Abstract
Gender is an important biological determinant of vulnerability to psychosocial stress. We used perfusion based functional magnetic resonance imaging (fMRI) to measure cerebral blood flow (CBF) responses to mild to moderate stress in 32 healthy people (16 males and 16 females). Psychological stress was elicited using mental arithmetic tasks under varying pressure. Stress in men was associated with CBF increase in the right prefrontal cortex (RPFC) and CBF reduction in the left orbitofrontal cortex (LOrF), a robust response that persisted beyond the stress task period. In contrast, stress in women primarily activated the limbic system, including the ventral striatum, putamen, insula and cingulate cortex. The asymmetric prefrontal activity in males was associated with a physiological index of stress responses—salivary cortisol, whereas the female limbic activation showed a lower degree of correlations with cortisol. Conjunction analyses indicated only a small degree of overlap between the stress networks in men and women at the threshold level of P < 0.01. Increased overlap of stress networks between the two genders was revealed when the threshold for conjunction analyses was relaxed to P < 0.05. Further, machine classification was used to differentiate the central stress responses between the two genders with over 94% accuracy. Our study may represent an initial step in uncovering the neurobiological basis underlying the contrasting health consequences of psychosocial stress in men and women.
Keywords: cerebral blood flow (CBF), arterial spin labeling (ASL), right prefrontal cortex (RPFC), left orbitofrontal cortex (LOrF), anterior cingulate cortex (ACC)
INTRODUCTION
Gender is an important determinant of human health, and there is a clear pattern for the sex-specific prevalence rates of several mental and physical disorders. Men are generally more susceptible to infectious diseases, hypertension (cardiovascular disease), aggressive behavior and abuse of alcohol or drugs. Women, on the other hand, have higher rates of autoimmune diseases, chronic pain, depression and anxiety disorders compared to men (Holden, 2005; Kudielka and Kirschbaum, 2005; Lundberg, 2005; Kajantie and Phillips, 2006). Some of these gender differences emerge during women's reproductive years, and gradually diminish after menopause (e.g. depression, cardiovascular disease), suggesting that the observed gender-specific disease pattern may be partly attributed to effects of sex hormones (Otte et al., 2005). Recently, individual differences in stress reactivity have been proposed as a potentially important risk factor for gender-specific health problems in men and women, in addition to genetic, socio-cultural, hormonal and developmental factors (Hamann and Canli, 2004; Young and Altemus, 2004; Goldstein et al., 2005; Kajantie and Phillips, 2006).
To date, assessing gender differences in stress reactivity primarily relied on measuring physiological responses to acute stressors in laboratory settings, including activities of the hypothalamic-pituitary-adrenal (HPA) axis (e.g. cortisol) and/or sympathetic nervous system (e.g. heart rate and blood pressure). A general trend has emerged suggesting greater acute HPA and autonomic responses in adult men compared to adult women using standard performance related psychosocial stressors such as public speaking and arithmetic tasks (Kudielka and Kirschbaum, 2005; Kajantie and Phillips, 2006). This greater sympathoadrenal responsiveness in males may be reasonably associated with the pathogenesis of cardiovascular disease, aggression and immune suppression (Segerstrom and Miller, 2004; Lundberg, 2005). In women, the phase of menstrual cycle, menopausal status and pregnancy were found to have marked effects on physiological stress responses. In particular, estrogen has been shown to buffer the sympathetic and HPA arousal (Goldstein et al., 2005; Kajantie and Phillips, 2006).
Other studies, however, reported either no gender difference in stress reactivity or greater cortisol elevation in females than males, when a social rejection task was adopted as the stressor instead of achievement tasks (Stroud et al., 2002; Dickerson and Kemeny, 2004). It has been proposed that women are more likely to be negatively affected by interpersonal events than men—a tentative factor underlying the emergence of gender differences in depression (Cyranowski et al., 2000). These differences in experimental findings and alternative theoretical models highlight the complex nature of the gender-specific stress response, which may be dependent on the type of stressor/challenge, experimental procedure, outcome measured and subject status (Dickerson and Kemeny, 2004). Furthermore, the probed peripheral physiological parameters typically reflect the integrative reaction of several biological systems, which are often delayed in time and modulated in magnitude by other stress mediators.
