Abstract
Early assessment of response to chemotherapy with fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) is becoming a routine part of management in patients with Hodgkin lymphoma (HL) and histologically aggressive non-Hodgkin lymphoma (NHL). Changes in FDG uptake can occur soon after the initiation of therapy and they precede changes in tumour volume. Recent studies in uniform populations of aggressive lymphomas (predominantly diffuse large B cell lymphomas) and HL have clarified the value of early response assessment with PET. These trials show that PET imaging after 2–3 chemotherapy cycles is far superior to CT-based imaging in predicting progression-free survival and can be at least as reliable as definitive response assessment at the end of therapy. This information is of great potential value to patients, but oncologists should be cautious in the use of early PET response in determining choice of therapy until some critical questions are answered. These include: When is the best time to use PET for response assessment? What is the best methodology, visual or quantitative? (For HL at least, visual reading appears superior to an SUV-based assessment). Can early responders be cured with less intensive therapy? Will survival be better for patients treated more intensively because they have a poor interim metabolic response? In the future, early PET will be crucial in developing response-adapted therapy but without further carefully designed clinical trials, oncologists will remain uncertain how best to use this new information.
Keywords: Positron emission tomography; Hodgkin lymphoma; non-Hodgkin lymphoma; prognosis, chemotherapy, radiotherapy
Introduction
Current treatment strategies for lymphoma
Hodgkin lymphoma (HL) and the non-Hodgkin lymphomas (NHL) represent a diverse group of neoplasms[1] that include some of the most treatment-responsive cancers currently known. More than 80% of patients with HL can be cured with current chemotherapy, radiation therapy (RT) or combined modality therapy regimens[2] and many patients with aggressive types of NHL can now be cured with appropriate intensive therapy[3]. Recent advances, including the use of monoclonal antibody-based therapies for B cell lymphomas[4] and the more widespread use of early high dose chemotherapy and haematopoietic stem-cell transplantation for patients who have failed initial therapy[5], have significantly improved the results of treatment for patients with lymphoma. Despite these therapeutic advances in HL and aggressive NHL that have increased cure rates, the intention of treatment in most cases of advanced stage low-grade NHL is induction of remission of disease rather than cure because of the high rate of relapse after systemic therapy for this disease. However, treatment with anti-CD-20 antibody therapy (Rituximab) is at last showing that survival in advanced low grade follicular lymphoma can be improved by better systemic therapy[6].
Curative therapy in HL and aggressive NHL involves the use of intensive cytotoxic chemotherapy in the great majority of patients. The overall treatment strategy, the type of chemotherapy to be used and the need for RT is usually determined prior to starting therapy using a range of prognostic factors specific for the type of lymphoma in question. In HL, the EORTC criteria are helpful in determining treatment choice in early stage disease[7] and the Hasenclever index is useful for advanced disease[8]. In aggressive NHL the International Prognostic Index (IPI) helps to determine treatment choice[9]. Patients with early-stage HL routinely receive involved-field RT in addition to limited chemotherapy and patients with advanced HL may receive RT to sites of bulky disease or to residual masses after completion of intensive chemotherapy[10]. In aggressive lymphomas, intensive chemotherapy, often combined with immunotherapy using a monoclonal antibody[11], is the most important component of therapy. Consolidative RT is often used in early stage diffuse large B cell lymphomas (DLBCL) and in cases with initially bulky tumours (variously defined as masses greater than 5–10 cm in diameter) or residual masses after completion of chemotherapy. RT is now used infrequently as the sole potentially curative therapy in HL or aggressive NHL but remains the standard treatment in early stage follicular lymphoma[12].
Because patients with aggressive NHL and with HL are commonly cured, there is an increasing emphasis on attaining long-term survival with the least acute and late toxicity from chemotherapy and RT. Acute and subacute toxicities of chemotherapy include myelosuppression, neuropathy, pulmonary fibrosis and cardiac damage. Later effects include risks of myelodysplasia and leukaemia, especially in patients treated with alkylating agents. RT can cause mucositis and xerostomia and can significantly increase the risks of second cancers, especially solid tumours including thyroid and breast carcinoma. For these reasons, when cure is the aim, it is desirable to treat patients with the least-toxic therapy that will achieve a durable complete remission of disease. That could mean limiting the number of cycles of chemotherapy to the optimum number for each individual patient and restricting the use of RT to those most likely to benefit from it. On the other hand, for those patients with more resistant disease it is important that ineffective therapy should be identified promptly and changed and that those patients who will ultimately need high-dose chemotherapy and stem-cell transplantation are identified as early as possible.
Potential role of early response assessment in lymphoma
Although treatment intensity in HL and NHL is determined to a significant degree by baseline prognostic indices of groups of apparently similar patients, it is likely that many individual patients are over-treated and receive more chemotherapy and RT than the minimum needed to attain cure. Similarly, many patients, especially those with advanced disease, receive initial treatment that is insufficient to induce a durable remission. The introduction of response-adapted therapy has been frustrated by the fairly crude methods previously available for assessment of early treatment response. However, there are now limited data suggesting that even with conventional imaging some useful information can be obtained[13]. If it were feasible to identify patients with a favourable response at an early stage in therapy, then it is possible that less intensive treatment than used in standard therapy would be sufficient to attain cure. Alternatively, if patients with a poor early response were identified, then steps could be taken to institute more intensive therapy at an early stage, before many more cycles of ineffective therapy were delivered, thereby minimizing unwarranted toxicity. The earlier a reliable response assessment could be made, the better.
