doses from the DPIs were double those from a pMDI.
Gradually, a new generation of novel DPIs became available with extensively different designs, operating characteristics and improved drug delivery to the lung. Some devices contained a reservoir of drug such as the Turbuhaler™ (Astra Zeneca), Clickhaler™ (Innovata Biomed) and Easyhaler™ (Orion), while other devices had individually sealed unit doses of drug, such as the Diskhaler™ (GlaxoSmithKline) and Diskus™ (GlaxoSmithKline) (otherwise known as the Accuhaler™ in the UK). Some DPIs, such as the Clickhaler™ and Easyhaler™ were designed to resemble pMDIs as closely as possible while other devices, such as Diskus™, were designed to facilitate easy use and patient acceptability. Other refinements such as integral dose counters to enable patients and their healthcarers to monitor their inhaler use have also been incorporated into the design of some DPIs. The importance of protecting the formulation, especially for those designed on the reservoir concept has also been considered.
Dry powder inhalers have now an established role in inhalation therapy. It is estimated that in 2004 approximately 113 million DPIs were dispensed worldwide (12). As the number of different types of DPIs on the market continues to increase, particularly with the advent of generic DPIs, the prescriber may experience some uncertainty in the selection of the optimal inhalation device for any given patient. This may be compounded by recent suggestions that because pMDIs are, in general, cheaper than DPIs, they should be prescribed as first-line treatments for all patients (13,14). Furthermore, it has been proposed that it is acceptable to switch patients from more expensive DPIs to pMDIs or generic DPIs without compromising treatment efficacy in asthmatic patients (14). However, the interchangeability of DPIs has been doubted (15).
Characteristics and performance of dry powder inhalers
The range of DPIs that are currently available falls into three device categories: single-unit dose inhalers in which each dose is loaded into the device before use; multi-dose reservoir inhalers in which a bulk supply of drug is preloaded into the device and multi-unit dose inhalers in which several single doses are individually sealed and discharged each time the device is actuated. Table 1 summarises the more common DPIs that fall into the three categories. In recent years, there has been a dramatic increase in the number of patent applications for new DPIs and it is anticipated that many more will be introduced in the future. Furthermore, attention has recently focussed on using the pulmonary route to deliver active compounds into the systemic circulation. For example, a DPI formulation of insulin (Exubera, Pfizer in collaboration with Nektar Therapeutics) will soon be available.
Table 1.
Examples of commercially available dry powder inhaler
| DPI type | Device name | Company | Drugs available |
|---|---|---|---|
| Single-unit dose | Aerolizer™ | Novartis | Formoterol |
| Cyclohaler™ | Pharmachemie | Salbutamol | |
| BDP | |||
| Budesonide Ipratropium bromide | |||
| Rotahaler™ | GlaxoSmithKline | Salbutamol | |
| BDP | |||
| Salbutamol + BDP | |||
| Spinhaler™ | Aventis | Sodium cromoglycate | |
| Inhalator™ | Boehringer-Ingelheim | Fenoterol | |
| Handihaler™ | Boehringer-Ingelheim | Tiotropium | |
| Multi-dose reservoir | Clickhaler™ | Innovata Biomed/ML Labs Celltech | Salbutamol |
| BDP | |||
| Easyhaler™ | Orion Pharma | Salbutamol | |
| BDP | |||
| Pulvinal™ | Chiesi | Salbutamol | |
| BDP | |||
| Turbuhaler™ | Astra Zeneca | Salbutamol | |
| Terbutaline | |||
| Formoterol | |||
| Budesonide | |||
| Formoterol/BUD | |||
| Twisthaler™ | Schering-Plough | Mometasone | |
| Novolizer™ | ASTA Medica | Budesonide | |
| Multi-unit dose | Aerohaler™ | Boehringer-Ingelheim | Ipratropium bromide |
| Fenoterol | |||
| Diskhaler™ | GlaxoSmithKline | Salbutamol* | |
| Salmeterol | |||
| BDP | |||
| FP | |||
| Diskus™/Accuhaler™ | GlaxoSmithKline | Salbutamol | |
| Salmeterol | |||
| FP | |||
| Salmeterol + FP |
Recently discontinued. BDP, beclometasone dipropionate; BUD, budesonide; FP, fluticasone propionate.
Single-unit dose devices
In single-unit dose devices, such as Spinhaler™, Rotahaler™ and Handihaler™, the drug, which is formulated as a micronised powder in a lactose excipient, is supplied in individual single-dose gelatin capsules which must be inserted into the inhaler before use. In the Spinhaler™, the capsule is placed into a holder located on top of a propeller. The walls of the capsule are pierced by two spears when the patient primes the device by sliding a cam, while in the Rotahaler™ the capsule is severed by a twisting action. Once the capsule has been broken, the patient inhales through the device causing the propeller to turn and vibrate dispersing the powder into the inspired airstream (5,16). After use, the remains of the gelatin capsule must be removed from the inhaler before the next capsule can be placed in the device. Consequently, devices such as these are inconvenient to use and have largely been superseded by multi-dose DPIs. However, a single-unit DPI, the Handihaler™, has recently been developed for the delivery of tiotropium to patients with COPD (17). The use of the Handihaler™ has been shown to be associated with a significantly high and age-related error rate, because of the complexity of its operation (18). In addition, the Handihaler™ has a high internal resistance and many COPD patients have problems achieving an adequate inhalation rate to emit the required dose to be deposited in the airways (19,20). As patients with COPD are generally older than asthmatics, the use of simpler multi-unit dose DPIs, which are easy to use and in which inhalation flow rate has minimal effect on dosage emission and ease, such as Diskus™, may well be beneficial in this population, (21,22) as well as those with asthma.
Multi-dose reservoir devices
Multi-dose DPIs, by definition, contain more than one dose of drug. There are two types of multi-dose DPI, reservoir and multi-unit dose devices. Multi-dose reservoir devices contain a bulk supply of drug from which individual doses are released with each actuation. The first such inhaler to be developed was the Turbuhaler™ (23) which is used to deliver β2-agonists and corticosteroids separately and in combination. The drug located within this inhaler is formulated as a pellet of a soft aggregate of micronised drug which may be formulated with or without any additional lactose excipient. To release a dose of drug, the patient twists the base of the device resulting in a dose of drug being shaved off the pellet while holding the inhaler in a vertical position. It is essential that this orientation is used when dose metering all reservoir DPIs, because they rely on gravity to fill the dose metering cup. The dose is then dispersed by turbulent airflow as the patient inhales through the device. This turbulent airflow creates the energy to disperse particles in the emitted dose that are small enough to have a high possibility of depositing in the conducting airways.
Other multi-dose reservoir devices have become available in recent years including the Easyhaler™ (24), Clickhaler™ (25) and Twisthaler™ (Schering-Plough) (26). The design of new multi-dose reservoir DPIs has focussed on minimising the flow-dependent dose emission that occurs with the Turbuhaler™. Also, attention has been directed to the protection of the formulation from moisture ingress during routine storage and patient use. For example, the Easyhaler™ has a protective case and the hopper is designed, so that it is impossible for the patient to blow into it. Furthermore, all multi-dose reservoir DPIs are packaged with a protective wrapper to prevent moisture ingress prior to dispensing and patient use. The majority of this type of DPIs are disposable and cannot be refilled with additional drug. However, the Novolizer™ (ASTA Medica) is rechargeable and designed to be used with cartridges that contain 200 doses of drug (27).
