Abstract
Common variable immunodeficiency (CVID) is the most frequent clinically manifested primary immunodeficiency. According to clinical and laboratory findings, CVID is a heterogeneous group of diseases. Recently, the defects of molecules regulating activation and terminal differentiation of B lymphocytes have been described in some patients with CVID. In this study, we show the overview of deficiencies of inducible costimulator, transmembrane activator and calcium-modulator and cytophilin ligand interactor, CD19 molecules, their genetic basis, pathogenesis and clinical manifestations.
Introduction
Common variable immunodeficiency (CVID) is a heterogeneous group of diseases characterized by hypogammaglobulinemia and repeated, predominantly, bacterial infections. Most of the patients have the normal number of peripheral T and B lymphocytes. B lymphocytes, however, show defects of differentiation and proliferation after antigen stimulation. The number of plasma and memory cells is reduced. As a result of this, the values of serum IgG and IgA are reduced and also irregularly the values of IgM (Di Renzo et al., 2004). The antibody response to protein and polysaccharide antigens is insufficient or completely missing in some cases. The attempts to classify CVID based on in vitro immunoglobulin (Ig) production and B lymphocyte subsets have confirmed marked heterogeneity of this antibody defects (Weiler & Bankers-Fulbright, 2005). Differentiation of precursor cells into mature B lymphocytes and then into plasma cells is a process regulated by products of a number of genes. Mutation of genes for inducible costimulator (ICOS), the first single gene defect, has been described in patients with CVID (Grimbacher et al., 2003). Subsequently, the mutations of genes encoding transmembrane activator and calcium-modulator and cytophilin ligand interactor (TACI) and CD19 have been found.
ICOS deficiency
The ICOS molecule was described by Hutloff et al. (1999). It ranks in the family of costimulation molecules similarly as CD28 and CTLA-4 (CD152) molecules. It is encoded by three genes that are mapped to chromosome 2q33. A great number of single nucleotide variants that participate in the ICOS polymorphisms have been reported. The comparison of the frequency of single nucleotide polymorphisms between patients and healthy controls showed no significant differences (Haaning Andersen et al., 2003; Guzman et al., 2005; Ohm-Laursen et al., 2005). The ICOS and CD28 molecules share up to 20% amino acid homology and both are type I transmembrane receptors. ICOS occurs as a homodimer, with an extracellular (Ig) V like domain. Unlike the CD28 molecule, which is expressed constitutively, the ICOS molecule is expressed only on activated T lymphocytes. The ICOS expression is up-regulated within hours after TcR engagement. Its membrane expression persists on recently activated as well as on memory Th1 and Th2 CD4+ lymphocytes (van Berkel & Oosterwegel, 2006; Warnatz et al., 2006). The ICOS expression appears to be higher on Th2 cells compared to Th1 CD4+ T cells (Coyle et al., 2000). The Th2-mediated response is more dependent on the ICOS function than Th1 response (Löhning et al., 2003; Grunig et al., 2005). The density of ICOS membrane expression correlates with cytokine production. In case of ICOS gene mutation, especially the synthesis of cytokine associated with high-level ICOS expression (IL-10, IL-2, IL-4, IL-5, IL-13) is disturbed. Within the course of viral or bacterial infections in knock-out ICOS−/− mice, large secondary germ centres fail to develop, and specific antibody response is missing. Both the reduced proliferation Th2 activity and the failure of T and B lymphocyte co-operation participate in the developed antibody defect (van Berkel & Oosterwegel, 2006; Warnatz et al., 2006) (Figure 1).
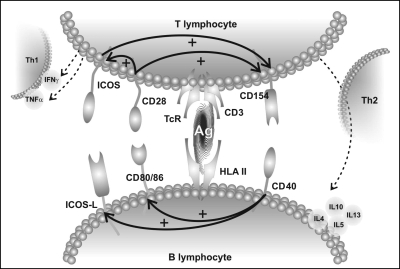
Figure 1.
The cascade of co-stimulation signals between T and B lymphocytes. The expression of CD154 (CD40L) and ICOS is up-regulated through constitutively surface expressed CD28 molecule and its CD80/CD86 ligands. CD40-CD154 ligand amplifies the expression of CD80/CD86 and ICOS-L. By this another support of co-stimulation ligands between T and B lymphocytes occurs through ICOS-ICOS-L which up-regulates CD154 expression.
