Abstract
Type 2 diabetes is characterised by a gradual decline in glycaemic control and progression from oral glucose-lowering monotherapy to combination therapy and exogenous insulin therapy. Functional decline of the insulin-secreting β-cells is largely responsible for the deterioration in glycaemic control. Preservation of β-cell functionality, in addition to maintaining glycaemic control and reducing insulin resistance, is now regarded as a key target for long-term management strategies. Early, aggressive intervention with combination therapy is emerging as a valid approach to optimise long-term outcomes and combining agents with differing modes of action and secondary effect profiles should prove valuable. Sulfonylureas and thiazolidinediones exert their glucose-lowering effect through differing mechanisms of action – the sulfonylureas by stimulating insulin secretion, whereas the thiazolidinediones are insulin sensitisers. Both agents offer excellent improvements in glycaemic control when given as monotherapy or in combination. The thiazolidinediones protect β-cell structural and functional integrity and functionality and complement the sulfonylureas by inducing and maintaining improvements in insulin resistance, the abnormal lipid profile associated with type 2 diabetes and other cardiovascular risk factors. Thus, there is a strong rationale to support the addition of thiazolidinediones to sulfonylureas as a treatment option for type 2 diabetes. This combination may be particularly effective in the early stages of the disease when β-cell function is at its highest, allowing maximal benefit to be obtained from the insulin secretion-promoting abilities of the sulfonylureas and the β-cell-protective effects of the thiazolidinediones.
Message for the Clinic
•The combination of a sulfonylurea and a thiazolidinedione may be a particularly effective treatment option in the early stages of type 2 diabetes when β-cell function is at its highest. This allows maximal benefit to be obtained from the insulin secretion-promoting abilities of the sulphonylureas and the β-cell-protective effects of the thiazolidinediones.
Review Criteria
•Evidence was identified through a search of MEDLINE and EMBASE from January 1996 to October 2006, using the search terms ‘thiazolidinedione’, ‘pioglitazone’, ‘rosiglitazone’, ‘sulfonylurea’, and ‘mechanism of action’, ‘pleiotropic’, ‘insulin resistance’ and ‘type 2 diabetes’.
Introduction
Patients with type 2 diabetes characteristically experience a gradual decline in glycaemic control and progression from oral glucose-lowering monotherapy to combination therapy and ultimately to exogenous insulin therapy (1,2). A decrease in the number of functional insulin-producing β-cells contributes to the pathological decline in glycaemic control typically seen in type 2 diabetes (3) and preserving β-cell function has emerged as a vital component of long-term management strategies.
Already at diagnosis of type 2 diabetes about 50% of insulin secretion capacity is lost (4). There is now increasing evidence available that near to normal glucose control prevents progressive deterioration of insulin secretion. Therefore, a move towards introducing combination therapy earlier in the course of type 2 diabetes with the aim of preserving glycaemic control and β-cell function and thereby improving long-term outcomes is considered as a better approach to meet the dual defect in pathophysiology of type 2 diabetes. Such an approach poses the challenge of defining optimal combination regimens and the point at which to introduce them. Recent studies suggest that early, aggressive dual or even triple combination therapy in newly diagnosed patients slows the decline in glycaemic control compared with standard monotherapies (5,6). Intervening before the onset of frank type 2 diabetes in high-risk groups, such as those with gestational onset diabetes (7,8) or individuals with impaired glucose tolerance (9,10), is also emerging as a relevant approach to reducing the long-term morbidity and mortality of this chronic disease.
Insulin resistance is a core feature of type 2 diabetes. Although not all individuals with insulin resistance will go on to develop type 2 diabetes, in those that do, overt type 2 diabetes develops when β-cells are no longer able to compensate for insulin resistance. Insulin resistance in itself may contribute to the decline in β-cell function by inducing endoplasmic reticulum stress as a consequence of an increased demand for insulin (11). This is in addition to the toxic effects of elevated glucose and lipid levels because of their impaired metabolic processing in the presence of insulin resistance (12).
Today, a variety of agents of differing modes of action are available to improve glycaemic control. The sulfonylureas (e.g. glipizide, glyburide, glimepiride and gliclazide) are insulin secretagogues that act to increase insulin secretion. Biguanides (principally metformin), reduce hepatic glucose output and increase the uptake of glucose by peripheral tissues. More recently, the thiazolidinediones (pioglitazone and rosiglitazone) have emerged as novel, effective glucose-lowering agents (13). The thiazolidinediones are peroxisome proliferator-activated receptor gamma (PPARγ)-stimulating insulin sensitisers that also act without increasing insulin secretion. Other, less widely used agents include the α-glucosidase inhibitor, acarbose, the incretin mimetic, exenatide, and the new short-acting insulin secretagogues, repaglinide and nateglinide. A variety of agents with novel mechanisms of action are currently in development and their role in future management regimens has yet to be defined. All these agents have varying, primary or secondary, effects on β-cell function. Recent research effort has focused on defining the effect of oral glucose-lowering agents in this respect to guide decisions on appropriate combination therapies to achieve long-term outcomes beyond glycaemic control.
This review examines the mechanistic distinctions in terms of glycaemic control and β-cell functional preservation and the latest clinical data that support the rationale for thiazolidinedione–sulfonylurea combination therapy in patients with type 2 diabetes.
