Abstract
Background/Aims
Liver regeneration after partial hepatectomy (PH) in retrorsine-exposed rats is accomplished through proliferation and differentiation of small hepatocyte-like progenitor cells (SHPCs). The cells of origin of SHPCs are not known. We investigated the possibility that SHPCs are directly derived from oval cells, a known liver progenitor cell, by combining the retrorsine/PH (RP) model with 2-acetamidofluorene (2-AAF), an anti-mitotic agent that elicits an oval cell reaction in response to liver deficit.
Methods
Male Fischer 344 rats were treated with retrorsine (30 mg/kg ip) at 6 and 8 weeks of age, with PH 5 weeks after the final treatment. Seven days prior to PH, a 21 day 2-AAF (50 mg) time-release pellet was inserted subcutaneously. Livers were harvested at 3, 7, 10, 14, and 21-days post-PH.
Results
Liver sections from animals treated with 2-AAF/retrorsine/PH (2-AAF/RP) contain significant numbers of proliferating oval cells, but no SHPCs at 7-days post-PH, while RP animals exhibit significant numbers of SHPCs and minimal oval cell reaction. Between 10 and 14-days post-PH, new hepatocyte clusters appear in 2-AAF/RP treated rats. Labeling of proliferating oval cells with BrdU at 6-days post-PH demonstrated that these new hepatocytes represent the progeny of differentiating oval cells.
Conclusions
The observed differences in progenitor cell responses between 2-AAF/RP and RP animals strongly suggest that SHPCs are not the progeny of oval cell precursors, but represent an independent liver progenitor cell population.
Keywords: pyrrolizidine alkaloid, oval cells, liver regeneration, liver stem cell
1. Introduction
The mammalian liver possesses an enormous capacity to replace cells that are lost to surgical resection or necrosis (1), and several different cell populations can be activated to repair or regenerate the liver depending upon the nature and extent of injury or tissue deficit (2). In an otherwise healthy liver, replacement of hepatocytes and biliary epithelia is achieved through the proliferation of mature (fully-differentiated) hepatocytes and biliary epithelial cells contained in the residual (viable) tissue (2, 3). Activation of undifferentiated stem cells does not occur when these mature cell types are capable of proliferating to restore liver mass and structure (4). However, in several experimental models, hepatocytes are rendered unable to proliferate through exposure to mitoinhibitory compounds, resulting in the emergence and proliferation of stem-like progenitor cells in response to liver deficit (3, 5). Numerous studies have appeared that describe the outgrowth of undifferentiated oval cells after hepatocellular injury (1, 5, 6). Oval cells respond to liver damage in the presence of mitoinhibitory agents that block hepatocyte proliferation [such as 2-acetamidofluroene (2-AAF)], as well as agents that cause necrosis (galactosamine) (7, 8). Our group and others described the cellular responses and time-course for liver regeneration after surgical partial hepatectomy (PH) in rats with retrorsine-induced hepatocellular injury (9, 10). Systemic exposure to retrorsine, a member of the pyrrolizidine alkaloid family, results in a severe inhibition of the replicative capacity of fully-differentiated hepatocytes (11, 12). When confronted with a strong proliferative stimulus such as PH (10–12), retrorsine-injured hepatocytes synthesize DNA, but are unable to complete mitosis, and arrest as non -proliferative giant cells (megalocytes) (10). In this model, the entire liver mass is reconstituted after PH through the emergence and expansion of a population of small hepatocyte-like progenitor cells (SHPCs) (10, 13), concurrent with apoptosis of retrorsine injured hepatocytes (10, 14).
The origin of SHPCs is commonly debated and remains unknown. Data from recent studies provide contradictory results suggesting that either oval cells or mature hepatocytes are the origin of the SHPCs (15, 16). We have investigated the possibility that SHPCs are the progeny of oval cells by combining the retrorsine/PH (RP) model of liver injury with 2-AAF treatment. We describe distinct differences in progenitor cell responses to liver deficit in animals exposed to (i) retrorsine and 2-AAF, (ii) retrorsine only, or (iii) 2-AAF only. Animals treated with both retrorsine and 2-AAF before PH contain robust oval cell proliferation by 3-days post-PH, with continued oval cell expansion between 3 and 10 days, and formation of new hepatocytes by 14-days post-PH. Animals exposed to retrorsine (in the absence of 2-AAF) never exhibit substantial oval cell proliferation, but clusters of SHPCs emerge as early as 3 days after PH and expand to regenerate the liver over a period of several weeks. Animals receiving 2-AAF only or retrorsine only never exhibit oval cells or SHPCs at any time point. These results suggest that exposure to 2-AAF impairs the emergence and outgrowth of SHPCs. Consistent with this suggestion, 2-AAF treatment 7-days post-PH in retrorsine-exposed rats results in the blockade of SHPC expansion. The results of this investigation fail to establish a precursor-product relationship between oval cells and SHPCs and suggest that SHPCs are susceptible to the mito-inhibitory effects of 2-AAF. Based upon these observations we conclude that SHPCs are not the progeny of oval cells, but represent a distinct liver progenitor cell population.