Multi-unit dose devices
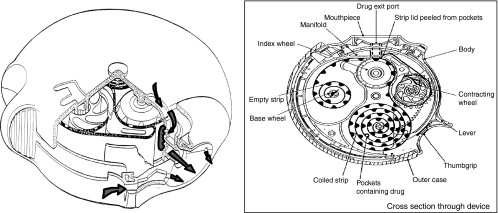
Multi-unit dose DPIs utilise individually prepared and sealed doses of drug. The first such DPI was the Aerohaler™ which contained six unit dose capsules as a magazine, each delivering one dose of drug. The device was used to deliver fenoterol and ipratropium bromide and was very similar in design to single-unit dose inhalers. The Diskhaler™ is used in conjunction with refill Rotadisks™ which house four or eight sealed blisters containing drug and lactose excipient (28). Excipients such as lactose improve dose uniformity by increasing the mass of powder for each dose thereby improving the accuracy of dose metering and minimising the effect of inhalation flow-dependent dose emission. The sealed blisters offer a high degree of protection against environmental factors such as humidity and because the premetered doses of drug are factory prepared and separately packaged, dose uniformity is assured. The patient operates the Diskhaler™ by lifting the mouthpiece lid which causes a blister in the Rotadisk™ to be pierced and the dose to be released as the patient inhales through the device. The Diskus™ or Accuhaler™, shown in Figure 2, was developed to enable the sealed dose uniformity achieved in the Diskhaler™ to be combined with a larger number of doses. Furthermore, the Diskus™ was designed to simplify use by providing up to 1 month's medication in one device without the need to manually replace spent cartridges or capsules. The Diskus™ houses a coiled strip of 60 double foil-wrapped individual doses. The patient operates the inhaler by sliding a lever which moves the next dose-containing blister into place. A ratchet within the inhaler causes the device to click when the next dose is properly positioned. Priming the device in this way simultaneously peels the two layers of foil apart exposing the dose ready for inhalation. The Diskus™ also incorporates a dose counter, which enables the patient to monitor the number of doses remaining in the device, and also has an integral outer case which serves to keep the device dust free and also resets the lever ready for the next dose.
Figure 2.
The Diskus inhaler
Performance of DPIs
As a result of the wide variation in design characteristics of the many DPIs available, their performance characteristics vary considerably and this may impact their suitability for use in different patient populations. Therefore, it is imperative that before prescribing a DPI for an individual patient, the characteristics of that DPI are known so that its suitability can be assessed. The main factors described in Figure 1 that must be taken into account are summarised in Table 2.
Table 2.
Factors affecting dry powder inhaler use
| Drug delivery | Consistent dose delivery throughout device life |
| Dose reproducibility across range of temperatures and humidities | |
| High proportion of dose available for inhalation over a range of inspiratory flow rates | |
| Large fine particle mass in relation to total emitted dose (see section Drug delivery) | |
| Low resistance of device to airflow during inhalation | |
| Ease of use | Small number of steps required to actuate device |
| Low inhaler technique training requirements | |
| Low degree of manual dexterity required to use device | |
| Patient preference | Appropriate size and low obtrusiveness |
| Incorporation of a dose counter | |
| Positive reinforcement of dose delivery |
Drug delivery
It is easy to assume that when an inhaler is actuated, the dose of drug delivered to the patient is the same as that cited on the package (label claim). However, this is not always the case. There are considerable differences in the proportions of the nominal dose (label claim) released from different DPIs which is defined as the total emitted dose (TED). European and American regulatory agencies have now put into place standards that specify output requirements in terms of the quantity and variability in the emitted doses from DPIs (29,30). These standards are designed to ensure consistent dosing from DPIs, both throughout the life of an individual DPI and also between inhalers of the same DPI make.
The TED is the term used to describe the quantity of drug that is released from an inhaler during a single actuation. Within the TED, therapeutic benefit is derived from the mass of drug particles that are small enough to reach the airways during inhalation. This parameter is described as the fine particle fraction (FPF) or fine particle mass (FPM) and refers to mass of particles released in an actuation that have an aerodynamic diameter of < 5 μm (31). These particles have the greatest potential to be deposited on to the airways during an inhalation. Larger aerosol particles tend to deposit in the oropharynx and are swallowed.
Both the TED and FPF are measured in the laboratory using in vitro pharmacopoeial methods. Using these methods, it has been demonstrated that there is a wide variation in performance, in terms of TED and FPF, from the range of DPIs available as a result of intrinsic design differences. These design differences affect parameters such as the internal resistance of a device which in turn affects the flow rate achieved through the DPI. For example, when the Turbuhaler™ and Diskus™ were compared for the delivery of budesonide and fluticasone propionate respectively, the Diskus™ delivered 87–93% of the label claimed dose while the Turbuhaler™ delivered 40–58% (32).
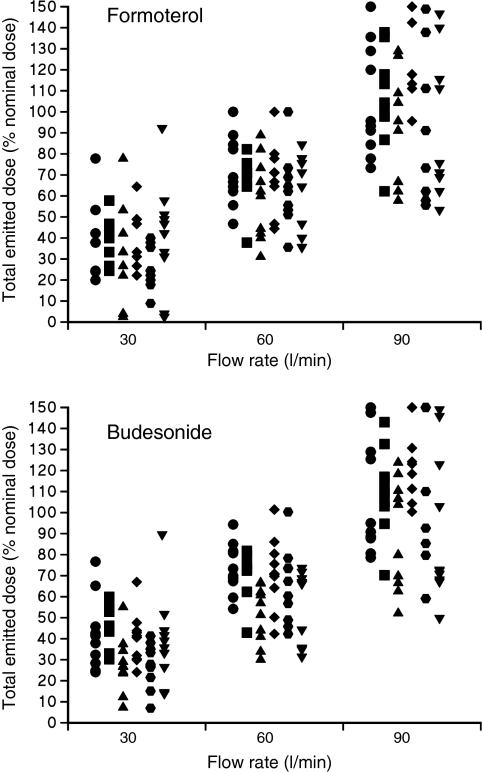
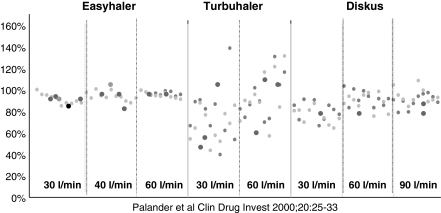
Flow rate has been shown to directly affect the proportion of the nominal dose (TED) and also the FPF of the aerosol released from different types of DPI. This effect is more significant with some DPIs than others. For example, at a constant sampling flow rate (60 l/min), the FPF varied by 40% of the nominal dose in the Turbuhaler™ and < 10% with the Spinhaler™ (33). Furthermore, even within a particular type of DPI, flow rate directly affects both TED and FPF. For example, increasing the flow rate of air through a budesonide/formoterol Turbuhaler™ from 30 to 60 l/min and 90 l/min resulted in increases in the budesonide TED from 37.5% to 64.4% and 107.4% respectively (34), with similar increases in formoterol TED, as shown in Figure 3. Increases in FPM were also observed in parallel with flow rate. Similarly, the FPM of both drugs more than doubled when the flow rate was increased from 30 to 60 l/min. Figure 3 also highlights that intra-inhaler dose emission can also be erratic. However, this phenomenon was not found for the Easyhaler™ or the Diskus™ when studied at different flow rates (35) as shown in Figure 4. Large intra- and interinhaler dose emission differences could have clinical consequences in the bronchodilator treatment of asthma exacerbations. Also, because of the absence of immediate therapeutic feedback when inhaling corticosteroids, these differences may also cause problems in achieving adequate asthma or COPD control. Therefore, it is important that a DPI should deliver a consistent dose irrespective of a patient's inspiratory flow rate.
Figure 3.
The amounts of budesonide and formoterol (expressed as a percentage of the labelled emitted dose) emitted from each dose of the six inhalers tested using in vitro inhalation flow rates of 30, 60 and 90 l/min (reproduced with permission)
Figure 4.