It would be desirable to be able to directly visualize the effects of psychological stress in the male and female brain. Functional neuroimaging studies have begun to shed light on the neuroanatomical substrates underlying human emotional processes tightly related to stress (Phan et al., 2002; Hamann and Canli, 2004). Nevertheless, the majority of emotional stimuli employed in existing functional magnetic resonance imaging (fMRI) studies (e.g. fearful faces) lack critical features of a standard psychosocial stress paradigm, which typically comprises motivated performance tasks along with social-evaluative threat and/or subjective feelings of uncontrollability (Dickerson and Kemeny, 2004). Using a quantitative fMRI method—arterial spin labeling (ASL) perfusion fMRI, we explored the neural correlates of psychological stress elicited by a mental arithmetic task under performance pressure (Wang et al., 2005a). The RPFC, which is affiliated with negative emotion, vigilance and goal-directed behavior, is activated in response to stress with concomitant suppression of the left prefrontal and orbitofrontal cortex. Brain activation in the limbic circuitry, including putamen, insula and anterior cingulate cortex (ACC), was also observed even after completion of stress tasks.
The goal of the present study was to further explore the gender-specific neural circuitry of psychological stress in the male and female brain. Perfusion fMRI was used as the neuroimaging tool due to its suitable spatiotemporal characteristics for visualizing stress effects (Detre and Wang, 2002). Recent evidence suggests that perfusion fMRI is resilient to effects of subject motion during overt speech production (Kemeny et al., 2005) and allows certain behavioral/pharmacological manipulations to be performed outside the MR scanner (Franklin et al., 2007), therefore may be suitable for many ecological paradigms in social cognitive and affective neuroscience.
MATERIALS AND METHODS
Subjects
Thirty-two healthy subjects (16 females and 16 males) were included in this study. The mean ages of the female and male group were 22.8 ± 2.4 (s.d.) and 24.3 ± 3.1 years (n.s.). The result of the general stress network based on the first 23 subjects has been reported (Wang et al., 2005a), and the present work focused on the specific stress network in each gender. All the subjects were native English speakers and screened for history of neurologic and psychiatric disease. Written informed consent was obtained prior to all human studies according to an Institutional Review Board approval from the University of Pennsylvania.
MRI experimental procedure
The experimental procedures have been described previously (Wang et al., 2005a). MRI experiments were carried out between three and five in the afternoon to control for diurnal fluctuations in salivary cortisol level. The scanning protocol consisted of four perfusion fMRI scans (8 min each) performed with a fixed order of Baseline 1, Low stress task, High stress task and Baseline 2 (Figure 1), followed by an anatomical scan (6 min) at the end. Such design allowed us to simultaneously study the acute (High vs Low stress task) and persistent (Baseline 2 vs 1) effects of psychological stress on brain activity, while controlling potential contamination of the control condition by increased emotional reactivity elicited by the high stress task. During the high stress task, subjects were instructed to perform serial subtraction of 13 from a four-digit number and respond verbally. To provide an element of harassment, we prompted the subjects for faster performance every two minutes and asked them to restart the task if an error occurred. As a low stress control condition, subjects counted backward aloud from 1000 without pressure.
Fig. 1.