Glycaemic control
The sulfonylureas and biguanides formed the mainstay of oral glucose-lowering therapy from their introduction in the early 1940s until the 1990s when thiazolidinediones became available. Sulfonylureas stimulate endogenous insulin secretion via their action at the KATP channel in the plasma membrane of pancreatic β-cells (14) and effectively decrease HbA1c levels by between 0.8% and 2.0% (15). As sulfonylureas act by enhancing insulin secretion, they are most effective in the early stages of type 2 diabetes when β-cell function is at its greatest. Despite their initial efficacy, glycaemic control is inevitably lost over time with the sulfonylureas, necessitating the introduction of combination therapy to regain control and prolong the time before progression to exogenous insulin therapy. In the United Kingdom Prospective Diabetes Study (UKPDS), 53% of newly diagnosed type 2 diabetics initially treated with sulfonylureas subsequently required additional treatment within 6 years to maintain glycaemic control (16). In the recently published ADOPT study, the proportion of the patients with type 2 diabetes exceeded the target fasting blood glucose level of 180 mg/dl after follow-up (17). Given the direct effects of the sulfonylureas on the β-cell and the potential for β-cell exhaustion, combination regimens with agents that improve glycaemia by different modes of action, such as the thiazolidinediones, are recommended. This combination may be particularly effective given the known positive effects of the thiazolidinediones on β-cell function discussed below.
The thiazolidinediones act as insulin-sensitising agents and increase the peripheral action of insulin. They act as ligands of PPARγ, which is involved with the regulation of genes that control glucose homeostasis and lipid metabolism and is found in high concentrations in adipose tissue, hepatocytes and skeletal muscle. These agents have also proved effective in reducing HbA1c levels and maintaining glycaemic control, with average reductions in HbA1c levels of between 0.5% and 1.5% (15,18). Recent evidence suggests that the thiazolidinediones may be more effective than sulfonylureas in maintaining glycaemic control over the long term (17,19). In a direct comparison of pioglitazone vs. gliclazide as monotherapy, significantly more pioglitazone-treated patients maintained their glycaemic control at 2 years than did patients treated with gliclazide (19).
Short- and long-term studies with thiazolidinediones and sulfonylurea in combination have shown that this combination offers sustained glycaemic control (20–27). Aljabri et al. (20) found that, compared with the addition of bedtime insulin to maximal doses of sulfonylurea or metformin, the addition of pioglitazone resulted in comparable improvements in glycaemic control and less hypoglycaemia after 16 weeks of treatment. Although this study did not report separate results for the sulfonylurea and metformin groups, there was an overall reduction in HbA1c of 1.9% compared with 2.3% for insulin (20). In another short-term study, Kipnes et al. (27) looked at the pioglitazone–sulfonylurea combination exclusively and reported decreases of 0.9% and 1.3% for pioglitazone doses of 15 and 30 mg, respectively, over 16 weeks.
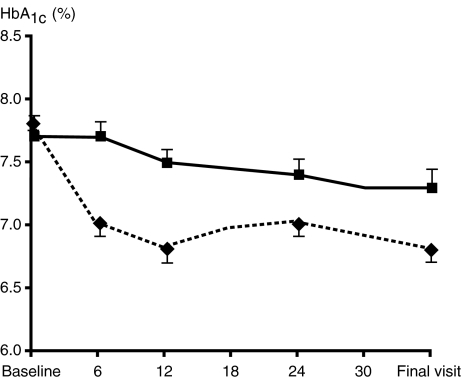
Long-term studies also support the sustained beneficial effects of this combination on glycaemic control (Table 1). In a 6-month study, Comaschi et al. (23) reported significant and sustained reductions in HbA1c when pioglitazone was added to either sulfonylurea or metformin. Four further studies of increasing duration have also confirmed the maintenance of glycaemic control following the addition of pioglitazone to sulfonylurea therapy. In the Quartet study, the addition of pioglitazone to existing sulfonylurea therapy resulted in a 1.2% reduction in HbA1c after 1 year in 319 patients with inadequately controlled type 2 diabetes (25). Derosa et al. (28) also reported significant (1.3%; p < 0.01 from baseline) and sustained reductions in HbA1c levels when either rosiglitazone or pioglitazone were added to failing glimepiride in 91 patients with type 2 diabetes and the metabolic syndrome after 1 year. In the 2-year end point of the Quartet study described above, comparing the addition of pioglitazone or metformin to failing sulfonylurea therapy, found that after a rapid decrease in HbA1c levels over the first 20 weeks of the study plateaued with overall reductions of 1.03% and 1.16% for the pioglitazone plus sulfonylurea and metformin plus sulfonylurea add-on groups, respectively, after 104 weeks of treatment (21). The recently presented glycaemic results of the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) study (22) of 5238 patients with type 2 diabetes and evidence of macrovascular disease further support the maintenance of glycaemic control following the addition of pioglitazone to existing sulfonylurea therapy. A subgroup analysis of the PROactive study looked at the 1001 patients who were managed with sulfonylurea therapy at baseline. Patients treated with pioglitazone experienced significant decreases from baseline in HbA1c levels that were sustained up to the 34.5-month assessment and fewer patients switched to or required addition of metformin or insulin compared with those who received placebo [Figure 1; (22)].
Table 1.