Standard methods for response assessment
The first widely-used standard response categories for HL in the era of CT imaging were those of the Cotswolds criteria[14]. In NHL the International Workshop Response criteria are the most commonly used[15]. These standard definitions of treatment response are based on changes in lesion dimensions with time. Curative treatment in lymphoma is initially directed toward attaining a remission, either complete remission (CR) in which there is no residual radiologic abnormality at any site of disease or complete remission unconfirmed (CRU) in which the sum of the diameters of tumour masses is reduced by >75% and any residual mass lesion remains stable or continues to regress for at least 1 month, or previously enlarged lymph nodes reduce in size to no more than 1.5 cm in transverse diameter. The CRU category reflects the fact that many patients with cured HL or DLBCL have residual mass lesions on completion of therapy. This is especially true of patients with HL who present with bulky mediastinal masses[16]. Only biopsy or subsequent evidence of disease progression can determine if residual masses contain active disease. Upon attainment of CR or CRU, treatment will often be continued for several further cycles of chemotherapy before ceasing and patients with CRU often receive RT to the residual masses, depending on the clinical situation.
Conventional assessment at, or near, the end of therapy is dichotomous with either CR/CRU or not CR/CRU. Less than CR/CRU at the end of a planned course of curative therapy usually is taken to mean that treatment has been unsuccessful and further therapy is needed. There are significant problems with the use of structural imaging for response assessment in lymphoma. They include the following: CT does not detect residual disease in normal sized nodes; patients with a residual mass after therapy may not have persistent disease (and biopsy may be false negative due to sampling error); serial CT scans may be required to evaluate residual masses, thereby delaying salvage therapy if there is truly active residual disease; there may be insufficient contrast between lymphoma and normal tissue at extranodal sites (e.g. salivary gland, bowel, liver, spleen[17]) to visualize disease.
When structural imaging is used to provide an early or interim assessment of treatment response, its value is limited by the slow rate of change of volume of many mass lesions as well as by the other limitations just described. CT assessment of bulky masses of HL and DLBCL after just one or two cycles of chemotherapy may show some tumour shrinkage but response is usually incomplete at that stage, especially in larger tumours. Such assessments are of limited value, although patients with a very poor treatment response or progressive disease may be identified early. Maximum prognostic value accuracy is obtained from structural imaging performed around the end of therapy, when tumours in responding patients have had sufficient time to reduce in volume.
Role of PET in early response assessment in lymphoma
Rationale for use of PET in response assessment
Most lymphomas have increased glucose utilization and are readily imaged using FDG, currently the radiopharmaceutical of choice for PET[18]. There is already a considerable literature on the use of FDG-PET in primary staging of HL[19] and NHL[20], clearly showing that PET adds to the sensitivity, specificity and overall accuracy in determining gross disease extent. Despite early suggestions that FDG-PET might not be useful in low grade NHL, more recent reports indicate that PET imaging adds significantly to the accuracy of staging in low grade follicular and other indolent histologies[21], although assessment of very indolent lymphoma may be limited by low FDG avidity. Intensity of FDG uptake in lymphoma is somewhat related to histological grade[22]. Higher levels of standardized uptake value (SUV) are typically observed in more aggressive NHL compared to lower grade NHL[23], although there is significant overlap of SUV levels between different grades of lymphoma. Morphological abnormality is not a requirement for diagnosis of disease involvement by PET and indeed, high uptake in structures of normal size increases the suspicion of involvement (Fig. 1). Detection of an especially FDG-avid lesion in the setting of documented low-grade NHL should raise the possibility of histological transformation to a higher grade lymphoma.
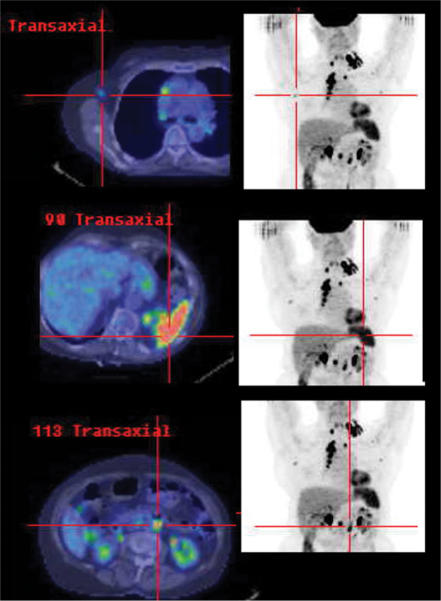
Figure 1.
In this patient with clinical and CT stage II NHL involving the left supraclavicular and superior mediastinal nodal stations, high FDG uptake in axillary and para-aortic nodes of normal size, and in a non-enlarged spleen, rendered the patient stage IIIS by PET criteria. These findings have potential management and prognostic implications. Due to lack of baseline radiological abnormality, CT is obviously of limited utility in monitoring response at these sites.