2. Materials & Methods
2.1. Animals
Male Fischer 344 rats were purchased from Charles River Laboratories (Wilmington, MA) or bred and maintained in the AAALAC-accredited animal facilities of the University of North Carolina at Chapel Hill. All procedures involving animals were carried out in accordance with federal and state guidelines put forth by the NIH and the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
2.2. Retrorsine and 2-Acetamidofluorene Administration, and Partial Hepatectomy
Male six week-old littermate Fischer 344 rats were randomized into retrorsine treatment (n=76) and control (n=110) groups at the outset of the experiment. Rats in the retrorsine treatment group received two treatments of retrorsine (30 mg/kg i.p.) two weeks apart (at 6 and 8 weeks of age). Retrorsine (12,18-Dihydroxysenecionan-11,16-dione; β-Longilobine, Sigma Chemical Company, St. Louis, MO) was added to distilled water at 10 mg/ml and titrated to pH 2.5 with 1 N HCl to completely dissolve the solid. Subsequently, the solution was neutralized using 1 N NaOH, and NaCl was added for a final concentration of 6 mg/ml retrorsine and 0.15 M NaCl (pH=7.0). The working solution was administered immediately after preparation. Four weeks following the second retrorsine treatment, experimental and control rats were randomized into the following experimental groups: retrorsine/PH (n=27), 2-AAF/retrorsine/PH (n=38), 2-AAF/PH (n=42), placebo/PH (n=31), 2-AAF only (n=18), placebo only (n=19), retrorsine/PH + 2-AAF (n=6), retrorsine/PH + placebo (n=5) (Fig. 1). Animals that were to receive 2-AAF treatment were implanted with 21-day controlled release 50 mg pellets which delivered an approximate dose of 10 mg/kg 2-AAF daily (Innovative Research, Sarasota, FL). Control animals were implanted with a placebo pellet (Innovative Research). One week later (5 weeks after the second retrorsine treatment), two-thirds surgical PH was performed on select experimental groups and on control rats of similar age (13 weeks old), essentially as originally described (17). Due to significant mortality in the 2-AAF/PH and 2-AAF/RP groups, only 130 (70%) rats survived the experimental protocol and these rats are included in the results presented. At 3, 7, 10, 14, and 21-days after PH liver tissue was harvested from n=3–7 rats (except for the 2-AAF/RP and 2-AAF/PH 21- day post-PH groups where n=2) and fixed in 10% neutral buffered formalin.
Figure 1.
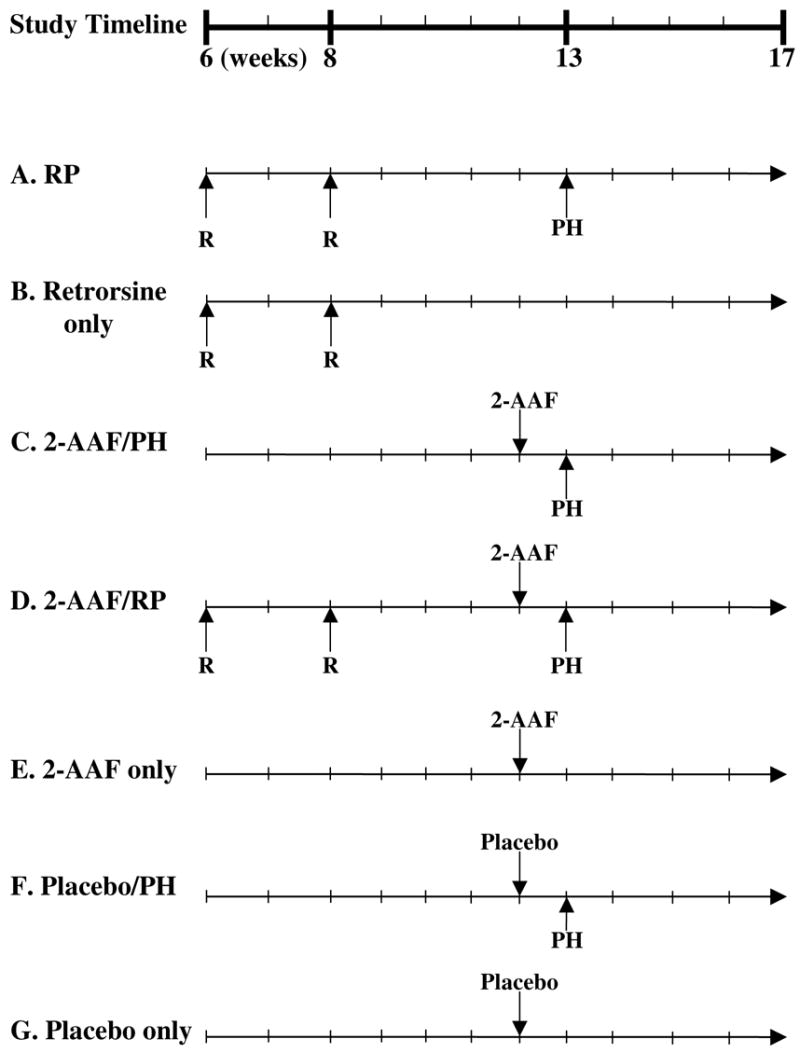
Experimental design timeline for retrorsine/2-AAF study. A timeline indicating the age of animals (weeks) at the various times of treatment in this study is provided. R indicates the time points for retrorsine injection (30 mg/kg each). 2-AAF indicates the time of insertion of a 21 day time-release 2-acetamidofluorene pellet (50 mg). Placebo indicates insertion of 21 day time-release placebo pellet (50 mg). (A) Retrorsine treatment + Partial Hepatectomy (RP). (B) Retrorsine treatment only (Retrorsine Only). (C) 2-AAF treatment + Partial Hepatectomy (2-AAF/PH). (D) 2-AAF treatment + Retrorsine treatment + Partial Hepatectomy (2-AAF/RP). (E) 2-AAF treatment only (2-AAF only). (F) Placebo treatment + Partial Hepatectomy (Placebo/PH). (G) Placebo treatment only (Placebo only).
2.3. Bromodeoxyuridine Treatment
Select animals were treated with 5-bromo-2′-deoxyuridine (100 mg/kg i.p.) 6-days post-PH (Sigma Chemical Company). The working solution was prepared by dissolving the solid into a saline solution at 33.3 mg/mL. This solution was heated using hot tap water and vortexed vigorously to dissolve the solid. The working solution was administered immediately following preparation.
2.4. Histology and Morphometry
Routine paraffin-embedding, sectioning, and preparation of H&E stained tissue sections from formalin-fixed liver tissue was performed by Histo-Scientific Research Laboratories (Mount Jackson, VA). Morphometric analysis was performed by digitally scanning H&E stained slides using an Aperio Scanscope T2 Virtual Microscope System (Vista, CA) at a resolution of 0.4667 μm/pixel. Images were analyzed using Aperio Imagescope v6.25 software. SHPC clusters were identified in the H&E sections based upon cell morphology and arrangement. Using the various tools provided by the Imagescope v6.25 software, SHPC clusters were enumerated and measured (area). All data obtained using Imagescope v6.25 was normalized to the cross sectional area of the tissue section, measured using Image J v1.36 software (National Institutes of Health, Bethesda, MD).