Uniformity of dose delivery from three salbutamol-containing multi-dose powder inhalers. Buventol Easyhaler® (200 μg/dose), Inspiryl Turbuhaler® (100 μg/dose) and Ventolin Diskus® (200 μg/dose) at different flow rates. The delivered dose is expressed as a percentage of the nominal labelled dose. Each data point represents a single-dose actuation (reproduced with permission)
The rationale for the presentation of drug as individually, factory-measured unit doses as supplied in the Diskus™ was to ensure a higher degree of dose consistency throughout the life of the device than that achieved in multi-dose reservoir devices (36). Consistent dosing from the Diskus™ has been found with salmeterol (37), fluticasone propionate (38) and the combination of both salmeterol and fluticasone propionate (39) and is therefore independent of the drug delivered. Figure 4 illustrates the minimal effect of inhalation flow rate on the dose emitted from the Diskus™. Furthermore, multi-unit dose inhalers, such as Diskus™ have been shown to deliver more consistent doses across a wide range of sampling flow rates than the Turbuhaler™ reservoir multi-dose device (40,41,32).
In vitro techniques such as those described above provide valuable data that gives an indication of where an aerosol may deposit in the airways (42). However, FPF measured under laboratory conditions cannot be extrapolated into a direct measure of drug deposition (43). For example, most inertial impaction devices, such as cascade impactors described in the pharmacopoeial methods, sample aerosol from an inhaler by drawing air through the device at a constant sampling flow rate (usually 60 l/min, or the flow rate achieved at a pressure drop of 4 KPa). This is not representative of in vivo use. In practice, the magnitude of airflow passing through the DPI during use is variable and controlled by the patient's ability to inhale. An individual patient's inspiratory capacity is affected by several parameters including lung size, degree of airway obstruction that is present and their inspiratory musculature. In addition to these patient factors, the resistance of the inhaler itself affects the flow rate a patient can achieve when inhaling through the device. Each type of DPI has its own resistance characteristics which are caused by the internal structure of the device and there is considerable variation in this parameter between available DPIs (44). The higher the internal resistance of a DPI, the lower the flow rate a patient can generate during inhalation at a given inspiratory pressure.
A modification of the aerosol sampling methodology used to obtain the TED and FPF measurements at variable flow rates has been developed. This technique, using the Electronic Lung, employs a variable sampling flow rate which is derived from in vivo recordings of patient breathing patterns. This ex vivo technique enables inhalation profiles from different patient groups to be recorded using a pressure-sensitive device. The recorded profiles are replayed via an electronic synthesiser which exactly copies the patient's inspiration through a sampling device while the aerosol is released from the DPI. Using this technique to simulate the breathing pattern of asthmatic children aged 4–8 years, the TED of fluticasone propionate via the Diskus™ was compared with that of budesonide delivered via the Turbuhaler™ (45). The results for TED were in general agreement with those obtained by pharmacopoeial methods and showed that 87–89% of the label claim was emitted from the Diskus™ compared with 56–62% from the Turbuhaler™. However, while the TED from the Diskus™ was greater than that from the Turbuhaler™, the FPF from the Diskus™ was slightly lower than that from the Turbuhaler™ (15–18% compared with 21–32% respectively). Overall, the results showed that Diskus™ delivered a more consistent dose across the varying inhalation patterns than the Turbuhaler™ (45).
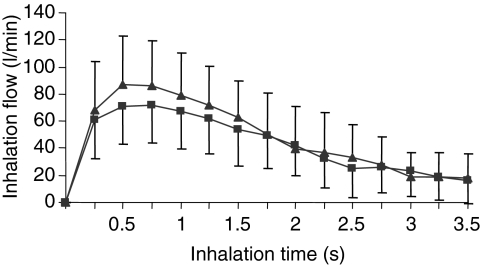
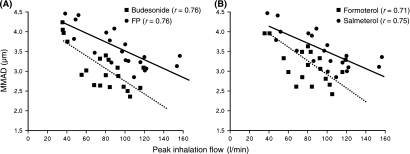
Recently, this technique has been used to estimate the dose that severe asthmatics would receive when inhaling from a Diskus™ (containing 500 mcg fluticasone propionate with 50 mcg salmeterol) and a Turbuhaler™ (containing 200 mcg budesonide and 6 mcg formoterol) (46). Figure 5 illustrates the range of inspiratory flow rates generated by the patients through the two devices and shows that while that the effect of inhalation flow rate on the dose emitted from the Diskus was minimal, it had a significant effect on that released from the Turbuhaler™. Figure 6 illustrates that there was an inverse relationship between inspiratory flow rate and mass median aerodynamic diameter (MMAD) of aerosol released from both DPIs. The change in the MMAD with inhalation flow rate was similar for both devices (46). This decrease in MMAD would counteract the greater potential for more central deposition of particles when using a faster inhalation flow rate.
Figure 5.
Mean (± SD) inhalation flow profile from the 20 severe asthmatics when they used a Diskus® (▴) and Turbuhaler® (▪) (reproduced with permission)
Figure 6.
The mass median aerodynamic diameter with a peak inhalation flow (A) budesonide and fluticasone propionate and (B) formoterol and salmeterol (the continuous line represents the line of regression for fluticasone and salmeterol, the dashed line for budesonide and formoterol) (reproduced with permission). FP, fluticosone propionate
Most DPIs are designed to be used by a spectrum of patients from children to the elderly people with a wide range of severities of asthma symptoms. This variety of patients inherently has different inhalation capacities and therefore generates varying inspiratory flow rates. As small children have smaller inspiratory capacities than adults in terms of both flow rate and volume, DPIs are generally not advocated for children under the age of 6 years. However, Diskus™ operates at low flow rates and has been shown to be effective for use in children aged as young as 4 years (47). The TED and FPF released by some DPIs have been shown to vary considerably and be affected by differences in inspiratory flow rate (48,33,44). A study conducted to compare the delivery of Seretide™ via the Diskus™ inhaler with that of Symbicort™ via the Turbuhaler™ showed that while the output from the Diskus™ was consistent across a range of flow rates, the output from the Turbuhaler™ varied considerably across the same range. Therefore, delivery from the Turbuhaler™ was directly affected by the inspiratory flow rate of the patient (49). Similarly, the FPF of formoterol delivered via Turbuhaler™ was found to be more dependent on inspiratory flow rate than that of salmeterol delivered via Diskus™ (41). A study was conducted to measure the ability of asthmatic children and older COPD patients to generate adequate inspiratory flow through three different DPIs [Diskus™, Turbuhaler™ and Aerolizer™ (Novartis)]. The results showed that all patients could generate adequate flow through the Diskus™. However, only 57% could generate adequate flow through the Turbuhaler™ and 21% through the Aerolizer™ (50). A further study in children highlighted the problems that some may have to generate sufficient inspiratory effort to receive the required dose from a Turbuhaler™ and recommended that this device should not be prescribed to preschool age children (51).
The use of radio-labelled medications allows the in vivo deposition of the aerosol to be observed and quantified using gamma-scintigraphic techniques. Using such methodologies, it has been possible to compare the lung delivery of inhaled medications delivered via different inhalers. For example, mean total lung deposition of 99 mTc-labelled salbutamol was calculated at 18% when delivered from a pMDI and 11% from a Diskhaler™ in asthmatic patients (52). Predictably, lung deposition was found to be higher in normal subjects without airflow obstruction, as illustrated by the study of Borgström et al. (53) who showed 27% and 28% lung deposition of 99 mTc-labelled terbutaline and budesonide respectively via the Turbuhaler™ when inhaled at approximately 60 l/min. However, this study also showed that lung deposition from the Turbuhaler™ was highly dependent on inspiratory flow rate and was halved to approximately 15% when the subject inhaled at 36 l/min. These in vivo studies highlight the in vitro and ex vivo results thereby showing the value of these laboratory methods to predict in vivo effects.