Before PET became available, planar and single photon emission computed tomography imaging with gallium-67 citrate (Ga-67 SPECT) was the most widely used functional imaging modality in lymphoma[24]. PET is rapidly displacing Ga-67 SPECT for staging lymphoma because of its very considerable advantages. Although there are limited comparative data, PET appears to be both more sensitive and more specific than Ga-67 SPECT for staging in lymphoma[25,26], and in both NHL and HL[27]. PET also appears to have more potential in treatment response assessment[28]. PET provides higher resolution images that are easier to interpret than Ga-67 SPECT and PET/CT fused images provide the best combination of structural and functional information currently available. PET is much speedier than a meticulous Ga-67 SPECT study and therefore more practical in situations where treatment decisions need to be made quickly. The cumulative radiation exposure from PET is less than from high-dose Ga-67 SPECT. This is of particular importance in pregnancy. Low-grade lymphomas usually have low gallium avidity and this limits the use of Ga-67 SPECT for both staging and the assessment of treatment response.
Response assessment methodology and terminology
Therapeutic response information provided by PET is more complex than that provided by structural imaging and researchers continue to search for the best way to use it. Uptake of FDG in lymphoma is influenced by many different biological factors, including substrate utilization, tumour perfusion[29], effects of hypoxia[30] and apoptosis, the viable cell fraction[31], the extent of inflammatory cell infiltrate (for example, in HL only 1% of cells may be tumour, the rest inflammatory) and oncogene expression[32]. It is sometimes forgotten that FDG-PET is a “glucose scan” and not a “cancer scan”. Acute effects on cellular metabolism may not necessarily be reflective of the residual viable cell fraction and therefore the timing of the PET scan in relation to factors that influence the tumour cell metabolism could prove to be crucial. It is likely that the results of scanning very soon after chemotherapy is administered are primarily influenced by changes in cellular metabolism but may also reflect rapid apoptotic cell death. Scans at later time-points, when more cell killing has occurred if treatment is effective, and when metabolic toxins have been eliminated, are likely to reflect predominantly the cytotoxic effects of therapy and the number of residual viable cells. Therefore, scan timing in relation to the administration of the drugs in each cycle, and the number of cycles after which the “early” response assessment is made, may both be important.
Whereas CT provides information only on tumour dimensions, PET/CT scanning allows the investigator to visualize and measure the intensity of residual metabolic activity within a lesion as well as estimating its size (Fig. 2). For lesions that are well-demarcated on CT, tumour volume assessment is more accurate when performed by CT than PET alone. For very intense lesions, spillover of activity into adjacent voxels can make them appear larger than they are. Conversely, in lesions within regions of high background activity or in small lesions, tumour size may be underestimated because of partial volume effects. Importantly, for lesions that are not seen at all on CT, the less well-defined PET images may provide the only indication of tumour size. The feature that really distinguishes PET from structural imaging is its ability to integrate the effects of residual tumour volume and residual tumour metabolic activity into a single response assessment. The best methodology for determining early PET response has not yet been established but there are two main approaches.
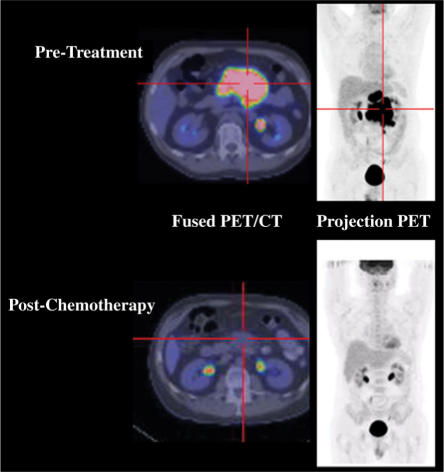
Figure 2.
PET/CT offers the ability to assess both the metabolic and structural characteristics of a tumoral mass and can also metabolically guide biopsy of heterogeneous masses to increase the likelihood of diagnostic pathological results. In this patient with a partial radiological response, PET demonstrated a sustained complete metabolic response.
Visual/qualitative methods
In lymphoma, most published clinical response data have been based on a visual estimate of response. In these studies, the authors have used absence of metabolic activity in a lesion, or reduction in intensity to no more than that contained in the blood pool, to denote a complete response. Due to the potential for this to be confused with a complete clinical or radiological response, we prefer to use the nomenclature of a complete metabolic response (CMR) for those with a normal PET scan following treatment. In most studies patients were separated into just two visual response categories, those with and those without PET evidence of persistent lymphoma (CMR and non-CMR). Mikhaeel and colleagues added a refinement to these categories by incorporating the category of MRU or “minimal residual uptake” to denote those difficult cases in which there was low level residual uptake in a lesion that would otherwise be considered to have responded completely[33]. Their recent publication, discussed in more detail below, suggests that the MRU category really represents an excellent partial response and carries a much worse prognosis than a CR, particularly in aggressive lymphoma.
When we analyzed our own prospective data on PET response in lung cancer, there were no published guidelines for qualitative PET response and we therefore developed and reported our own simple visual assessment system, including a PET CR category as defined above[34]. We also included separate categories of PET PR and PET progressive disease (PD) and found that these had profound prognostic significance. Because of its superior prognostic stratification compared to conventional response, we suggest that it is appropriate to designate those evaluations of response based on PET by the term “metabolic response”. A PET CR then becomes “complete metabolic response” or CMR, distinguishing it from the less reliable structural response assessment. This nomenclature is also applicable to lymphoma.
