Abstract
Multiple brain disorders that show serotonergic imbalances have a developmental onset. Experimental models indicate a role for serotonin as a morphogen in brain development. To selectively study the effects of serotonin depletions on cortical structural development and subsequent behavior, we developed a mouse model in which a serotonin neurotoxin, 5,7-dihydroxytryptamine (5,7-DHT), is injected into the medial forebrain bundle (mfb) on the day of birth. Littermates with saline injections into the mfb and age matched mice served as controls. This study characterized the extent and duration of serotonergic denervation after the selective neonatal lesion and investigated effects on exploratory behavior, spatial learning and anxiety in mice of both sexes. We report significant decreases in the serotonergic (5-HT) innervation to cortex and hippocampus, but not to subcortical forebrain structures in 5,7-DHT-lesioned mice. The depletion of 5-HT fibers in cortical areas was long lasting in lesioned mice but autoradiographic binding to high affinity 5-HT transporters was only transiently reduced. Male but not female lesioned mice reduced their exploration significantly in response to spatial rearrangement and object novelty, suggesting increased anxiety in response to change but normal spatial cognition. Our data show that developmental disruptions in the serotonergic innervation of cortex and hippocampus are sufficient to induce permanent, sex specific, behavioral alterations. These results may have significant implications for understanding brain disorders presenting with cortical morphogenetic abnormalities and altered serotonin neurotransmission, such as autism, schizophrenia and affective disorders.
Keywords: Serotonin, cerebral cortex, ontogenesis, mouse, autism, behavior
1. Introduction
Serotonergic forebrain projections from the brainstem are involved in modulating aggression, anxiety, sleep, locomotion and cognition (Brown and Linnoila, 1990; Spoont, 1992; Hobson et al., 1998; Lucki, 1998; Birger et al., 2003; Mogensen et al., 2003). Disruptions of this 5-HT circuitry are implicated in mental health disorders including depression, autism and schizophrenia (Brown and Linnoila, 1990; Cook and Leventhal, 1996; Aghajanian and Marek, 2000; Chandana et al., 2005), most of which have a developmental onset. Thus, 5-HT dysfunction in development may be involved in disease etiology.
The morphogenetic role of serotonin in central nervous system development has long been recognized in various animal models (Lauder, 1983; Lauder, 1993; Shuey et al., 1993; Azmitia, 1999). In cerebral cortical development, early manipulations of the serotonergic innervation lead to altered development and plasticity in sensory areas in a variety of species (Gu and Singer, 1995; Osterheld-Haas and Hornung, 1996; Janusonis et al., 2004). In rat, neonatal systemic depletion of the cortical serotonergic innervation delays thalamocortical patterning (Blue et al., 1991) and diminishes the size of the barrel field area during the first postnatal week (Bennett-Clarke et al., 1994). Interestingly, increased serotonin neurotransmission during the critical period also alters barrel field development (Vitalis et al., 1998; Boylan et al., 2000b; Salichon et al., 2001). The importance of serotonin in thalamocortical maturation is underscored by the transient expression of the high affinity serotonin uptake sites (SERTs), and the 5-HT1B receptor in thalamocortical afferents in the barrel field area during the first postnatal weeks (Bennett-Clarke et al., 1991; Bennett-Clarke et al., 1993; Bruning and Liangos, 1997; Hansson et al., 1998; Lebrand et al., 1998; Mansour-Robaey et al., 1998). Disruptions of SERTs in neonatal development lead to abnormal thalamocortical connectivity (Xu et al., 2004). Serotonergic 5-HT2A receptors also are transiently over-expressed in the barrel field and have been implicated in the trophic actions of serotonin (Mansour-Robaey et al., 1998) that are observed in cultured thalamic neurons (Lieske et al., 1999). Serotonergic effects on dendritic and spine growth are not limited to the barrel field of the somatosensory cortex. Neonatal 5-HT depletion by administration of parachloroamphetamine (PCA) or 5,7-dihydroxytryptamine (5,7-DHT) leads to fewer spines and a trend for decreased dendritic tree size in dentate gyrus neurons (Yan et al., 1997). PCA-induced serotonergic depletion on postnatal days 10-20 leads to permanent decreases in MAP-2 expression in the hippocampus, suggesting altered dendritic formation (Mazer et al., 1997). Effects on the neurochemical development of cortical interneurons (Durig and Hornung, 2000) and augmentation in hippocampo-medial prefrontal cortex LTP (Ohashi et al., 2003) have also been observed following neonatal serotonergic depletions. Together, these morphological data provide strong support that altered morphogenesis and plasticity may be implicated in mental health disorders with serotonergic involvement.
In contrast to consistently observed effects on morphogenesis in cortical and hippocampal areas, behavioral reports on early postnatal serotonin manipulations are few and contradictory. Early studies showed behavioral effects of the neonatal 5-HT lesions only when subsequent pharmacological challenges were given (Breese et al., 1978). On the other hand, Mazer et al. reported substantial spatial learning deficits in rats administered PCA on postnatal days 10-20 (Mazer et al., 1997). The same lab also reported significantly impaired passive avoidance behavior and “autism like” behaviors, after in utero exposure to increased serotonin levels induced respectively via MAO inhibition or serotonergic agonists. Similar in utero exposures precipitate post-birth serotonergic depletion in the pups (Whitaker-Azmitia, 2005).
We hypothesize that the serotonergic innervation to neocortex serves as a modulator of morphogenesis, shaping thalamocortical and cortico-cortical synaptic development and plasticity. Appropriate synaptic connections in turn provide the substrate for behavioral, particularly cognitive functioning in adulthood. Thus, if the normal ontogeny of the cortical serotonergic innervation is altered, abnormal cortical function and behavioral pathologies will result.
The lack of uniformity in the manipulations employed in previous studies and the fact that in all cases serotonin levels were altered in the entire brain, has made it difficult to establish a relationship between morphological changes in cortical or other specific forebrain structures and behavioral effects. We have developed a mouse model for focal serotonergic depletions of the developing cortex, at birth, via neonatal injections of 5,7-DHT, directly into the medial forebrain bundle (mfb). Previous data have shown that this lesion, by adulthood, significantly impairs passive avoidance retention but does not affect locomotor activity or performance of a simple odor discrimination task in adult mice (Berger-Sweeney et al., 1998). In fact, performance of a non-matched to sample odor discrimination task was improved in the lesioned mice (Berger-Sweeney et al., 1998). The neonatal, mfb injections of 5,7-DHT also had significant and permanent effects on cortical thickness in the frontal and parietal cortex (Hohmann et al., 2000). Our present study was designed to characterize the extent and duration of serotonergic denervation after the neonatal mfb injections of 5,7-DHT. Moreover, we here investigate the consequences of the lesion on exploratory behavior, spatial learning and anxiety in mice of both sexes. Since previously reported morphometric assessments of cortex (Hohmann et al., 2000) were performed in behaviorally naïve, young adult mice, we also reassessed cortical cytoarchitectonic changes, after the conclusion of behavioral testing, in older mice.
2. Results
2.1 Morphological Findings: Serotonin
Examination of the immunocytochemical staining pattern in male and female normal BALB/CbyJ mice showed a similar distribution of 5-HT axons in cortex to that reported in other rodents (Blue et al., 1991; Dori et al., 1996; Boylan et al., 2000a; Zhou et al., 2000; Donovan et al., 2002). 5-HT fibers were distributed throughout all layers of cortex with regionally specific changes in innervation density. The 5-HT innervation exhibited a rostral to caudal gradient; with frontal areas having higher densities while more caudal regions had lower densities of 5-HT axons (Fig. 1). In contrast, the density of 5-HT axons in cortex was markedly diminished in mice that received neonatal, mfb 5,7-DHT lesions (Figs. 1 and 2). At postnatal day (PND) 7, very few axons were present; only light patches of diffuse 5-HT immunoreactivity were observed in the barrel field area. These patches comprise thalamocortical axons that transiently express serotonin uptake sites (Lebrand et al., 1996; Bruning and Liangos, 1997; Boylan et al., 2000a) (Figs. 1 and 2). One month after the lesion, in PND 30 lesioned mice, the density of 5-HT axons had increased in frontal areas of cortex, but few 5-HT axons were present in more caudal areas of cortex (Fig. 1). In the adult mice, 5-HT axon density in the cortex of lesioned animals showed further recovery in frontal and caudal regions of cortex. Nevertheless, the overall 5-HT innervation density in the cortex of 5,7-DHT lesioned mice was less than in age normal controls. Interestingly, the axons showing regrowth into denervated areas appeared to be thicker than in controls (Fig. 2).
Fig. 1.
The 5-HT innervation to cerebral cortex and hippocampus is greatly diminished after neonatal injections of 5,7-DHT into the mfb. Dark-field photomicrographs illustrate the 5-HT innervation in parasagittal sections of PND 7, PND 30 and adult mice. An arrow demarcates the remaining 5-HT staining in layer IV of barrel filed cortex, where thalamocortical axons take up serotonin transiently. Note the gradual recovery of innervation in the frontal cortical areas of the PND 30 and adult compared to PND 7 5,7-DHT lesioned subject.
Fig 2.
Higher power images of the immunostained 5-HT fibers from parietal cortex at PND 7 (dark field images at 20x and bright field images at 40x) and adult parietal and frontal cortex (20x). At postnatal day 7, both serotonergic and thalamocortical axons contribute to the dense pattern of immunostaining in normal controls but in 5,7-DHT lesioned cortex serotonergic axons are depleted and thalamocortical input to the barrels remains. In the adult recovery of serotonergic axons has occurred. The axons that have regrown in the lesioned mice appear to be coarser than in the age-matched controls.
The hippocampus showed a marked loss of 5-HT axons in PND 7 mice and a gradual recovery of 5-HT innervation density with age (Fig. 1). In contrast to the cortex and hippocampus, other regions of the brain in lesioned mice had similar 5-HT innervation densities to controls at all ages (Fig. 1).