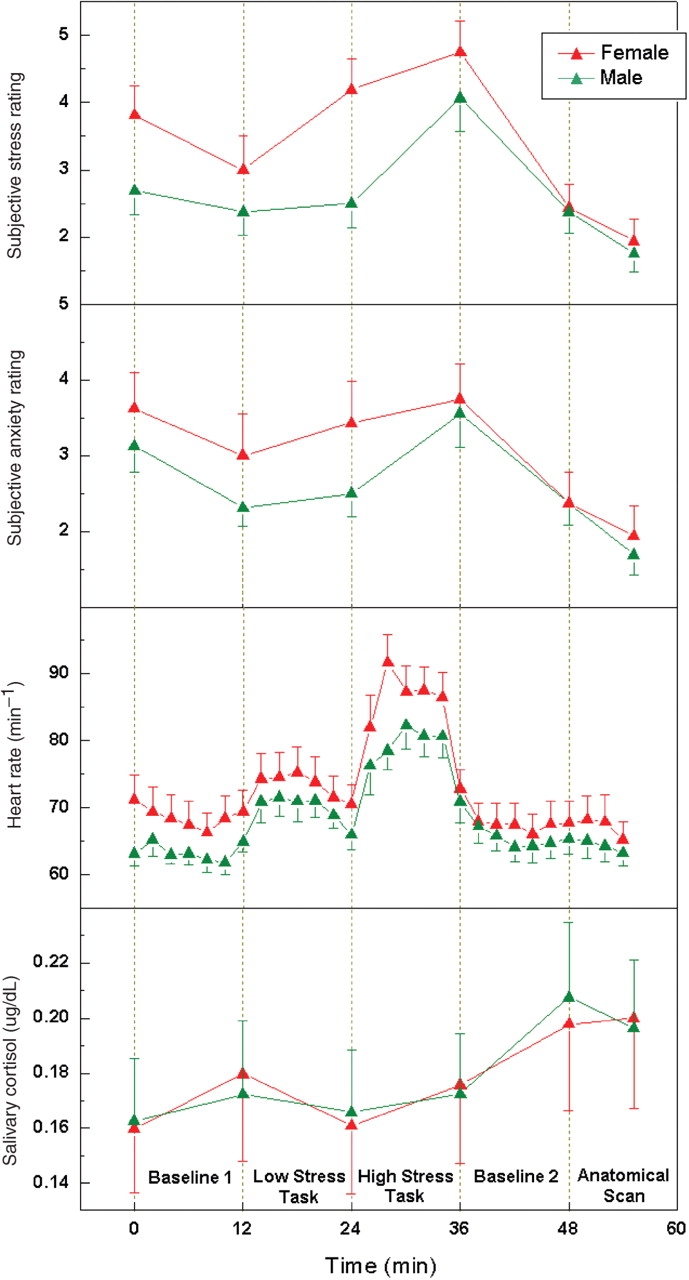
Average subjective ratings of stress, anxiety, heart rate and salivary cortisol level during the time course of the stress experiment in the male and female group. All the behavioral and physiological measures are significantly increased after the high stress task. The error bars indicate standard error. In regression analyses of CBF data, the differences between perceived stress and anxiety reported at the end of the tasks are used as covariates.
Self report of stress and anxiety level (on the scale of 1 to 9) as well as saliva samples were collected immediately after the subjects entered the MR scanner and after each MR scan. Subjects were instructed to put (without chewing) a cotton swab inside the mouth for 2 min, until it became saturated with saliva. Throughout the experiment, heart rate was recorded every 2 min based on a pulse-oxymetry reading. Subject's self evaluation of effort required and task difficulty were recorded after the stress tasks. In the last 21 of the 32 subjects (11 females, 10 males), we also recorded performance data including the number of errors and completed subtractions before committing an error during the serial subtraction task.
MR scanning was conducted on a Siemens 3.0T Trio whole-body scanner (Siemens Medical Solution, Erlangen, Germany), using a standard Transmit/Receive head coil. A continuous arterial spin labeling (CASL) technique (Wang et al., 2005b) was used for perfusion fMRI scans, along with a 3D MPRAGE volumetric scan for high resolution T1-weighted anatomic images. Imaging parameters were identical to those described previously (Wang et al., 2005a).
Data analysis
Salivary samples were spun in a centrifuge, the inner tube and swab were discarded, and salivary cortisol concentrations were determined using enzyme immunoassay kit # 1-3002 (Salimetrics LLC. State College, PA, USA). Samples were analyzed in duplicate. Behavioral and physiological measurements were analyzed using the repeated measures general linear model (GLM) of the SPSS 12.0 software package (SPSS Inc. Chicago, IL, USA) to assess the effect of experimental condition (within-subject effect) and gender (between-subject effect). For performance data, we used the non-parametric Mann–Whitney and Wilcoxon method to test whether there was significant difference between the two genders. In addition, we measured the area-under-the-curve (AUC) of the salivary cortisol level.
