Abstract
Spinal γ-aminobutyric acid (GABA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors have been implicated in the mechanisms of neuropathic pain after nerve injury; however, how these two receptors interact at the spinal level remains unclear. Here we show that intrathecal midazolam through activation of spinal GABAA receptors attenuated the expression and function of spinal AMPA receptors in rats following peripheral nerve injury. Thermal hyperalgesia and mechanical allodynia induced by chronic constriction nerve injury (CCI) in rats were attenuated by the short-acting benzodiazepine midazolam (20=10>5 μg > vehicle) administered intrathecally once daily for seven postoperative days. CCI-induced upregulation of AMPA receptors within the spinal cord dorsal horn was also significantly reduced by the intrathecal midazolam (10, 20 μg) treatment. The inhibitory effects of midazolam (10, 20 μg) on neuropathic pain behaviors and AMPA receptor expression were prevented by co-administration of midazolam with the GABAA receptor antagonist bicuculline (3 μg), whereas intrathecal treatment with bicuculline (1 or 3 μg) alone in naive rats induced the upregulation of spinal AMPA receptor expression and nociceptive responses, indicating a tonic regulatory effect from endogenous GABAergic activity on the AMPA receptor expression and spinal nociceptive processing. These results indicate that modulation of spinal AMPA receptor expression and function by the GABAergic activity may serve as a mechanism contributory to the spinal nociceptive processing.
Keywords: AMPA receptor, GABA receptor, Midazolam, Nerve injury, Neuropathic pain, Intrathecal
Introduction
Neuropathic pain after peripheral nerve injury is a chronic pain condition, which remains very difficult to treat. Spinal glutamatergic and γ-aminobutyric acid (GABA)ergic systems are among several proposed mechanisms of neuropathic pain and have been extensively investigated over the last two decades (Dubner, 1991; Dougherty and Willis, 1991; Yamamoto and Yaksh, 1992; Mao et al., 1995; Woolf and Mannion, 1999; Hammond 2001). Evidence exists indicating that either activation of the glutamatergic system or a possible loss of GABAergic activity, or both, after peripheral nerve injury contributes to the development and maintenance of neuropathic pain behaviors in rats (Mao et al., 1995; 1997; Bridges et al., 2001; Cronin et al., 2004; Polgar et al., 2005; Scholz et al., 2005). These earlier studies suggest a possible interaction between these regulatory systems within the central nervous system.
Ionotropic glutamate receptors including alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors are localized both presynaptically and postsynaptically within superficial laminae of spinal cord dorsal horn. AMPA receptors have been shown to play a significant role in the mechanisms of neuropathic pain and spinal nociceptive processing (Mao et al., 1992a,b; Leem et al., 1996; Kondo et al, 2002; Garry et al., 2003). The AMPA receptor consists of several subunits and previous studies have indicated that spinal AMPA receptor expression was altered after either peripheral nerve injury or inflammation, which contributed to the development of pathological pain conditions in rats (Harris et al., 1996; Alvarez et al., 2000).
On the other hand, it has been demonstrated that activation of spinal GABAergic system negatively regulated spinal nociceptive processing (Kontinen and Dickenson, 2000; Hammond 2001; Nishiyama et al., 2003), suggesting an important role for the GABAergic system in the balance between spinal excitatory and inhibitory elements after peripheral nerve injury. Benzodiazepines activate GABAA receptors, reduce excitatory transmitter release presynaptically as well as excitatory activity postsynaptically in spinal dorsal horn neurons (Haefely, 1988). Indeed, intrathecal midazolam (a benzodiazepine-GABAA receptor agonist) attenuated neuropathic pain behaviors (Hwang and Yaksh, 1997). Recently, presynaptic AMPA receptors on GABAergic terminals have been shown to have a bidirectional role in neuronal activity with the superficial spinal cord dorsal horn and contributed to the mechanisms of central sensitization and hyperalgesia (Lu et al, 2005). Moreover, the effect on GABA receptors may interact with that of AMPA receptor antagonists in rats (Nishiyama et al, 1999). Thus, it is possible that there might be interactions at the spinal level between the GABA and AMPA receptors and such interactions may have a functional role in neuropathic pain behaviors.
Utilizing a rat model of chronic constriction nerve injury (CCI) (Bennett and Xie, 1988), we examined whether midazolam (a clinically available short-acting benzodiazepine and GABA analogue) given intrathecally would modulate the expression of spinal AMPA receptors and neuropathic pain behaviors in CCI rats through activation of spinal GABAA receptors.
Material and Methods
CCI model
Adult male Sprague Dawley rats weighing 275–325 gm (Charles River Laboratories, Wilmington, MA) were used. The animal room was artificially lighted from 7 AM to 7 PM. The experimental protocol was approved through our Institutional Animal Care and Use committee. Rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally). CCI was produced by loosely ligating one common sciatic nerve (using 4-0 chromic gut sutures, 4 ligatures) according to the method of Bennett and Xie (1988), and sham operation was performed following the same surgical procedure for CCI except for nerve ligation.
Intrathecal catheterization and drug delivery
An intrathecal catheter was implanted in each rat under the same surgical condition. A PE-10 catheter was inserted onto the lumbar enlargement (about 8.5 cm from the incision site for this rat size) according to the method described previously (Yaksh and Rudy, 1976; Mao et al., 2002). Those rat exhibiting postoperative neurological deficits (e.g., paralysis) or poor grooming and eating were excluded from experiments as described previously (Mao et al., 2002). Midazolam was purchased from Sigma (St. Louis, MO) and bicuculline and S-AMPA were purchased from Tocris (Ellisville, MO). Bicuculline was dissolved in 10% DMSO diluted in normal saline (vehicle) and S-AMPA was dissolved in normal saline. Midazolam was diluted in normal saline as indicated. All drugs were injected intrathecally in a 10-μl volume followed by a 10-μl saline flush. The doses for each agent used in this study are: 1) midazolam (a GABA agonist, 5, 10, 20 μg), bicuculline (a GABAA receptor antagonist, 0.3, 1, 3 μg), S-AMPA (an AMPA receptor agonist, 0.1 μg).
Behavioral test and statistical analysis
Rats were habituated to the test environment (one 60 min session per day) for two consecutive days before the baseline testing. Testing for thermal hyperalgesia was performed according to a previously published method (Hargreaves et al., 1988). Radian heat was adjusted to ensure the baseline latency at 10–12 sec and the cutoff time of 20 sec. At least two trials were made in each rat. Mechanical allodynia was examined by applying a von Frey filament to the plantar surface of each hindpaw until the filament was bended and threshold force was established by examining the bending force that resulted in at least two clear paw-withdrawals out of five applications (Tal and Bennett, 1994). The cutoff force was 20 gm. All testing was conducted between 9 AM and 1 PM.