Triggering of TcR with appropriate costimulation leads to gene transcription in the nucleus. The intracellular tail of the ICOS molecule contains two tyrosine residues, one of which lies within the YTyr180MEM motive, which is associated with adapter molecule grow factor receptor-bound protein-2 (Grb-2). One amino acid replacement at the position in this motive includes explaining of the lack of capability to recruit Grb-2. This molecule plays a key role during the initiation of IL-2 synthesis (Harada et al., 2003). This finding explains why activating signals coming through defective ICOS molecules do not lead to synthesis of IL-2 (Itoh et al., 2002).
Activated T lymphocytes amplify the ICOS-L expression on B lymphocytes through IL-4, IL-5, IL-6, granulocyte macrophage colony-stimulating factor (GM-CSF), IFNγ and tumour necrosis factor (TNF)α cytokines (Bayry et al., 2005). ICOS-L expression on B lymphocytes is also up-regulated through CD40 and CD154 ligands or bacterial superantigens (Salzer et al., 2004; Janke et al., 2006). The ICOS: ICOS-L interaction is necessary for IL-10, IL-17 synthesis (Chtanova et al., 2004; Warnatz et al., 2006). The ICOS-L molecule is encoded by one gene, which is found on the long arm of chromosome 21 (mapped 21q22.3). It exists in two splice variants that differ in cytoplasmatic tail. The B7H2 variant is encoded by exon 1–7 and appears on both lymphoid and non-lymphoid cells. The hGL-50 variant is encoded by exon 1–6 and 8, and is found only on lymphoid cells (Ohm-Laursen et al., 2005; Warnatz et al., 2006). The ICOS-L is the receptor only for ICOS. Interaction of ICOS — ICOS-L molecules is important for differentiation of naive B lymphocytes into memory and plasma cells. ICOS-L ligand shares up to 20% homology with CD80 and CD86 molecules (receptors for CD28 and CTLA-4 molecules) (Salzer et al., 2004; van Berkel & Oosterwegel, 2006).
The homozygous deletion of ICOS causes an antibody deficiency syndrome in affected individuals. The clinical phenotype includes recurrent and serious bacterial infections, autoimmune phenomena, splenomegaly, lymphadenopathy and malignancy. Patients with CVID, based on ICOS defect, have mild B lymphopaenia and significantly reduced number of memory CD19+/CD27+ B lymphocytes (Warnatz et al., 2002; Piqueras et al., 2003). This laboratory finding is not, however, characteristic for ICOS mutations because up to 75% of patients with CVID have a reduced number of memory isotype-switched CD19+/CD27+/IgM− B lymphocytes (Schejbel et al., 2005). However, patients with ICOS deficiency belong to CVID type I group (Freigburg classification) (Warnatz et al., 2002; Carsetti et al., 2005). ICOS deficiency, based on one point mutation, was the first example of an autosomal recessive disorder that occurs only in a small amount of patients with CVID (approximately 2%) (Warnatz et al., 2006).
TACI deficiency
In order to ensure physiological antibody response after antigen stimulation, other activation signals mediated by both intracellular interactions and soluble factors are important (Kroczek et al., 2004). Class switch recombination (CSR) of Ig synthesis requires four signals. The first one is mediated by IL-3, IL-4, IL-5, IL-6, IL-13 cytokines, which participate in CH genes transcription regulation. In case of a T-cell–dependent antibody response, the interaction of CD40L (CD154) molecule expressed on activated T lymphocytes with CD40 molecule on B lymphocytes is necessary (McHeyzer-Williams, 2003). Costimulatory ICOS — ICOS-L interaction constitutes the third necessary stimulus for sufficient antibody production. The fourth signal is mediated by other interactions of molecules, which belong to the superfamily of TNF molecules (B-cell–acting factor of the TNF family (BAFF), A proliferation-inducing ligand (APRIL)), and their receptors (B-cell maturation antigen (BCMA), BAFF receptor (BAFF-R), TACI).
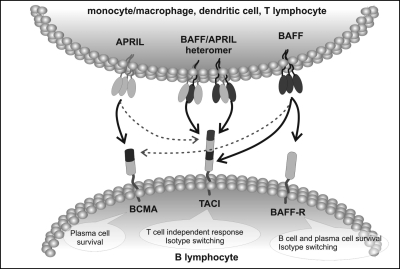
The BAFF and APRIL are among these molecules (Castigli et al., 2005a). BAFF is present predominantly on monocytes/macrophages, dendric cells and T lymphocytes (Mackay & Ambrose, 2003). On the contrary, it is not expressed on B lymphocytes although this molecule has been detected on B-cell–derived chronic lymphatic leukaemia (Novak et al., 2002). The APRIL molecule is expressed on a large variety of cells, monocytes/macrophages, dendritic cells and activated T lymphocytes. BAFF and APRIL molecules rank as type II membrane receptors. They exist as membrane molecules and are in soluble form. BAFF and APRIL molecules create both homotrimers and heteromers capable of stimulating B lymphocyte differentiation (Wu et al., 2000; Locksley et al., 2001). It has been proved that APRIL and BAFF molecules interfere with CSR of T-independent antibody response (Litinskiy et al., 2002; Martin & Vishva, 2005).