Long-term glycaemic and lipidaemic effects of combined sulfonylurea and thiazolidinedione therapy in patients with type 2 diabetes
| Study | Comparator groups | N | Duration | Glycaemic effects | Lipidaemic effects |
|---|---|---|---|---|---|
| Comaschi et al., 2006 (23) | Pioglitazone + sulfonylurea | 6 months | Significant and comparable improvementsin HbA1c at 12 months (p = 0.0001) | Significant improvement across alllipid parameters | |
| Pioglitazone + metformin | |||||
| Torre et al., 2006 (79) | Pioglitazone + sulfonylurea | 77 | 6 months | Not reported | Significant decrease in triglyceridelevels in the pioglitazone + sulfonylurea group only Significant improvement inHDL-cholesterol levels |
| Pioglitazone + metformin | 103 | ||||
| Derosa et al., 2004 (85) | Glimepiride + pioglitazone | 45 | 1 year | Significant and comparable improvementsin HbA1c at 12 months (p < 0.01) | Significant improvement across alllipid parameters |
| Glimepiride + rosiglitazone | 44 | Worsening of multiple lipid parameters | |||
| Ginis et al., 2006 (24) | Pioglitazone + sulfonylurea | 791 | 1 year | Significant improvement in both groupsfrom baseline Pioglitazone + sulfonylurea: 6.65% Pioglitazone + metformin: 6.61% | Triglycerides decreased significantlyin both groups (p < 0.0001) Overall reduction of 12.8% for bothgroups combined for total cholesterol Significant improvement in HDL- andLDL-cholesterol levels in both groups |
| Pioglitazone + metformin | 705 | ||||
| PROactive Charbonnel and Scheen,2006 (22); Spanheimeret al., 2006 (78) | Pioglitazone + sulfonylurea | 508 | 3 years | Significantly greater improvements overtime in HbA1c vs. placebo (p < 0.001at final visit) | Significant improvement in triglycerideand HDL-cholesterol levels vs. placebo |
| Placebo + sulfonylurea | 493 | ||||
| Quartet Charbonnel et al., 2005 (21) | Pioglitazone + sulfonylurea | 319 | 2 years | HbA1c reduced by 1.03% | Significant reduction in triglyceridesand greater increase inHDL-cholesterol (p ≤ 0.001) vs.metformin + sulfonylurea |
| Metformin + sulfonylurea | 320 | HbA1c reduced by 1.16% |
Figure 1.
Maintenance of long-term glycaemic control with combination sulfonylurea and pioglitazone therapy [Charbonnel and Scheen, 2006 (22)]. The placebo group is shown by the solid line and squares. The pioglitazone group is shown by the dashed line and diamonds. Copyright © 2005 American Diabetes Association from Diabetes, vol. 55, 2006; A478. Reprinted with permission from the American Diabetes Association
Effects of thiazolidinediones and sulfonylureas on β-cell stress
Thiazolidinediones and sulfonylureas exert opposing effects on β-cell functionality over time. β-cell stress is reduced with thiazolidinedione therapy, but enhanced with sulfonylurea therapy (29).
Studies have shown that chronic exposure to glibenclamide can lead to an acceleration of β-cell apoptosis and β-cell exhaustion or desensitisation (30–34). The precise mechanism by which glibenclamide initiates β-cell apoptosis remains unclear, but may involve closure of the inwardly rectifying K+ sulfonylurea receptor subtype of the ATP-sensitive potassium channel (32,34), the sustained enhancement of Ca2+ influx mediated by glibenclamide leading to elevated and toxic cytosolic Ca2+ levels (33) and nitric oxide production (30). In addition, prolonged exposure to sulfonylurea renders β-cells less responsive to subsequent sulfonylurea stimulation. Davalli et al. (31) found that prolonged in vitro exposure of human pancreatic islets to various insulin secretagogues, including sulfonylureas, resulted in a reduced capacity to respond to further secretory stimulation. Interestingly, certain sulfonylureas may offer a protective effect from hydrogen peroxide-induced β-cell damage (35).
Several lines of evidence support the beneficial effects of thiazolidinediones in terms of improving insulin secretion, preserving β-cell mass and islet structure and also protecting β-cells from oxidative stress (36–42). How the thiazolidinediones exert these protective effects remains unclear. In the presence of chronically elevated glucose levels (glucotoxicity), elevated free fatty acid (FFA) levels (lipotoxicity) appear to play an important role in β-cell damage during the early stages of type 2 diabetes, impairing their functionality and inducing cell death (43,44). In the presence of both chronic hyperglycaemia and elevated FFA levels, β-cells synthesise less insulin and lose the glucose-stimulated insulin secretion response (45,46). Thiazolidinediones may protect against the toxic effects of chronically elevated lipid levels by limiting the exposure of β-cells to circulating FFAs (47) and by their effects on triglyceride partitioning in tissues. They may also protect against β-cell apoptosis and facilitate β-cell proliferation more directly by preventing Nuclear Factor (NF)kB activation in β-cells (37,48). Amyloid deposition has also been associated with increased β-cell apoptosis (49) and a reduction in amyloid deposition with thiazolidinediones has been demonstrated in vivo (50). The protective effects of the thiazolidinediones in this respect may be exerted via activation of a phosphatidylinositol 3′-kinase (PI3K) Akt cascade, which acts to inhibit the human islet amyloid polypeptide (h-IAPP) induction of apoptosis (51). Recently a strong effect of pioglitazone on low-grade-inflammation in patients with cardiovascular disease (CVD) without diabetes has been described, which may be another way to protect the β-cells (52).