Data from the thermal hyperalgesia (withdrawal latency in sec) test were analyzed by using repeated measure two-way ANOVA across testing time points to detect overall differences among treatment groups. Whenever applicable, the data were also examined by using repeated measure two-way ANOVA across treatment group to examine overall difference among testing time points. When significant main effects were observed, post hoc Newman-Keuls’s test were followed. Data from the mechanical allodynia (threshold bending force in gm) test were analyzed using the non-parametric Mann-Whitney test. The statistically significant level was set at α = 0.05.
Western blot
Rats were decapitated rapidly (<1 min) under pentobarbital anesthesia and lumbar spinal cord segments (lumbar 5) were removed. Spinal segments were separated into the ipsilateral and contralateral side as well as the dorsal and ventral horn and homogenized in SDS sample buffer containing a mixture of proteinase inhibitors (Sigma). Lumbar segments were harvested because CCI has a major impact at these sites. Protein samples were separated on SDS-PAGE gel (4–15% gradient gel; Bio-Rad, Herculed, CA) and transferred to polyvinylidene difluoride filters (Millipore, Bedford, MA). The filters were blocked with 3% milk and incubated overnight at 4 °C with a primary antibody (GluR1 - rabbit polyclonal, 1:100, Chemicon, Temecula, CA; GluR2 - mouse monoclonal, 1:250, BD, Franklin Lakes, NJ; GluR3 - mouse monoclonal, 1:300, Chemicon) and then 1 hr at room temperature with HRP-conjugated secondary antibody (1:700, Amersham, Arlington Heights, IL). The blots were visualized in ECL solution (NEN, Boston, MA) for 1 min and exposed onto hyperfilms (Amersham) for 1–10 min. The blots were then incubated in a stripping buffer (67.5 mM Tris, pH 6.8, 2% SDS, 0.7% β-mercaptoethanol) for 30 min at 50 °C and reprobed with a polyclonal rabbit anti-β-actin antibody (1:20,000; Alpha Diagnostic International, San Antonio, TX) as loading control. The Western analysis was made in triplicates. The density of a specific band was measured against a corresponding loading control, and differences were compared using repeated measure one-way ANOVA followed by post hoc Newman-Keuls’s tests.
Results
Effects of midazolam on neuropathic pain behaviors: reduction by bicuculline
The effects of midazolam on neuropathic pain behaviors were examined in seven groups of rats including (1) CCI plus vehicle, (2 – 4) CCI plus 5, 10 or 20 μg midazolam, (5) CCI plus 10 μg midazolam and 3 μg bicuculline, (6) sham plus 10 μg midazolam, and (7) sham plus vehicle. Each agent was given once daily (intrathecally) for seven consecutive postoperative days, beginning immediately after operation. Midazolam (20 = 10μg > 5μg>vehicle) reduced thermal hyperalgesia and mechanical allodynia in the hindpaw ipsilateral to CCI, as compared with vehicle-treated CCI rats on all of postoperative days (Fig. 1, p< 0.05; n=6–7). In contrast, midazolam (10 or 20 μg) did not change baseline thermal and mechanical nociceptive responses in sham rats, nor did midazolam (5–20 μg) change thermal and mechanical nociceptive responses in the hindpaw contralateral to CCI (Fig. 1, P> 0.05; n=5–7).
Figure 1. Effects of midazolam and bicuculline on mechanical allodynia and thermal hyperalgesia of CCI rat.
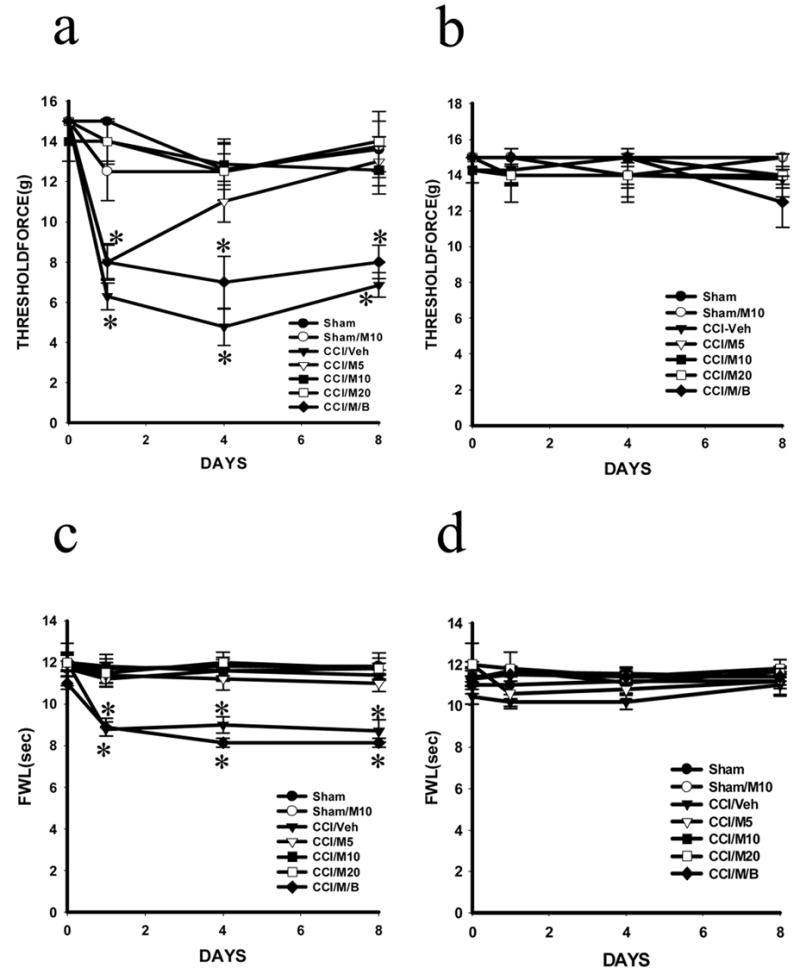
Mechanical allodynia (a, b) was dose-dependently attenuated in the ipsilateral hindpaw (a) in CCI rats treated with midazolam (5, 10 and 20 μg; CCI/M5, CCI/M10 and CCI/M20) but not vehicle (CCI/Veh) given intrathecally once daily for postoperative day 1–7. Thermal hyperalgesia (c, d) were attenuated in the ipsilateral hindpaw (c) in CCI rats treated with midazolam (same as above) but not vehicle (CCI/Veh) given intrathecally once daily for postoperative day 1–7. Midazolam did not alter baseline nociceptive response in sham rats (Sham/M10). There were no changes in nociceptive responses in the contralateral hindpaw (b, d). The attenuation by midazolam (10 μg) of mechanical allodynia (a) and thermal hyperalgesia (c) in the ipsilateral hindpaw of CCI rats was reduced by bicuculline (3 μg; CCI/M/B), given intrathecally once daily for postoperative day 1–7. The combined treatment with midazolam and bicuculline did not alter the nociceptive responses in the contralateral hindpaw * P< 0.05 as compared with the corresponding sham group. FWL: foot-withdrawal latency; Threshold force: a measurement for mechanical allodynia (also in other figures). For all figures, the number of rats per group was shown in the main text.