2.5. Immunohistochemistry
Colorimetric immunoperoxidase analysis was carried out on paraffin-embedded tissue sections using standard procedures. Tissue sections were deparaffinized in xylene, washed through a gradient of ethanol solutions (100%, 95%, and 70%), and rehydrated in a phosphate buffered saline (PBS) solution (1.54 mM KH2PO4, 155.17 mM NaCl, 2.71 mM NaH2PO4-7H2O; pH 7.2). Endogenous peroxidase activity was quenched for 10 minutes using a 0.3% H202 solution diluted in 100% methanol. Antigen retrieval was achieved by placing the slides in a heated 10 mM citrate antigen retrieval buffer (DakoCytomation) and subsequently placing the slide chamber (with buffer) in a steamer for 30 minutes. Non-specific antibody binding was blocked with a serum-free protein block (DakoCytomation) and primary antibodies were detected with the DakoCytomation Labelled Streptavidin-Biotin2, Horseradish Peroxidase (LSAB2®, HRP) system (DakoCytomation). The secondary antibody was detected using a substrate containing diaminobenzidine (DakoCytomation). Tissue sections were counterstained using Mayer’s hematoxylin (Sigma Chemical Company). Primary antibodies were diluted using antibody diluent with background reducing components (DakoCytomation). Mouse anti-rat BrdU antibody (Abcam, Cambridge, MA) and mouse anti-human cytokeratin 19 antibody (DakoCytomation) were used at a dilution of 1:25.
2.6. Data Analysis
The numerical data presented in figures and tables represent the mean ± standard error of the mean (SEM). All statistical analyses were performed using Kaleidograph v4.0 (Synergy Software, Reading, PA). Mean SHPC cluster sizes were calculated using each individual cluster as a separate event. All additional analyses were performed using individual animals as separate events. Significance of quantitative data was calculated using Student’s two-tailed t-test with unequal variance among groups. A statistically significant difference in data was defined by a P<0.05.
3. Results
3.1. Regenerative responses after PH in rats exposed to retrorsine alone and retrorsine in combination with 2-AAF
At 3-days following PH, RP animals have liver weights and liver/body weight ratios comparable to that observed in 2-AAF/PH animals (P>0.05 for RP versus 2-AAF/PH) (Fig. 2B, 2C). RP animals have low liver weights and liver/body weight ratios through 14-days post-PH, and then show a moderate increase in liver weight at 21-days post-PH (Fig 2B, 2C). Animals in the 2-AAF/RP group have liver weights that are not significantly different (P>0.05) from those observed in RP animals for the first 14-days post-PH (Fig. 2B). The lack of increasing liver weights among these animals during the first 14-days post-PH does not reflect a lack of regenerative activity. Rather, retrorsine-injured hepatocytes undergo megalocytosis following PH, and then apoptosis as new cells are generated in response to liver deficit (10). Animals in the 2-AAF/RP group show a dramatic increase in liver weight between 14 and 21-days post-PH and have liver weights that are indistinguishable from placebo/PH control animals at 21-days post-PH (P>0.05 for 2-AAF/RP versus placebo/PH) (Fig. 2B). In contrast, 2-AAF/PH animals have low liver weights through 7-days post-PH, with a moderate increase in liver size at 10-days post-PH, and becoming indistinguishable (P>0.05 for 2-AAF/PH versus placebo/PH) from control (placebo/PH) by 14-days post-PH (Fig 2B). Likewise, 2-AAF/PH animals demonstrate an increase in liver/body weight ratios by 14-days post-PH and attain a control liver/body weight ratio by 21-days post-PH (P>0.05 for 2-AAF/PH versus placebo/PH) (Fig. 2C). Together, these results suggest that the timing and kinetics of liver regeneration after PH in retrorsine-exposed animals treated with 2-AAF is similar to that observed in 2-AAF/PH animals.
Figure 2.
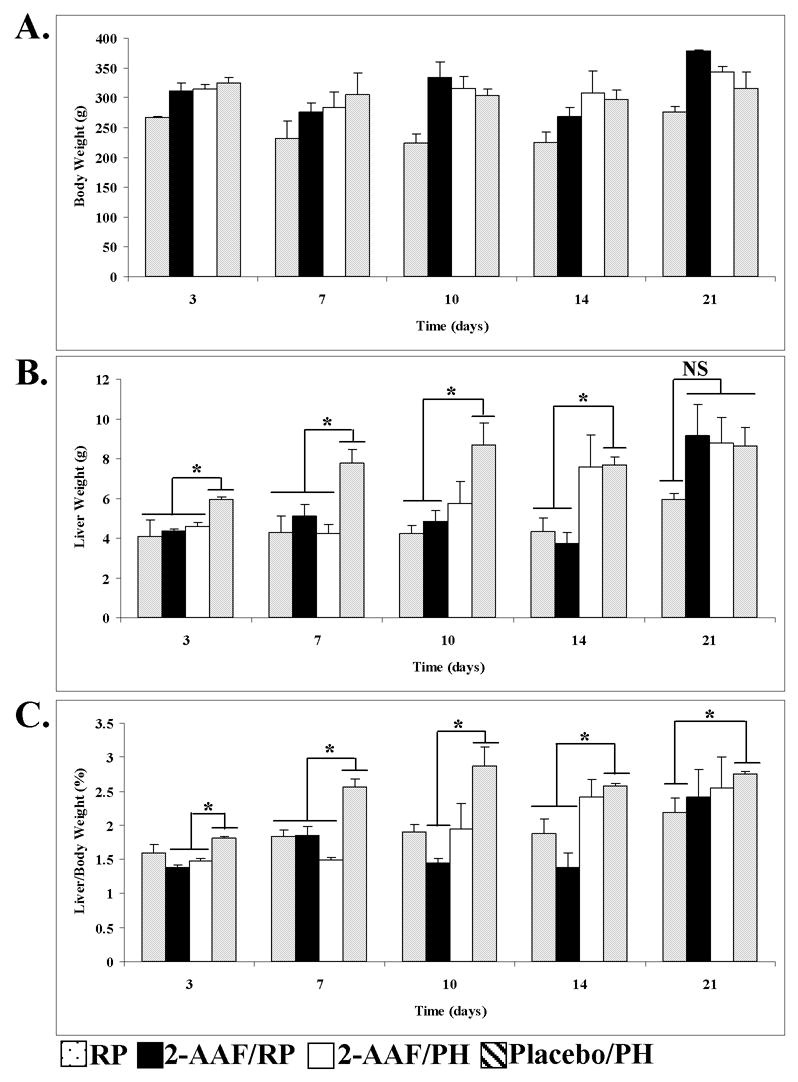
Treatment with 2-AAF blocks the SHPC regenerative response but not liver regeneration in retrorsine-exposed rats. The effects of 2-AAF treatment on (A) average body weight, (B) average liver weight, and (C) average liver/body weight ratios are shown. Each bar represents mean calculated from all surviving animals in the various experimental groups (± SEM, n=3–7 per time point for all groups except for 21 day 2-AAF/RP and 2-AAF/PH where n=2). The asterisks denote statistically significant differences (P<0.05) in liver weight (B) or liver/body weight ratios (C) for the designated comparisons. NS denotes no significant difference (P>0.05) for the designated comparison.