Just as tablet formulations of medications are subject to pharmaceutical deterioration when exposed to excessively high temperatures and humidity, the pharmaceutical performance of inhalers may also be adversely affected. The manufacturers of Diskus™ and Turbuhaler™ recommend in their Summary of Product Characteristics for Seretide™ and Symbicort™ respectively that they should not be stored at temperatures above 30 °C. Regulatory agencies require that inhalers should be tested for pharmaceutical stability over the long term (3 months) at 25 °C and 60% relative humidity (RH) and should also show short-term (1 month) stability at 40 °C and 75% RH.
Conditions of high temperature and humidity have been shown in in vitro studies to negatively affect the efficacy of the Turbuhaler™ plastic reservoir device (54). The effect of hot and humid storage conditions on the FPF from the terbutaline Turbuhaler™ has been studied in vitro. It was found that there was considerable variation in output from the Turbuhaler™ over the range of test conditions and FPM was reduced to near zero when it was used at 5 °C (55). The Turbuhaler™ has been equipped with a detachable cap that forms a seal on the outlet of the inhaler when it is closed properly. This, of course, relies on the co-operation of the patient to put the lid firmly back on to the device after each use. In the Diskus™, each dose of drug is protected from the environment by moisture proof, sealed aluminium foil units, which are peeled back to expose each dose just as it is used. This has been shown to provide a high level of dose protection (37). In addition to the foil strip, the Diskus™ is also supplied with an outer foil wrapper which provides extra protection from the environment during storage prior to use. A recent study showed that the FPF (but not TED) from the Diskus™ was shown to be reduced by 50% after 3 months of storage at high temperature and humidity (40 °C/75% RH), while that from the Turbuhaler™ was not affected to the same extent (56). However, in this study Diskus™ inhalers were used that had limited unexpired shelf life and no assurance was given regarding the storage conditions of these inhalers prior to their use in this study. Furthermore, the conditions of temperature and humidity under which the aerosol was sampled from the inhalers differed considerably from the conditions under which the inhalers had been stored. It is, therefore, difficult to assess whether the observed changes in FPM were due to the experimental conditions, ambient conditions during sampling or previous storage conditions of the inhalers.
Ease of use
Arguably, the most important criterion in the selection of a DPI is its ease of use. Even if a DPI is shown to have an excellent pharmaceutical performance in terms of drug output, if it is not used correctly then it can be rendered ineffective. The results of a recent survey conducted in 169 patients with asthma or COPD showed that patients rated ‘ease of use during an attack’ as the most important feature of an ideal inhaler (57). If patients cannot use an inhaler correctly, their treatment is compromised which may have extremely serious consequences.
Dry powder inhaler devices have an inherent advantage over pMDIs, because the dose of drug is only released from the inhaler as the patient inhales. Therefore, it is not necessary for the patient to co-ordinate inspiration with actuation of the device. However, it is still necessary for the patient to provide a sustained inspiration of adequate flow rate through the inhaler in order for the complete dose to be released. The inspiratory flow rate required for optimum delivery depends on the individual DPI and the ease with which a patient can generate this flow rate depends on the resistance of the individual DPI.
The diverse characteristics of patients who require inhaled medications are such that simplicity of operation is of paramount importance. One study assessed patients’ use of their own DPI or pMDI and found that nearly 90% made at least one mistake in their inhalation technique (58). It is therefore imperative to provide training to patients so that they learn to use their DPIs correctly. It has been shown that effective training increases patients’ ability to use a DPI correctly (57,59). Table 3 summarises the instructions for use of some popular DPIs (60).
Table 3.
Instructions for dry powder inhaler use from Patient Information Leaflets (60)
| Device | Loading dose | Preparation for inhaling the dose | Inhaling dose |
|---|---|---|---|
| Clickhaler™ | Hold upright, shake, press | Breathe out as far as comfortable | Steadily and deeply |
| Diskhaler™ | Insert disk, slide tray, pierce disk | Breathe out as far as comfortable | Suck in quickly and deeply |
| Diskus™ | Open, slide | Breathe out as far as comfortable | Suck in quickly and deeply |
| Easyhaler™ | Hold upright, shake, press | Breathe out | Strongly and deeply |
| Pulvinal™ | Hold upright, press button, twist | Breathe out deeply | As quickly and deeply as possible |
| Turbuhaler™ | Hold upright, twist base | Breathe out gently | Deeply and as hard as possible |
It has been demonstrated that after a single instruction, 74% patients were able to use the Diskus™ correctly compared with only 32% patients who were able to use the Turbuhaler™ correctly after one instruction (61). After two training sessions, 99% patients could use the Diskus™ correctly and 88% patients used the Turbuhaler™ correctly. However, 12% of the patients studied required further training before they could operate the Turbuhaler™.
Many studies have highlighted the incorrect use of inhalers and have shown that some inhalers are easier to use than others. However, many such clinical studies require patients to have a good inhalation technique as an inclusion criterion. Therefore, real-life studies such as observational studies may be more relevant in evaluating DPI use. For example, an observational study in 3811 patients compared correct use of five types of inhaler (Aerolizer™, Autohaler™, Diskus™, Turbuhaler™ and MDI) (62). There were clear differences between devices in the percentage of patients who made at least one mistake during use. Fewer patients made a mistake when using the Diskus™ compared with the other devices. The percentage of patients who made errors that would result in ineffective dosing was also lowest when they used the Diskus™ (11%) and highest during use of the Turbuhaler™ (32%). Similar figures of incorrect use of the Turbuhaler™ have been found in other studies in adults and also in children (59,63–67). A study conducted in elderly patients with COPD demonstrated that patients made significantly fewer critical errors when they used the Diskus™ inhaler than when they used the single-unit dose Handihaler™ inhaler (22). DPIs may also be used to deliver other medications such as antiviral drugs to elderly patients who have not used any inhalers before. A study conducted to determine whether elderly people could use Relenza™ Diskhaler™ as effectively as Turbuhaler™ found that 74% of patients had difficulty to load and prime the Diskhaler™ compared with 43% of patients with difficulty using the Turbuhaler™ (68). Clearly, it is important to maximise simplicity of use for elderly patients who are unfamiliar with inhaled therapy.
The design of a DPI is critical in facilitating correct use. It should be easy to hold by all patients including the elderly people who may have concomitant conditions such as arthritis that can affect manual dexterity. Similarly, a DPI should be easy to operate with a minimal number of required steps. The mouthpiece should be designed to be comfortable during use. Furthermore, the device should offer minimal resistance to airflow so that it is easy for the patient to inhale through the device. Some DPIs (e.g. Clickhaler™, Easyhaler™) have been designed to resemble pMDIs as closely as possible, in terms of size and unobtrusiveness. However, because the mode of operation of a DPI is so different from that of a pMDI, swapping a patient from a pMDI to such a DPI may cause confusion to the patient and result in incorrect use.
Several clinical studies have shown that the Diskus™ is easier to use than the Turbuhaler™ in elderly people, adult and paediatric patients (69–75). Similarly, the Diskus™ has been found to be easier to use than the Diskhaler™ (76,77).
Patient preference
Patient preference has been identified as the second most important consideration for device selection after ease of use (78). The recognition that patients have distinct personal preferences regarding the inhaler that they use and are able to make informed choices is particularly important in establishing an effective disease management partnership between the healthcare professional and the patient. Compliance with the treatment is improved when patient and physician work together to achieve effective disease control (79). Many studies have been conducted to establish patient preference for one device over others. The results show that there is enormous variation in preferred devices. Table 4 summarises the findings of some of the studies in which patient preference for one DPI over another was compared.
Table 4.