Insulin resistance
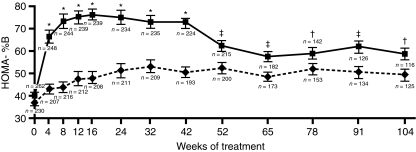
Clinical studies have also provided indirect support for the preservation of β-cell function with thiazolidinedione therapy. Homeostasis model assessment (HOMA) is a mathematical tool that models the glucose–insulin feedback loop in the homeostatic state. It permits estimations of insulin sensitivity (%S) and β-cell function (%B) from pairs of fasting glucose and insulin (or C-peptide) measurements. A 23-week study of pioglitazone monotherapy showed that there was an increase in HOMA-%B (53). In a separate study in drug-naïve patients with type 2 diabetes, Wallace et al. (54) reported an increase in HOMA-%B during 3 months of pioglitazone therapy compared with a small decrease among patients receiving placebo. In addition, they reported a significant decrease in the pro-insulin/insulin ratio suggesting that the β-cells were under less stress. This observation has also been reported for rosiglitazone (55). Other studies have also reported improvements in the insulinogenic index (provides a measure of β-cell function during an oral glucose tolerance test) and the disposition index (in which the value is corrected for underlying insulin resistance) (56–58). These improvements in β-cell function appear to be maintained during long-term studies. For example, in studies comparing pioglitazone and gliclazide either as monotherapies (19) or as add-on treatments to existing therapy (59), both agents were associated with an initial improvement in the HOMA-%B. However, only patients treated with pioglitazone were able to maintain this improvement during 2 years of treatment, while those treated with gliclazide experienced a continued gradual decline, despite a greater initial improvement [Figure 2; (19,59)].
Figure 2.
Improvement in β-cell function over 2 years of treatment with either pioglitazone or gliclazide [Tan et al. 2005 (19)]. The gliclazide group is shown by the solid line and squares. The pioglitazone group is shown by the dashed line and diamonds; *p < 0.0001, ‡p < 0.01, †p < 0.05. Copyright © 2005 American Diabetes Association. From Diabetes Care, vol. 28, 2005, 544–550. Reprinted with permission from the American Diabetes Association
Studies support an effect for both thiazolidinediones and sulfonylureas in improving insulin resistance in the short term in type 2 diabetes using techniques such as the hyperinsulinaemic/euglycaemic clamp (which allows the determination of the amount of glucose necessary to compensate for an increased insulin level without causing hypoglycaemia) and HOMA-%S (54,60–64). However, direct comparisons during long-term treatment suggest that the thiazolidinediones may be better able to improve and maintain improvements in insulin sensitivity (19,65). During 1 year of treatment, pioglitazone therapy was associated with significant reductions in HOMA-%S (p = 0.002 from baseline), whereas no significant decrease was recorded for gliclazide in patients with type 2 diabetes (65). Similarly, in a 2-year study, Tan et al. (19) found that pioglitazone was associated with an improvement in HOMA-%S, while gliclazide was associated with a worsening in this measure of insulin resistance. Combining sulfonylurea therapy with thiazolidinediones may ensure long-term benefits in terms of insulin resistance (66).
In the ADOPT study, comparing rosiglitazone with glibenclamide and with metformin in early type 2 diabetes, rosiglitazone improved insulin resistance and β-cell function to a significantly greater extent than both competitors did (17).
Control of dyslipidaemia and other cardiovascular risk factors
In addition to the toxic effects of elevated FFAs on β-cells, the abnormal lipid profile – including elevated small dense LDL, lowered HDL-cholesterol and elevated triglycerides – associated with type 2 diabetes poses a further burden in terms of increasing the risk of CVD. Thus, most studies of glucose-lowering therapy now also include an assessment of lipid effects.
Older sulfonylureas appear to be associated with adverse cardiovascular risks, possibly due to their binding to ATP-sensitive potassium channels in cardiomyocytes and vascular smooth muscle cells (67,68). However, how these effects might translate into clinical implications is poorly defined and the newer sulfonylureas appear to be associated with a lower risk of adverse cardiovascular events, such as myocardial infarction and may in fact inhibit atheromatous plaque formation (69,70). Patients with pre-existing CVD may be particularly sensitive to the effects of sulfonylureas in this respect (71,72), as studies in which such patients were excluded showed no increased risk of cardiovascular mortality (73). No primary effects on lipid profile induced by sulfonylureas are seen.
Pioglitazone has been shown to improve the lipid profile in patients with type 2 diabetes by increasing HDL-cholesterol levels, decreasing triglyceride levels and increasing the lipoprotein particle size of LDL in combination regimens with sulfonylurea (20,27,74). Evidence is emerging of a differential effect between the thiazolidinediones in this respect (75,76). In the study reported by Peters Harmel et al. (76) described above, the addition of pioglitazone to metformin and/or sulfonylurea therapy was associated with a decrease in triglyceride levels and an increase in HDL-cholesterol levels. The addition of rosiglitazone also resulted in an increase in HDL-cholesterol, but with no significant improvement in triglyceride levels and an overall increase in total cholesterol levels (76). More recently, Khan et al. (75) reported on the improvement in lipid parameters in patients switched from rosiglitazone–sulfonylurea combination therapy to pioglitazone–sulfonylurea therapy in addition to continued stable statin therapy. After 17 weeks of treatment, replacement of rosiglitazone with pioglitazone was associated with significant improvements in triglyceride and total cholesterol levels and more modest improvements in LDL-cholesterol (75). Long-term studies have shown that the beneficial effects associated with pioglitazone therapy are maintained over months and years [Table1; (21,24,74,77–79)].
Thiazolidinediones have other advantages over traditionally employed oral agents, including additional potential cardioprotective effects, such as improving the pro-thrombotic state and blood pressure. When given in combination with the sulfonylurea, glimepiride, both rosiglitazone and pioglitazone have been shown to improve key components of the prothrombotic state associated with type 2 diabetes [including plasminogen activator inhibitor 1 levels] (28). In a study of patients with type 2 diabetes and the metabolic syndrome failing on initial therapy with a sulfonylurea or metformin, combination therapy with glimepiride and either pioglitazone or rosiglitazone was associated with significant improvements in both systolic and diastolic blood pressure over 12 months (80). In addition, thiazolidinediones have been shown to lower the levels of a number of inflammatory parameters, including high sensitivity C-reactive protein and matrix metalloproteinase (52,81–84).