The effects of midazolam on neuropathic pain behaviors were primarily mediated through the GABAA receptor, because co-administration of midazolam (10 μg) with the GABAA receptor antagonist bicuculline (3 μg), given once daily for postoperative day 1–7, reversed the attenuation of thermal hyperalgesia and mechanical allodynia by midazolam (Fig. 1, P< 0.05, n=5–7). The bicuculline dose was referenced from previous studies (Hwang and Yaksh, 1997; Kaneko and Hammond, 1997; Malan et al., 2002) and a lower dose of bicuculline (0.3 μg) was ineffective in our pilot experiment. The combined treatment of midazolam (10 μg) and bicuculline (3 μg) did not alter the thermal and mechanical nociceptive responses in the hindpaw contralateral to CCI (Fig. 1, p>0.05; n=5–7). These results indicate that intrathecal midazolam attenuated neuropathic pain behaviors in CCI rats, which was mainly mediated through the GABAA receptor within the spinal cord dorsal horn.
Effects of midazolam on spinal AMPA receptor expression: reduction by bicuculline
The effects of midazolam on the expression of spinal AMPA receptors after CCI were examined using Western blot. CCI but not sham operation induced an upregulation of AMPA receptors (GluR1, GluR2, GluR3) within the spinal cord dorsal horn ipsilateral to CCI on postoperative day 4 and 8 (not day 1) (Fig. 2, p< 0.05, n=4–6), while there were no significant changes in the AMPA receptor expression within the contralateral spinal cord dorsal horn (data not shown). Intrathecal treatment with midazolam (10 = 20 μg, but not 5μg, once daily for seven postoperative days) reduced the upregulation of AMPA receptors in CCI rats as compare with CCI rats treated with a vehicle (Fig. 3, p<0.05, n=4–6). Similar to its effects on neuropathic pain behaviors, the GABAA receptor antagonist bicuculline reduced the effect of midazolam on spinal AMPA receptor expression when bicuculline (3 μg) was co-administered intrathecally with midazolam (10μg) once daily for postoperative day 1–7 (Fig. 4, p< 0.05, n=5–6). These results indicate that the upregulation of spinal AMPA receptor after CCI was mediated at least in part through the GABAA receptor within the spinal cord dorsal horn.
Figure 2. Time course of AMPA receptor expression after CCI.
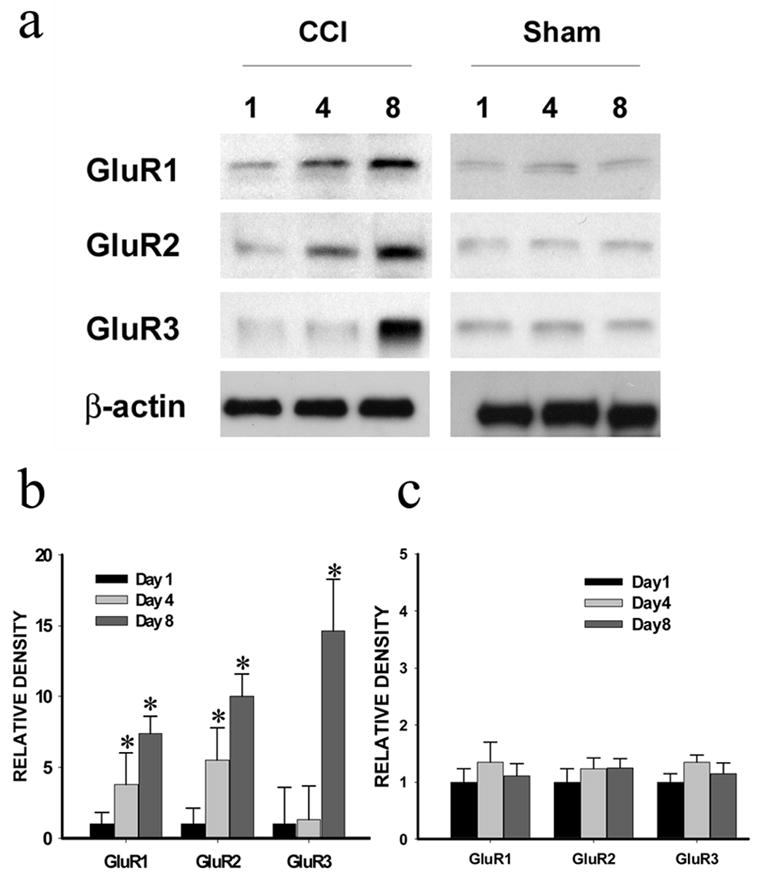
The AMPA receptor (GluR1, GluR2, GluR3) expression (Western blot) within the ipsilateral spinal cord dorsal horn was upregulated in CCI rats but not in sham rats on postoperative day 4 and 8 (not day 1). a: Western blots; β-actin is a loading control. B, c: Statistical data analysis. * P < 0.05 as compared with the corresponding sham control.
Figure 3. Effects of midazolam on spinal AMPA receptor expression.