Semiquantitative or SUV-based methods
Although many lymphoma patients will have complete responses after 2–3 cycles of chemotherapy, it is possible that a very early “quantitative” measurement of metabolic response could provide valuable prognostic information, perhaps after only one cycle of chemotherapy or even after just one day[35]. If assessment is performed before a majority of patients have attained a CMR, then the presence of, or quality of the PMR attained could be of prognostic significance. There are very many potential methods for quantitative assessment of response to chemotherapy based on PET[36,37]. None of the more complex methods has been widely adopted and the only parameter with widespread acceptance as a measure of lesion intensity is the SUV, most commonly SUVmax. This is derived from tissue activity at a single point in time and uses the voxel, or cluster of voxels with highest lesion activity. This analysis is not dependent on acquisition of dynamic information of the type required for kinetic modelling approaches. SUV measurement is affected by a variable time between injection and scan, by variation in time to equilibrium and by variation in uptake curve slope before and after therapy. Additionally it may be affected by blood glucose concentration, differs if weight or body surface area is used as a correction factor and may not give the same results if different scanners used. Additionally, inflammatory reactions (infection, radiation) in normal tissues may produce SUV in “malignant” range (SUV >2.5). However, the absolute accuracy of the SUV measurement may not be a confounding factor in assessment of treatment response using FDG-PET if the pre- and post-treatment scans are performed on the same scanner under identical conditions. So far, no large trial using SUV criteria to measure early response has been performed in a uniform cohort of NHL patients but some data exist for HL. The most authoritative SUV-based response criteria are currently those proposed by the EORTC[38]. A simplified method for measuring the metabolic rate of glucose has shown promise in assessment of response to chemotherapy in lung cancer and could have applicability in lymphoma[39].
Studies of early PET response assessment in patients with mixed lymphoma populations
Soon after clinical PET first became available, its potential value for staging and restaging of lymphoma was apparent[40]. Accumulating evidence has shown that PET at the end of therapy adds significantly to the accuracy of measurement of the overall success of treatment. These studies have involved patients with HL alone[41–43], NHL alone[44,45], and mixed populations of HL and NHL[16,46–50]. The presence of residual FDG uptake at sites of prior disease at the conclusion of treatment has a very high positive predictive value for future disease progression in the absence of further therapy (>90%), although it is also clear that the potential confounding factors of inflammatory disorders, including infection, and radiation-induced FDG accumulation in normal tissues need to be considered. We have also noted that persisting metabolic abnormality can be associated with sites of focal bone involvement, probably reflecting bone healing. We now discount these abnormalities when all soft tissue sites of disease have had a CMR. While attainment of a CMR at the end of systemic therapy is a powerful positive prognostic factor, it does not guarantee that a cure has been attained, nor does it imply that further treatment, such as consolidative RT, should be withheld (Fig. 3). Because most patients with lymphoma attain a CMR at the end of initial therapy, it was suggested that an earlier response assessment would have greater power to discriminate between high-and low-risk patients and that the rate of attainment of response could also be a prognostic factor.
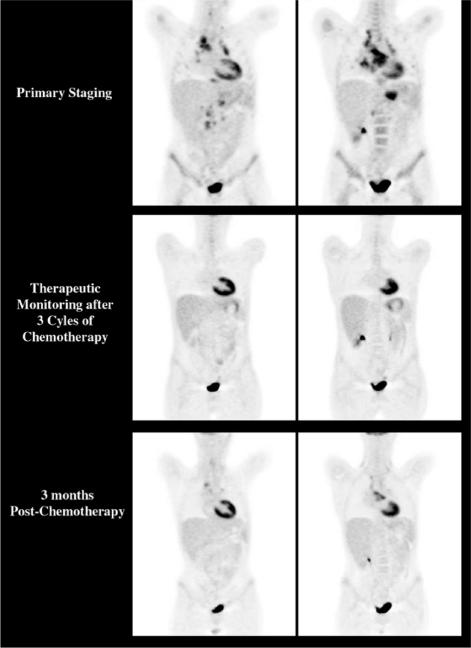
Figure 3.
Despite evidence that a complete metabolic response (normalization of baseline abnormalities) indicates a substantially better prognosis than lack thereof, it should not be assumed that this represents cure as demonstrated by this case where an interim CMR was clearly followed by local relapse in the mediastinum. Of note, interim CT showed only a partial response whereas the recurrent disease detected by post-treatment PET was not apparent on CT at that time with the patient being classified as having had a complete response.
Earlier studies of PET for interim and definitive response assessment in lymphoma[13,51–54] necessarily included diverse histological types of NHL and often also included HL patients in order to gain enough statistical power. Interim response assessments were made after differing numbers of cycles of chemotherapy of different types and using PET technology of varying quality. Kostakoglu and colleagues showed that response assessment after only one cycle could have profound prognostic significance, but these data were based on use of a scanner gamma camera operating in coincidence mode[55]. The spatial and contrast resolution of such scanners is far inferior to modern PET/CT scanners. Consequently, a coincidence scanner may be more likely to show a CMR in a given patient than a modern dedicated PET machine. Nevertheless, despite the diversity of trial designs used in these early studies, they supported the case for early response assessment and showed that larger scale prospective trials with well-defined cohorts of patients with similar types of lymphoma and therapies would be worthwhile. Results of the first of these larger more uniform trials are now becoming available.