Analysis of H&E stained liver sections showed that the nature of the regenerative responses observed in RP and 2-AAF/RP animals differ significantly. Liver tissue harvested from RP animals at 3-days post-PH contain emerging SHPC clusters and modest numbers of oval cells (Fig. 3A). At this same time point post-PH in animals treated with 2-AAF, oval cells begin to appear in large numbers and SHPCs are not observed (Fig. 3B–C). By 7-days post-PH, liver from RP animals show a robust SHPC response with numerous proliferating cell clusters and very little oval cell proliferation (Fig. 3E). In contrast, livers harvested at this time point from 2-AAF/RP and 2-AAF/PH rats exhibit marked proliferation of oval cells, but SHPCs are not observed (Fig. 3F–G). At 10-days post-PH RP animals exhibit large numbers of proliferating SHPC clusters (Fig. 3I). In contrast, SHPCs are not observed in livers harvested from 2-AAF/RP (Fig. 3J) and 2-AAF/PH (Fig. 3K) animals at this time point. At 14-days post-PH a population of phenotypically ‘small’ hepatocytes appear in the 2-AAF/RP and 2-AAF/PH animals (Fig. 3N–O). These hepatocytes are found in close proximity to proliferating oval cells suggesting that these cells are the differentiated progeny (i.e. new hepatocytes) of the oval cells (Fig. 3N–O). Livers from RP animals at this time-point did not contain significant numbers of oval cells, but contained large numbers of expanding SHPC clusters (Fig. 3M). Neither SHPC clusters nor oval cells are observed at any time point after PH in placebo-treated animals (Fig. 3D, 3H, 3L, and 3P). Likewise, SHPC clusters and oval cells are not observed in 2-AAF only, placebo only, and retrorsine only animals that were not surgically manipulated (data not shown). The differences between progenitor cell responses observed during liver regeneration in RP and 2-AAF/RP animals suggest that although the SHPCs (and their progenitors) are resistant to the mito-inhibitory effects of retrorsine, they may be susceptible to 2-AAF poisoning.
Figure 3.
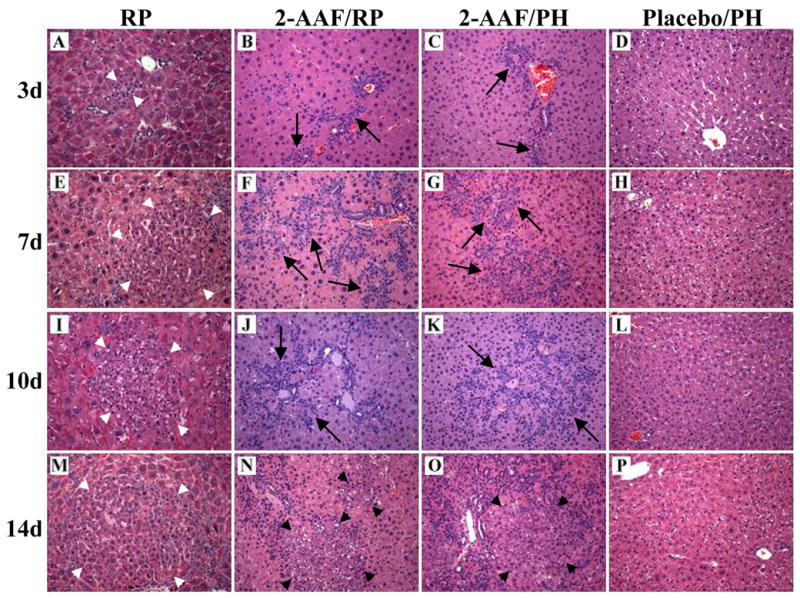
SHPCs do not respond to liver deficit in retrorsine-exposed animals treated with 2-AAF. Animals receiving standard retrorsine treatment and PH exhibit expanding SHPC clusters at (A) 3-days, (E) 7-days, (I) 10-days, and (E) 14-days post-PH. Animals receiving both retrorsine and 2-AAF exhibit large numbers of oval cells at (B) 3-days, (F) 7-days, and (J) 10-days post-PH, and new hepatocyte formation is observed at (N) 14-days post-PH. Likewise, animals receiving only 2-AAF exhibit large numbers of proliferating oval cells new at (C) 3-days, (G) 7-days, or (K) 10-days post-PH, and demonstrate some new hepatocyte cluster formation by (O) 14 days after PH. Animals receiving placebo treatment do not exhibit oval cells or SHPC clusters at (D) 3-days, (H) 7-days, (L) 10-days, or (P) 14-days post-PH. Short white arrows indicate SHPC clusters, long black arrows indicate oval cells, and short black arrows indicate new hepatocyte clusters. (Original objective lens magnification 10x).