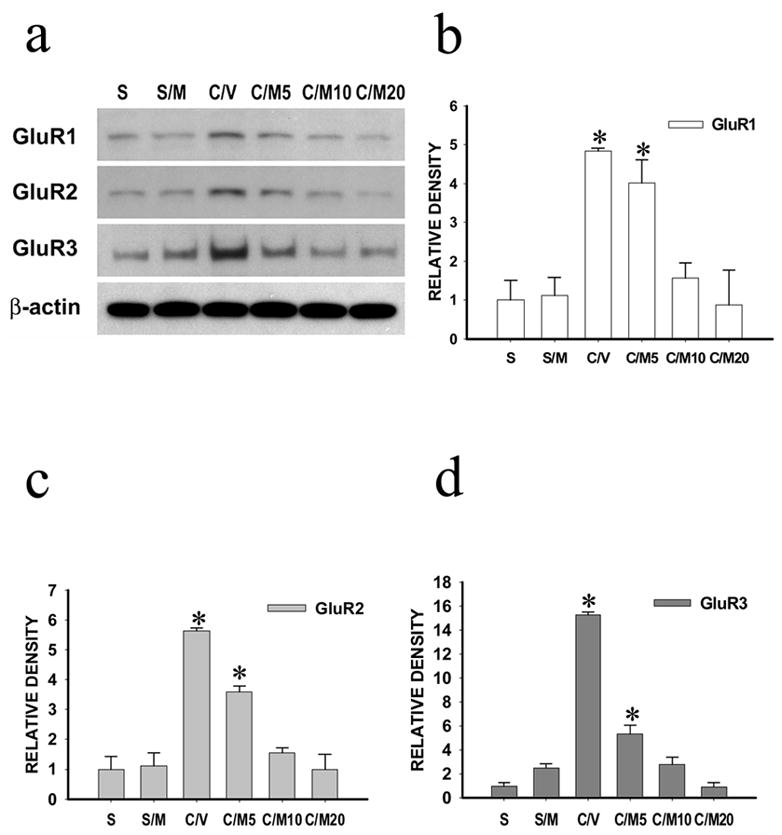
The AMPA receptor (GluR1, GluR2, GluR3) expression (Western blot) within the ipsilateral spinal cord dorsal horn was upregulated in CCI rats, which was reduced by intrathecal midazolam (10 or 20 μg, not 5μg) given once daily for postoperative day 1–7. Samples were taken on day 8. a: Western blots; β-actin is a loading control. b-d: Statistical data analysis. S: sham; S/M; sham rats with 10 μg midazolam; C/M5, C/M10 or C/M20: CCI rats with 5, 10 or 20 μg midazolam; C/V: CCI rats with vehicle. * P < 0.05 as compared with the corresponding sham group.
Figure 4. Effects of bicuculline on spinal AMPA receptor expression.

The effect of midazolam (10 μg) on the AMPA-R expression within the ipsilateral spinal cord dorsal horn after CCI was attenuated by co-administration of bicuculline (3 μg) once daily for seven days. Samples were taken on day 8. C/M/B: CCI rats with midazolam and bicuculline; C/V: CCI rats with vehicle. * P< 0.05 as compared with the corresponding sham group.
Effects of bicuculline on nociceptive behaviors and spinal AMPA receptor expression in naïve rats
In order to examine whether inhibition of GABAA receptor activity within the spinal cord would alter thermal and mechanical nociceptive responses as well as spinal AMPA receptor expression in naïve rats, four groups of rats were used including naïve rats treated with 0.3, 1 or 3 μg bicuculline. Each dose was given once daily to naïve rats for seven consecutive days. Bicuculline (3 μg but not 0.3 μg or 1μg) induced nociceptive responses to thermal and mechanical stimulation in naïve rats (Fig. 5, P< 0.05, n=5), similar to that observed after CCI. Interestingly, bicuculline (3 μg but not 0.3 or 1μg) treatment also induced an upregulation of AMPA receptors (GluR1, GluR2, GluR3) within the spinal cord dorsal horn of naïve rats in the absence of CCI (Fig. 6, p< 0.05, n=5). Collectively, these results demonstrate a tonic influence from spinal GABA activity on both AMPA receptor expression and nociceptive responses.
Figure 5. Effect of bicuculline on nociceptive behaviors in naïve rats.

Intrathecal treatment with bicuculline (3 μg but not 0.3 μg and 1μg) given once daily for seven days induced nociceptive responses to mechanical (a) and thermal (b) stimulation. The behavioral tests were made on day 8. * P< 0.05 as compared with the naïve group.
Figure 6. Effect of bicuculline on spinal AMPA receptor expression in naïve rats.
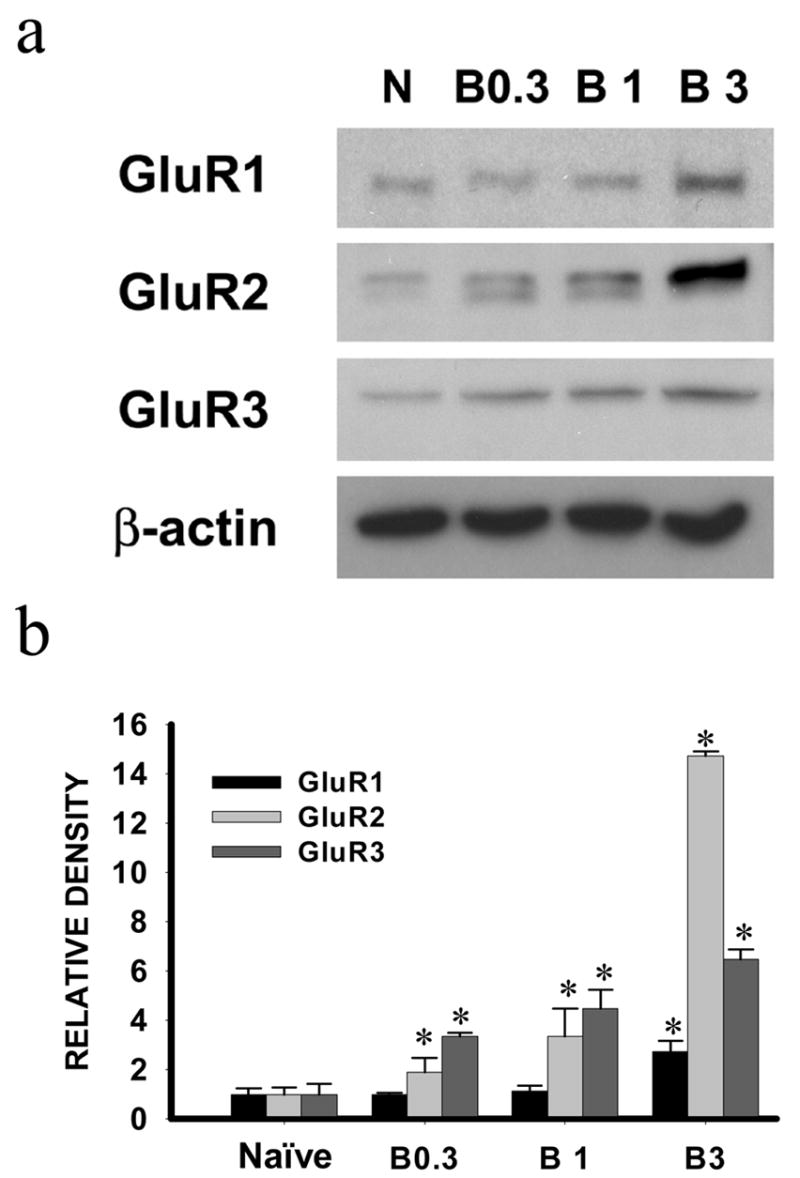
Intrathecal treatment with bicuculline (3 μg but not 0.3 or 1μg) given once daily for seven days induced the AMPA receptor (GluR1, GluR2, GluR3) upregulation within the spinal cord dorsal horn of naïve rats. Samples were taken on day 8. a: Western blot; b: statistical analysis. * P< 0.05 as compared with the naïve rat group.