Quantitative analysis of 5-HT fiber densities, in lesioned and age matched control mice, at PND 15, 30 and 60 confirmed qualitative visual evaluations. Highly significant (p≤ 0.002) reductions of 5-HT fiber densities in frontal cortex were measurable through PND 60, however parietal cortical fiber densities were only significantly reduced through PND 30 (p≤ 0.002) and differences in innervation density were no longer statistically significant at PND 60. Fiber densities in hippocampus were significantly reduced (p≤ 0.05) at all ages. In the thalamus and cerebellum, 5-HT fiber densities did not differ significantly between control and lesioned mice at any age.
To visualize the distribution of SERT sites, [3H]-citalopram binding was assessed in age normal, vehicle injected and 5,7-DHT lesioned mice at PND 7, 15 and 30. Normal age matched control mice showed high levels of SERTs at PND 7 and 15 followed by an approximately 40% decline at PND 30 (Fig. 3). In the neonatal 5,7-DHT lesioned group, uptake sites were decreased by 30% at PND 7 and 15 but had recovered by PND 30. In fact, at that age, citalopram binding was increased in lesioned mice by 40% above normal control levels. Interestingly, vehicle injected littermate control mice also showed a small decline at PND 7 and 15 followed by a substantial increase above normal levels by PND 30.
Fig. 3.
[3H]-citalopram binding to serotonin uptake sites in cerebral cortex. Neonatally 5,7-DHT lesioned mice had significantly decreased numbers of [3H]-citalopram binding sites in the cerebral cortex than age normal mice at P 7 (P<0.05). In contrast, at P30, the 5,7-DHT-lesioned mice had significantly higher numbers of [3H]-citalopram binding sites than age normal controls (P < 0.001).
2.2 Morphometric Findings: Cortical Width
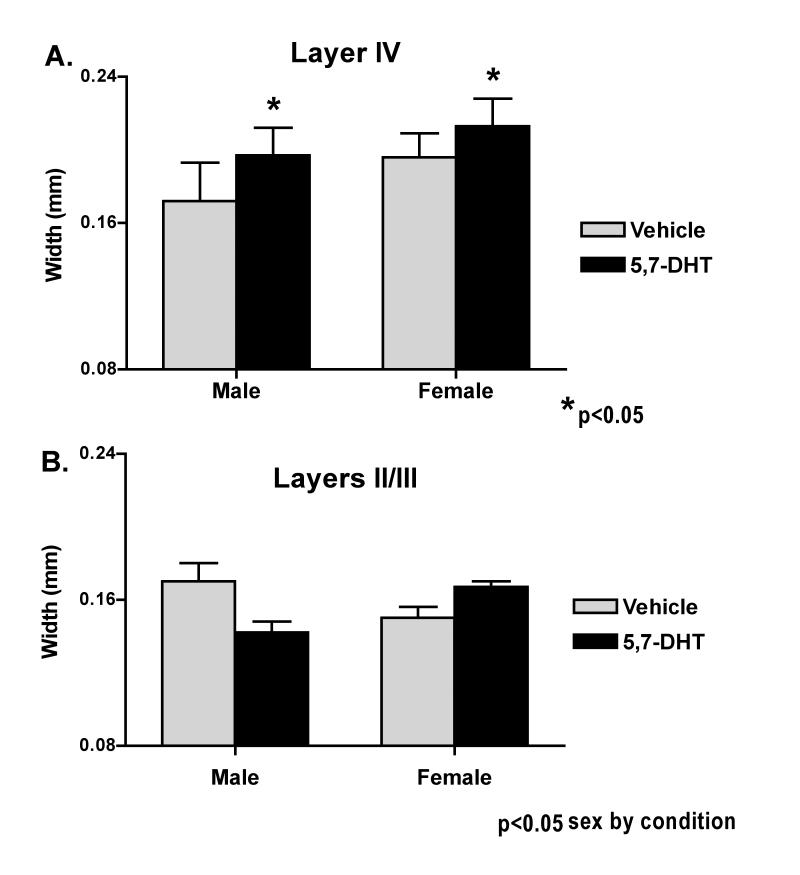
Qualitative observations of Nissl stained tissue showed normal cytoarchitecture throughout frontal and parietal cortex of lesioned and vehicle control mice. Morphometric analysis revealed a significant increase in the width of layer IV in both lesioned males and females (P = 0.026; Fig. 4A) that was consistent across all cortical levels. In addition, there were significant sex differences across conditions (P = 0.027) with females showing greater increases in layer IV width compared to males. In contrast, analysis of the anterior barrel field only, revealed significant sex by condition differences (P = 0.013) for layers II/III, with males showing decreased cortical width after the neonatal lesion but females still showing an increase (Figure 4B). Similar dimorphisms were absent from the more posterior somatosensory cortex.
Fig. 4.
Cortical widths in dorsal sensorimotor cortex: A. Layer IV widths in male and female neonatally 5,7-DHT lesioned mice is significantly increased over control values (P = 0.026) in both anterior barrel field and more posterior sensorimotor cortex. Width in females (lesioned combined with non-lesioned) was significantly greater than in males (combined lesioned and anon-lesioned) (P = 0.027, ANOVA main effect by sex). B. The width of cortical layers II/III is decreased in male anterior barrel field cortex while female cortical width is still increased (P = 0.013, sex by condition in the ANOVA main effect). This dimorphism was limited to this layer and cortical area only.
2.3 Behavioral Findings
A neurological test battery performed prior to the behavioral studies, did not reveal significant differences between neonatally lesioned or vehicle injected control mice of either sex. All animals scored within the normal range for BALB/CByJ mice of their age group in righting reflex, placing reflex, and grip strength. Beam crossing scores for males and females of both groups did differ significantly (P = 0.042, ANOVA main effect). In this task, mice have to walk across a small (about 1cm diameter) round “beam” to return from a platform to their home cage. Female mice had higher scores than males, indicating a greater propensity to fall off the beam (see Table 1). Interestingly, lesioned males scored higher in the beam-crossing task than male control animals but took a shorter time than control males (see Table 1). While the differences were not statistically significant, they did suggest that male lesioned mice crossed the beam with less hesitation than controls but had more incidents of falling off.
Table 1.
Table 1 compares several measures of motor activity and anxiety in neonatally lesioned and vehicle control male and female mice. While there is a trend for the 5,7-DHT lesioned males to differ from vehicle males in most measures, none of these differences reached statistical significance. Note: anxiety scores in the OFOR task were measured by the ratio of the time spent in the periphery of the enclosure divided by the time spent in the middle of the enclosure. More anxious mice spend more time close to the perimeter, leading to higher anxiety scores.
| Male vehicle | Male 5,7-DHT | Female vehicle | Female 5,7-DHT | |
|---|---|---|---|---|
| Beam Score | 2.17 ± 0.6 | 3.4 ± 0.2 | 3.5 ± 0.2* | 3.4 ± 0.3* |
| Beam time (sec) | 75.7 ± 18.6 | 55.3 ± 15.0 | 63.4 ± 11.0 | 62.1 ± 15.4 |
| Locomotion (OFOR) | 56.3 ± 8.3 | 47.0 ± 10.1 | 50.3 ± 7.8 | 51.3 ± 6.9 |
| Rearing (OFOR) | 1.6 ± 0.5 | 2.1 ± 0.5 | 2.4 ± 0.6 | 2.2 ± 0.6 |
| Periphery (OFOR) | 83.8 | 86.0 | 79.0 | 81.0 |
| Middle (OFOR) | 16.2 | 14.0 | 20.9 | 18.9 |
| Anxiety Score (P/M) | 5.2 | 6.1 | 3.8 | 4.3 |
P = 0.042 (sex difference)
Overall locomotion, as assessed in session 1 (S1) of the open field object recognition (OFOR) task, did not reveal significant differences between any of the groups (Table 1). However, both lesioned and control females and lesioned males moved at a similar rate that was slightly slower than control males. A similar trend was observed in rearing frequency. Anxiety scores, calculated as the ratio between times spent in the periphery to that in the center of the enclosure in S1, tended to be lower in female lesioned and control mice than male controls. This was compatible with the observation that females also had shorter beam times than male controls, as both were measures of anxiety. On the other hand, anxiety scores in the OFOR trended highest in male lesioned mice, despite previous observations of relatively short beam times.
Overall exploratory activity was similar in control males and females as well as lesioned male mice (Fig. 5). However, female 5,7-DHT lesioned mice, displayed lower levels of exploration throughout all sessions; these differences were statistically significant in sessions 2 through 4 (P ≤ 0.05). All groups habituated to the task as indicated by a general decline in exploration over the course of S2-4. Female lesioned mice showed a steeper slope in the decline of exploratory contact with objects, indicating that they habituated at a slightly faster rate than other groups (Fig. 5).
Fig. 5.
Habituation to the various objects placed in the OFOR environment. Neonatally 5,7-DHT lesioned females explored objects significantly less (P ≤ 0.05) in sessions 2 through 4 than any other group. The female lesioned mice also habituated faster to the objects than any other group.