Both APRIL and BAFF molecules bind two type III membrane receptors, BCMA, TACI (Schneider et al., 1999). Both receptors belong to a superfamily of TNF receptors. BCMA is expressed exclusively on B lymphocytes while TACI is expressed both on B lymphocytes and activated T lymphocytes. The third receptor belonging to the TNF-R family is the BAFF-R, which is the unique receptor for BAFF (Bodmer et al., 2002; Losi et al., 2005). This membrane molecule is predominantly expressed on B lymphocytes and only on part of activated T lymphocytes (Schneider & Tschopp, 2003; Martin & Vishva, 2005). Interactions of APRIL and BAFF, expressed on monocytes and macrophages, with their receptors on B lymphocytes are evidence of the significance of natural immunity for the correct antibody response (McHeyzer-Williams, 2003; de Durana et al., 2006). The absence of any of the previously mentioned signals is the cause for defect of terminal differentiation of a mature B lymphocyte into plasmatic and memory cells, which are manifested by antibody immunodeficiency (Salzer & Grimbacher, 2005) (Figure 2).
Figure 2.
TNF receptor family members, BAFF-R, BCMA and TACI, and their ligands BAFF and APRIL check terminal differentiation of B lymphocytes and/or CSR of Ig synthesis. TNF receptors and their ligands regulate both the survival and the apoptosis during immune system development and immune responses. Dashed lines show the interactions with limited functions.
Receptor TACI molecules are encoded by the TNFRSF13b gene, which is situated at the short arm of chromosome 17 (17p11.2). The described mutations affect TACI molecule in its extracellular (C104R, S144X), transmembrane (A181E) and intracellular parts (S194X, R202H, Ins204) (Castigli et al., 2005b). Both homozygous and heterozygous TNFRSF13b mutations are associated with antibody deficiencies (Salzer et al., 2005). This suggests that TACI deficiency may manifest in autosomal dominant as well as in recessive traits in familial and in sporadic CVID. In the case of heterozygous mutations, the clear evidence that they are the cause of humoral deficiencies has not been shown. It is not possible to exclude that heterogeneous mutations may be the only modifying factor that leads to the manifestation of other defects. The TACI mutations show a range of clinical symptoms from no infection to very severe infections, suggesting that other genetic and environmental factors contribute to the variable disease spectrum. In addition, TACI deficiency may also represent a common genetic defect for CVID and selective IgA deficiency (sIgAD), which has been long proposed to be based on the clinical observations in CVID/sIgAD families (Salzer & Grimbacher, 2006).
The numbers of B cells in homozygous TACI deficiency are normal or tend to be slightly reduced. Patients with homozygous mutations in TACI showed selective impairment of APRIL and BAFF-induced B-cell proliferation and CSR. They have low numbers of CD19+/CD27+/IgM− B lymphocytes and suffer from severe infections caused by encapsulated microbes. The vaccination with non-conjugated vaccines is not effective. In the case of homozygous TACI mutations, polysaccharide antigens are followed by a disturbance in the proliferation of B lymphocytes (Castigli et al., 2005b).
Unlike knock-out TACI−/− mice, in which spontaneous development of lymphomas occur, only one case of malignant lymphoma has been described in patients with TACI mutation (Seshasayee et al., 2003). The increased incidence of lupus-like disease seen in TACI−/− mice was not observed in CVID patients with the same defect. In case of patients with CVID, the incidence of autoimmune manifestations in patients with and without TACI defect is the same. The incidence of TNFRSF13b mutations is estimated to be in 5–10% of patients with CVID (Salzer & Grimbacher, 2006).