Effects of midazolam on nociceptive behaviors induced by S-AMPA in naïve rats
In order to confirm the modulatory effect of spinal GABA activity on AMPA receptor-mediated nociceptive behaviors, the effects of midazolam on nociceptive responses induced by an exogenous AMPA receptor agonist (S-AMPA) were examined in the following groups of naïve rats including (1) 0.1 μg S-AMPA alone, (2) 0.1 μg S-AMPA and 10 μg midazolam, (3) vehicle alone. S-AMPA dose was referenced from a previous study (Kontien and Meert, 2002). Each agent was given intrathecally once daily for seven consecutive days.
S-AMPA (0.1 μg) induced nociceptive responses to thermal or mechanical stimulation, as compared with the vehicle group, when examined on day 8 (Fig. 7, P< 0.05, n=5–7). The S-AMPA-induced nociceptive behaviors were attenuated by intrathecal co-administration of S-AMPA (0.1 μg) with midazolam (10 μg) once daily for seven days, when also examined on day 8 (Fig. 7, P< 0.05, n=5–7). Midazolam (10 μg) alone did not alter nociceptive behaviors in sham rats (see Fig. 1). Moreover, S-AMPA (0.1 μg) induced an upregulation of AMPA receptor (GluR1, GluR2, GluR3) within the spinal cord dorsal horn of naïve rats when examined on day 8, which was also significantly reduced by co-administering midazolam (10 μg) with S-AMPA for seven days (Fig 8, p< 0.05, n=5–7). Similar to that seen in sham rats (Fig. 3), Midazolam (10 μg) alone did not change the baseline AMPA receptor expression (data not shown). These results support a functional relationship between spinal GABA activity and AMPA receptor-mediated nociceptive responses in naïve rats as well.
Figure 7. Effect of S-AMPA and midazolam on nociceptive behaviors in naïve rats.
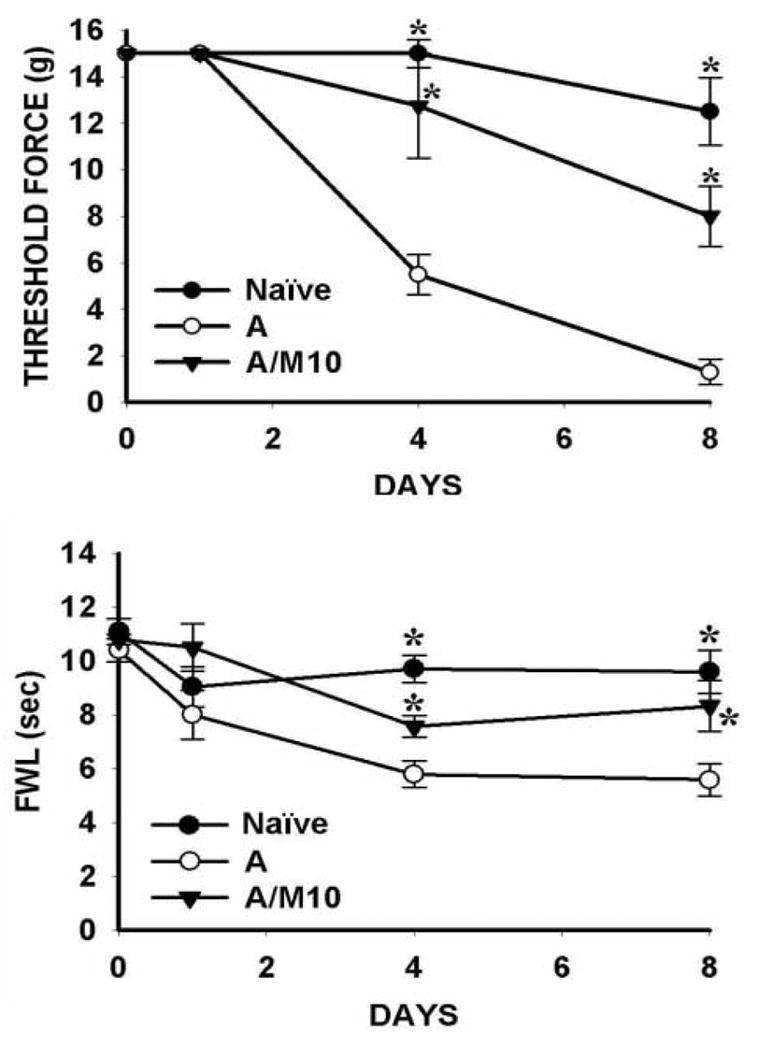
Intrathecal treatment with the AMPA receptor agonist S-AMPA (0.1 μg) given once daily for seven days induced nociceptive responses to mechanical (a) and thermal (b) stimulation, when examined on day 8. The S-AMPA-induced nociceptive responses were attenuated by intrathecal co-administration of S-AMPA (0.1 μg) with midazolam (10 μg) given once daily for seven days. * P< 0.05 as compared with the S-AMPA alone group. A/M: S-AMPA+midazolam; A: S-AMPA alone.
Figure 8. Effect of S-AMPA and midazolam on spinal AMPA receptor expression in naïve rats.