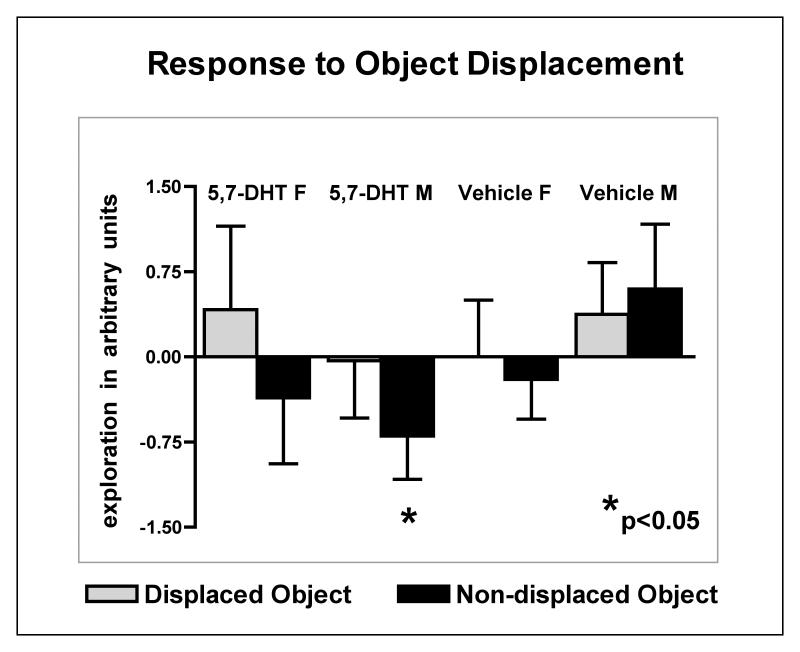
In response to changing the position of two of the objects (object displacement) in Session 5 (S5), male lesioned mice significantly differed from all other groups. Direct comparisons of the exploration of all objects in S5 between male lesioned and male control mice showed a 30% drop in exploration in the lesioned group that was significant (P = 0.024). The 15% decline in exploration in female lesioned mice was not significantly different from female control mice. Moreover, male and female lesioned mice showed opposite exploratory patterns of the displaced (DO) vs. the non-displaced objects (NDO). Female lesioned mice increased exploration of the displaced objects and slightly decreased exploration of the familiar objects, a pattern that indicates recognition of the object displacement and appropriately increased curiosity towards the spatially displaced objects. In contrast, male lesioned mice responded to the change in object placement by decreasing their exploration of all objects (Fig. 6).
Fig. 6.
Response to the displacement of two objects in the OFOR environment. Note that numbers above 0 indicate an increase in object exploration and numbers below 0 mark a decrease in object exploration compared to prior sessions. Neonatally 5,7-DHT lesioned males decreased exploration of all objects in this changed environment, although ANOVAs of the displaced object vs. the non-displaced object across all groups did not reveal significant differences. However, direct comparisons of the exploration of all objects in S5 between male lesioned and male control mice showed a 30% drop in exploration in the lesioned group that was significant (P = 0.024).
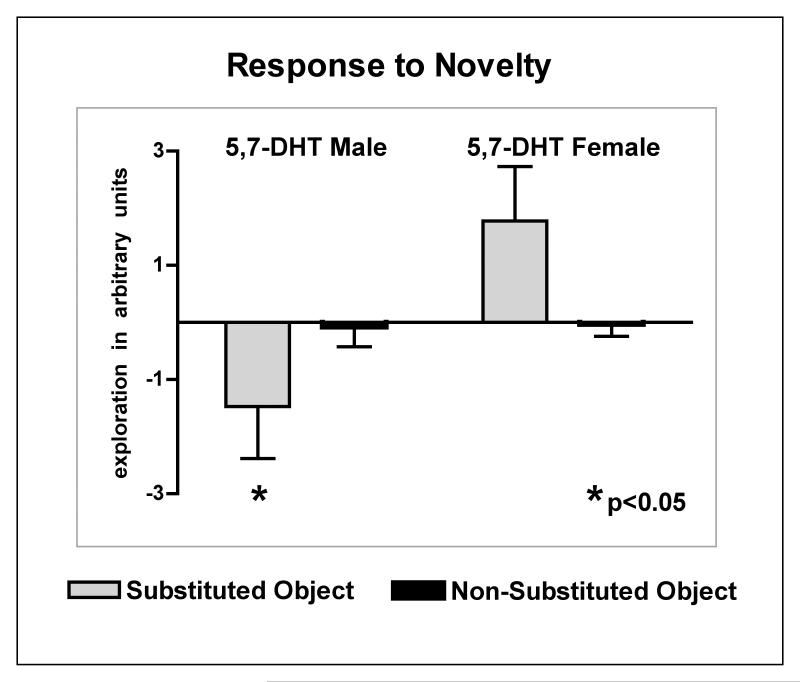
Similarly, in Session 7 (S7), with the introduction of a novel object, male lesioned mice significantly decreased exploration of all objects (P = 0.022; Fig. 7). In contrast to lesioned males, female lesioned mice explored the novel, substituted object (SO) more than the other objects (NSO) in the OFOR; exploration of the novel object in female lesioned mice did not significantly differ from control females.
Fig. 7.
Response to a novel (substituted) object. Note that, as for Fig. 6, numbers above 0 indicate an increase of object exploration and numbers below 0 mark a decrease of object exploration compared to prior sessions. Male neonatally 5,7-DHT lesioned mice responded to the novel object by decreasing their exploration to all objects while female lesioned mice showed increased exploration of the novel object (P = 0.022 for male lesioned vs. female lesioned post-hoc).
Taken together, these data indicate that that male and female lesioned mice respond in opposite ways to alterations of their environment. Such sex differences are absent in the control mice. The lesioned males’ responses to object displacement suggest that these mice recognized the spatial displacement but found changes in their environment aversive and anxiety producing.
3. Discussion
Our data demonstrate that focal 5,7-DHT injections at birth in BALB/CByJ mice result in selective depletions of the cortical and hippocampal serotonin innervation, during a critical time period in cortical morphogenesis, and precipitate permanent behavioral changes. Although considerable decreases in 5-HT immunohistochemistry persisted into adulthood in some cortical areas, citalopram binding studies suggest a substantial recovery and illustrate the transient nature of the serotonergic depletion. The neonatal serotonin depletion alters exploratory behavior in a sex dependent fashion, compared to vehicle control mice. Increases in cortical width, while still evident, have substantially attenuated compared to previous assessments in young mice and now also display sex dimorphism. These data suggest that neonatal serotonin depletions of cognitive brain areas result in behavioral changes based on morphogenetic alterations during early postnatal cortical development. This supports our hypothesis that altered serotonergic ontogeny may be instrumental in generating the behavioral pathologies associated with some developmental brain disorders.
3.1 Selective Serotonergic Depletions
As illustrated in Fig. 1, the neonatal injections of 5,7-DHT into the mfb produced a focused depletion of 5-HT fibers in neocortex and hippocampus leaving deeper forebrain regions, the diencephalon and brainstem unaffected. Thus, subsequent behavioral changes in our lesioned mice are not likely to be the result of secondary impact on other neuromodulatory systems in the brain stem or basal forebrain. Commensurate with this, and consistent with our previous observations (Berger-Sweeney et al., 1998), we do not see gross motor abnormalities or locomotor problems in the lesioned mice that would suggest subcortical functional changes. On the other hand, a slight lack of coordination, as indicated by the increased beam score in male lesioned mice, is consistent with a disruption of fine motor control as would occur following impairment of cortical motor modulation.
The restricted pattern of 5-HT fiber depletion did not differ between the sexes and continued into adulthood in an attenuated form. Lesion induced 5-HT innervation decreases were most dramatic in frontal cortex, a region that ordinarily contains a high density of 5-HT afferents from the raphe nuclei (Molliver, 1987; Molliver et al., 1990; Mamounas et al., 1991; Dori et al., 1996; Donovan et al., 2002). Remnants of 5-HT patches in layer IV of somatosensory cortex remained at PND 7, indicating that sequestration of serotonin by thalamocortical axons (Bennett-Clarke et al., 1991; Bennett-Clarke et al., 1994; Lebrand et al., 1996; Bennett-Clarke et al., 1997; Bruning and Liangos, 1997; Mansour-Robaey et al., 1998; Boylan et al., 2000a) persists after the neonatal 5,7-DHT lesions, despite the substantial reductions in cortical 5-HT innervation. Accumulation of 5-HT into thalamocortical terminals via SERT is likely the reason that citalopram binding was only mildly reduced in PND 7 and PND 15 mice after the lesion. At the younger ages, a preponderance of SERTs is associated with thalamocortical afferents rather than with the afferent 5-HT fibers (Bennett-Clarke et al., 1996; Lebrand et al., 1996). With maturation, thalamocortical SERTs decrease and this is paralleled by the decrease in citalopram binding in normal mice at PND 30. The significant overshoot of citalopram in lesioned and 5,7-DHT mice by PND 30 suggests, however, an increase of SERTs on serotonergic fibers and thus, likely increased 5-HT availability in the existing/remaining fibers. The increased density of SERTs, despite persisting reductions in serotonergic fiber innervation, might explain why previous HPLC analysis had indicated a more substantial recovery of 5-HT levels after the surgery (Berger-Sweeney et al., 1998) and suggests that serotonergic tone may only be impaired slightly, if at all, in the mature cortex, particularly the parietal cortex.
3.2 Cortical Alterations
The current morphological data suggest that the increases in cortical width attenuate with age. Our earlier data, generated in approximately three month old, behaviorally naïve, adult Balb/CbyJ mice (Hohmann et al., 2000), demonstrated significant increases in total cortical width, based on increases in multiple cortical areas throughout the fronto-parietal cortex. The width increases were evident and significant in all measured areas but varied by region and sex in how substantially particular cortical layers were affected. In contrast, our present data in much older mice (10-12 months) showed significant increases in the width of only cortical layer IV that was consistent for all brain regions analyzed. Moreover, dimorphic changes became apparent for layers II/III of the anterior parietal cortex with males now showing decreased with while females were still maintaining a width increase. In conjunction, these findings suggest that changes in cortical plasticity continue to occur in adulthood in mice depleted of serotonin. It is well known that young adult mice at the ages used in our previous study are likely still susceptible to substantial environmentally mediated morphological plasticity, including pruning (Walsh, 1981; Rosenzweig and Bennett, 1996; Kolb and Whishaw, 1998). Consistent with this, neonatal depletion of serotonin in the cortex of kittens increased the number of callosal projection neurons (Djavadian et al., 2003).