In a recently published study comparing the efficacy of simvastatin, pioglitazone and the combination of both in patients with CVD, but without diabetes, the combination of pioglitazone and statin had additive and complimentary effects on a broad spectrum of inflammatory parameters and the lipoprotein profile independent of HbA1c level (52).
Conclusions
Type 2 diabetes manifests in an insulin-resistant individual when pancreatic β-cells are unable to produce sufficient insulin to overcome insulin resistance in the muscles and liver. Intervening early to attain glycaemic control and protect β-cell function is now regarded as central to improving long-term outcomes for patients with type 2 diabetes. The insulin secretagogue sulfonylureas and biguanides represented the mainstay of oral glucose-lowering therapy from their development in the 1940s to the 1990s. Today, these agents are still used and are proving especially useful in early combination therapy regimens with agents that improve glycaemic control by different molecular mechanisms. In patients treated with a sulfonylurea, there is a strong rationale to support the early combination with thiazolidinediones in type 2 diabetes. In addition to improving and maintaining glycaemic control, the thiazolidinediones reduce β-cell stress, improve insulin resistance and modify a variety of cardiovascular risk factors, including the abnormal lipid profile and increased low-grade inflammation activity associated with type 2 diabetes. The combined benefits of thiazolidinediones and sulfonylureas may delay the progression of type 2 diabetes and the need for exogenous insulin therapy, and may also offer benefits in terms of reduced risk of CVD.
Acknowledgments
I would like to thank Takeda for supporting this supplement with an educational grant. The views presented in this article are entirely my own and have not been influenced in any way by Takeda, nor has Takeda been involved in its preparation.
References
- 1.Fox KM, Gerber Pharmd RA, Bolinder B, et al. Prevalence of inadequate glycaemic control among patients with type 2 diabetes in the United Kingdom general practice research database: a series of retrospective analyses of data from 1998 through 2002. Clin Ther. 2006;28:388–95. doi: 10.1016/j.clinthera.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulphonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. Progressive requirement for multiple therapies (UKPDS 49). The UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047–58. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 4.UKPDS Study Group. UK Prospective Diabetes Study V. Characteristics of newly presenting type 2 diabetic patients: estimated insulin sensitivity and islet β-cell function. Diabetic Med. 1988;5:444–8. [PubMed] [Google Scholar]
- 5.Bell DS, Ovalle F. Outcomes of initiation of therapy with once-daily combination of a thiazolidinedione and a biguanide at an early stage of type 2 diabetes. Diabetes Obes Metab. 2004;6:363–6. doi: 10.1111/j.1462-8902.2004.00357.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Sugimoto D, Strange P, et al. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naïve patients. Diabetes Care. 2006;29:554–9. doi: 10.2337/diacare.29.03.06.dc05-0695. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic B-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 8.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–22. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. J Am Med Assoc. 2003;290:486–94. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 10.Ratner RE. An update on the diabetes prevention program. Endocr Pract. 2006;12:20–4. doi: 10.4158/EP.12.S1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden MR, Tyagi SC, Kerklo MM, Nicolls MR. Type 2 diabetes mellitus as a conformational disease. JOP. 2005;6:287–302. [PubMed] [Google Scholar]
- 12.Boden G, Laakso M. Lipids and glucose in type 2 diabetes: what is the cause and effect. Diabetes Care. 2004;27:2253–9. doi: 10.2337/diacare.27.9.2253. [DOI] [PubMed] [Google Scholar]
- 13.Nobel J, Baerlocher MO, Silverberg J. Management of type 2 diabetes mellitus. Role of thiazolidinediones. Can Fam Physician. 2005;51:683–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Lebovitz HE. Oral therapies for diabetic hyperglycemia. Endocrinol Metab Clin North Am. 2001;30:909–33. doi: 10.1016/s0889-8529(05)70221-8. [DOI] [PubMed] [Google Scholar]
- 15.Luna B, Feinglos M. Oral agents in the management of type 2 diabetes mellitus. Am Fam Physician. 2001;63:1747–56. [PubMed] [Google Scholar]
- 16.Wright A, Burden AC, Paisley RB, et al. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57) Diabetes Care. 2002;25:330–6. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- 17.Kahn SE, Haffner SM, Heise MA, et al. for the ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 18.Van Gaal L, Scheen A. Are all glitazones the same. Diabetes Metab Res Rev. 2002;18:S1–S4. [PubMed] [Google Scholar]
- 19.Tan MH, Baksi A, Krahulec B, et al. Comparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care. 2005;28:544–50. doi: 10.2337/diacare.28.3.544. [DOI] [PubMed] [Google Scholar]