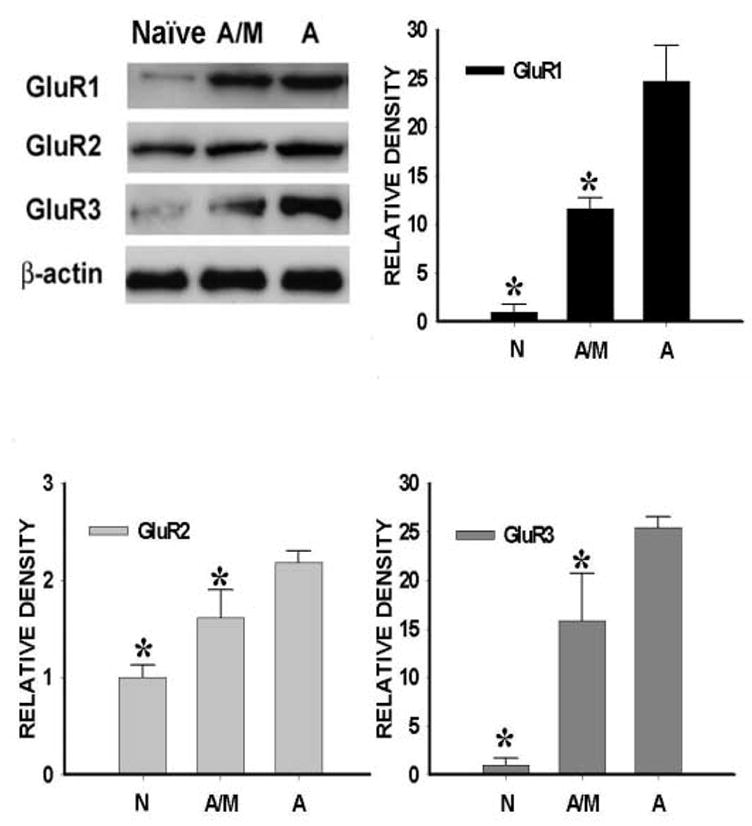
The AMPA receptor upregulation induced by S-AMPA (0.1 μg) was attenuated by intrathecal co-administration of S-AMPA (0.1 μg) with midazolam (10 μg) given once daily for seven days. Samples were taken on day 8. a: Western blot; b–d: statistical analysis. * P< 0.05 as compared with the S-AMPA treated group. A/M: S-AMPA+midazolam; A: S-AMPA alone.
Discussion
We have demonstrated that 1) intrathecal treatment with midazolam attenuated the development of thermal hyperalgesia and mechanical allodynia in CCI rats, which was mediated primarily through spinal GABAA receptors, 2) CCI induced an upregulation of spinal AMPA receptor (GluR1, GluR2, and GluR3 subunits) expression, which was also attenuated by midazolam through activation of spinal GABAA receptors, 3) inhibition of spinal GABAA receptors with bicuculline in naïve rats induced an upregulation of spinal AMPA receptor expression and nociceptive responses to thermal and mechanical stimulation, and 4) activation of spinal AMPA receptors with S-AMPA (an exogenous AMPA receptor agonist) induced both nociceptive responses and the upregulation of spinal AMPA receptor expression in naïve rats, preventable by intrathecal midazolam treatment. These results indicate a functional relationship between spinal GABAA and AMPA receptors under both physiological and pathological (nerve injury) conditions, which contributed to the development of neuropathic pain behaviors in rats.
A technical concern is an appropriate intrathecal dose range of midazolam. Previous studies have shown that the effect of intrathecal midazolam on nociceptive behaviors was dose-dependent such that a low dose range (2–4 μg) did not produce significant antinociceptive effects (Taira et al., 2000), whereas a high dose range (10–100 μg) was effective in antinociception (Nishiyama et al., 1999). Previous studies have also shown that intrathecal 30 μg midazolam induced motor dysfunction (Nishiyama et al., 1999) and midazolam at an even higher dose (e.g., 100 μg/day for 20 consecutive days) caused spinal neurotoxicity (Svenson et al., 1995). Considering these potential confounding factors, we used a lower but effective dose range (5, 10, 20 μg) in the present studies and did not observe any motor dysfunction and abnormal behavioral responses in both sham and CCI rats.
The role of spinal AMPA receptors in neuropathic pain behaviors after nerve injury, as demonstrated in the present study, is consistent with previous studies (Mao et al., 1992a,b; Leem et al., 1996; Kondo et al, 2002; Garry et al., 2003). For instance, activation of spinal AMPA receptors has been shown to induce nociceptive behaviors including thermal hyperalgesia (Yamamoto and Sakashita, 1998; Menéndez et al., 2003) and vocalization (Kontinen and Meert, 2002). Our previous study has indicated that non-NMDA receptors within the spinal cord are necessary for the development of neuropathic pain behaviors after CCI, because inhibition of non-NMDA receptors alone prevented the development of thermal hyperalgesia and spontaneous pain behaviors in CCI rats (Mao et al., 1992a,b). The role of non-NMDA receptor activation in this process may be in part related to its ability to depolarize neuronal membrane potentials thereby facilitating the activation of NMDA receptors (Dubner, 1991; Mao, et al., 1995; Garry et al., 2003).
GABA is an important inhibitory neurotransmitter (Sosnowski and Yaksh, 1990; Hammond 2001). Spinal GABAergic activity mediates synaptic inhibition and diminishes AMPA receptor-mediated acute nociception (Nishiyama et al., 1998), thereby modulating spinal nociceptive processing (Simmons et al., 1998). A growing body of evidence has suggested a functional relationship between GABA and AMPA receptor-mediated activities in spinal nociceptive processing (Izzo et al., 2001; Kovacs et al., 2003; Sanchez et al., 2005). For example, there is a presynaptic modulatory role of AMPA receptors in the GABA release (Engelman et al., 2006) and midazolam regulates excitatory transmission in spinal cord dorsal horn neurons of adult rats (Kohno et al., 2006). In addition, colocalization between GABA and AMPA receptors has been demonstrated in several spinal and supraspinal regions (Kondo et al., 1995; Kerr et al., 1998; Bergersen et al., 2003), and ionotropic glutamate receptors are expressed in GABAergic terminals in the rat superficial dorsal horn (Lu et al., 2006).
In the present study, a short-acting benzodiazepine (midazolam) effectively prevented the development of neuropathic pain behaviors in CCI rats after repeated administration, which was blocked by the GABAA receptor antagonist bicuculline. These data provides additional evidence for a regulatory mechanism regarding the expression and function of spinal AMPA receptors through activation of spinal GABAA receptors. It should be pointed out that the present study only examined the regulatory role of spinal GABAA activity on the AMAP receptor-mediated development of neuropathic pain behaviors after CCI, these results do not rule out the possibility that spinal GABA activity may also regulate the maintenance of neuropathic pain behaviors.