3.3 Behavioral Effects
Our studies are the first to examine the effects of selective cortical 5-HT depletions in early postnatal development on behavioral consequences in male and female mice in adulthood. The present data show some interesting sex differences. Female mice with neonatal depletion of cortical serotonin show an accelerated habituation in overall exploratory behavior but otherwise normal responses to spatial environmental change and novelty. Male lesioned mice, on the other hand, displayed significantly decreased exploratory behaviors in response to spatial environmental change and novelty even though their initial level of object exploration was similar to controls. The lower levels of exploratory activity in 5,7-DHT lesioned females are not a function of altered locomotor activity, as that did not differ between the groups (Table 1), nor could it be attributed to increased anxiety, which also does not significantly differ among lesioned females, control females or control males.
Previous work in mice and rats, using paradigms similar to the OFOR task used by us, show that normal rodents habituate to objects, as indicated by a gradual decrease in exploration between S2 and S4; but they increase their exploration in response to spatial object displacement and object novelty (Poucet, 1989; Thinus-Blanc et al., 1996; Ricceri et al., 1999; Frick and Gresack, 2003). To what extent animals increase their exploration of all objects or only that of the displaced objects, relative to the unchanged objects, appears to depend on sex and strain variables (Poucet, 1989; Thinus-Blanc et al., 1996; Frick and Gresack, 2003). Lack of response to spatial change is generally associated with damage to, or abnormalities in, brain centers involved in spatial learning and memory such as the hippocampus and neocortex (Poucet, 1989; Save et al., 1992; Thinus-Blanc et al., 1996; Hohmann et al., 1999; Ricceri et al., 2002). However, the response to object novelty is usually left intact in these paradigms (Save et al., 1992; Ricceri et al., 1999).
In our present study, both male and female serotonin depleted mice clearly responded to the object displacement, suggesting that spatial learning and memory is intact. Yet, the response to spatial change was qualitatively abnormal in the lesioned males, showing dramatically decreased explorations instead of increased exploration. This is suggestive of altered affect and/or impulsivity and, along with similar decreases in exploratory responses to novelty, conveys the impression of anxiety or phobia in response to the change in spatial environment. In contrast, the response of the serotonin depleted female mice to object displacement and replacement was similar to that in normal males and females from a variety of strain backgrounds and ages in this task (Roullet and Lassalle, 1990; Frick and Gresack, 2003). The interpretation that spatial and object novelty elicits anxiety in the serotonin depleted males is consistent with the observation that this group also has the highest anxiety scores in the open field exploration (Table 1). On the other hand, increased anxiety levels were not apparent in the beam time, indicating that increased anxiety may not be a general feature in the lesioned males but may directly relate to the exploration of a spatial environment.
These present findings in male lesioned mice are consistent with our previous behavioral observations in similarly lesioned mice (Berger-Sweeney et al., 1998). In those studies, neonatally 5-HT lesioned mice, compared to vehicle controls, displayed normal performance on a simple odor discrimination task, improved performance of a delayed non-matched to sample odor discrimination task but impairments on a passive avoidance task. While the first two paradigms test positively (food) motivated cognitive performance, the latter task requires the mouse to withhold a strongly motivated response to an environmental feature, that is, seeking the comfort of the dark compartment in the passive avoidance chamber.
3.4 Structure -Function Relationships
The behavioral effects of the neonatal serotonin depletions are most likely a consequence of altered cortical/hippocampal development. There is presently no evidence that adult 5-HT depletion increases anxiety-like responses to spatial and object novelty. On the contrary, serotonin depletion in mature mice or rats, using 5,7-DHT or other pharmacological disruptions of serotonin neurotransmission, may result in behavioral effects including decreased anxiety (Briley et al., 1990), increased aggression (Hole et al., 1977), impaired cognition such as place recall in the Morris water maze (Mogensen et al., 2003) or disrupted timing behavior (Morrisey et al., 1994). On the other hand, several transgenic and knock-out mutants with constitutive, and thus developmentally altered, expression of components of the 5-HT system show behavioral modifications more akin to what we see after neonatal serotonin depletions of cortical structures. For instance, serotonin transporter gene knock-out mice had a 50% reduction in serotonin and exhibited decreased feeding behavior in a novel situation, despite the absence of a generalized increase in anxiety (Lira et al., 2003). In another study of 5-HT transporter knockout mice, anxiety and impaired exploration were reported and attributed to 5-HT1A receptors (Holmes et al., 2003). This notion receives support from studies on 5-HT1A knockout mice, which showed decreased exploratory activity and increased fear of aversive environments (Ramboz et al., 1998). In addition, 5-HT4 receptor knockout mice displayed attenuated exploratory activity as adults and abnormal feeding behavior in response to novelty (Compan et al., 2004). Mice transgenic for S100beta, which developed with reduced brain serotonin levels, showed impaired habituation to novel stimuli. This response was consistent with an aberrant (anxious) behavioral response repertoire to a novel environment (Bell et al., 2003). Similar to us, Kostowski et al. recently reported normal locomotor performance in rats intracisternally injected with 5,7-DHT on postnatal day three (Kostowski and Krzascik, 2003); these rats also displayed decreased activity in a forced swim test, an indicator of a depressed affect.
3.5 Sex differences
The morphological or molecular basis for the observed behavioral sex differences is presently a matter of conjecture. As mentioned in the results, the persistent morphological changes in layer IV occur in both male and female lesioned mice, although they are more pronounced in the males. In contrast, region dependent sex differences become apparent in anterior barrel field but not in more posterior sensorimotor cortex. We have recently described sex differences in the early development of the cortical 5-HT innervation (Connell et al., 2004). Unlike the males, female BALB/CbyJ mice do not show a very pronounced early postnatal peak in serotonin levels in neocortex. These results suggest that the 5-HT innervation may not have as critical a role in postnatal development of female cortex. Moreover, in males, testosterone modulates serotonin levels and with that levels of aggression (Blanchard et al., 1993; Birger et al., 2003). Thus, other cognitive-emotional behavioral features might be under the control of sex hormones as well. Substantial sex dimorphism does exist in several disorders that involve 5-HT imbalances, including autism, depression and anxiety disorders (Earls, 1987; Halbreich and Kahn, 2001; Muhle et al., 2004). Serotonin may not have the same saliency in male and female cortical development and later plasticity, rendering each sex differentially susceptible to 5-HT imbalances in various brain regions. It is already well established that autism is a developmental disorder and there is suggestive evidence that depression and other affective disorders might have developmental roots. It is intriguing to speculate that developmental disruptions of early 5-HT forebrain innervation might lead to different clinical phenotypes, depending, among other things, on the gender of the affected individual, the timing and the level (increase vs. decrease) of the serotonergic alteration.
3.6 Neonatal serotonin depletions as an animal model for autism
Our current findings may be of particular relevance to understanding the etiology of autism. The morphological and behavioral changes we show here are consistent with the hypothesis that decreased serotonin, during a critical, early postnatal period in cortical development, may be responsible for altered cortical structure and function in autism. Although current dogma holds that initial gene changes in autism occur in early embryogenesis, Chugani and colleagues have found significant differences in early postnatal serotonin synthesis in frontal, temporal, parietal and occipital cortex between children with autism and control subjects (Chugani et al., 1999; Chandana et al., 2005). Serotonin synthesis in normal human cortex, as in rodents, is highest shortly after birth and thereafter decreases with age up to sexual maturity (Chugani et al., 1999; Chandana et al., 2005). Young children with autism have a significantly attenuated peak in serotonin synthesis compared to age matched controls (Coyle and Molliver, 1977; Hohmann et al., 1988; Chugani et al., 1999; Connell et al., 2004; Chandana et al., 2005). With age, those with autism show a flatter curve of decline that might result in the slightly elevated serotonin levels observed by young adulthood. (Chugani et al., 1999; Chandana et al., 2005). A serotonergic (5-HT) deficit during cortical development, moreover, is compatible with reports of treatment successes using serotonin uptake blockers as well as with genetic linkage studies suggesting impairments in genes linked to proper serotonin neurotransmission (Cabelli et al., 1995; Klauck et al., 1997; Anderson et al., 2002; Veenstra-VanderWeele et al., 2002; Conroy et al., 2004; Coutinho et al., 2004; McCauley et al., 2004; Muhle et al., 2004; Murphy et al., 2004; Nabi et al., 2004; Coon et al., 2005; Mulder et al., 2005; Scott and Deneris, 2005; Whitaker-Azmitia, 2005). It is interesting in this context to note that a defective serotonergic innervation is also compatible with alterations of brainstem segmentation genes such as engrailed or Hox 2, which have been implicated in autism (Polleux and Lauder, 2004; Bartlett et al., 2005; Benayed et al., 2005). Serotonergic projection neurons develop in the brainstem areas under control of these segmentation genes (Davis and Joyner, 1988; Gavalas et al., 2003). Yet, serotonergic afferents only innervate cortex just before birth and thus begin to exert their effects on cortical maturation predominantly after birth in both rodent and human (Lidov and Molliver, 1982; Dori et al., 1996; Connell et al., 2004).
The age dependent increases in cortical size observed in autism (Courchesne, 2004), may be analogous to the significant width increases in our lesioned mice (Hohmann et al., 2000) which also attenuate with age. Head circumference and cortical volume are increased in young children (2-6 years of age) with autism (Piven et al., 1996; Courchesne et al., 2001; Aylward et al., 2002; Sparks et al., 2002) during the same time that cortical 5-HT synthesis lags behind normal. Moreover, numerous studies show structural alterations in the cerebral cortex of children with autism (Bailey et al., 1998; Casanova et al., 2002; Herbert et al., 2002; Levitt et al., 2003). Such changes in the size and architecture of important cortical processing areas may underlie functional deficits in the development of cortical sound processing (Gage et al., 2003), visuo-motor maps (Mueller et al., 2003) and face recognition (Pierce et al., 2001; Dawson et al., 2002) that are observed in autism. Clearly, stereological studies will have to confirm that the width changes we have measured are truly representative of volume changes and they will have to ascertain the relative participation of white and gray matter areas in any volume change. Such studies are currently under way.