3.2. Immunohistochemical analysis of 2-AAF-treated retrorsine-exposed rat liver
Immunostaining of the oval cell and biliary tract marker cytokeratin 19 (ck19) clearly labeled proliferating oval cells (along with biliary epithelia) in 2-AAF/PH and 2-AAF/RP rats at 7-days post-PH (Fig. 4A, 4C). At 14-days post-PH in 2-AAF/PH animals, ck19-positive oval cells surround new hepatocyte clusters (Fig. 4B). The new hepatocytes were weakly positive for ck19 (Fig. 4B). This observation suggests that the new hepatocytes represent the progeny of proliferating/differentiating oval cells, as observed by others (18). Likewise, ck19-positive oval cells surround new hepatocyte clusters in livers of 2-AAF/RP animals at 14-days post-PH (Fig. 4D). In contrast, ck19 immunostaining of livers from RP animals at 7 days and 14-days post-PH decorated the modest oval cell response, but failed to reveal ck19-positive oval cells in close proximity to SHPC clusters (Fig. 4E–F). Rather ck19 immunostaining exclusively labeled biliary tracts in these animals (Fig. 4E–F). These results provide additional evidence that the oval cell response in RP rats is modest and that identifiable oval cells do not obviously feed into SHPC clusters.
Figure 4.
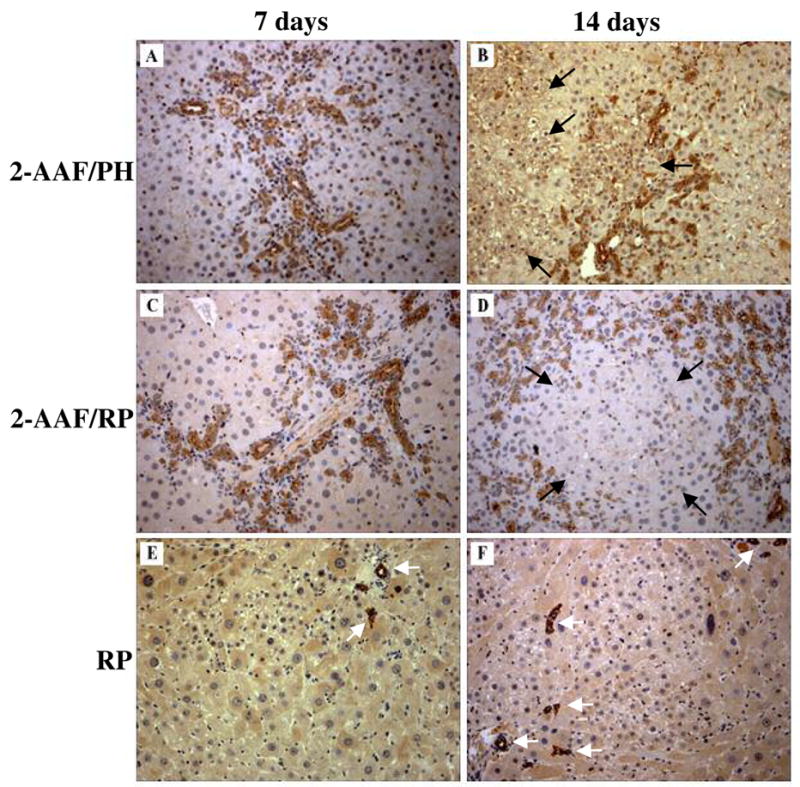
Immunostaining with oval cell marker cytokeratin 19. Cytokeratin 19 (ck19) staining in 2-AAF/PH animals at (A) 7-days post-PH labels biliary epithelial cells and oval cells. and (B) 14-days post-PH labels biliary epithelial cells, oval cells and new hepatocyte clusters. Likewise, ck19 immunostaining in 2-AAF/RP animals at (C) 7-days and (D) 14-days post-PH labels biliary epithelial cells and oval cells surrounding new hepatocyte clusters. Ck19 immunostaining in RP animals at (E) 7-days and (F) 14-days post-PH only labels biliary tracts in these animals. White arrows indicate ck19 positive biliary tracts, black arrows indicate new hepatocyte clusters. (Original objective lens magnification 10x).
3.3. BrdU labeling demonstrates that new hepatocyte clusters observed in 2-AAF-treated animals are the progeny of oval cells
To investigate the lineage relationship between oval cells and hepatocytes observed in 2-AAF-treated animals we treated 2-AAF/RP and 2-AAF/PH rats with BrdU (100 mg/kg i.p.) 6-days after PH. As previously described (19–21), treatment with BrdU 6-days post-PH exclusively labels oval cells in animals receiving 2-AAF before PH (Fig. 5A, 5C). By 14-days post-PH BrdU-positive cells are observed in 100% of the newly formed hepatocyte clusters in 2-AAF/PH (Fig. 5B) and 2-AAF/RP animals (Fig. 5D). These results demonstrate that new hepatocyte clusters observed in 2-AAF/PH and 2-AAF/RP animals are the progeny of proliferating/differentiating oval cells.
Figure 5.