Summary of studies to identify patient preference for dry powder inhalers
| Comparisons | Comments | References |
|---|---|---|
| BDP Easyhaler™ vs. Diskhaler™ | N = 185. Easyhaler™ rated more acceptablethan Diskhaler™ | Wettengel et al. (84) |
| Salmeterol or FP Diskus™ vs. Diskhaler™ | Diskus™ preferred to Diskhaler™ | Boulet et al. (77) |
| Stallaert et al. (97) | ||
| Diskus™ vs. previous device | Diskus™ preferred to previous device (53% vs.Turbuhaler™; 82% vs. Diskhaler™; 83% vs.Rotahaler™; 92% vs. Spinhaler™) | Le Soeuf and Clay (98) |
| Diskus™ vs. Easyhaler™ vs. Turbuhaler™ | Adult stable asthmatics scored Easyhaler™ 75 (of 90); Diskus™ 67 and Turbuhaler™ 65 | Giner et al. (83) |
| Diskus™ vs. Handihaler™ | 67% COPD patients preferred Diskus™ | Moore and Stone (21) |
| Diskus™ vs. Turbuhaler™ | More patients prefer Diskus™ (60–82%) to Turbuhaler™ (8–18%). Turbuhaler™ preferredin one study (50% vs. 34%) | Luyt et al. (74) |
| Arossa et al. (71) | ||
| Chapman et al. (72) | ||
| Serra-Batlles et al. (57) | ||
| Manjra et al. (99) | ||
| van der Palen et al. (75) | ||
| Willingness to use Diskus™ vs. Turbuhaler™ | More patients happy to be prescribed Diskus™again (78–91%) than Turbuhaler™ (37–65%) | Backman et al. (70) |
| Williams and Richard (100) | ||
| Chapman et al. (72) | ||
| Burdon et al. (73) |
COPD, chronic obstructive pulmonary disease.
Although comparative studies are generally designed to show overall preference for one device over another, in reality patients may prefer certain features of one inhaler over those of another inhaler, but not necessarily other features. Therefore, studies have been conducted to identify the most important characteristics or features of an ‘ideal’ DPI for different groups of patient (80–83). The findings of these studies show that different groups of patients or parents have different priorities. For example, parents of children who administer the dose of medication to their child rated that it was more important to them to feel that the child had received the dose than it was for children or adults who self-administer the medication (83). Similarly, children stated that they preferred a small device that was easy to carry around, while this characteristic was not as important to adults or parents, and elderly patients may well wish for a larger device that is easier to see and hold. Clearly, different patient populations have different priorities and ‘wish lists’ for an ideal inhaler. A recent study set out to identify the ‘wish list’ among 250 patients with COPD (21). The three most important features of an ideal inhaler were being quick to use when needed, overall ease of use and having a counter to show how many doses remained in the device. The study found that the Diskus™ was rated significantly higher than the Handihaler™ for each of these features.
Interchangeability of DPI devices
The findings of some studies have suggested that clinical equivalence can be achieved when generic inhalers are substituted for other DPIs (84). However, such studies do not accurately reflect real-life situations and the effectiveness of inhaled medication to achieve disease control is the result of a combination of device design, pharmaceutical performance and patient behaviour.
The findings of this review have shown that there are wide differences between currently available DPIs in terms of pharmaceutical performance, ease of use and patient preference. It is clear that ‘one inhaler does not fit all’ and several factors should be taken into consideration when prescribing a dry powder inhaler device. However, two recent reviews have compared the clinical effectiveness of pMDIs with DPIs in the delivery of bronchodilators and corticosteroids in asthma (13,14). The conclusion of each review was that ‘no evidence was found that alternative inhaler devices (DPIs, breath-actuated pMDI) are more effective than the pMDI for the delivery of β2-agonist or corticosteroids in asthma. pMDI remain the most cost-effective delivery devices’. In some ways, the findings of these reviews are not surprising. This is because both reviews concentrated on the results of clinical studies in which patients were selected whose asthma symptoms were stable, and who demonstrated a good inhaler technique and were compliant with the dosing regimen of the study. Furthermore, the treatment periods in many of the studies cited were of short duration and because many of the studies were designed to show equivalence, doses were selected that were at the top of the dose–response curve. Consequently, many of the studies cited in these reviews demonstrated clinical equivalence between a standard pMDI and the alternative inhaler device.
The proportion of a dose of medication from a DPI that deposits in the lung during inhalation varies as a result of the device used, the drug delivered and the patient characteristics (85). A study was conducted in healthy volunteers that compared the lung deposition of 99 mTc radio-labelled budesonide when delivered via Clickhaler™ and Turbuhaler™. Lung deposition was assessed by the use of scintigraphy. The results showed that delivery of the budesonide to the lungs was directly affected by the device used and was significantly lower from the Turbuhaler™ (15.8%) compared with the Clickhaler™ (26.8%) (p < 0.001) (86). Similar findings were obtained when the deposition of sodium cromoglycate was compared from four different DPIs (87). Such differences in deposition have been shown to affect the safety profiles of inhaled corticosteroids. For example, in a 6-month study of children aged 5–10 years, delivery of budesonide via Turbuhaler™ was found to cause a significantly slower growth rate in these children than when the same drug was delivered via Easyhaler™ (88). Therefore, the pharmaceutical performance of a DPI, and consequent systemic absorption of the drug, has been shown to directly affect the safety profile of an inhaled medication. Similar differences in systemic absorption have been found when salbutamol was delivered from Diskus™, Turbuhaler™, Easi-breathe™ and Diskhaler™ (89). This therefore indicates that changing a delivery device can have adverse effects on both the safety and efficacy of an inhaled drug and that DPIs should not be regarded as interchangeable (15).
It is recognised that patient factors such as ease of use and patient preference directly affect treatment compliance. Therefore, if patients are switched from devices that they find simple to operate and like to use, then compliance with therapy may well deteriorate. This, in turn, will result in a loss of symptom control and consequent increases in morbidity and also healthcare costs. Furthermore, substitution of a familiar inhaler with a generic inhaler could confuse the patient causing them to make additional appointments to see their doctor or nurse thereby negating any possible cost savings.
The availability of a DPI that can be used to deliver a range of drugs is important as patients become used to using a particular device effectively. The Diskus™ is available with salmeterol, fluticasone propionate and also the combination of both drugs at three different strengths. Analysis of prescribing habits, by managed care organisations in the USA, has shown that refill rates are higher when patients are prescribed combination inhalers compared with two separate inhalers each containing a single agent (90). Improving compliance with therapy regimens results in less reliever use, lower exacerbation rates and reduces the overall healthcare costs of respiratory disease (91).
Conclusions
National and international guidelines state that the aim of asthma management is to achieve optimal disease control (92–94). Poorly controlled asthma results in increased exacerbations of symptoms and resultant healthcare costs (95) and also negatively impacts quality of life (96). Poorly controlled COPD also results in an increased rate of disease progression, exacerbations requiring hospitalisation and ultimately mortality. Fundamental to achieving optimal disease control in both asthma and COPD is the provision of effective and reliable treatment. DPIs have become popular for the delivery of inhaled medications for both asthma and COPD. Improvements in technology and pharmaceutical science have facilitated the development of DPIs that are simple to use, provide consistent dosing of drug and are liked by patients. However, there are still wide variations in these properties among the different makes of DPI available. The prescriber must therefore ensure that the selection of a delivery device is appropriate for the individual patient needs. While it has been suggested that it is acceptable to swap a patient to the cheapest device available without compromising disease control, this is not supported by the clinical evidence that clearly demonstrates that there are wide differences between the quality of treatment delivered by different DPIs. The ‘ideal’ dry powder inhaler is the one that delivers consistent and reliable doses and is the one that the patient trusts, finds easy to use and prefers over others. While there is no single ‘ideal’ DPI that fulfils all those criteria for the entire spectrum of patients who use inhaled medications, the evidence suggests that multi-unit dose DPIs such as Diskus™ offer the most reliable and consistent pharmaceutical performance, and are preferred by patients who rate them as the easiest to use.