Large studies of PET in early response assessment of high grade NHL
Haioun and colleagues from Paris[56] reported their experience of early interim PET in 90 patients with aggressive lymphoma, 94% of whom had DLBCL. The treatment delivered in the study was determined by patient age and IPI score. In 37 patients the IPI score was low and in 53 it was high. Patients aged over 60 received R-CHOP and those aged 60 or less received the more intensive ACVBP or ACE, or R-ACVBP. Thirty-six patients, who attained at least a conventionally determined PR and with age-adjusted IPI of 2–3, proceeded directly to high-dose therapy and autologous peripheral blood stem-cell transplantation. A prospectively defined PET scoring system was used in the study. All foci were scored separately for extent and intensity: 1, low; 2, moderate; or 3, high. A CR was defined as no increased uptake in any lesion, or grade 1 intensity and extent in a single lesion (equivalent to a CMR or MRU by previously described criteria). A non-CR was defined as one residual site (with an extent score of 1) associated with an intensity score of 2, or as 2 or more residual sites with any extent and intensity scores.
The authors found that early PET was negative in 54 patients and positive in 36 patients. After completion of induction therapy, 83% of PET-negative patients achieved a conventional CR compared to only 58% of PET-positive patients. Actuarial 2 years event-free survival was 82% for PET-negative and 43% for PET-positive patients respectively (P < 0.001). The 2-year overall survival was 90% and 61%, respectively (P = 0.006). The prognostic significance of early PET response was independent of IPI and identified patients at higher risk of treatment failure. Both early and late PET responses correlated well with event-free survival and overall survival.
Mikhaeel and colleagues from Guy's and St. Thomas’ Hospital, London, reported a study in “high-grade” NHL in which all 121 eligible patients had a baseline PET and a further PET after 2–3 cycles[33]. Conventional imaging response to treatment was not analyzed. The IPI score was not given but more than 50% had stage I or II disease. Histology was as follows: DLBCL 52%, mediastinal BCL 10%, “high-grade” B-cell lymphoma not otherwise specified 17% and T-cell lymphoma 10.7%. Treatment was with CHOP or CHOP-R in 74% of cases and consolidative RT was given in 39%. PET scans were scored visually as “negative”, “minimal residual uptake” (MRU, as described above) or “positive”. After 2–3 cycles of chemotherapy, 50 PET scans were negative, 19 showed MRU and 52 were scored as positive. Actuarial 5-year progression-free survival was 88.8% for PET negative, 59.3% for MRU and 16.2% for PET positive patients. Interim PET results were significantly correlated with both progression-free survival (P < 0.0001) and overall survival (P < 0.01). Early interim PET response was independent of B symptoms, age, stage, the presence of extranodal disease, and bulk. It was also the strongest single prognostic factor. One particularly interesting finding was that patients with MRU and stage III–IV disease had a similar poor prognosis to those who had positive interim PET scans but MRU patients with stage I–II disease had a good prognosis, similar to those with negative interim PET scans. This difference could be explained by the fact that most stage I–II patients received consolidative RT to known sites of lymphoma and RT may have eradicated residual localized chemotherapy-resistant disease.
Results of studies of PET in early response assessment in Hodgkin lymphoma
Hutchings and colleagues from Denmark investigated the prognostic significance of early PET response assessment in 77 patients with HL after two cycles of chemotherapy (principally ABVD)[57]. After two cycles, 61 patients had negative PET scans and 16 patients had positive scans. By the time of last follow-up, 11 of 16 FDG-PET-positive patients had progressed and two had died. Only three of 61 FDG-PET-negative patients progressed and all were alive. Early FDG-PET was very strongly correlated with progression-free survival (P < 0.0001) and overall survival (P < 0.01). For prediction of progression-free survival, early interim PET was as accurate after two cycles as was PET performed after four cycles, or at the end of treatment, and it was superior to CT assessment at all times. The authors found that both a SUVmax based response assessment and a visual assessment had prognostic significance. Receiver operating characteristics tables showed that a cut-off SUVmax of 4 gave the optimum balancing point between sensitivity and specificity for prediction of progression. Nevertheless, the visual reading of PET response after two cycles gave more useful prognostic information (HR 43.82, P < 0.0001) than the corresponding SUV measurement (HR 1.131, P = 0.001).
In a separate study, Hutchings, this time with colleagues from Guy's and St. Thomas’ Hospital, London, investigated early PET response in 85 patients with HL[58]. After two or three cycles of chemotherapy, 63 patients had negative FDG-PET scans, nine patients had MRU as defined above and 13 patients had positive scans. Three PET-negative (CMR) patients and one patient from the MRU group relapsed. In the PET-positive group, nine patients progressed and two died. Survival analyses showed highly significant associations between early interim PET and progression-free survival (P < 0.0001) and overall survival (P < 0.03). All advanced-stage patients with positive interim FDG-PET relapsed within 2 years. The authors concluded that an early interim PET scan is an accurate and independent predictor of progression-free survival and overall survival in HL and that a positive interim FDG-PET is highly predictive of relapse in advanced-stage disease.
Gallamini and colleagues from Cuneo, Italy, have more recently reported the results of a study of 108 patients with newly diagnosed HL who had stage IIA with adverse prognostic factors, or stage IIB–IVB, and were re-staged with FDG-PET after two cycles of ABVD chemotherapy[57]. Eighty-eight patients attained CR by conventional imaging while 20 showed disease progression during therapy or within 6 months after having reached CR; one patient relapsed. Interim PET was positive in 20 patients of whom 17 progressed during therapy, one relapsed and two remained in CR. By contrast, 85/88 (97%) patients with a negative PET-2 remained in CR; three progressed or relapsed early after the end of chemotherapy. Thus, the positive predictive value of a PET-2 for relapse was 90% and the negative predictive value was 97%. The sensitivity, specificity and overall accuracy of PET-2 were 86%, 98% and 95%, respectively. The 2-year probability of failure-free survival for PET-2 negative and for PET-2 positive patients was 96% and 6%, respectively (log rank test = 116.7, P < 0.01). It is important to note that, in this study, the early PET result did not influence therapy.