Our mouse studies show behavioral alterations in male, neonatally lesioned mice that resemble behavioral pathologies seen in autism. Individuals with autism are very sensitive to any change in their routine or in their personal environment. They frequently will respond to environmental alterations with anxiety and by either increasing or decreasing activity levels (Kootz et al., 1982; van Engeland et al., 1991; Lord et al., 1994; Williams et al., 1999; Lord et al., 2000). Likewise, in this study, male lesioned mice responded to changes in object position and to object novelty by decreasing exploration of all objects. Similar to many high functioning individuals with autism, the 5,7-DHT lesioned mice do not have pronounced spatial learning or memory deficits since they did clearly notice the spatial re-arrangement of the objects and in our previous studies excelled in the delayed non-matched-to-sample odor discrimination task. Thus, we conclude that neonatal, focal 5-HT depletion provides a suitable model to further examine the relationship between the cortical neurochemical, morphological and behavioral pathologies involved in autism. Although an altered cortical 5-HT innervation is unlikely the primary deficit in autism, it may well be a major contributor to neuropathologies underlying the sensorimotor and cognitive disruptions that characterize the disorder.
4. Experimental Procedure
4.1 Lesion Surgery
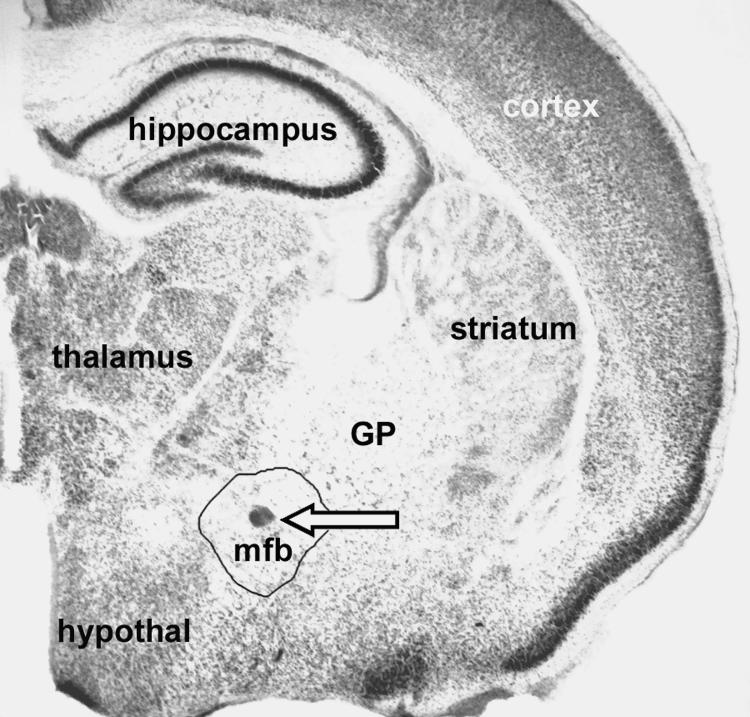
BALB/cByJ mice of both sexes were removed from their mothers within the first 12-20 hours after birth and divided into two groups with approximately equal sex ratios. Under hypothermia anesthesia, bilateral injections of 5,7-DHT (0.6 microliters of a 5 micogram/microliter solution) or saline, for controls, were made into the mfb near the Diagonal Band of Broca, horizontal limb as described previously by Hohmann et al.1998 and Hohmann et al, 2000 (Hohmann and Berger-Sweeney, 1998a; Hohmann et al., 2000). Briefly, the neonatal mice were placed into a specially designed mouse pup holder that was placed into the ear-bars of a small animal stereotaxic apparatus (Kopf), which immobilized the head of the pup. Coordinates for the injection were measured using the sinuses underlying the midline and frontonasal skull suture and 5,7-DHT in sterile physiological saline, or of saline alone (vehicle control) were injected bilaterally. On each side, a 27-gauge needle was lowered into the brain through skin and skull. Approximately 0.2 microliters of a 5 microgram/microliter 5,7-DHT solution or saline were made at three different sites along the anterior to posterior trajectory of the needle tract in the forebrain. After the injections, pups were re-warmed to normal body temperature on a heating pad, returned to their mothers and allowed to survive until the day of sacrifice. Fig. 8 shows the lesion site as it appears in a PND 7 mouse brain.
Fig. 8.
5,7-DHT lesion site. An arrow demarcates the lesion site, a small needle track scar in the center of the mfb in a Methylene Blue-Azure II stained coronal section from a PND 7 mouse that was neonatally lesioned with 5,7-DHT. Other abbreviations are GP for Globus Pallidus, and hypothal for hypothalamus. (Anatomical localization according to “The Mouse Brain” Paxinos, G. and Franklin, K.B.J., Academic Press.)
4.2 Histological Analysis
4.2.1 Serotonin Immunocytochemistry
On postnatal day (PND) 7, 30, 60 and in the behaviorally tested adults, male and female mice were perfusion fixed and their brains prepared for immunocytochemical localization of serotonin as reported previously (Donovan et al., 2002) or for Nissl staining. Specifically, mice were anesthetized with chloral hydrate, weighed and perfused transcardially with phosphate buffered saline (PBS) followed by 4% paraformaldehyde in 0.15 M phosphate buffer (pH 7.4); flow volume and rate varied with age. After perfusion, the brains were removed and post-fixed in the same solution for 4-8 hours. The brains were then immersed in 30% sucrose in PBS for cryoprotection, and the brains frozen and stored at -80°C before immunocytochemical processing.
Coronal and parasagittal sections (cut at 40-50 μm) were stained for serotonin as previously described (Donovan et al., 2002). Free-floating sections were incubated for one hour in blocking solution (5% normal goat serum, 0.3% Triton, 5% non-fat dry milk and PBS) followed by incubation with anti-serotonin antibodies (Diasorin, Stillwater, MN) diluted 1:20,000 in blocking solution for 24 hours at 4°C. Sections then were processed by the avidinbiotin method, which included a three-hour incubation in biotinylated goat anti-rabbit serum (1:200) followed by an one-hour incubation in ABC (1:50; Vector Laboratories, Burlingame, CA). The tissue was then reacted with a diaminobenzidine tetrachloride (DAB) solution for 10-15 minutes. Sections were mounted on gelatin-subbed slides and allowed to dry overnight. The DAB reaction product was subsequently enhanced using a silver gold enhancement protocol (Kitt et al., 1985). All glassware used was cleaned with Ultra Clean (Krackeler Scientific, Albany, NY) 24 hours before silver-gold enhancement. Slides were incubated in 1.42% silver nitrate (Sigma, St. Louis, MO) solution at 56°C for 1 hour on a shaking water bath. After rinsing in running deionized water for 15 minutes, the slides were incubated in the dark in a 0.2% gold chloride (Sigma, St. Louis, MO) solution for 10 minutes at room temperature. Slides then were rinsed and dipped in a 5% sodium thiosulfate (Sigma, St. Louis, MO) solution for 5 minutes, followed by a final rinse in deionized water. After enhancement, the sections were dehydrated and coverslips applies with DPX mounting medium (Fluka; Sigma, St. Louis, MO).
Quantitative analysis of 5-HT immunoreactive fiber density was performed in parasagittal sections at PND 15, 30 and in adulthood as described previously in Mamounas et al. (Mamounas et al., 2000) for different cortical areas, hippocampus, thalamus and cerebellum. Three to seven animals per were analyzed statistically using GraphPad Prism. Density values for each region and age were compared between normal and 5,7-DHT-lesioned mice by t-tests.
4.2.2 Morphometric Analysis
Morphometric analysis was performed on a subset of the behaviorally tested mice (4 each, 5,7-DHT lesioned and vehicle control males, five 5,7-DHT lesioned females and 6 vehicle-injected females). Coronal sections (cut at 50 μm) were stained with Methylene Blue-Azure II (Sigma). Quantitative morphological assessments were performed as described in detail in Hohmann and Berger-Sweeney 1998 and Hohmann et al., 2000 (Hohmann and Berger-Sweeney, 1998b; Hohmann et al., 2000). Briefly, using the MCID, AIS imaging system (St. Catherine’s, Ontario) images of the tissue were digitized and cortical width was measured at two separate levels through the anterior (anterior barrel field area) and posterior somatosensory cortex (torso and limb areas). In each section, bilateral cortical and laminar thickness measurements were taken at four separate medial to lateral positions within the somatosensory cortex. Means of these individual measurements for each section were used for statistical analysis using factorial analysis of variance (ANOVA) with Fisher post-hoc tests (StatView). Statistical analysis was performed for each cortical area and hemisphere separately as well as for all combined.
4.3 Autoradiography
Animals from each treatment group were deeply anesthetized, killed by rapid decapitation and their brains quickly removed and frozen on dry ice. Brains were stored at -80°C prior to sectioning. Tissues were cut into 20 μm thick coronal sections on a cryostat set at -20°C, thaw mounted onto Superfrost Plus slides (Fisher Scientific) and stored at -80°C in plasticcovered microscope slide boxes. For each animal, adjacent sections through the forebrain and midbrain were used to examine autoradiographic binding to serotonin uptake sites (SERTs). Six animals at each age, PD-15, 30 and 60, and one section per animal were used for analysis. Tissues were stained with cresyl violet after x-ray film exposure to delineate cellular elements and anatomic boundaries. Autoradiographic binding to SERTs was performed using [3H]-citalopram (Amersham Biosciences), as described previously (Bennett-Clarke et al., 1996; Bruning and Liangos, 1997; Boylan et al., 2000a).