- 20.Aljabri K, Kozak SE, Thompson DM. Addition of pioglitazone or bedtime insulin to maximal doses of sulfonylurea and metformin in type 2 diabetes patients with poor glucose control: a prospective, randomised trial. Am J Med. 2004;116:230–5. doi: 10.1016/j.amjmed.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Charbonnel B, Schernthaner G, Brunetti P, et al. Long-term efficacy and tolerability of add-on pioglitazone therapy to failing monotherapy compared with addition of gliclazide or metformin in patients with type 2 diabetes. Diabetologia. 2005;48:1093–104. doi: 10.1007/s00125-005-1751-1. [DOI] [PubMed] [Google Scholar]
- 22.Charbonnel B, Scheen A. Combination therapy with pioglitazone and either metformin or sulfonylurea: glycemic results from PROactive [abstract] Diabetes. 2006;55:A478. [Google Scholar]
- 23.Comaschi MA, Di Pietro C, Fionda A, et al. Efficacy and safety of pioglitazone in addition to SU or Met vs FC with Met plus glyburide in T2 diabetes Pts [abstract] Diabetes. 2006;55:A473–4. [Google Scholar]
- 24.Ginis AA, Karamanos B, Charalampidou E, et al. Evaluation of changes of glycaemic control and lipid parameters in patients with T2DM after one year of treatment with pioglitazone in combination with metformin or sulfonylurea in a Greek observational study [abstract] Diabetologia. 2006;49:490. [Google Scholar]
- 25.Hanefeld M, Charbonnel BH, Brunetti P, et al. One-year glycemic control with a sulfonylurea plus pioglitazone vs. a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care. 2004;27:141–7. doi: 10.2337/diacare.27.1.141. [DOI] [PubMed] [Google Scholar]
- 26.Hohberg C, Luebben G, Pfutzner A, et al. Conversion of type 2 diabetic patients treated with a conventional insulin regimen to oral treatment with pioglitazone and glimepiride improves insulin resistance and does not result in an overall deterioration of metabolic control [abstract] Diabetes. 2006;55:A481. [Google Scholar]
- 27.Kipnes MS, Krosnick A, Rendell MS, et al. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111:10–7. doi: 10.1016/s0002-9343(01)00713-6. [DOI] [PubMed] [Google Scholar]
- 28.Derosa G, Cicero AFG, Gaddi A, et al. A comparison of the effects of pioglitazone and rosiglitazone combined with glimepiride on prothrombotic state in type 2 diabetic patients with the metabolic syndrome. Diabetes Res Clin Pract. 2005;69:5–13. doi: 10.1016/j.diabres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Del Prato S, Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism. 2006;55:S20–7. doi: 10.1016/j.metabol.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Ansar MM, Ansari M. Nitric oxide involvement in pancreatic beta cell apoptosis by glibenclamide. Nitric Oxide. 2006;14:39–44. doi: 10.1016/j.niox.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Davilli AM, Pontiroli AE, Socci C, et al. Human islets chronically exposed in vitro to different stimuli become unresponsive to the same stimuli given acutely: evidence supporting specific desensitization rather than beta-cell exhaustion. J Clin Endocrinol Metab. 1992;74:7900–4. doi: 10.1210/jcem.74.4.1548342. [DOI] [PubMed] [Google Scholar]
- 32.Hambrock A, de Oliveira Franz CB, Hiller S, Osswald H. Glibenclamide-induced apoptosis is specifically enhanced by expression of the sulfonylurea receptor isoform SUR1 but not by expression of SUR2B or the mutant SUR1 (M1289T) J Pharmacol Exp Ther. 2006;316:1031–7. doi: 10.1124/jpet.105.097501. [DOI] [PubMed] [Google Scholar]
- 33.Iwakura T, Fujimoto S, Kagimoto S, et al. Sustained enhancement of Ca(2+) influx by glibenclamide induces apoptosis in RINm5F cells. Biochem Biophys Res Commun. 2000;271:422–8. doi: 10.1006/bbrc.2000.2616. [DOI] [PubMed] [Google Scholar]
- 34.Maedler K, Carr RD, Bosco D, et al. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501–6. doi: 10.1210/jc.2004-0699. [DOI] [PubMed] [Google Scholar]
- 35.Kimoto K, Suzuki K, Kizaki T, et al. Gliclazide protects pancreatic beta-cells from damage by hydrogen peroxide. Biochem Biophys Res Commun. 2003;303:112–9. doi: 10.1016/s0006-291x(03)00310-3. [DOI] [PubMed] [Google Scholar]
- 36.Diani AR, Sawada G, Wyse B, et al. Pioglitazone preserves pancreatic islet structure and insulin secretory function in three murine models of type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;286:E116–22. doi: 10.1152/ajpendo.00331.2003. [DOI] [PubMed] [Google Scholar]
- 37.Finegood DT, McArthur MD, Kojwang D, et al. β-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–9. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 38.Higa M, Zhou YT, Ravazzola M, et al. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci USA. 1996;96:11513–28. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida H, Takizawa M, Ozawa S, et al. Pioglitazone improves insulin secretory capacity and prevents the loss of beta-cell mass in obese diabetic db/db mice: possible protection of beta cells from oxidative stress. Metabolism. 2004;53:488–94. doi: 10.1016/j.metabol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Kawasaki F, Matsuda M, Kanada Y, et al. Structural and functional analysis of pancreatic islets preserved by pioglitazone in db/db mice. Am J Physiol Endocrinol Metab. 2005;288:E510–8. doi: 10.1152/ajpendo.00128.2004. [DOI] [PubMed] [Google Scholar]