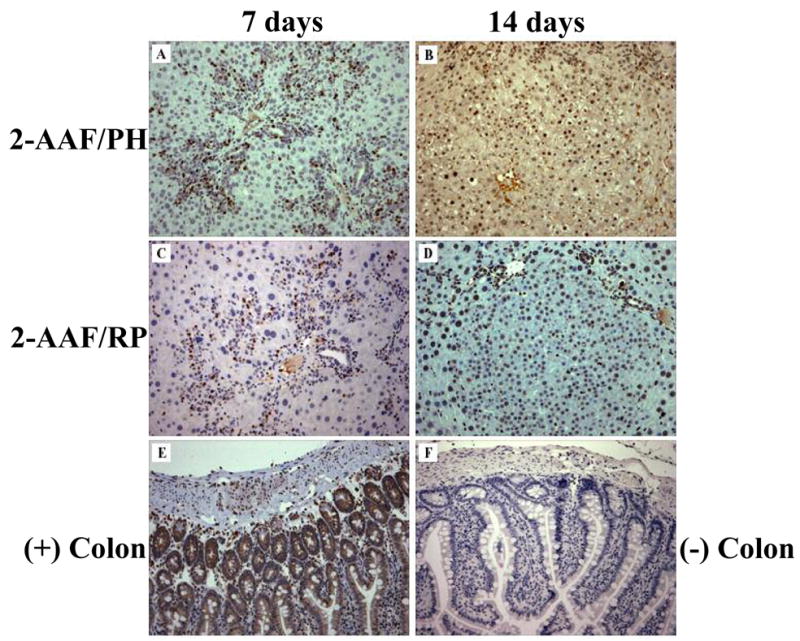
Bromodeoxyuridine labeling demonstrates a precursor-product relationship between oval cells and new hepatocyte clusters in 2-AAF-treated animals. Treatment with BrdU at 6-days post-PH specifically labels oval cells in liver tissue collected at 7-days post-PH from 2-AAF treated animals both in the (A) absence and (C) presence of retrorsine. By 14-days post-PH in animals receiving only 2-AAF, BrdU-positive oval cells give rise to new hepatocytes clusters (B). A similar result is seen at 14-days post-PH in retrorsine-exposed animals also treated with 2-AAF (D). Colon taking from a BrdU-treated animal serves as a positive control (E) and colon taken from an animal that was not treated with BrdU serves as a negative control (F). (Original objective lens magnification 10x)
3.4. Treatment with 2-AAF after PH blocks SHPC cluster expansion
To directly address the possibility that SHPCs are susceptible to the mito-inhibitory effects of 2-AAF poisoning, we treated rats with the standard RP protocol followed by the addition of 2-AAF after the initiation of SHPC proliferation (Fig. 6). One week following PH, rats were treated with either a placebo (n=5) or a 2-AAF (n=6) controlled time release pellet and livers were collected 7 days later (14-days post-PH). Morphometric analysis of H&E stained tissue from these animals demonstrated that SHPC clusters in animals receiving 2-AAF ceased to expand after administration of 2-AAF, suggesting that the proliferation of SHPC was inhibited by 2-AAF exposure (Fig 6D and Table 1). The size of SHPC clusters observed in RP + 2-AAF animals at 14-days post-PH were indistinguishable (P>0.05 for RP 7-day post-PH versus RP + 2-AAF 14-day post-PH) from those observed in RP animals at 7-days post-PH (Fig. 6D and Table 1). In contrast, SHPC clusters in RP + placebo animals continue to expand and by 14-days post-PH are approximately two-fold larger than those observed in RP + 2-AAF animals (Table 1 and Fig 6E). There were no differences (P>0.05 for RP versus RP + placebo at 14-days post-PH) in the size of SHPC clusters observed in RP animals receiving placebo pellets and RP animals (in the absence of placebo) at 14-days post-PH (Table 1). These data directly demonstrate that SHPCs are susceptible to 2-AAF poisoning and suggest that SHPCs are not the progeny of 2-AAF resistant oval cells.
Figure 6.
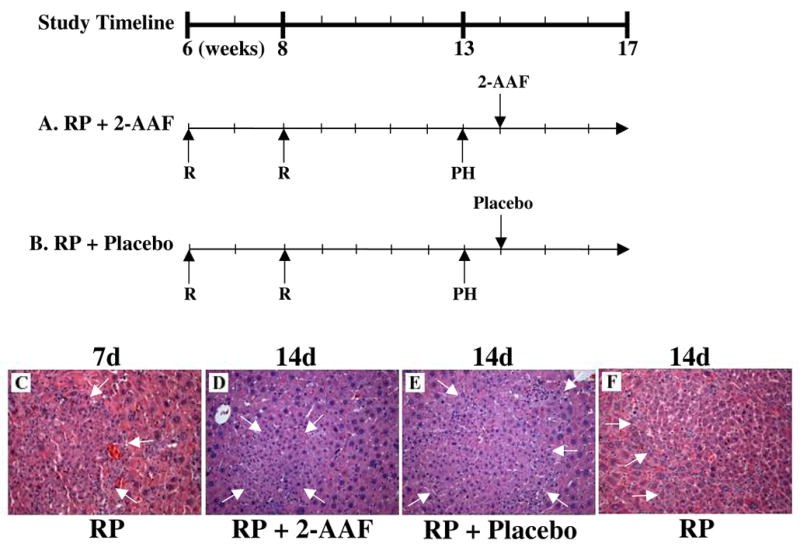
Treatment with 2-AAF after PH in retrorsine-exposed rats blocks SHPC expansion. A timeline indicating the age of animals (weeks) at the various times of treatment in this study is provided. R indicates the time points for retrorsine injection (30 mg/kg each). 2-AAF indicates the time of insertion of a 21 day time-release 2-acetamidofluorene pellet (50 mg). Placebo indicates insertion of 21 day time-release placebo pellet (50 mg). (A) Retrorsine + parital hepatectomy + 2-AAF treatment 7-days post-PH (RP + 2-AAF). (B) Retrorsine + partial hepatectomy + placebo treatment 7-days post-PH (RP + Placebo). H&E stained liver harvested from at 14-days post-PH from (D) RP + 2-AAF animals exhibit SHPC clusters comparable in size to those observed in (C) RP animals at 7-days post-PH. However, livers taken from (E) RP + Placebo animals at 14-days post-PH exhibit SHPC clusters comparable to (F) RP animals at 14-days post-PH. Arrows indicate SHPC clusters. (Original objected lens magnification 10x).
Table 1.
Treatment with 2-AAF 7d post-PH Blocks Expansion of SHPCs in Retrorsine-Exposed Rats
| SHPC Cluster Area (μm2) | |
|---|---|
| RP 7d Post-PH* | 4.82 × 104 ± 1.76 × 103 (n=100) |
| RP + 2-AAF 14d Post-PH*** | 4.39 × 104 ± 2.87 × 103 (n=100) |
| RP + Placebo 14d Post-PH** | 8.00 × 104 ± 3.81 × 103 (n=100) |
| RP 14d Post-PH**** | 8.65 × 104 ± 4.12 × 103 (n=100) |
P=0.2059 for RP + 2-AAF 14d versus RP 7d
P<0.0001 for RP + Placebo 14d versus RP at 7d post-PH
P<0.0001 for RP + Placebo versus RP + 2-AAF at 14d post-PH
P=0.2462 for RP + Placebo versus RP at 14d post-PH
4. Discussion
In the past the presence of a stem cell population in the adult liver has been the topic of debate. This controversy is largely due to the lack of high cellular turnover in the liver that is observed in other organs that are known to contain a classic, stem-cell-fed lineage system (i.e. colon, bone marrow, etc.) (22–24). The presence of a liver stem cell was further questioned because mature hepatocytes are capable of restoring lost tissue mass after surgical resection or injury, making a liver stem cell appear unnecessary. However, mounting evidence over the past several years has led to the general acceptance of the presence of cell population(s) with stem-like properties in the livers of adult rodents (3). These cells vary from (i) propagable multipotential stem-like cells (i.e. WB-F344, RLE-13, and others derived from liver epithelial cells) capable of differentiating into cells of both hepatic and extrahepatic tissues (2), (ii) bipotential stem-like cells (i.e. oval cells) capable of differentiating into mature hepatocytes and biliary epithelia (1, 6), and (iii) unipotential cells (i.e. SHPCs) capable of differentiating into mature hepatocytes (3). Our focus is on the regenerative responses mediated by bipotential and unipotential stem-like cells.