Autoradiographic films were analyzed by densitometric analysis using a video based image analysis system (Imaging Research, Inc., St. Catherines, Ontario). A set of [3H] standards was exposed with the tissue sections for quantification of binding density. A relative optical density value was measured for each standard and these were then fit to an interpolation function. In these studies, the best fit was provided by a second degree polygonal function. Using this function, optical densities of the autoradiographic sections were compared to densities produced by the [3H] standards and transformed to nCi/mg tissue. The Nissl-stained sections were helpful in delineating the cerebral cortical boundaries. The densities of age normal controls, saline injected, and 5,7-lesioned mice were compared statistically by factorial ANOVA with Tukey′s Multiple Comparison Post Test (GraphPad Prism). Values are shown as means ± standard error (s.e.m.).
4.4 Behavioral Testing
Behavioral studies were conducted on 9 male and 7 female 5,7-DHT lesioned mice and 6 male and 12 female saline injected control littermates. Mice were derived from more than three litters each for 5,7-DHT and vehicle controls and age matched controls respectively; pups were weaned at four weeks of age and housed in small, same sex, littermate groups of 2-4 mice per cage until the start of behavioral testing. Testing began at three months postnatal. For eight days prior to the start of the behavioral assessments, the experimenter (person performing the tests) handled all mice for the purpose of habituation.
4.4.1 Neurological Test Battery
The Neurological Testing Battery is akin to a clinical neurological examination for mice and assesses basic sensorimotor abilities such as the righting reflex, placing reflex, grip strength and ability to walk across a narrow round beam. For all assessments, the experimenter was blind to the condition of the mouse (lesioned or vehicle-injected littermate). All tests were performed in triplicate on all mice prior to the onset of behavioral testing and the average scores for each animal were used for subsequent analysis.
For the righting reflex, a mouse was lowered by its tail toward a flat horizontal surface while the experimenter attempted to place the mouse’s back onto this surface. The ability of the mouse to turn around and land on its paws was rated on a scale of 0 - 2. If a mouse could not be turned on its back it was given a score of 2, a more sluggish response in righting itself was given a score of 1; mice unable to right themselves or respond with disorientation after righting themselves were given a score of 0. Impairments in the righting reflex would suggest deficits in proprioception, equilibrium or cerebellar problems.
For the placing reflex, mice were lowered by their tails toward the grid of the cage top; their readiness to stretch out their front paws towards the surface as they neared it was rated on a scale from 0 - 2. A score of 2 was given to a mouse that immediately stretched its paws as it neared the surface; a score of 1 indicated a delayed response and a score of 0 signified that the animal landed head first on the surface. Deficits in the placing reflex would suggest substantial sensorimotor rather than visual impairment, as the animal’s whiskers should touch the surface prior to the body.
For grip strength, mice were allowed first to attach to the grid of the cage top and were then pulled away by their tail; the strength of attachment to the cage top was assessed on a scale of 0 - 2. A strong resistance to being pulled away from the cage top equaled a score of 2, a weak resistance a score of 1 and no resistance a score of 0. Reduced grip strength could be an indicator of reduced muscle strength or overall ill health.
For performance on a beam-walking task, the “beam” (a 5 ml pipette) was stretched between the home cage and a support at the opposite end of the beam. The mouse was placed on a platform near the far end of the beam and allowed to move toward the home cage (strong motivator!). We measured the amount of time, up to a maximum of 125 seconds, taken by each mouse to cross the beam and enter the home cage. Mice that did not cross to their home cage by 125 seconds were removed from the task and given the maximum score of 4. In addition, we rated the ability of the mouse to cross smoothly without losing grip on a scale from 1-4 with 4 being the worst score. Each time a mouse fell off the beam or just held on to the beam by one or two paws, its score increased by one point up to a maximum of 4. The beam-walking test assessed both motor coordination and anxiety (reluctance to cross the beam). In addition to time measurements and scoring, the experimenter also recorded notes on the mouse overall behavior during the task. The semi-quantitative scores for all assessments were statistically assessed using factorial ANOVAs with Fisher post-hoc tests (StatView).
4.4.2 Open Field Object Recognition Task
This task was adapted from a task developed by Ricceri et al. (Ricceri et al., 1999) in accordance with parameters introduced by Poucet (Poucet, 1989). The OFOR task was performed in a round enclosure of approximately 3 feet in diameter surrounded by walls of about ten inches in height that lean outward at a slight angle of about 15 degrees (commercial plastic baby pool). The entire enclosure was painted black to increase visibility of the white mice; quadrants and annuli demarcating periphery vs. center were marked on the floor with white lines. Mice were videotaped during the performance of all aspects of the task and all measurements were conducted on a Dell Pentium computer using Observer Video-Pro (Noldus) software. The objects referred to below consisted of Lego constructions of various shapes and colors. The apparatus was wiped with an alcohol/water solution between subjects to eliminate all possible odor cues.
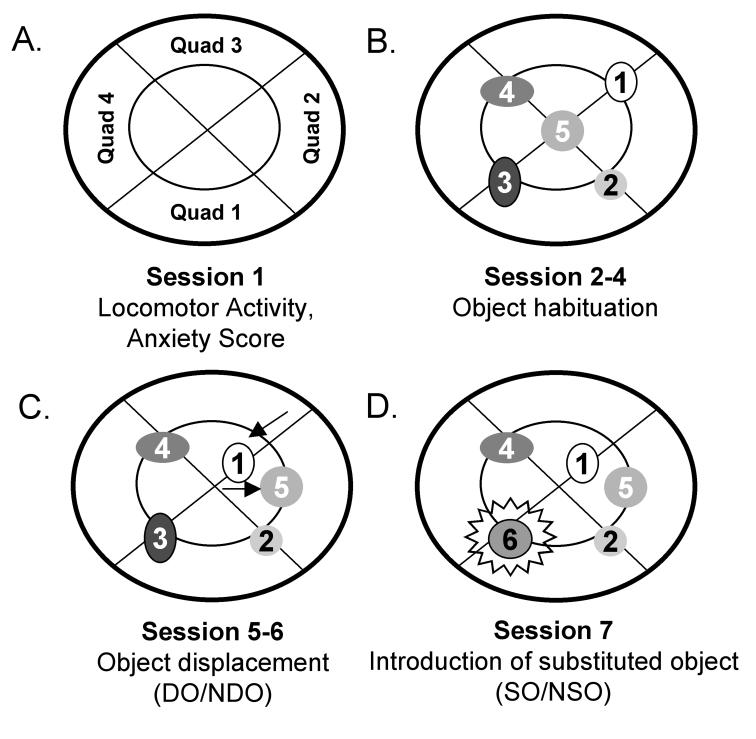
In the first session (S1), mice were placed into the enclosure without objects to assess open field behaviors including locomotor activity (number of quadrant crossings), rearing and time spent exploring the perimeter versus the center of the field (indicators of anxiety level; Fig. 9A). Anxiety scores were calculated by dividing time spent in the periphery by time spent in the center. During sessions 2-4, 5 objects of different shapes and colors were placed at set locations in the enclosure and the amount of time a mouse explored the objects was scored. Object exploration consisted of either “whisking” or sniffing the object, exploration with forepaws or climbing onto the object (Fig. 9B). In session 5 (S5), objects 1 and 5 (displaced objects) were moved to a new location; their position was maintained in session 6 (S6) (Fig. 9C). In session 7 (S7), object 3 was replaced with novel object 6 (substituted object; Fig. 9D). Mice were placed into the enclosure for 6 minutes with 3-minute intervals between sessions. The experimenter recorded the time spent with each object.
Fig. 9.
Schematic representation of the Open Field Recognition Task. A. In session 1, anxiety scores were calculated by dividing time spent in the periphery (outer annulus) by time spent in the center (inside annulus). Locomotor activity was determined by counting the number of times the mouse crossed from one quadrant into another. B. Numbers 1 through 6 represent objects of different shapes and colors (all constructed from Legos). Contact with objects 1-5 was assessed in sessions 2-4. C. In sessions 5 and 6, objects 1 and 5 were moved to a new location (arrows) to test spatial memory. D. In session 7, object 3 was replaced by object 6 to assess the novelty response.
In S5-7, mice normally increase exploration, especially of the displaced or novel objects, compared to the prior sessions. Thus, time spent with the displaced object compared to the other objects (non-displaced object) in S5 is ordinarily used to measure the mouse’ spatial recognition and memory. In S7, time spent with the novel, substituted object compared to the other objects (non-substituted objects) signals the animal’s ability to recognize novelty. DO/NDO and NO/NSO are calculated according to a formula devised by Ricceri et al. (Ricceri et al., 1999): DO = mean time spent with displaced objects in S5 minus mean time spent with the same objects in session 4 (S4); NDO = mean time spent with non-displaced objects in S5 minus mean time with the same objects in S4; SO = mean time spent with the novel object (object 6) in S7 minus mean time spent with the object (object 3) previously in this position in S6; NSO = mean time spent with non-substituted objects in S7 minus mean time with the same objects in S6.
To compare object displacement and novelty responses, due to considerable data spread within each group, exploration times were normalized on a scale of 1 to 5. The maximum time a mouse spent with any of the toys in a given session was scored as a 5; all other exploratory times were calculated as relative ratios of this maximum value. Group values were compared statistically by a factorial ANOVA with Fisher Post-hoc tests (StatView).
Acknowledgements
This work was supported by NAAR, G12 RR0175811 and SO6 GM051971 to C. Hohmann and 1 U54 MH066417 to M.E. Blue and C.F. Hohmann.