CD19 molecule deficiency
The CD19 molecule is detectable in the cytoplasm of B lymphocyte precursors, and from the stage of immature B lymphocytes it is imbedded in the cell membrane. It participates in feedback regulation of signals which come through BcR. On the other hand, it facilitates the recognition of antigen-antibody complexes that link to BcR and the CD21 molecule. Together with the CD21 molecule, it ensures another communication between natural immunity and B lymphocytes. van Zelm described the homozygous mutation in the CD19 gene in four members of a family with antibody deficiency that complies with classification criteria for CVID. The encoding gene is situated on the short arm of chromosome 16 (mapped 16p11.2) (van Zelm et al., 2006). The defect is autosomally recessive. Its real frequency is unknown in patients with CVID because the findings discovered up to now are rare. The numbers of peripheral B lymphocytes are normal or only slightly reduced in affected individuals. CD19 expression on B lymphocytes is missing and as a result of this, the expression of CD21 surface molecules is reduced. Calcium influx into cells is disturbed (McHeyzer-Williams, 2003). The hypersomatic mutation process proceeds in a normal way; however, CD27+ B lymphocytes are missing. Post-activation antibody response to rabies vaccine is insufficient. In diagnosed individuals, the defect was manifested by repeated bacterial infections. Autoimmune and lymphoproliferative symptoms were not observed (van Zelm et al., 2006).
Conclusion
In 1953 Janeway described a case of a 39-year-old woman with hypogammaglobulinaemia, which is considered the first mention of CVID. More than 50 years have passed during which separate pathological units, as for example hyper IgM syndrome linked and unlinked to X-chromosome and autosomal recessive agammaglobulinaemia, have been excluded. In the last 3 years, mutations of genes encoding ICOS, TACI and CD19 have been described. These defects are found in less than 15% of the patients and for this reason the etiology of most of the patients with CVID remains unknown. The described mutations and findings of other expected gene defects will probably cause disintegration of this heterogeneous antibody deficiency syndrome.
Acknowledgments
This study has been supported by the research project number MZO 00179906 from the Ministry of Health.
References
- Bayry J, Hermine O, Webster DA, Levy Y, Kaveri SV. Common variable immunodeficiency: the immune system in chaos. Trends in Molecular Medicine. 2005;11:370. doi: 10.1016/j.molmed.2005.06.005. [DOI] [PubMed] [Google Scholar]
- van Berkel MEAT, Oosterwegel MA. CD28 and ICOS: similar or separate costimulators of T cells? Immunology Letters. 2006;105:115. doi: 10.1016/j.imlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends in Biochemical Sciences. 2002;27:19. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, Plebani A, Aiuti F, Quinti I. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. Journal of Allergy and Clinical Immunology. 2005;115:412. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, Geha RS. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nature Genetics. 2005a;37:829. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. Journal of Experimental Medicine. 2005b;201:35. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. Journal of Immunology. 2004;173:68. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S. The CD 28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- Di Renzo M, Pasqui AL, Auteri A. Common variable immunodeficiency: a review. Clinical and Experimental Medicine. 2004;3:211. doi: 10.1007/s10238-004-0027-2. [DOI] [PubMed] [Google Scholar]
- de Durana YD, Mantchev GT, Bram RJ, Franco A. TACI-BlyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107:594. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbacher B, Hutloff A, Schlesier M. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nature Immunology. 2003;4:261. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- Grunig G, Banz A, de Waal Malefyt R. Molecular regulation of Th2 immunity by dendritic cells. Clinical Pharmacology and Therapeutics. 2005;106:75. doi: 10.1016/j.pharmthera.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Guzman VB, Morgun A, Shulzhenko N. Characterization of CD28, CTLA4, and ICOS polymorphisms in three Brazilian ethnic groups. Human Immunology. 2005;66:773. doi: 10.1016/j.humimm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Haaning Andersen AD, Lange M, Lillevang ST. Allelic variation of the inducible costimulator (ICOS) gene: detection of polymorphisms, analysis of the promoter region, and extended haplotype estimation. Tissue Antigens. 2003;61:276. doi: 10.1034/j.1399-0039.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K, Toma H, Altman A, Abe R. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) Journal of Experimental Medicine. 2003;197:257. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Itoh S, Itoh M, Nishida K, Yamasaki S, Yoshida Y, Narimatsu M, Park SJ, Hibi M, Ishihara K, Hirano T. Adapter molecule Grb2-associated binder 1 is specifically expressed in marginal zone B cells and negatively regulates thymus-independent antigen-2 responses. Journal of Immunology. 2002;168:5110. doi: 10.4049/jimmunol.168.10.5110. [DOI] [PubMed] [Google Scholar]