Consistent with previous studies, we observed a tonic GABAergic influence on spinal AMPA receptor expression and spinal nociceptive processing because inhibition of intrinsic GABA activity in naive rats induced mechanical allodynia and thermal hyperalgesia. However, this endogenous inhibitory GABAergic activity seen in naïve rats must be limited or insufficient under pathological conditions, because 1) both thermal hyperalgesia and mechanical allodynia did develop in CCI rats despite this intrinsic inhibitory GABA activity and 2) activation of spinal AMPA receptors with exogenous AMPA receptor agonist S-AMPA was able to induce nociceptive behaviors and upregulation of spinal AMPA receptor expression in naïve rats, similar to the results observed after CCI. While the endogenous GABAergic activity may play a role in the regulation of spinal nociceptive processing, it is possible that this function could be diminished after nerve injury presumably due to a potential loss of spinal GABAergic interneurons (Sugimoto et al., 1990; Mao et al., 1997; Scholz et al., 2005). These findings add to the discussion concerning the role of spinal GABAergic neurons in the mechanisms of neuropathic pain (Polgar et al., 2005; Scholz et al., 2005). It seems reasonable to suggest that preserving endogenous spinal GABAergic activity alone after nerve injury might be insufficient to counter-balance spinal excitation induced by glutamatergic activities, as indicated by our data showing that AMPA receptor-mediated spinal excitation induced nociceptive responses in naïve rats even in the presence of an intact endogenous GABAergic system (i.e., without nerve injury).
Benzodiazepine receptors are coupled with a subpopulation of GABA receptors and benzodiazepines can enhance spinal GABA activity by increasing Cl− conduction (Unnerstall et al., 1981) and reducing glutamate release in the spinal cord (Haefely, 1988). These interactions between benzodiazepines and GABA receptors may be a pharmacological basis for the midazolam effect that was preventable by the GABAA receptor antagonist bicuculline. Besides its antinociceptive effects (Kontinen and Dickenson, 2000; Nishiyama et al., 2003), intrathecal midazolam exhibited a potent synergistic effect with AMPA receptor antagonists in rats (Nishiyama et al., 1999; Nishiyama et al., 2001). Thus, our data suggest that a combined treatment with a GABA receptor agonist and an AMPA receptor antagonist may be an effective approach for managing clinical neuropathic pain.
Acknowledgments
This work was supported by PHS RO1 grants DA08835, NS42661, and NS45681.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez FJ, Fyffe RE, Dewey DE, Haftel VK, Cope TC. Factors regulating AMPA-type glutamate receptor subunit changes induced by sciatic nerve injury in rats. J Comp Neurol. 2000;426:229–42. doi: 10.1002/1096-9861(20001016)426:2<229::aid-cne5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Ruiz A, Bjaalie JG, Kullmann DM, Gundersen V. GABA and GABAA receptors at hippocampal mossy fibre synapses. Eur J Neurosci. 2003;18:931–41. doi: 10.1046/j.1460-9568.2003.02828.x. [DOI] [PubMed] [Google Scholar]
- Bridges D, Thompson SW, Rice ASC. Mechanisms of neuropathic pain. Br J Anesth. 2001;87:12–26. doi: 10.1093/bja/87.1.12. [DOI] [PubMed] [Google Scholar]
- Cronin JN, Bradbury EJ, Lidierth M. Laminar distribution of GABAA- and glycine-receptor mediated tonic inhibition in the dorsal horn of the rat lumbar spinal cord: effects of picrotoxin and strychnine on expression of Fos-like immunoreactivity. Pain. 2004;112:156–163. doi: 10.1016/j.pain.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Willis WD. Modification of the responses of primate spinothalamic neurons to mechanical stimulation by excitatory amino acids and an N-methyl-D-aspartate antagonist. Brain Res. 1991;542:15–22. doi: 10.1016/0006-8993(91)90991-4. [DOI] [PubMed] [Google Scholar]
- Dubner R. Neuronal plasticity and pain following peripheral tissue inflammation or nerve injury. In: Bond M, Charlton E, Woolf CJ, editors. Pain Research and Clinical Management; Proceedings of Vth World Congress on Pain; Amsterdam: Elsevier; 1991. pp. 263–276. [Google Scholar]
- Engelman HS, Anderson RL, Daniele C, Macdermott AB. Presynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors modulate release of inhibitory amino acids in rat spinal cord dorsal horn. Neuroscience. 2006;139:539–553. doi: 10.1016/j.neuroscience.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Garry EM, Moss A, Rosie R, Delaney A, Mitchell R, Fleetwood-Walker SM. Specific involvement in neuropathis pain of AMPA receptors and adapter proteins for the GluR2 subunite. Mol Cell Neurosci. 2003;24:10–22. doi: 10.1016/s1044-7431(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Haefely WE. Benzodiazepines. Int A nesthesiol Clin. 1988;26:262–272. doi: 10.1097/00004311-198802640-00005. [DOI] [PubMed] [Google Scholar]
- Hammand DL. J.J. Bonica Lecture--2001: role of spinal GABA in acute and persistent nociception. Reg Anesth Pain Med. 2001;26:551–557. doi: 10.1053/rapm.2001.27835. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1998;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harris JA, Corsi M, Quartaroli M, Arban R, Bentivoglio M. Upregulation of spinal glutamate receptors in chronic pain. Neuroscience. 1996;74:7–12. doi: 10.1016/0306-4522(96)00196-0. [DOI] [PubMed] [Google Scholar]
- Hwang J, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- Izzo E, Auta J, Impagnatiello F, Pesold C, Guidotti A, Costa E. Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. Proc Natl Acad Sci U S A. 2001;98:3483–8. doi: 10.1073/pnas.051628698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hammond DL. Role of spinal receptors in formalin-induced nociceptive in the rat. J Pharmacol Exp Ther. 1997;282:928–938. [PubMed] [Google Scholar]
- Kerr RC, Maxwell DJ, Todd AJ. GluR1 and GluR2/3 subunits of the AMPA-type glutamate receptor are associated with particular types of neurone in laminae I-III of the spinal dorsal horn of the rat. Eur J Neurosci. 1998;10:324–33. doi: 10.1046/j.1460-9568.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Guo T, Agashe GS, Davies MF, Clark DJ, Maze M. Glucocorticoid inhibition of neuropathic limb edema and cutaneous neurogenic extravasation. Brain Res. 2001;913:140–148. doi: 10.1016/s0006-8993(01)02763-9. [DOI] [PubMed] [Google Scholar]
- Kohno T, Wakai A, Ataka T, Ikoma M, Yamakura T, Baba H. Actions of midazolam on excitatory transmission in dorsal horn neurons of adult rat spinal cord. Anesthesiology. 2006;104:338–343. doi: 10.1097/00000542-200602000-00020. [DOI] [PubMed] [Google Scholar]