Acknowledgments
Many thanks to Michelle Clay for help with the preparation of this manuscript.
References
- 1.Freedman T. Medihaler therapy for bronchial asthma: a new type of aerosol therapy. Postgrad Med J. 1956;20:667–73. doi: 10.1080/00325481.1956.11691366. [DOI] [PubMed] [Google Scholar]
- 2.Brompton Hospital/MRC Collaborative trial. Double-blind trial comparing two dosage schedules of beclomethasone dipropionate aerosol in the treatment of chronic bronchial asthma. Lancet. 1974;2:303–7. [PubMed] [Google Scholar]
- 3.Boyd G. The continued need for MDIs. J Aerosol Med. 1995;8(Suppl. 1):S9–12. doi: 10.1089/jam.1995.8.suppl_1.s-9. [DOI] [PubMed] [Google Scholar]
- 4.Ross DL. Advances in metered dose inhaler technology with the development of a chlorofluorocarbon-free drug delivery system. J Aerosol Med. 1999;12:151–60. doi: 10.1089/jam.1999.12.151. [DOI] [PubMed] [Google Scholar]
- 5.Bell JH. Dry powder aerosols 1: a new powder inhalation device. J Pharm Sci. 1971;10:1559–64. doi: 10.1002/jps.2600601028. [DOI] [PubMed] [Google Scholar]
- 6.Amirav I. What do paediatricians know about the correct use of inhalers and spacer devices? J Allergy Clin Immunol. 1994;94:669–75. doi: 10.1016/0091-6749(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 7.Cochrane GM. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117:542–50. doi: 10.1378/chest.117.2.542. [DOI] [PubMed] [Google Scholar]
- 8.Noakes TJ. CFCs, their replacements and the ozone layer. J Aerosol Med. 1995;8(Suppl. 1):S3–7. doi: 10.1089/jam.1995.8.suppl_1.s-3. [DOI] [PubMed] [Google Scholar]
- 9.Hetzel M. Comparison of salbutamol Rotahaler with conventional pressurized aerosol. Clin Allergy. 1977;7:563–8. doi: 10.1111/j.1365-2222.1977.tb01486.x. [DOI] [PubMed] [Google Scholar]
- 10.Edmunds AT. A clinical comparison of beclomethasone dipropionate delivered by pressurized aerosol and as a powder from a Rotahaler. Arch Dis Child. 1979;54:233–5. doi: 10.1136/adc.54.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pakes GE. Ipratropium bromide: a review of its pharmacological properties and therapeutic efficacy in asthma and chronic bronchitis. Drugs. 1980;20:237–66. doi: 10.2165/00003495-198020040-00001. [DOI] [PubMed] [Google Scholar]
- 12.Taylor A. Do all dry powder inhalers show the same pharmaceutical performance? Int J Clin Pract. 2005;59(Suppl. 149):7–12. doi: 10.1111/j.1368-504X.2005.00721.x. [DOI] [PubMed] [Google Scholar]
- 13.Brocklebank D The National Health Technology Assessment Inhaler Review Group. Systematic review of clinical effectiveness of pressurised metered dose inhalers versus other hand held inhaler devices for delivering corticosteroids in asthma. BMJ. 2001;323:1–7. doi: 10.1136/bmj.323.7318.896. on behalf of. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ram FSF The National Health Technology Assessment Inhaler Review Group. Systematic review of clinical effectiveness of pressurised metered dose inhalers versus other hand held inhaler devices for delivering β2-agonists bronchodilators in asthma. BMJ. 2001;323:1–7. doi: 10.1136/bmj.323.7318.901. on behalf of. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrystyn H. Do patients show the same level of adherence with all dry powder inhalers? Int J Clin Pract. 2005;59:19–25. doi: 10.1111/j.1368-504X.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 16.Hallworth GW. An improved design of powder inhaler. Br J Clin Pharmacol. 1977;4:689–90. [Google Scholar]
- 17.Chodosh S. Effective delivery of particles with the Handihaler® dry powder inhalation system over a range of chronic obstructive pulmonary disease severity. J Aerosol Med. 2001;14:309–15. doi: 10.1089/089426801316970268. [DOI] [PubMed] [Google Scholar]
- 18.Dahl R, et al. Assessment of patient performance of the Handihaler® compared with the metered dose inhaler four weeks after instruction. Respir Med. 2003;97:1126–33. doi: 10.1016/s0954-6111(03)00162-8. [DOI] [PubMed] [Google Scholar]
- 19.Tarsin W. Peak inhalation rates (PIFRs) of COPD patients when using a metered dose inhaler (MDI) and recently introduced dry powder inhalers (DPI) Thorax. 2003;58(Suppl. 111):ii73. [Google Scholar]
- 20.Al-Fadhl SA. Tiotropium dose emission is influenced by the inhalation flow and the recommended two inhalations for each dose. Eur Respir J. 2005;26(Suppl. 49):125s–6. [Google Scholar]
- 21.Moore AC. Meeting the needs of patients with COPD: patients’ preference for the Diskus compared with the Handihaler. Int J Clin Pract. 2004;58:444–50. doi: 10.1111/j.1368-5031.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- 22.Franks M. Use of a cognitive ergonomics approach to compare usability of a mutidose dry powder inhaler and capsule dry powder inhaler: an open-label, randomized, controlled study. Clin Ther. 2004;26:1791–9. doi: 10.1016/j.clinthera.2004.11011. [DOI] [PubMed] [Google Scholar]
- 23.Wetterlin K. Turbuhaler: a new powder inhaler for administration of drugs to the airways. Pharm Res. 1988;5:506–8. doi: 10.1023/a:1015969324799. [DOI] [PubMed] [Google Scholar]
- 24.Vidgren M. Easyhaler multiple dose powder inhaler – practical and effective alternative to the pressurized MDI. Aerosol Sci Technol. 1995;22:335–45. [Google Scholar]
- 25.Warren S. Effect of inhalation flow profiles on the deposition of radiolabelled BDP from a novel dry powder inhaler (DPI. Clickhaler), a conventional metered dose inhaler (MDI), and MDI plus spacer. In: Dalby RN, editor. Respiratory Drug Delivery VI. Buffalo Grove, IL: Interpharm Press; 1998. pp. 453–5. [Google Scholar]
- 26.Yang TT. Evaluation of the new mometasone furoate dry powder inhaler agglomerate formulation and deagglomeration during inhalation. Chest. 2000;118:98S. [Google Scholar]
- 27.Berner B. Asta Medica multidose dry powder inhaler. In: Dalby RN, editor. Respiratory Drug Delivery VI. Buffalo Grove, IL: Interpharm Press; 1998. pp. 475–7. [Google Scholar]
- 28.Sumby BS, et al. Dose reliability of the Serevent Diskhaler system. Pharm Tech Int. 1993;5 (June):20–7. [Google Scholar]
- 29.European Pharmacopeia. Preparations for Inhalation. European Pharmacopeia Supplement; 2001. 2.9.18. [Google Scholar]
- 30.FDA US. Guidance for Industry: Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Drug Products. Rockville, US: US Dept of Health and Human Sciences, FDA Center for Drug Evaluation and Research (CDER), 1998; Chemistry, Manufacturing and Controls Documentation. [Google Scholar]
- 31.Brain JD. Mechanisms of particle deposition and clearance. In: Moren F, editor. Aerosols in Medicine: Principles, Diagnosis and Therapy. Amsterdam: Elsevier; 1993. pp. 351–74. [Google Scholar]
- 32.Hill LS. A comparison of the performance of two modern multidose dry powder asthma inhalers. Respir Med. 1998;92:105–10. doi: 10.1016/s0954-6111(98)90040-3. [DOI] [PubMed] [Google Scholar]