Abbreviations
- 5-HT
serotonergic
- SERTs
Serotonin uptake sites
- PCA
parachloroamphetamine
- 5,7-DHT
5,7-dihydroxydopamine
- mfb
medial forebrain bundle
- PND
postnatal day
- OFOR
Open Field Object Recognition
- S1
Session 1
- S2
Session 2
- S4
Session 4
- S5
Session 5
- S6
Session 6
- S7
Session7
- DO
mean time spent with displaced objects in S5 minus mean time spent with the same objects in S4
- NDO
mean time spent with non-displaced objects in S5 minus mean time with the same objects in S4
- SO
mean time spent with the novel object (object 6) in S7 minus mean time spent with the object (object 3) previously in this position in S6
- NSO
mean time spent with non-substitute objects in S7 minus mean time with the same objects in S6
- PBS
phosphate buffered saline
- DAB
diaminobenzidine tetrahydrochloride
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Gutknecht L, Cohen DJ, Brailly-Tabard S, Cohen JH, Ferrari P, Roubertoux PL, Tordjman S. Serotonin transporter promoter variants in autism functional effects and relationship to platelet hyperserotonemia. Mol Psychiatry. 2002;7:831–836. doi: 10.1038/sj.mp.4001099. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology. 1999;21:33S–45S. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bartlett CW, Gharani N, Millonig JH, Brzustowicz LM. Three autism candidate genes a synthesis of human genetic analysis with other disciplines. Int J Dev Neurosci. 2005;23:221–234. doi: 10.1016/j.ijdevneu.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bell K, Shokrian D, Potenzieri C, Whitaker-Azmitia PM. Harm avoidance, anxiety, and response to novelty in the adolescent S-100beta transgenic mouse role of serotonin and relevance to Down syndrome. Neuropsychopharmacology. 2003;28:1810–1816. doi: 10.1038/sj.npp.1300242. [DOI] [PubMed] [Google Scholar]
- Benayed R, Gharani N, Rossman I, Mancuso V, Lazar G, Kamdar S, Bruse SE, Tischfield S, Smith BJ, Zimmerman RA, Dicicco-Bloom E, Brzustowicz LM, Millonig JH. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am J Hum Genet. 2005;77:851–868. doi: 10.1086/497705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Chiaia NL, Crissman RS, Rhoades RW. The source of the transient serotoninergic input to the developing visual and somatosensory cortices in rat. Neuroscience. 1991;43:163–183. doi: 10.1016/0306-4522(91)90425-n. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Chiaia NL, Rhoades RW. Thalamocortical afferents in rat transiently express high-affinity serotonin uptake sites. Brain Res. 1996;733:301–306. doi: 10.1016/0006-8993(96)00791-3. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Chiaia NL, Rhoades RW. Contributions of raphe-cortical and thalamocortical axons to the transient somatotopic pattern of serotonin immunoreactivity in rat cortex. Somatosens Mot Res. 1997;14:27–33. doi: 10.1080/08990229771196. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proc Natl Acad Sci U S A. 1993;90:153–157. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW. Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat′s somatosensory cortex. J Neurosci. 1994;14:7594–7607. doi: 10.1523/JNEUROSCI.14-12-07594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Sweeney J, Libbey M, Arters J, Junagadhwalla M, Hohmann CF. Neonatal monoaminergic depletion in mice (Mus musculus) improves performance of a novel odor discrimination task. Behav Neurosci. 1998;112:1318–1326. [PubMed] [Google Scholar]
- Birger M, Swartz M, Cohen D, Alesh Y, Grishpan C, Kotelr M. Aggression the testosterone-serotonin link. Isr Med Assoc J. 2003;5:653–658. [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress behavioral, brain and neuroendocrine correlates. Behav. Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Blue ME, Erzurumlu RS, Jhaveri S. A comparison of pattern formation by thalamocortical and serotonergic afferents in the rat barrel field cortex. Cereb Cortex. 1991;1:380–389. doi: 10.1093/cercor/1.5.380. [DOI] [PubMed] [Google Scholar]
- Boylan CB, Bennett-Clarke CA, Chiaia NL, Rhoades RW. Time course of expression and function of the serotonin transporter in the neonatal rat′s primary somatosensory cortex. Somatosens Mot Res. 2000;17(a):52–60. doi: 10.1080/08990220070292. [DOI] [PubMed] [Google Scholar]
- Boylan CB, Bennett-Clarke CA, Crissman RS, Mooney RD, Rhoades RW. Clorgyline treatment elevates cortical serotonin and temporarily disrupts the vibrissaerelated pattern in rat somatosensory cortex. J Comp Neurol. 2000;427(b):139–149. doi: 10.1002/1096-9861(20001106)427:1<139::aid-cne9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Breese GR, Vogel RA, Mueller RA. Biochemical and behavioral alterations in developing rats treated with 5,7-dihydroxytryptamine. J Pharmacol Exp Ther. 1978;205:587–595. [PubMed] [Google Scholar]
- Briley M, Chopin P, Moret C. Effect of serotonergic lesion on “anxious” behaviour measured in the elevated plus-maze test in the rat. Psychopharmacology (Berl) 1990;101:187–189. doi: 10.1007/BF02244124. [DOI] [PubMed] [Google Scholar]
- Brown GL, Linnoila MI. CSF serotonin metabolite (5-HIAA) studies in depression, impulsivity, and violence. J Clin Psychiatry. 1990;51(Suppl):31–41. discussion 42-33.
- Bruning G, Liangos O. Transient expression of the serotonin transporter in the developing mouse thalamocortical system. Acta Histochem. 1997;99:117–121. doi: 10.1016/S0065-1281(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of occular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1665. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Chandana SR, Behen ME, Juhasz C, Muzik O, Rothermel RD, Mangner TJ, Chakraborty PK, Chugani HT, Chugani DC. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci. 2004;24:412–419. doi: 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell S, Karikari C, Hohmann CF. Sex-specific development of cortical monoamine levels in mouse. Brain Res Dev Brain Res. 2004;151:187–191. doi: 10.1016/j.devbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Conroy J, Meally E, Kearney G, Fitzgerald M, Gill M, Gallagher L. Serotonin transporter gene and autism a haplotype analysis in an Irish autistic population. Mol Psychiatry. 2004;9:587–593. doi: 10.1038/sj.mp.4001459. [DOI] [PubMed] [Google Scholar]
- Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Coon H, Dunn D, Lainhart J, Miller J, Hamil C, Battaglia A, Tancredi R, Leppert MF, Weiss R, McMahon W. Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2) Am J Med Genet B Neuropsychiatr Genet. 2005 doi: 10.1002/ajmg.b.30168. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism early overgrowth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Coutinho AM, Oliveira G, Morgadinho T, Fesel C, Macedo TR, Bento C, Marques C, Ataide A, Miguel T, Borges L, Vicente AM. Variants of the serotonin transporter gene (SLC6A4) significantly contribute to hyperserotonemia in autism. Mol Psychiatry. 2004;9:264–271. doi: 10.1038/sj.mp.4001409. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Molliver ME. Major innervation of newborn rat cortex by monoaminergic neurons. Science. 1977;196:444–447. doi: 10.1126/science.850788. [DOI] [PubMed] [Google Scholar]
- Davis CA, Joyner AL. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988;2:1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- Dawson G, Carver L, Melzhoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay and typical development. Child Devel. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavadian RL, Wielkopolska E, Turlejski K. Neonatal depletion of serotonin increases the numbers of callosally projecting neurons in cat visual areas 17 and 18. Neurosci Lett. 2003;351:91–94. doi: 10.1016/j.neulet.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Donovan SL, Mamounas LA, Andrews AM, Blue ME, McCasland JS. GAP-43 is critical for normal development of the serotonergic innervation in forebrain. J Neurosci. 2002;22:3543–3552. doi: 10.1523/JNEUROSCI.22-09-03543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dori I, Dinopoulos A, Blue ME, Parnavelas JG. Regional differences in the ontogeny of the serotonergic projection to the cerebral cortex. Exp Neurol. 1996;138:1–14. doi: 10.1006/exnr.1996.0041. [DOI] [PubMed] [Google Scholar]
- Durig J, Hornung JP. Neonatal serotonin depletion affects developing and mature mouse cortical neurons. Neuroreport. 2000;11:833–837. doi: 10.1097/00001756-200003200-00035. [DOI] [PubMed] [Google Scholar]
- Earls F. Sex differences in Psychiatric disorders. Psychiat. Devel. 1987;5:1–23. [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral responses to spatial stimuli and object novelty in adult C57BL/6 mice. Behav. Neurosci. 2003;117:735–744. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Roberts TP. Cortical auditory system maturational abnormalities in children with autism disorder an MEG investigation. Brain Res Dev Brain Res. 2003;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Ruhrberg C, Livet J, Henderson CE, Krumlauf R. Neuronal defects in the hindbrain of Hoxa1, Hoxb1 and Hoxb2 mutants reflect regulatory interactions among these Hox genes. Development. 2003;130:5663–5679. doi: 10.1242/dev.00802. [DOI] [PubMed] [Google Scholar]
- Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7:1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998;83:1185–1201. doi: 10.1016/s0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J, Takeoka M, Tager-Flusberg H, Caviness VS., Jr. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Stickgold R, Pace-Schott EF. The neuropsychology of REM sleep dreaming. Neuroreport. 1998;9:R1–14. doi: 10.1097/00001756-199802160-00033. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, Berger-Sweeney J. Cholinergic regulation of cortical development and plasticity. New twists to an old story. Perspect Dev Neurobiol. 1998;5(a):401–425. [PubMed] [Google Scholar]
- Hohmann CF, Berger-Sweeney J. Sexually dimorphic responses to neonatal basal forebrain lesions in mice II. Cortical morphology. J Neurobiol. 1998;37(b):595–606. [PubMed] [Google Scholar]
- Hohmann CF, Boomer A, Simmons Q, Ricceri L. Environmental influences on sex differences in cortical structure and function; Proceedings of the International Behaviora Neuroscience Society Annual Meeting; Nancy, France. 1999. [Google Scholar]