- 41.Smith SA, Lister CA, Toseland CD, et al. Rosiglitazone prevents the onset of hyperglycaemia and proteinuria in the Zucker diabetic fatty rat. Diabetes Obes Metab. 2000;2:363–72. doi: 10.1046/j.1463-1326.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- 42.Yajima K, Hirose H, Fujita H, et al. Combination therapy with PPARgamma and PPARalpha agonists increases glucose-stimulated insulin secretion in db/db mice. Am J Physiol Endocrinol Metab. 2003;284:E966–71. doi: 10.1152/ajpendo.00149.2002. [DOI] [PubMed] [Google Scholar]
- 43.Bell DSH. B-cell rejuvenation with thiazolidinediones. Am J Med. 2003;115:20S–3S. doi: 10.1016/j.amjmed.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Rhodes CJ. Type 2 diabetes – a matter of beta cell life and death. Science. 2005;307:380–4. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 45.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–74. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995;80:1584–90. doi: 10.1210/jcem.80.5.7745004. [DOI] [PubMed] [Google Scholar]
- 47.Buse JB, Tan MH, Prince MJ, et al. The effects of oral anti-hyperglycaemic medication on serum lipid profiles in patients with type 2 diabetes. Diabetes Obes Metab. 2004;6:133–56. doi: 10.1111/j.1462-8902.2004.00325.x. [DOI] [PubMed] [Google Scholar]
- 48.Zeender E, Maedler K, Bosco D, et al. Pioglitazone and sodium salicylate protect human beta-cells against apoptosis and impaired function induced by glucose and interleukin-1beta. J Clin Endocrinol Metab. 2004;89:5059–66. doi: 10.1210/jc.2004-0446. [DOI] [PubMed] [Google Scholar]
- 49.Butler AE, Jang J, Gurlo T, et al. Diabetes due to a progressive defect in β-cell mass in rats transgenic for human islet amyloid polypeptide (HIP rat). A new model for type 2 diabetes. Diabetes. 2004;53:1509–16. doi: 10.2337/diabetes.53.6.1509. [DOI] [PubMed] [Google Scholar]
- 50.Hull RL, Shen ZP, Watts MR, et al. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does no prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005;54:2235–44. doi: 10.2337/diabetes.54.7.2235. [DOI] [PubMed] [Google Scholar]
- 51.Lin CY, Gurlo T, Haataja L, et al. Activation of peroxisome proliferator-activated receptor-gamma by rosiglitazone protects human islet amyloid polypeptide toxicity by a phosphatidylinositol 3′-kinase-dependent pathway. J Clin Endocrinol Metab. 2005;90:6678–86. doi: 10.1210/jc.2005-0079. [DOI] [PubMed] [Google Scholar]
- 52.Hanefeld M, Marx N, Pfutzner A, et al. Anti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular-risk patients with elevated high sensitivity-CRP: the PIOSTAT study. JACC. 2007;49:290–7. doi: 10.1016/j.jacc.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 53.Rosenblatt S, Miskin B, Glazer NB, et al. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron Artery Dis. 2001;12:413–23. doi: 10.1097/00019501-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Wallace TM, Levy JC, Matthews DR. An increase in insulin sensitivity and basal beta-cell function in diabetic subjects treated with pioglitazone in a placebo-controlled randomized study. Diabet Med. 2004;21:568–76. doi: 10.1111/j.1464-5491.2004.01218.x. [DOI] [PubMed] [Google Scholar]
- 55.Smith SA, Porter LE, Biswas N, et al. Rosiglitazone, but not glyburide, reduces circulating proinsulin and the proinsulin:insulin ratio in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:6048–53. doi: 10.1210/jc.2004-0705. [DOI] [PubMed] [Google Scholar]
- 56.Buchanan TA, Xiang AH, Peters RK, et al. Response of pancreatic beta-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes. 2000;49:782–8. doi: 10.2337/diabetes.49.5.782. [DOI] [PubMed] [Google Scholar]
- 57.Cavaghan MK, Ehrmann DA, Byrne MM, et al. Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. J Clin Invest. 1997;100:530–7. doi: 10.1172/JCI119562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ovalle F, Bell DSH. Effect of rosiglitazone vs. insulin on the pancreatic β-cell function of subjects with type 2 diabetes. Diabetes Care. 2004;27:2585–9. doi: 10.2337/diacare.27.11.2585. [DOI] [PubMed] [Google Scholar]
- 59.Urquhart R, Tan MH, Mariz S, et al. Long-term (2-year) effects on HOMA-%S, an estimate of insulin sensitivity, of pioglitazone, gliclazide and metformin as add-on therapies in patients with T2DM [abstract] Diabetes. 2004;53:A150. [Google Scholar]
- 60.Clark HE, Matthews DR. The effect of glimerpiride on pancreatic beta-cell function under hyperglycaemic clamp and hyperinsulinaemic, euglycaemic clamp conditions in non-insulin-dependent diabetes mellitus. Horm Metab Res. 1996;28:445–50. doi: 10.1055/s-2007-979835. [DOI] [PubMed] [Google Scholar]
- 61.Kawamori R, Matsuhisa M, Kinoshita J, et al. Pioglitazone enhances splanchic glucose uptake as well as peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1998;41:35–43. doi: 10.1016/s0168-8227(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 62.Miyazaki Y, Ferrannini E, Mahankali A, et al. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care. 2001;24:710–9. doi: 10.2337/diacare.24.4.710. [DOI] [PubMed] [Google Scholar]
- 63.Tan MH, Glazer NB, Johns D, et al. Pioglitazone as monotherapy or in combination with sulfonylurea or metformin enhances insulin sensitivity (HOMA-S or QUICKI) in patients with type 2 diabetes. Curr Med Res Opin. 2004;20:723–8. doi: 10.1185/030079904125003386. [DOI] [PubMed] [Google Scholar]
- 64.Yamasaki Y, Kawamori R, Wasada T, et al. Pioglitazone (AD-4833) ameliorates insulin resistance in patients with NIDDM. Tohoku J Exp Med. 1997;183:173–83. doi: 10.1620/tjem.183.173. [DOI] [PubMed] [Google Scholar]