- 33.de Boer AH. Inhalation characteristics and their effects on in vitro drug delivery from dry powder inhalers. Part 2: Effect of peak flow rate (PIFR) and inspiration time on the in vitro drug release from three different types of commercial dry powder inhalers. Int J Pharm. 1996;138:45–56. [Google Scholar]
- 34.Tarsin W. In vitro and inter-inhaler flow rate-dependent dosage emission from a combination of budesonide and formoterol in a dry powder inhaler. J Aerosol Med. 2004;17:25–32. doi: 10.1089/089426804322994433. [DOI] [PubMed] [Google Scholar]
- 35.Palander A. In vitro comparison of three salbutamol-containing multidose dry powder inhalers. Clin Drug Invest. 2000;20:25–33. [Google Scholar]
- 36.Fuller R. The Diskus™: a new multi-dose powder device – efficacy and comparison with Turbuhaler™. J Aerosol Med. 1995;8(Suppl. 2):S11–7. doi: 10.1089/jam.1995.8.suppl_2.s-11. [DOI] [PubMed] [Google Scholar]
- 37.Brindley A. Design, manufacture and dose consistency of the Serevent Diskus inhaler. Pharm Technol Eur Biopharm. 1995;7:14–22. [Google Scholar]
- 38.Prime D. Assessing dose delivery from the Flixotide Diskus inhaler – a multi-dose powder inhaler. Pharm Tech Eur. 1996;8:23–36. [Google Scholar]
- 39.Ashurst IC. Development of a dry powder inhaler device containing a combination of salmeterol and fluticasone propionate. Eur Respir J. 1998;12(Suppl. 28):93s. [Google Scholar]
- 40.Prime D. Evaluation of the pharmaceutical performance of two dry powder inhalers: a comparison of Diskus and Turbuhaler inhalers. Am J Respir Crit Care Med. 1996;153:A62. [Google Scholar]
- 41.Malton A. A comparison of in vitro drug delivery from two multidose powder inhalation devices. Eur J Clin Res. 1995;7:177–93. [Google Scholar]
- 42.Martonen TB. Use of analytically defined estimates of aerosol respirable fraction to predict lung deposition patterns. Pharm Res. 1992;9:1634–9. doi: 10.1023/a:1015880828704. [DOI] [PubMed] [Google Scholar]
- 43.Newman SP. How well do in vitro particle size measurements predict drug delivery in vivo? J Aerosol Med. 1998;11(Suppl. 1):S97–104. [PubMed] [Google Scholar]
- 44.Steckel H. In vitro evaluation of dry powder inhalers 1: drug deposition of commonly used devices. Int J Pharm. 1997;154:19–29. [Google Scholar]
- 45.Bisgaard H. Fine particle mass from the Diskus inhaler and Turbuhaler in children with asthma. Eur Respir J. 1998;11:1111–5. doi: 10.1183/09031936.98.11051111. [DOI] [PubMed] [Google Scholar]
- 46.Tarsin WY. Emitted dose estimates from Seretide Diskus and Symbicort Turbuhaler following inhalation by severe asthmatics. Int J Pharm. 2006;316:131–7. doi: 10.1016/j.ijpharm.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 47.Bracamonte T. Efficacy and safety of salmeterol/fluticasone propionate combination delivered by the Diskus™ or pressurised metered dose inhaler in children with asthma. Clin Drug Invest. 2005;25:1–11. doi: 10.2165/00044011-200525010-00001. [DOI] [PubMed] [Google Scholar]
- 48.Hindle M. Dose emissions from marketed dry powder inhalers. Int J Pharm. 1995;116:169–77. [Google Scholar]
- 49.Gustaffson P. Comparison of total and fine drug particle mass generated from two dry powder inhalers (DPIs) by 4 year old children with asthma. Eur Respir J. 2002;20(Suppl. 38):429s. [Google Scholar]
- 50.Kokot M. Selection of a personal dry powder inhaler based on peak inspiratory flow measurements in selected groups of patients with bronchial obstruction. Pol Merkur Lekarski. 2000;9:672–6. [PubMed] [Google Scholar]
- 51.Pederson S. Influence of inspiratory flow rate upon the effect of a Turbuhaler. Arch Dis Child. 1990;65:308–10. doi: 10.1136/adc.65.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melchor R. Lung deposition patterns of directly labelled salbutamol in normal subjects and in patients with reversible airflow obstruction. Thorax. 1993;48:506–11. doi: 10.1136/thx.48.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borgström L. Lung deposition of budesonide inhaled via Turbohaler: a comparison with terbutaline sulphate in normal subjects. Eur Respir J. 1994;7:69–73. doi: 10.1183/09031936.94.07010069. [DOI] [PubMed] [Google Scholar]
- 54.Lindsay DA, et al. A multicentre comparison of the efficacy of terbutaline turbuhaler and salbutamol pressurized metered dose inhaler in hot, humid regions. Eur Respir J. 1994;7:342–5. doi: 10.1183/09031936.94.07020342. [DOI] [PubMed] [Google Scholar]
- 55.Meakin BJ. Simulated ‘in-use’ and ‘mis-use’ aspects of the delivery of terbutaline sulphate from Bricanyl Turbohaler™ dry powder inhalers. Int J Pharm. 1995;119:103–8. [Google Scholar]
- 56.Borgström L. An in vivo and in vitro comparison of two powder inhalers following storage at hot/humid conditions. J Aerosol Med. 2005;18:304–10. doi: 10.1089/jam.2005.18.304. [DOI] [PubMed] [Google Scholar]
- 57.Serra-Batlles J. Patient perception and acceptability of multidose dry powder inhalers: A randomized crossover comparison of Diskus/Accuhaler with Turbuhaler. J Aerosol Med. 2002;15:59–64. doi: 10.1089/08942680252908584. [DOI] [PubMed] [Google Scholar]
- 58.Van Beerendonk I. Assessment of the inhalation technique in outpatients with asthma or chronic obstructive pulmonary disease using a metered dose inhaler or dry powder device. J Asthma. 1998;35:273–9. doi: 10.3109/02770909809068218. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira MB. Turbutest in the training of asthmatic Turbuhaler users. Allergy. 1999;54:375–9. doi: 10.1034/j.1398-9995.1999.00969.x. [DOI] [PubMed] [Google Scholar]
- 60.Gustafsson P. Can patients use all dry powder inhalers equally well? Int J Clin Pract. 2005;59(Suppl. 149):13–8. doi: 10.1111/j.1368-504X.2005.00722.x. [DOI] [PubMed] [Google Scholar]
- 61.Clay MM. Ease of handling and clinical efficacy of a new multi-dose powder inhaler for the delivery of salmeterol in adult asthmatics. Eur Respir J. 1994;7(Suppl. 18):163s. [Google Scholar]
- 62.Molimard M, et al. Assessment of handling of inhaler device in real life: an observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249–54. doi: 10.1089/089426803769017613. [DOI] [PubMed] [Google Scholar]
- 63.Kaneko N. The efficacy of turbuhaler dry powder inhaler in Japanese asthma – measurement of peak inspiratory flow when using Turbuhaler. Jpn J Allergol. 1996;45:649–54. [PubMed] [Google Scholar]
- 64.Hawksworth GM. Characterisation of the inspiratory manoeuvre when asthmatics inhale through a Turbuhaler pre- and post- counselling in a community pharmacy. Respir Med. 2000;94:501–4. doi: 10.1053/rmed.1999.0768. [DOI] [PubMed] [Google Scholar]
- 65.Cimas JE. The validation of checklists for the inhalation technique for the pressurized cartridge and the Turbuhaler. Arch Bronconeumol. 1999;35:15–9. doi: 10.1016/s0300-2896(15)30319-7. [DOI] [PubMed] [Google Scholar]