Bipotential stem-like cells are thought to respond to tissue deficit when mature hepatocytes are damaged and incapable of proliferating to restore lost liver mass. One example of a bipotential progenitor cell with stem-like properties is the oval cell, which is generally believed to reside in the bile ducts or periportal region of the liver (25). Oval cells exhibit a less differentiated phenotype than mature hepatocytes, expressing markers of fetal hepatoblasts, hematopoietic stem cells, and biliary epithelia (6). These cells are closely associated with liver injury models where animals are exposed to mito-inhibitory agents that block hepatocyte division (i.e. 2-AAF) (8). Several studies have demonstrated that oval cells are capable of differentiating into hepatocytes in a short time course after PH in a dose dependent manner (18, 21, 26).
In addition to oval cells there are other potential stem-like progenitor cell populations in the adult rodent liver that respond to toxic liver injury. Several studies have reported the presence of a population of cells termed small hepatocyte-like progenitor cells (SHPCs) that are closely associated with exposure to the pyrrolizidine alkaloid retrorsine (9, 10, 13, 15, 16). The ability of SHPCs to differentiate into cells of the biliary lineage has not been shown. Therefore, SHPCs are considered an example of unipotential stem-like progenitor cells that can differentiate into mature hepatocytes. Recently, the cellular origins of SHPCs have been the subject of controversy and some investigators have suggested that SHPCs and oval cells might represent the same (or closely related) population of cells (6). There are several potential sources for SHPCs (Fig. 7) and recent studies were conducted to address the cellular origins of SHPCs. These studies produced extremely varied results. Avril et al. employed a retroviral-based model to genetically label mature hepatocytes with the β-galactosidase gene in retrorsine-exposed Sprague-Dawley rats before PH to determine the contribution of mature hepatocytes to the formation of SHPC clusters in this model of liver injury (15). This study showed that a significant number of SHPC clusters expressed β-galactosidase and based upon this evidence concluded that mature hepatocytes are the source of SHPCs (15). However, this study failed to rule out the potential contributions of other cell types. For example, the investigators never specify the percentage of biliary epithelial cells and other extrahepatic cells that were labeled with β-galactosidase gene using this retroviral-based method (13). Therefore, it is impossible to determine if oval cells or some other cell type contributed to the formation of SHPC clusters in these animals (13). A more recent study by Vig et al. used a hepatitis B surface antigen (HBsAg-tg) mouse model of chronic liver injury to study the origins of the SHPCs (16). This study used three dimensional mapping techniques to demonstrate that livers of retrorsine-exposed HBsAg-tg mice exhibit SHPC proliferation, and that these clusters were both surrounded and infiltrated by proliferating oval cells (16). However, the investigators note that not all of the SHPCs observed are positive for oval cell markers and conclude that other cell types may possibly contribute to the formation of these cell clusters (16). The original model of retrorsine-induced liver injury is based upon acute injury and regeneration in rats (10, 11). Thus, it is difficult to discern if the cell clusters observed in the chronic HBsAg-tg mouse model are the same cell type (i.e. SHPCs) observed in RP rats. Moreover, it is known that oval cells differentiate into phenotypically ‘small’ hepatocytes before becoming mature hepatocytes (18). Thus, it is possible that the cells observed in this model are not SHPCs, but new hepatocyte progeny of proliferating oval cells. Combined, these published studies leave numerous unanswered questions about the origins of the SHPCs. Are SHPCs derived from a population of retrorsine-resistant hepatocytes? Are these cells the progeny of oval cells? Do these cells represent an independent reserve progenitor cell population?
Figure 7.
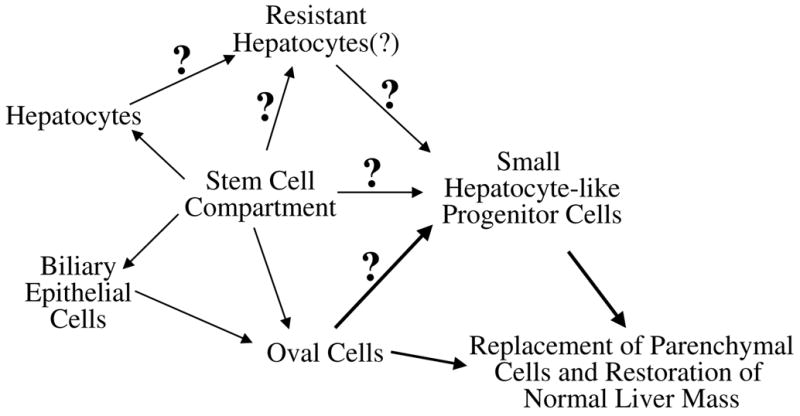
Potential origins of the small hepatocyte-like progenitor cells. It is well established that oval cells are derived from a periportal stem cell population. However, the origin of SHPCs is not known and several cell populations could potentially be the progenitor cell giving rise to these cells. These potential progenitor cell populations include a non-hepatocytic retrorsine-resistant cells located in the liver parenchyma, a population of retrorsine-resistant mature hepatocytes, or oval cells.