Discussion
The two recently published studies on early PET response in higher grade NHL from Paris and London and the three studies on early PET in HL, from the UK, Denmark and Italy, show striking similarities. All of these studies indicate that FDG-PET scanning after 2–3 cycles of chemotherapy provides valuable prognostic information at a time when changes could be made in the patient's first-line treatment protocol. This information appears to be as reliable, or more reliable than definitive response assessment at the end of therapy. A positive PET scan after 2–3 cycles of chemotherapy is a powerful negative prognostic factor in aggressive NHL and in HL and predicts for worse progression-free survival and overall survival in patients treated on current protocols. Clinical trials may be justified for such patients to investigate the effects of early intensification of therapy in an effort to improve their relatively poor outcomes with current treatment strategies. Such a trial has recently been initiated by the Australasian Leukaemia and Lymphoma Group (ALLG). On the other hand, it is not possible to say with confidence that a negative PET scan after 2–3 cycles of chemotherapy can be used to identify patients who have such a good prognosis that they could safely be given a less intensive form of treatment whilst maintaining their excellent prognosis. It is possible that this kind of information could eventually be derived from the results of future randomized studies of PET scanning performed very early in the treatment course. For example, these could involve PET response assessment after a single cycle of chemotherapy or even within a few days of commencing chemotherapy with patients then allocated to standard or less-aggressive treatment based on metabolic response. Further well-designed clinical trials will be required to investigate this possibility and any routine application of less intensive therapy in responsive patients must first be validated in well-designed randomized trials. The optimum time for early interim response requires investigation and could potentially vary between different histological types of lymphoma and with different treatment schedules. In some patients with localized disease, treatment with RT may overcome the negative prognostic effect of an incomplete PET response.
The best method for assessment of early PET response remains unclear. However, qualitative visual methods, for which the greatest weight of evidence exists, appear to be robust and highly predictive of outcomes. It is uncertain if a simple division into CMR or “not CMR” is the best qualitative approach or if the addition of the additional category of MRU enhances the value of PET. The results of the Guy's and St. Thomas’ Hospital group suggest that this latter category may have independent prognostic value. Our own schema, which includes a category with progressive metabolic disease, may help to flag those unfortunate patients with primary refractory disease who have an especially poor prognosis and who generally cannot be cured without high dose chemotherapy and stem-cell transplantation. There is limited evidence for the utility of SUV-based response assessment methodology in lymphoma, although it has clear value in malignancies that are less sensitive to chemotherapy, such as epithelial solid tumours. In the Danish HL study, visual response assessment was clearly better than SUV-based measurement when the two were compared. It is possible that integration of PET and structural imaging data might provide more information than PET alone but this approach may only be useful at the end of therapy when tumour masses have had enough time to shrink. Juweid and colleagues[59] have reported that a response classification, at the end of therapy, based on integration of FDG PET into the International Workshop Criteria (IWC) provides a more accurate response assessment than IWC alone in patients with NHL. The optimum method for combining PET and structural imaging information into an intuitive and simple response classification has, in the view of the authors of this review, not yet been attained. In the meantime, it will be important in clinical trials to identify whether clinical stage was defined using conventional techniques or involved a baseline PET scan and to also identify whether response rates were based on PET, IWC criteria or a combination of both. Since PET tends to be more sensitive than CT, its use will tend to suggest more advanced disease in PET-staged cohorts than those staged conventionally (Fig. 1) but will also tend to decrease the apparent time to progression in patients followed by PET in whom cure is not achieved (Fig. 3). Balanced against this, apparent response rates are likely to be superior compared to CT-assessment (Figs. 2 and 3).
Conclusion
There is now powerful evidence that early interim response assessment with FDG-PET provides invaluable prognostic information in patients with histologically aggressive NHL and HL treated with induction chemotherapy. Further clinical trials are needed to define the best way to use this important new prognostic factor in designing true response-adapted therapies. On the basis of existing evidence we recommend that patients with positive interim PET scans after 2–3 cycles of therapy should be considered for more aggressive treatment while CMR patients should continue with standard treatment at this time.