- Kondo E, Iwata K, Ogawa A, Tashiro A, Tsuboi Y, Fukuoka T, Yamanaka H, Dai Y, Morimoto T, Noguchi K. Involvement of glutamate receptors on hyperexcitability of wide dynamic range neurons in the gracile nucleus of the rats with experimental mononeuropathy. Pain. 2002;95:153–63. doi: 10.1016/s0304-3959(01)00392-x. [DOI] [PubMed] [Google Scholar]
- Kondo E, Kiyama H, Yamano M, Shida T, Ueda Y, Tohyama M. Expression of glutamate (AMPA type) and gamma-aminobutyric acid (GABA)A receptors in the rat caudal trigeminal spinal nucleus. Neurosci Lett. 1995;186:169–72. doi: 10.1016/0304-3940(95)11316-o. [DOI] [PubMed] [Google Scholar]
- Kontinen V, Dickenson AH. Effects of midazolam in the spinal nerve ligation model of neuropathic pain in rats. Pain. 2000;85:425–431. doi: 10.1016/S0304-3959(99)00298-5. [DOI] [PubMed] [Google Scholar]
- Kontinen VK, Meert TF. Vocalization responses after intrathecal administration of ionotropic glutamate receptor agonists in rats. Anesth Analg. 2002;95:997–1001. doi: 10.1097/00000539-200210000-00038. [DOI] [PubMed] [Google Scholar]
- Kovacs AD, Cebers G, Cebere A, Liljequist S. Loss of GABAergic neuronal phenotype in primary cerebellar cultures following blockade of glutamate reuptake. Brain Res. 2003;977:209–20. doi: 10.1016/s0006-8993(03)02682-9. [DOI] [PubMed] [Google Scholar]
- Leem JW, Choi EJ, Park ES, Paik KS. N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neurosci Lett. 1996;211:37–40. doi: 10.1016/0304-3940(96)12714-2. [DOI] [PubMed] [Google Scholar]
- Lu CR, Willcockson HH, Phend SL, Darstein M, Valtschanoff JG, Rustioni A. Ionotropic glutamate receptors are expressed in GABAergic terminals in the rat superficial dorsal horn. J Comp Neurol. 2005;486:169–178. doi: 10.1002/cne.20525. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Mata HP, Porreca F. Spinal GABAA and GBBAB receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ, Lu J, Hayes RL. Intrathecal MK-801 and local nerve anesthesia synergistically reduce nociceptive behaviors in rats with experimental peripheral mononeuropathy. Brain Res. 1992a;576:254–262. doi: 10.1016/0006-8993(92)90688-6. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hypera lgesia in rats with painful peripheral neuropathy. Brain Res. 1992b;598:271–278. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and opiate tolerance: A current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Zhu J, Lu J, Mayer DJ. The inhibition of nitric oxide-activated poly(ADP-ribose) synthetase attenuates trans-synaptic alteration of spinal cord dorsal horn neurons and neuropathic pain in the rat. Pain. 1997;72:355–366. doi: 10.1016/s0304-3959(97)00063-8. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces down-regulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menéndez L, Lastra A, Villanueva N, Hidalgo A, Baamonde A. Spinal nociceptin inhibits AMPA-induced nociceptive behavior and Fos expression in rat spinal cord. Pharmacology, Biochemistry and Behavior. 2003;74:657–661. doi: 10.1016/s0091-3057(02)01042-0. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Yaksh TL, Wber E. Effects of intrathecal NMDA and non-NMDA antagonists on acute thermal nociception and their interaction with morphine. Anesthesiology. 1998;89:715–722. doi: 10.1097/00000542-199809000-00023. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Gyermek L, Lee C, Kawasaki-Yatsugi S, Yamaguchi T. Analgesic interaction between intrathecal midazolam and glutamate receptor antagonists on thermal-induced pain in rats. Anesthesiology. 1999;91:531–537. doi: 10.1097/00000542-199908000-00028. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Gyermek L, Lee C, Kawasaki-Yatsugi S, Yamaguchi T. Synergistic analgesic effects of intathecal midazolam and NMDA or AMPA receptor antagonists in rats. Can J Anaesth. 2001;48:288–294. doi: 10.1007/BF03019761. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Hanaoka K. Midazolam can potentiate the analgesic effects of intrathecal bupivacaine on thermal- or inflammatory-induced pain. Anesth Analg. 2003;96:1386–1391. doi: 10.1213/01.ANE.0000057606.82135.7D. [DOI] [PubMed] [Google Scholar]
- Polgar E, Hughes DI, Arhan AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RM, Dai W, Levada RE, Lippman JJ, Jensen FE. AMPA/kainate receptor-mediated downregulation of GABAergic synaptic transmission by calcineurin after seizures in the developing rat brain. J Neurosci. 2005;25:3442–51. doi: 10.1523/JNEUROSCI.0204-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RMA, Li DL, Hoo KH, Deveril M, Ornstein PL, Iyengar S. Kainate GluR 5 formalin in the rat. Neuropharmacology. 1998;37:25–36. doi: 10.1016/s0028-3908(97)00188-3. [DOI] [PubMed] [Google Scholar]
- Sosnowski M, Yaksh TL. Spinal administration of receptor-selective drugs as analgesics:new horizons. J Pain Sympt Manage. 1990;5:204–213. doi: 10.1016/0885-3924(90)90010-h. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Bennett GJ, Kajander K. Strychnine-enhanced transsynaptic degeneration of dorsal horn neurons in rats with an experimental painful peripheral neuropathy. Neurosci Lett. 1990;98:139–143. doi: 10.1016/0304-3940(89)90499-0. [DOI] [PubMed] [Google Scholar]
- Svenson BA, Welin M, Gordh TJ, Westman J. Chronic subarachnoid midazolam in the rat: morphological evidence of spinal cord neurotoxicity. Reg Anesth. 1995;20:426–434. [PubMed] [Google Scholar]
- Taira Y, Nakakimura K, Matsumoto M, Sakabe T. Spinal and supraspinal midazolam potentiates antinociceptive effects of isoflurane. Br J Anesth. 2000;85:881–886. doi: 10.1093/bja/85.6.881. [DOI] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:275–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Unnerstall JR, Kuhar MJ, Niehoff DL, Palacios JM. Benzodiazepine receptors are coupled to a subpopulation of –aminobutyric acid (GABA) receptors: evidence from a quantitative autoradiographic study. J Pharmacol Exp Ther. 1981;218:797–804. [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sakashita Y. COX-2 inhibitor prevents the development of hyperalgesia induced by intrathecal NMDA or AMPA. NeuroReport. 1998;9:3869–3873. doi: 10.1097/00001756-199812010-00019. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Spinal pharmacology of thermal hyperalgesia induced by constriction injury of sciatic nerve. Excitatory amino acid antagonists. Pain. 1992;49:121–128. doi: 10.1016/0304-3959(92)90198-K. [DOI] [PubMed] [Google Scholar]