In the current study we investigated the contribution of oval cells to the formation of SHPCs by combining the RP model of liver injury (10, 11) with 2-AAF treatment. Based on the CYP450 expression profile observed in SHPCs (9, 27) and differences in the requirements for retrorsine and 2-AAF metabolism we hypothesized that retrorsine-resistant SHPCs (or their precursors) would be susceptible to 2-AAF poisoning. In fact, we observed that SHPCs do not represent a regenerative cell population in retrorsine-exposed animals treated with 2-AAF, but that the livers of these animals are reconstituted through the outgrowth, expansion, and differentiation of oval cells. Two lines of evidence support the suggestion that SHPCs (or their precursors) are sensitive to 2-AAF treatment: (i) no SHPCs are observed in 2-AAF/RP animals and (ii) administration of 2-AAF to RP rats inhibits SHPC proliferation. In contrast, oval cells readily emerge and proliferate in animals treated with retrorsine (2-AAF/RP). Furthermore, the timing and nature of the cellular responses in 2-AAF/PH and 2-AAF/RP animals was indistinguishable, and consistent with published studies (18, 28). These results provide significant new evidence that SHPCs are not derived from oval cells. We suggest that SHPCs represent a novel parenchymal stem-like progenitor cell that participates in liver regeneration following specific forms of liver injury.
Acknowledgments
Supported by NIH grant CA 78343. DHB was supported, in part, by NIH training grant T32 ES 07017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 2.Coleman WB, Grisham JW. Epithelial stem-like cells of the rodent liver. In: Strain AJ, Diehl AM, editors. Liver Growth and Repair. London: Chapman and Hall; 1998. pp. 50–99. [Google Scholar]
- 3.Coleman WB, Grisham JW, Malouf NN. Adult liver stem cells. In: Turksen K, editor. Adult Stem Cells. Totowa, NJ: Humana Press; 2004. pp. 101–148. [Google Scholar]
- 4.Dabeva MD, Alpini G, Hurston E, Shafritz DA. Models for hepatic progenitor cell activation. Proc Soc Exp Biol Med. 1993;204:242–252. doi: 10.3181/00379727-204-43660. [DOI] [PubMed] [Google Scholar]
- 5.Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. Oval cell-mediated liver regeneration: Role of cytokines and growth factors. J Gastroenterol Hepatol. 2003;18:4–12. doi: 10.1046/j.1440-1746.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- 6.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 7.Lemire JM, Shiojiri N, Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- 8.Sell S, Leffert HL, Shinozuka H, Lombardi B, Gochman N. Rapid development of large numbers of alpha-fetoprotein-containing “oval” cells in the liver of rats fed N-2-fluorenylacetamide in a choline-devoid diet. Gann. 1981;72:479–487. [PubMed] [Google Scholar]
- 9.Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157:771–786. doi: 10.1016/S0002-9440(10)64591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon GJ, Coleman WB, Hixson DC, Grisham JW. Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am J Pathol. 2000;156:607–619. doi: 10.1016/S0002-9440(10)64765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laconi S, Curreli F, Diana S, Pasciu D, De Filippo G, Sarma DS, et al. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol. 1999;31:1069–1074. doi: 10.1016/s0168-8278(99)80320-1. [DOI] [PubMed] [Google Scholar]
- 13.Coleman WB, Best DH. Cellular responses in experimental liver injury: possible cellular origins of regenerative stem-like progenitor cells. Hepatology. 2005;41:1173–1176. [Google Scholar]
- 14.Gordon GJ, Coleman WB, Grisham JW. Bax-mediated apoptosis in the livers of rats after partial hepatectomy in the retrorsine model of hepatocellular injury. Hepatology. 2000;32:312–320. doi: 10.1053/jhep.2000.9144. [DOI] [PubMed] [Google Scholar]
- 15.Avril A, Pichard V, Bralet MP, Ferry N. Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J Hepatol. 2004;41:737–743. doi: 10.1016/j.jhep.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Vig P, Russo FP, Edwards RJ, Tadrous PJ, Wright NA, Thomas HC, et al. The sources of parenchymal regeneration after chronic hepatocellular liver injury in mice. Hepatology. 2006;43:316–324. doi: 10.1002/hep.21018. [DOI] [PubMed] [Google Scholar]
- 17.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 18.Paku S, Nagy P, Kopper L, Thorgeirsson SS. 2-acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology. 2004;39:1353–1361. doi: 10.1002/hep.20178. [DOI] [PubMed] [Google Scholar]
- 19.Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 20.Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- 21.Paku S, Schnur J, Nagy P, Thorgeirsson SS. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol. 2001;158:1313–1323. doi: 10.1016/S0002-9440(10)64082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fausto N. Liver Stem Cells. The Liver: Biology and Pathobiology. In: Arias IM, Boyer JL, Fausto N, Jakoby WB, Shachter D, Shafritz DA, editors. The Liver: Biology and Pathobiology. 3. New York: Raven Press; 1994. pp. 1501–1518. [Google Scholar]
- 23.Orkin SH. Hematopoietic Stem Cells: molecular diversification and developmental interrelationships. In: Marshak DR, Gardner RL, Gottlieb D, editors. Stem Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2001. pp. 515–536. [Google Scholar]
- 24.Winton DJ. Stem cells in epithelium of the small intestine and colon. In: Marshak DR, Gardner RL, Gottlieb D, editors. Stem Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2001. pp. 515–536. [Google Scholar]
- 25.Fougere-Deschatrette C, Imaizumi-Scherrer T, Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, et al. Plasticity of hepatic cell differentiation: bipotential adult mouse liver clonal cell lines competent to differentiate in vitro and in vivo. Stem Cells. 2006;24:2098–2109. doi: 10.1634/stemcells.2006-0009. [DOI] [PubMed] [Google Scholar]
- 26.Nagy P, Bisgaard HC, Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation. J Cell Biol. 1994;126:223–233. doi: 10.1083/jcb.126.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon GJ, Coleman WB, Grisham JW. Induction of cytochrome P450 enzymes in the livers of rats treated with the pyrrolizidine alkaloid retrorsine. Exp Mol Pathol. 2000;69:17–26. doi: 10.1006/exmp.2000.2308. [DOI] [PubMed] [Google Scholar]
- 28.Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol. 1997;26:343–352. doi: 10.1016/s0168-8278(97)80051-7. [DOI] [PubMed] [Google Scholar]