References
- 1.Jaffe ES, Harris NL, Vardiman JW, editors. World Health Organization classification of tumours. Lyon, France: IARC Press; 2001. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. [Google Scholar]
- 2.Raemaekers J, Kluin-Nelemans H, Teodorovic I, et al. The achievements of the EORTC Lymphoma Group. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;38(Suppl 4):S107–13. doi: 10.1016/s0959-8049(01)00446-4. [DOI] [PubMed] [Google Scholar]
- 3.Zinzani PL. Lymphoma: diagnosis, staging, natural history, and treatment strategies. Semin Oncol. 2005;32:S4–10. doi: 10.1053/j.seminoncol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Maloney DG. Immunotherapy for non-Hodgkin's lymphoma: monoclonal antibodies and vaccines. J Clin Oncol. 2005;23:6421–8. doi: 10.1200/JCO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Josting A, Sieniawski M, Glossmann JP, et al. High-dose sequential chemotherapy followed by autologous stem cell transplantation in relapsed and refractory aggressive non-Hodgkin's lymphoma: results of a multicenter phase II study. Ann Oncol. 2005;16:1359–65. doi: 10.1093/annonc/mdi248. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–52. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 7.Eghbali H, Raemaekers J, Carde P. The EORTC strategy in the treatment of Hodgkin's lymphoma. Eur J Haematol. 2005;(Suppl):135–40. doi: 10.1111/j.1600-0609.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 8.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339:1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 9.A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med; The International Non-Hodgkin's Lymphoma Prognostic Factors Project; 1993. pp. 987–94. [DOI] [PubMed] [Google Scholar]
- 10.Yahalom J. Transformation in the use of radiation therapy of Hodgkin lymphoma: new concepts and indications lead to modern field design and are assisted by PET imaging and intensity modulated radiation therapy (IMRT) Eur J Haematol. 2005;(Suppl):90–7. doi: 10.1111/j.1600-0609.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 11.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 12.MacManus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J Clin Oncol. 1996;14:1282–90. doi: 10.1200/JCO.1996.14.4.1282. [DOI] [PubMed] [Google Scholar]
- 13.Carde P, Koscielny S, Franklin J, et al. Early response to chemotherapy: a surrogate for final outcome of Hodgkin's disease patients that should influence initial treatment length and intensity? Ann Oncol. 2002;13(Suppl 1):86–91. doi: 10.1093/annonc/13.s1.86. [DOI] [PubMed] [Google Scholar]
- 14.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–6. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 16.Naumann R, Vaic A, Beuthien-Baumann B, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin's disease and non-Hodgkin's lymphoma. Br J Haematol. 2001;115:793–800. doi: 10.1046/j.1365-2141.2001.03147.x. [DOI] [PubMed] [Google Scholar]
- 17.Rini JN, Leonidas JC, Tomas MB, Palestro CJ. 18F-FDG PET versus CT for evaluating the spleen during initial staging of lymphoma. J Nucl Med. 2003;44:1072–4. [PubMed] [Google Scholar]
- 18.Jerusalem G, Hustinx R, Beguin Y, Fillet G. Positron emission tomography imaging for lymphoma. Curr Opin Oncol. 2005;17:441–5. doi: 10.1097/01.cco.0000174041.29557.5c. [DOI] [PubMed] [Google Scholar]
- 19.Munker R, Glass J, Griffeth LK, et al. Contribution of PET imaging to the initial staging and prognosis of patients with Hodgkin's disease. Ann Oncol. 2004;15:1699–704. doi: 10.1093/annonc/mdh426. [DOI] [PubMed] [Google Scholar]
- 20.Hicks RJ, MacManus MP, Seymour JF. Initial staging of lymphoma with positron emission tomography and computed tomography. Semin Nucl Med. 2005;35:165–75. doi: 10.1053/j.semnuclmed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Blum RH, Seymour JF, Wirth A, MacManus M, Hicks RJ. Frequent impact of [18F]fluorodeoxyglucose positron emission tomography on the staging and management of patients with indolent non-Hodgkin's lymphoma. Clin Lymphoma. 2003;4:43–9. doi: 10.3816/clm.2003.n.013. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez M, Rehn S, Ahlstrom H, Sundstrom C, Glimelius B. Predicting malignancy grade with PET in non-Hodgkin's lymphoma. J Nucl Med. 1995;36:1790–6. [PubMed] [Google Scholar]
- 23.Schoder H, Noy A, Gonen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:4643–51. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 24.Even-Sapir E, Israel O. Gallium-67 scintigraphy: a cornerstone in functional imaging of lymphoma. Eur J Nucl Med Mol Imaging. 2003;30(Suppl 1):S65–81. doi: 10.1007/s00259-003-1164-7. [DOI] [PubMed] [Google Scholar]
- 25.Friedberg JW, Fischman A, Neuberg D, et al. FDG-PET is superior to gallium scintigraphy in staging and more sensitive in the follow-up of patients with de novo Hodgkin lymphoma: a blinded comparison. Leuk Lymphoma. 2004;45:85–92. doi: 10.1080/1042819031000149430. [DOI] [PubMed] [Google Scholar]
- 26.Wirth A, Seymour JF, Hicks RJ, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography, gallium-67 scintigraphy, and conventional staging for Hodgkin's disease and non-Hodgkin's lymphoma. Am J Med. 2002;112:262–8. doi: 10.1016/s0002-9343(01)01117-2. [DOI] [PubMed] [Google Scholar]
- 27.Naumann R, Beuthien-Baumann B, Reiss A, et al. Substantial impact of FDG PET imaging on the therapy decision in patients with early-stage Hodgkin's lymphoma. Br J Cancer. 2004;90:620–5. doi: 10.1038/sj.bjc.6601561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zijlstra JM, Hoekstra OS, Raijmakers PG, et al. 18FDG positron emission tomography versus 67Ga scintigraphy as prognostic test during chemotherapy for non-Hodgkin's lymphoma. Br J Haematol. 2003;123:454–62. doi: 10.1046/j.1365-2141.2003.04617.x. [DOI] [PubMed] [Google Scholar]