Perfusion fMRI data were analyzed offline using the VoxBo (www.voxbo.org) and SPM2 software packages (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). MR image series were first realigned to correct for head movements, coregistered with each subject's anatomical MRI, and smoothed in space with a 3D 12 mm FWHM (full width at half maximum) Gaussian kernel. Subjects who showed excessive head motion (>5 mm along the x, y or z axis) were excluded from further analysis (Kim et al., 2006). Perfusion weighted image series were generated by pair-wise subtraction of the label and control images, followed by conversion to absolute CBF image series. Voxel-wise analyses of the cerebral blood flow (CBF) data were conducted in each subject, utilizing a GLM including the global time course as a covariate (first-level analysis). No temporal filtering or smoothing was involved. Two contrasts were defined in the GLM analysis, namely the CBF difference between the two stress tasks (High stress–Low stress) and the CBF difference between the two baseline conditions (Baseline 2–1).
Individual contrast images (β maps for each contrast) were normalized into a canonical space (Montreal Neurological Institute standard brain). Linear regression analyses were carried out on these normalized individual maps to obtain the activation pattern correlated with differences in perceived stress and anxiety (reported at the end of tasks) between the high and low stress tasks (second-level analysis), respectively. In addition, we used the AUC measure of salivary cortisol as the independent variable, and changes in baseline CBF (Baseline 2–1) as the dependent variable for regression analyses, given that cortisol reflects cumulative physiological changes by undergoing the two stress tasks. These regression analyses were performed in the two subgroups of male and female subjects, respectively.
Normalized individual contrast maps were further analyzed using regression analyses with gender as the reference function, to detect the differences in the average brain activation pattern for the two defined contrasts between the male and female subjects. In addition, changes in perceived effort/difficulty between the high and low stress tasks were included as a covariate in the GLM. Behavioral measures of effort and difficulty were highly correlated across subjects (R = 0.5, P = 0.003), and were averaged to form one index of effort/difficulty. One-sample t-tests were used to obtain the statistical significance in all above regression analyses. Areas of significant activation were defined at the cluster level for the P-value <0.005 (uncorrected) and the cluster extent size larger than 15 voxels (2 × 2 × 2 mm3). Regions of interest (ROIs) based on activation clusters were generated using the SPM Marsbar toolbox. CBF changes in these ROIs were extracted and entered into a univariate GLM analysis using the SPSS software to investigate the effect size of each covariate. The correlation coefficients of ROI based CBF data with perceived stress or AUC cortisol were converted into Z-scores using Fisher's transformation, and then compared between the male and female groups (Blalock, 1972).
To estimate the separation ability of the stress related brain regions for gender, we employed a linear support-vector-machine (SVM) classifier that is able to find a separating hyperplane with large margin in the original feature space (Vapnik, 1999). The corresponding CBF changes in stress related brain regions demonstrating gender differences were directly used as features for classification. The importance of each feature was then estimated by its corresponding component of the weighting vector in defining the linear separating hyperplane. In order to explore the regions of significant overlap between group activations for males and females, we performed conjunction analyses using the SPM minimum T-statistic (Friston et al., 2005). At the group level, three uncorrected threshold levels (P < 0.005, 0.01, 0.05) were used to form a mask from one group (e.g. the male), respectively, and then applied to the other group (e.g. the female). This procedure was repeated with swapped order of male and female groups. Inference was based on P-values adjusted for the search volume using random field theory. Voxels exceeding threshold in both procedures were considered significant for conjunction analyses.
RESULTS
Behavioral and physiological stress responses
The measured behavioral and physiological data indicated that the experimental paradigm successfully elicited a mild to moderate level of psychological stress in both male and female subjects. The main effect of experimental condition was significant for perceived stress [F(5, 26) = 17.47, P < 0.001], perceived anxiety [F(5, 26) = 19.55, P < 0.001] and heart rate [F(4, 27) = 41.76, P < 0.001], which were immediately elevated in response to the stress tasks, as well as for salivary cortisol [F(5, 26) = 3.22, P = 0.021], which showed a delayed response to the high stress task (Figure 1). The main effect of gender was not significant for perceived stress/anxiety, heart rate or salivary cortisol measures. However, the interaction of experimental condition and gender was significant for perceived stress [F(5, 26) = 5.52, P = 0.001]. Post hoc analyses indicated that males reported a greater acute response in perceived stress from the low to high stress task [F(1, 30) = 4.39, P = 0.045] compared to females. This effect was not observed for perceived anxiety, although self ratings of stress and anxiety were correlated (R = 0.76, P < 0.001). Despite a higher level of task difficulty [F(1, 30) = 7.20, P = 0.012] and effort required [F(1, 30) = 4.93, P = 0.034] reported by females during the stress tasks (Supplementary Figure 1), men and women performed equally well for the serial subtraction task. There was no significant difference between the two sexes in the recorded number of errors made (male: mean ± s.e.m. = 5.7 ± 1.0, female: 6.2 ± 1.3, Z = 0.17, P = 0.87) and completed subtractions before committing an error (male: 19.2 ± 3.4, female: 15.3 ± 4.8, Z = 1.35, P = 0.18).