- Janke M, Witsch EJ, Mages HW. Eminent role of ICOS costimulation for T cells interacting with plasmacytoid dendritic cells. Immunology. 2006;118:353. doi: 10.1111/j.1365-2567.2006.02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Current Opinion in Immunology. 2004;16:321. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Litinskiy MB, Nardelli B, Hilbert DM. DCs induce CD40-independent immunoglobulin class switching through BlyS and APRIL. Nature Immunology. 2002;3:822. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Löhning M, Hutloff A, Kallinich T. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. Journal of Experimental Medicine. 2003;197:181. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi CG, Silini A, Fiorini C, Soresina A, Meini A, Ferrari S, Notarangelo LD, Lougaris V, Plebani A. Mutational analysis of human BAFF receptor TNFRSF13C (BAFF-R) in patients with common variable immunodeficiency. Journal of Clinical Immunology. 2005;25:496. doi: 10.1007/s10875-005-5637-2. [DOI] [PubMed] [Google Scholar]
- Mackay F, Ambrose Ch. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Reviews. 2003;14:311. doi: 10.1016/s1359-6101(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Martin F, Vishva MD. Unraveling TACIt functions. Nature Genetics. 2005;37:793. doi: 10.1038/ng0805-793. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams MG. B cells as effectors. Current Opinion in Immunology. 2003;15:354. doi: 10.1016/s0952-7915(03)00046-3. [DOI] [PubMed] [Google Scholar]
- Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B-lymphocyte stimulator by chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100:2973. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- Ohm-Laursen L, Schjebel L, Jacobsen K. Normal ICOS, ICOSL and AID alleles in Danish patients with common variable immunodeficiency. Scandinavian Journal of Immunology. 2005;61:566. doi: 10.1111/j.1365-3083.2005.001603.x. [DOI] [PubMed] [Google Scholar]
- Piqueras B, Lavenu-Bombled C, Galicier L, Bergeron-van der Cruyssen F, Mouthon L, Chevret S, Debre P, Schmitt C, Oksenhendler E. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. Journal of Clinical Immunology. 2003;23:385. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- Salzer U, Grimbacher B. TACItly changing tunes: farewell to a yin and yang of BAFF receptor and TACI in humoral immunity? New genetic defects in common variable immunodeficiency. Current Opinion in Allergy and Clinical Immunology. 2005;5:496. doi: 10.1097/01.all.0000191887.89773.cc. [DOI] [PubMed] [Google Scholar]
- Salzer U, Grimbacher B. Monogenetic defects in common variable immunodeficiency: what can we learn about terminal B cell differentiation? Current Opinion in Rheumatology. 2006;18:377. doi: 10.1097/01.bor.0000231906.12172.6e. [DOI] [PubMed] [Google Scholar]
- Salzer U, Chapel HM, Webster ADB, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nature Genetics. 2005;37:820. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- Salzer U, Maul-Pavicic A, Cunningham-Rundles C, Urschel S, Belohradsky BH, Litzman J, et al. ICOS deficiency in patients with common variable immunodeficiency. Clinical Immunology. 2004;113:234. doi: 10.1016/j.clim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Schejbel L, Marquart H, Andersen V, Permin H, Andersen P, Svejgaard A, Barington T. Deficiency of somatic hypermutation of immunoglobulin G transcripts is a better predictor of severe respiratory tract infections than lack of memory B cells in common variable immunodeficiency. Journal of Clinical Immunology. 2005;25:392. doi: 10.1007/s10875-005-5034-x. [DOI] [PubMed] [Google Scholar]
- Schneider P, Tschopp J. BAFF and the regulation of B cell survival. Immunology Letters. 2003;88:57. doi: 10.1016/s0165-2478(03)00050-6. [DOI] [PubMed] [Google Scholar]
- Schneider P, Mackay F, Steiner V. BAFF, a novel ligand of the tumor necrosis factor (TNF) family, stimulates B cell growth. Journal of Experimental Medicine. 1999;189:1747. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshasayee D, Valdez P, Yan M. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BlyS receptor. Immunity. 2003;18:279. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, van Dongen JJ, Orlowska-Volk M, Knoth R, Durandy A. Human ICOS-deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107:3045. doi: 10.1182/blood-2005-07-2955. [DOI] [PubMed] [Google Scholar]
- Warnatz K, Denz A, Dräger R, Braun M, Groth C, Wolff-Vorbeck G, Eibel H, Schlesier M, Peter HH. Severe deficiency of switched memory B cells (CD27+IgM−IgD−) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- Weiler CR, Bankers-Fulbright JL. Common variable immunodeficiency: test indications and interpretations. Mayo Clinic Proceedings. 2005;80:1187. doi: 10.4065/80.9.1187. [DOI] [PubMed] [Google Scholar]
- Wu Y, Bressette D, Carrell JA, Kaufman T, Feng P, Taylor K, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BlyS. Journal of Biological Chemistry. 2000;275:35478. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- van Zelm MC, Reisli I, van der Burg M. An antibody-deficiency syndrome due to mutations in the CD19 gene. New England Journal of Medicine. 2006;354:1901. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]