- 29.Miles KA, Griffiths MR, Keith CJ. Blood flow-metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:22–8. doi: 10.1007/s00259-005-1932-7. [DOI] [PubMed] [Google Scholar]
- 30.Rajendran JG, Wilson DC, Conrad EU, et al. [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 31.Spaepen K, Stroobants S, Dupont P, et al. [(18)F]FDG PET monitoring of tumour response to chemotherapy: does [(18)F]FDG uptake correlate with the viable tumour cell fraction? Eur J Nucl Med Mol Imaging. 2003;30:682–8. doi: 10.1007/s00259-003-1120-6. [DOI] [PubMed] [Google Scholar]
- 32.Crippa F, Seregni E, Agresti R, et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998;25:1429–34. doi: 10.1007/s002590050319. [DOI] [PubMed] [Google Scholar]
- 33.Mikhaeel NG, Hutchings M, Fields PA, O'Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–23. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- 34.MacManus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 35.Yamane T, Daimaru O, Ito S, et al. Decreased 18F-FDG uptake 1 day after initiation of chemotherapy for malignant lymphomas. J Nucl Med. 2004;45:1838–42. [PubMed] [Google Scholar]
- 36.Hoekstra CJ, Paglianiti I, Hoekstra OS, et al. Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med. 2000;27:731–43. doi: 10.1007/s002590050570. [DOI] [PubMed] [Google Scholar]
- 37.Hoekstra CJ, Hoekstra OS, Stroobants SG, et al. Methods to monitor response to chemotherapy in non-small cell lung cancer with 18F-FDG PET. J Nucl Med. 2002;43:1304–9. [PubMed] [Google Scholar]
- 38.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 39.Hoekstra CJ, Stroobants SG, Smit EF, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:8362–70. doi: 10.1200/JCO.2005.01.1189. [DOI] [PubMed] [Google Scholar]
- 40.Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–48. [PubMed] [Google Scholar]
- 41.Hutchings M, Eigtved AI, Specht L. FDG-PET in the clinical management of Hodgkin lymphoma. Crit Rev Oncol Hematol. 2004;52:19–32. doi: 10.1016/j.critrevonc.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Panizo C, Perez-Salazar M, Bendandi M, et al. Positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual Hodgkin's disease mediastinal masses. Leuk Lymphoma. 2004;45:1829–33. doi: 10.1080/1042819042000223813. [DOI] [PubMed] [Google Scholar]
- 43.Guay C, Lepine M, Verreault J, Benard F. Prognostic value of PET using 18F-FDG in Hodgkin's disease for posttreatment evaluation. J Nucl Med. 2003;44:1225–31. [PubMed] [Google Scholar]
- 44.Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin's lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–9. doi: 10.1200/JCO.2001.19.2.414. [DOI] [PubMed] [Google Scholar]
- 45.Filmont JE, Czernin J, Yap C, et al. Value of F-18 fluorodeoxyglucose positron emission tomography for predicting the clinical outcome of patients with aggressive lymphoma prior to and after autologous stem-cell transplantation. Chest. 2003;124:608–13. doi: 10.1378/chest.124.2.608. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer NG, Hany TF, Taverna C, et al. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging – do we need contrast-enhanced CT? Radiology. 2004;232:823–9. doi: 10.1148/radiol.2323030985. [DOI] [PubMed] [Google Scholar]
- 47.Zinzani PL, Fanti S, Battista G, et al. Predictive role of positron emission tomography (PET) in the outcome of lymphoma patients. Br J Cancer. 2004;91:850–4. doi: 10.1038/sj.bjc.6602040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cremerius U, Fabry U, Neuerburg J, et al. Prognostic significance of positron emission tomography using fluorine-18-fluorodeoxyglucose in patients treated for malignant lymphoma. Nuklearmedizin. 2001;40:23–30. [PubMed] [Google Scholar]
- 49.Mikhaeel NG, Timothy AR, Hain SF, O'Doherty MJ. 18-FDG-PET for the assessment of residual masses on CT following treatment of lymphomas. Ann Oncol. 2000;11(Suppl 1):147–50. [PubMed] [Google Scholar]
- 50.Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood. 1999;94:429–33. [PubMed] [Google Scholar]
- 51.Torizuka T, Nakamura F, Kanno T, et al. Early therapy monitoring with FDG-PET in aggressive non-Hodgkin's lymphoma and Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging. 2004;31:22–8. doi: 10.1007/s00259-003-1333-8. [DOI] [PubMed] [Google Scholar]
- 52.Schot B, van Imhoff G, Pruim J, Sluiter W, Vaalburg W, Vellenga E. Predictive value of early 18F-fluoro-deoxyglucose positron emission tomography in chemosensitive relapsed lymphoma. Br J Haematol. 2003;123:282–7. doi: 10.1046/j.1365-2141.2003.04593.x. [DOI] [PubMed] [Google Scholar]
- 53.Romer W, Hanauske AR, Ziegler S, et al. Positron emission tomography in non-Hodgkin's lymphoma: assessment of chemotherapy with fluorodeoxyglucose. Blood. 1998;91:4464–71. [PubMed] [Google Scholar]
- 54.Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin's lymphoma. Ann Oncol. 2002;13:1356–63. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- 55.Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin's disease. J Nucl Med. 2002;43:1018–27. [PubMed] [Google Scholar]
- 56.Haioun C, Itti E, Rahmouni A, et al. [18F]Fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–81. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 57.Hutchings M, Loft A, Hansen MT, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin Lymphoma. Blood. 2005;107:52–9. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 58.Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16:1160–8. doi: 10.1093/annonc/mdi200. [DOI] [PubMed] [Google Scholar]
- 59.Juweid ME, Stroobants S, Hoekstia OS, et al. Use of position emission tomography for response assessment of lymphoma: Consensus of the Imaging subcommittee of International Harmonization project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]