- 65.Periello G, Pampanelli S, Di Pietro C, et al. Comparison of glycaemic control over 1 year with pioglitazone or gliclazide in patients with type 2 diabetes. Diabet Med. 2006;23:246–52. doi: 10.1111/j.1464-5491.2006.01801.x. [DOI] [PubMed] [Google Scholar]
- 66.Ceriello A, Eckland DJ, Johns D, et al. Comparison of effect of pioglitazone with metformin or sulfonylurea (monotherapy and combination therapy) on postload glycemia and composite insulin sensitivity index during an oral glucose tolerance test in patients with type 2 diabetes. Diabetes Care. 2005;28:266–72. doi: 10.2337/diacare.28.2.266. [DOI] [PubMed] [Google Scholar]
- 67.Quast U, Stephan D, Bieger S, Russ U. The impact of ATP-sensitive K+ channel subtype selectivity of insulin secretagogues for the coronary vasculature and the myocardium. Diabetes. 2004;53:S156–64. doi: 10.2337/diabetes.53.suppl_3.s156. [DOI] [PubMed] [Google Scholar]
- 68.Simpson SH, Majumdar SR, Tsuyuki RT, et al. Dose-response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ. 2006;174:169–74. doi: 10.1503/cmaj.050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnsen SP, Monster TB, Olsen ML, et al. Risk and short-term prognosis of myocardial infarction among users of antidiabetic drugs. Am J Ther. 2006;13:134–40. doi: 10.1097/00045391-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Ueba H, Kuroki M, Hashimoto S, et al. Glimepiride induces nitric oxide production in human coronary artery endothelial cells via a PI3-kinase-Akt dependent pathway. Atherosclerosis. 2005;183:35–9. doi: 10.1016/j.atherosclerosis.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 71.Garratt KN, Brady PA, Hassinger NL, et al. Sulfonylurea drugs increase early mortality in patients with diabetes mellitus after direct angioplasty for acute myocardial infarction. J Am Coll Cardiol. 1999;33:119–24. doi: 10.1016/s0735-1097(98)00557-9. [DOI] [PubMed] [Google Scholar]
- 72.Scognamiglio R, Avogar A, Vigili Kreutzenberg de S, et al. Effect of treatment with sulfonylurea drugs or insulin on ischemia-induced myocardial dysfunction in type 2 diabetes. Diabetes. 2002;51:808–12. doi: 10.2337/diabetes.51.3.808. [DOI] [PubMed] [Google Scholar]
- 73.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 74.Perez A, Khan M, Johnson T, Karunaratne M. Pioglitazone plus a sulfonylurea or metformin is associated with increased lipoprotein particle size in patients with type 2 diabetes. Diabetes Vasc Dis Res. 2004;1:44–50. doi: 10.3132/dvdr.2004.006. [DOI] [PubMed] [Google Scholar]
- 75.Khan M, Spanheimer R, Shinall S. Lipid and apolipoprotein levels improve with pioglitazone plus sulfonylurea after treatment conversion from rosiglitazone in subjects with type 2 diabetes and dyslipidemia who continue stable statin therapy [abstract] Diabetologia. 2006;49:704. [Google Scholar]
- 76.Peters Harmel AL, Kendall DM, Buse JB, et al. Impact of adjunctive thiazolidinedione therapy on blood levels and glycemic control in patients with type 2 diabetes. Curr Med Res Opin. 2004;20:215–23. doi: 10.1185/030079903125002937. [DOI] [PubMed] [Google Scholar]
- 77.Betteridge DJ, Vergés B. Long-term effects on lipids and lipoproteins of pioglitazone vs. gliclazide addition to metformin and pioglitazone vs. metformin addition to sulfonylurea in the treatment of type 2 diabetes. Diabetologia. 2005;48:2477–81. doi: 10.1007/s00125-005-0034-1. [DOI] [PubMed] [Google Scholar]
- 78.Spanheimer R, Tan M, Yates J, et al. The effects of long-term pioglitazone therapy on lipid profiles in high-risk type 2 diabetes patients: results from PROactive [abstract] Diabetologia. 2006;49:69–70. [Google Scholar]
- 79.Torre E, Ponzani P, Menozzi F, Mariz S, Corsi A, Comaschi M. The efficacy and safety of pioglitazone in addition to SU or metformin vs fixed combination of metformin plus glibenclamide on diabetic dyslipidaemia [abstract] Diabetologia. 2006;49:493–4. [Google Scholar]
- 80.Derosa G, Cicero AFG, D'Angelo A, et al. Thiazolidinedione effects on blood pressure in diabetic patients with metabolic syndrome treated with glimepiride. Hypertens Res. 2005;28:917–24. doi: 10.1291/hypres.28.917. [DOI] [PubMed] [Google Scholar]
- 81.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–84. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 82.Marx N, Imhof A, Froehlich J, et al. Effect of rosiglitazone treatment on soluble CD40L in patients with type 2 diabetes and coronary artery disease. Circulation. 2003;107:1954–7. doi: 10.1161/01.CIR.0000069272.06194.91. [DOI] [PubMed] [Google Scholar]
- 83.Mohanty P, Aljada A, Ghanim H, et al. Evidence for a potent anti-inflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–35. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 84.Pfutzner A, Marx N, Lubben G, et al. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J Am Coll Cardiol. 2005;45:1925–3. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 85.Derosa G, Cicero AF, Gaddi A, et al. Metabolic effects of pioglitazone and rosiglitazone in patients with diabetes and metabolic syndrome treated with glimepiride: a twelve-month, multicenter, double-blind, randomized, controlled, parallel-group trial. Clin Ther. 2004;26:744–54. doi: 10.1016/s0149-2918(04)90074-4. [DOI] [PubMed] [Google Scholar]