- 66.Goren A, et al. Assessment of the ability of young children to use a powder inhaler device (Turbuhaler) Ped Pulmonol. 1994;18:77–80. doi: 10.1002/ppul.1950180204. [DOI] [PubMed] [Google Scholar]
- 67.de Boeck K. Is the correct use of a dry powder inhaler (Turbuhaler) age dependent? J Allergy Clin Immunol. 1999;103:763–7. doi: 10.1016/s0091-6749(99)70417-3. [DOI] [PubMed] [Google Scholar]
- 68.Diggory P. Comparison of elderly people's technique in using two dry powder inhalers to deliver zanamivir: randomised controlled trial. BMJ. 2001;322:1–4. doi: 10.1136/bmj.322.7286.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burdon J. A comparison of fluticasone propionate 250 mcg bd via MDPI and budesonide 600 mcg bd via Turbuhaler in adult asthmatics. Aust N Z J Med. 1997;27:247. [Google Scholar]
- 70.Backman R. Fluticasone propionate via Diskus inhaler at half the microgram dose of budesonide via Turbuhaler inhaler. Clin Drug Invest. 2001;21:735–43. [Google Scholar]
- 71.Arossa W. Patient perception of the Diskus inhaler: a comparison with the turbuhaler inhaler. Eur Respir J. 1998;12(Suppl. 28):41s. [Google Scholar]
- 72.Chapman KR, et al. Handling of the multi-dose powder inhalers Diskus versus Turbuhaler in elderly asthmatics. Am J Respir Crit Care Med. 1996;153(4)(part 2):A61. [Google Scholar]
- 73.Burdon J. Comparison of the efficacy and ease of handling of salmeterol xinafoate delivered via a new multi-dose powder inhaler and terbutaline sulphate delivered via a breath-actuated metered dose inhaler. Eur Respir J. 1995;8(Suppl. 19):P1050. [Google Scholar]
- 74.Luyt D. A comparison of the ease of handling of the Diskus/Accuhaler inhaler and the turbuhaler inhaler in children aged 6–12 years with asthma. J Aerosol Med Depos Clear Eff Lung. 1995;8:P144. 105. [Google Scholar]
- 75.van der Palen J. Comparison of the new multidose powder inhaler (Diskus/Accuhaler) and the Turbuhaler regarding preference and ease of use. J Asthma. 1998;35:147–52. doi: 10.3109/02770909809068202. [DOI] [PubMed] [Google Scholar]
- 76.Gunawardena KA. Salmeterol delivered from a new multi-dose powder inhaler (Diskus/Accuhaler inhaler) or Diskhaler inhaler in adult asthmatics. Am J Respir Crit Care Med. 1994;149(4)(part 2):A219. [Google Scholar]
- 77.Boulet LP. Comparison of Diskus inhaler, a new multidose powder inhaler, with Diskhaler inhaler for the delivery of salmeterol to asthmatic patients. J Asthma. 1995;32:429–36. doi: 10.3109/02770909409077754. [DOI] [PubMed] [Google Scholar]
- 78.Fletcher M. Effects of asthma training on inhaler device selection and utilisation in general practice. Eur Respir J. 2004;24(Suppl. 48):257s. [Google Scholar]
- 79.Everard ML. Inhaler devices in infants and children: challenges and solutions. J Aerosol Med. 2004;17:186–95. doi: 10.1089/0894268041457129. [DOI] [PubMed] [Google Scholar]
- 80.Moore AC. patient preference for the Diskus over the Handihaler in patients with COPD. Am J Respir Crit Care Med. 2003;167:A96. [Google Scholar]
- 81.Sharma RK. Perception among paediatric patients of the Diskus inhaler, a novel multidose powder inhaler for use in the treatment of asthma. Clin Drug Invest. 1996;11:145–53. [Google Scholar]
- 82.Schlaeppi M. Patient perception of the Diskus inhaler: a comparison with the Turbuhaler inhaler. Br J Clin Pract. 1996;50:14–9. [PubMed] [Google Scholar]
- 83.Giner J, et al. Patient preference in the choice of dry powder inhalers. Arch Bronconeumol. 2004;40:106–9. doi: 10.1016/s1579-2129(06)70074-x. [DOI] [PubMed] [Google Scholar]
- 84.Wettengel R. Therapeutic equivalence and acceptability of two multidose powder inhalers in the treatment of asthma. Respiration. 2000;67:77–82. [Google Scholar]
- 85.Bisgaard H. Drug delivery from inhaler devices. Lung deposition, clinical effect and cost-effectiveness vary. Br Med J. 1996;13:895–6. doi: 10.1136/bmj.313.7062.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warren S. Gamma scintigraphic evaluation of a novel budesonide dry powder inhaler using a validated radiolabeling technique. J Aerosol Med. 2002;15:15–25. doi: 10.1089/08942680252908548. [DOI] [PubMed] [Google Scholar]
- 87.Vidgren M, et al. Effect of powder inhaler design on drug deposition in the respiratory tract. Int J Pharm. 1988;42:211–6. [Google Scholar]
- 88.Vanto T. Comparison of two budesonide dry powder inhalers in the treatment of asthma in children. J Aerosol Med. 2004;17:15–24. doi: 10.1089/089426804322994424. [DOI] [PubMed] [Google Scholar]
- 89.Aswania O, et al. Intra-subject variability in lung dose in healthy volunteers using five conventional portable inhalers. J Aerosol Med. 2004;17:231–8. doi: 10.1089/jam.2004.17.231. [DOI] [PubMed] [Google Scholar]
- 90.Stoloff SW. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol. 2004;113:245–51. doi: 10.1016/j.jaci.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Stempel DA. Asthma related ED/hospitalisation risk and asthma costs of fluticasone propionate/salmeterol in a single inhaler compared to fluticasone propionate and salmeterol in separate inhalers. J Allergy Clin Immunol. 2004;113:S272. doi: 10.1016/j.jaci.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 92.GINA (Global Initiative for Asthma) Pocket Guide for Asthma Management and Prevention. http://www.ginasthma.org(accessed May 2007)
- 93.BTS/SIGN (British Thoracic Society/Scottish Intercollegiate Guidelines Network) British guideline on the management of asthma. Thorax. 2003;58(Suppl. 1):i1–94. doi: 10.1136/thorax.58.suppl_1.1i. http://www.brit-thoracic.org.uk.asthma-guide-download.html (accessed April 2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.National Institutes of Health, National Heart, Lung and Blood Institute. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2004. [Google Scholar]
- 95.Barnes PJ. The costs of asthma. Eur Respir J. 1996;9:636–42. doi: 10.1183/09031936.96.09040636. [DOI] [PubMed] [Google Scholar]
- 96.Seemungal TA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–22. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 97.Staellaert RALM, et al. Evaluation of a new dry powder device, the Diskus/Accuhaler versus the Diskhaler inhaler for the delivery of fluticasone propionate in adult asthmatics. J Aerosol Med Depos Clear Eff Lung. 1995;8(1):140. [Google Scholar]
- 98.Le Soeuf P. A multicentre study to evaluate ease of handling and patient preference for the Diskus/Accuhaler multi-dose dry powder inhaler. ISAM Nov. 1994:abs 5. [Google Scholar]
- 99.Manjra AI. Device handling comparison between Diskus (D) and Turbuhaler (TH) by paediatric patients and their parents. Eur Respir J. 1998;12 (Suppl 28):86s. [Google Scholar]
- 100.Williams J. Ease of handling and clinical efficacy of fluticasone propionate Accuhaler/Diskus inhaler compared wirg Turbuhaler inhaler in paediatric patients. Br J Clin Pract. 1997;51:147–53. [PubMed] [Google Scholar]