- Hohmann CF, Hamon R, Batshaw ML, Coyle JT. Transient postnatal elevation of serotonin levels in mouse neocortex. Brain Res. 1988;471:163–166. doi: 10.1016/0165-3806(88)90163-0. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, Richardson C, Pitts E, Berger-Sweeney J. Neonatal 5,7-DHT lesions cause sex-specific changes in mouse cortical morphogenesis. Neural Plast. 2000;7:213–232. doi: 10.1155/NP.2000.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hole K, Johnson GE, Berge OG. 5,7-Dihydroxytryptamine lesions of the ascending 5-hydroxitryptamine pathways habituation, motor activity and agonistic behavior. Pharmacol. Biochem. Behav. 1977;7:205–210. doi: 10.1016/0091-3057(77)90135-6. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells relevance for cortical development. J Neurosci. 2004;24:1652–1659. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitt CA, Struble RG, Cork LC, Mobley WC, Walker LC, Joh TH, Price DL. Catecholaminergic neurites in senile plaques in prefrontal cortex of aged nonhuman primates. Neuroscience. 1985;16:691–699. doi: 10.1016/0306-4522(85)90202-7. [DOI] [PubMed] [Google Scholar]
- Klauck SM, Poustka F, Brenner A, Lesh KP, Poustka A. Serotonin transporter (5-HTT) gene variaants associated with autism? Hum. Mol. Genet. 1997;6:2223–2228. doi: 10.1093/hmg/6.13.2233. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Brain plasticity and behavior. Annu Rev Psychol. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- Kootz JP, Marinelli B, Cohen DJ. Modulation of response to environmental stimulation in autistic children. J. Autism Dev. Disord. 1982;12:185–193. doi: 10.1007/BF01531308. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Krzascik P. Neonatal 5-hydroxytryptamine depletion induces depressivelike behavior in adult rats. Pol J Pharmacol. 2003;55:957–963. [PubMed] [Google Scholar]
- Lauder JM. Hormonal and humoral influences on brain development. Psychoneuroendocrinology. 1983;8:121–155. doi: 10.1016/0306-4530(83)90053-7. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases o., Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J. Comp. Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Levitt JG, Blanton RE, Smalley S, Thompson PM, Guthrie D, McCracken JT, Sadoun T, Heinichen L, Toga AW. Cortical sulcal maps in autism. Cereb Cortex. 2003;13:728–735. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- Lidov HGW, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat Ascending pathways and terminal fields. Brain Res. Bull. 1982;8:389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- Lieske V, Bennett-Clarke CA, Rhoades RW. Effects of serotonin on neurite outgrowth from thalamic neurons in vitro. Neuroscience. 1999;90:967–974. doi: 10.1016/s0306-4522(98)00501-6. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Mullen CA, O′Hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J Comp Neurol. 1991;314:558–586. doi: 10.1002/cne.903140312. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Mechawar N, Radja F, Beaulieu C, Descarries L. Quantified distribution of serotonin transporter and receptors during the postnatal development of the rat barrel field cortex. Brain Res Dev Brain Res. 1998;107:159–163. doi: 10.1016/s0165-3806(98)00016-9. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borelia A, Whitacker-Azmitia P. serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat. a possible model of nerodevelopmental disorders with cognitive deficits. Brain res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, Folstein SE, Haines JL, Sutcliffe JS. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. Am J Med Genet B Neuropsychiatr Genet. 2004;127:104–112. doi: 10.1002/ajmg.b.20151. [DOI] [PubMed] [Google Scholar]
- Mogensen J, Svendsen G, Lauritsen KT, Ermens P, Hasman A, Elvertorp S, Plenge P, Mellerup E, Wortwein G. Associative and nonassociative learning after chronic imipramine in rats. Pharmacol Biochem Behav. 2003;76:197–212. doi: 10.1016/s0091-3057(03)00220-x. [DOI] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- Molliver ME, Berger UV, Mamounas LA, Molliver DC, O′Hearn E, Wilson MA. Neurotoxicity of MDMA and related compounds anatomic studies. Ann N Y Acad Sci. 1990;600:649–661. doi: 10.1111/j.1749-6632.1990.tb16916.x. [DOI] [PubMed] [Google Scholar]
- Morrisey G, Ho MY, Wogar MA, Bradshaw CM, Szabadi E. Effects of lesion of the ascending 5-hydroxytryptamine pathway on timing behavior investigated with the fixed-inteval peak procedure. Psychopharmacology. 1994;114:463–468. doi: 10.1007/BF02249337. [DOI] [PubMed] [Google Scholar]
- Mueller R-A, Kleinhans N, Kemmotsu N, Courchesne E. Abnormal variability and distribution of functional maps in autism. Am J. Psychiat. 2003;160:1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, Brugman AM, Ketelaars CE, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Serotonin transporter intron 2 polymorphism associated with rigid-compulsive behaviors in Dutch individuals with pervasive developmental disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;133:93–96. doi: 10.1002/ajmg.b.30122. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Nabi R, Serajee FJ, Chugani DC, Zhong H, Huq AH. Association of tryptophan 2,3 dioxygenase gene polymorphism with autism. Am J Med Genet. 2004;125B:63–68. doi: 10.1002/ajmg.b.20147. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Togashi H, Ueno K, Yoshioka M. The serotonergic modulation of synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway. Neurosci Lett. 2003;342:179–182. doi: 10.1016/s0304-3940(03)00293-3. [DOI] [PubMed] [Google Scholar]
- Osterheld-Haas MC, Hornung JP. Laminar development of the mouse barrel cortex effects of neurotoxins against monoamines. Exp Brain Res. 1996;110:183–195. doi: 10.1007/BF00228550. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism a magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Poucet B. Object exploration, habituation, and response to a spatial change in rats following septal or medial frontal cortical damage. Behav Neurosci. 1989;103:1009–1016. doi: 10.1037//0735-7044.103.5.1009. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout an animal model of anxietyrelated disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri l., Hohmann CF, Berger-Sweeney J. A role for the cholinergic basal forebrain system in early cortical development and behavior in male and female rats. Devel. Brain Res. 2002;954:160–172. doi: 10.1016/s0006-8993(02)03172-4. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Usiello A, Valanzano A, Calamandrei G, Frick K, Berger-Sweeney J. Neonatal 192 IgG-saporin lesions of basal forebrain cholinergic neurons selectively impair response to spatial novelty in adult rats. Behav. Neurosci. 1999;113:1204–1215. doi: 10.1037//0735-7044.113.6.1204. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Roullet P, Lassalle JM. Genetic variation, hippocampal mossy fibres distribution, novelty reactions and spatial representation in mice. Behav Brain Res. 1990;41:61–70. doi: 10.1016/0166-4328(90)90054-i. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Buhot M-C, Foreman N, Thinus-Blanc C. Exploratory activity and response to a spatial change in rats with hippocampal or posterior parietal cortical lesions. Behavioral Brain Res. 1992;47:113–127. doi: 10.1016/s0166-4328(05)80118-4. [DOI] [PubMed] [Google Scholar]
- Scott MM, Deneris ES. Making and breaking serotonin neurons and autism. Int J Dev Neurosci. 2005;23:277–285. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Shuey DL, Sadler TW, Tamir H, Lauder JM. Serotonin and morphogenesis. Transient expression of serotonin uptake and binding protein during craniofacial morphogenesis in the mouse. Anat Embryol (Berl) 1993;187:75–85. doi: 10.1007/BF00208198. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Spoont MR. Modulatory role of serotonin in neural information processing implications for human psychopathology. Psychol Bull. 1992;112:330–350. doi: 10.1037/0033-2909.112.2.330. [DOI] [PubMed] [Google Scholar]
- Thinus-Blanc C, Save E, Rossi-Arnaud C, Tozzi A, Ammassari-Teule M. The difference shown by C57BL/6 and DBA/2 inbred mice in detecting spatial novelty are subserved by a different hippocampal and parietal cortex interplay. Behav. Brain Res. 1996;80:33–40. doi: 10.1016/0166-4328(96)00016-2. [DOI] [PubMed] [Google Scholar]
- van Engeland H, Roelofs JW, Verbaten MN, Slangen JL. Abnormal electrodermal reactivity to novel visual stimuli in autistic children. Psychiatry Res. 1991;38:27–38. doi: 10.1016/0165-1781(91)90050-y. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Kim SJ, Lord C, Courchesne R, Akshoomoff N, Leventhal BL, Courchesne E, Cook EH., Jr. Transmission disequilibrium studies of the serotonin 5-HT2A receptor gene (HTR2A) in autism. Am J Med Genet. 2002;114:277–283. doi: 10.1002/ajmg.10192. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Callebert J, Launay JM, Price DJ, Seif I, Gaspar P. Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex determination of a sensitive developmental period. J Comp Neurol. 1998;393:169–184. doi: 10.1002/(sici)1096-9861(19980406)393:2<169::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Walsh RN. Effects of environmental complexity and deprivation on brain anatomy and histology a review. Int J Neurosci. 1981;12:33–51. doi: 10.3109/00207458108990671. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development a role in autism. Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Williams E, Costall A, Reddy V. Children with autism experience problems with both objects and people. J. Autism Dev. Disord. 1999;29:367–378. doi: 10.1023/a:1023026810619. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sari Y, Zhou FC. Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res Dev Brain Res. 2004;150:151–161. doi: 10.1016/j.devbrainres.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Yan W, Wilson CC, Haring JH. Effects of neonatal serotonin depletion on the development of rat dentate granule cells. Brain Res Dev Brain Res. 1997;98:177–184. doi: 10.1016/s0165-3806(96)00176-9. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Brain Res Dev Brain Res. 2000;119:33–45. doi: 10.1016/s0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]