Neural pathways associated with perceived stress in men and women
The neural correlates of subjects’ own experience of stress were probed using voxel-wise linear regression analyses of the perfusion fMRI data with perceived stress. First, acute stress responses during the performance of stress tasks were identified by correlating changes in regional CBF and perceived stress from the low to high stress task (High–Low stress task). Second, lasting stress effects after task completion were identified by correlating baseline CBF variations (Baseline 2–1) with changes in perceived stress from the low to high stress task. We first examined the right and left prefrontal cortex based on main findings from our prior study (Wang et al., 2005a).
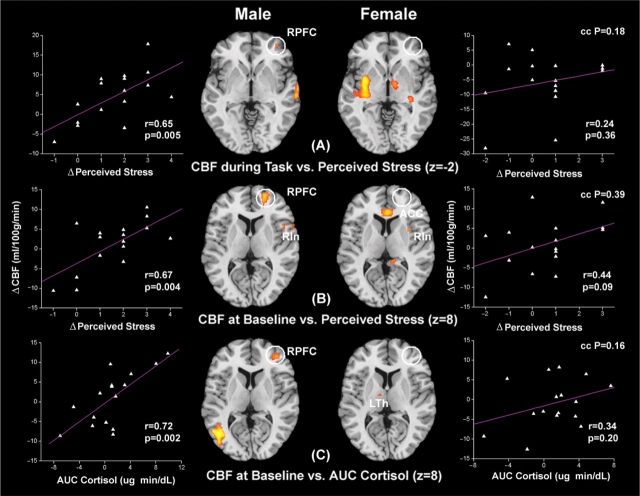
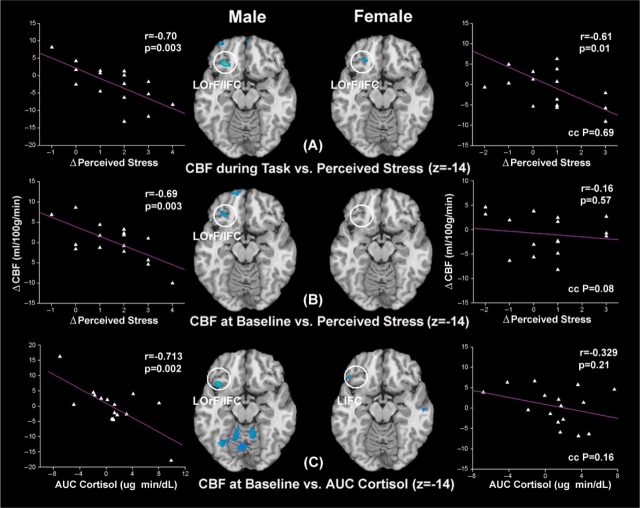
Performing the two regression analyses in each gender revealed that, in the male group, CBF in the right prefrontal cortex (RPFC) was elevated both during the performance of stress tasks and at baseline after task completion in subjects experiencing stress. However, no significant correlation between RPFC activation and perceived stress was observed in the female group either during tasks or at baseline (Figure 2A and B). This gender difference in RPFC activation was especially evident during the performance of stress tasks, wherein the RPFC CBF showed the lowest correlation with subjective stress ratings in the female group (R = 0.24, P = 0.36, Figure 2A). We further observed that, in the male group, CBF in the left orbitofrontal cortex (LOrF) inferior frontal cortex (IFC) was suppressed both during the performance of stress tasks and at baseline after task completion in subjects experiencing stress (Figure 3A and B). For females, the association of CBF reduction in LOrF/IFC and perceived stress was only significant during the performance of stress tasks (Figure 3A). These results suggest that the stress response in men is primarily characterized by RPFC activation accompanied by LOrF/IFC inhibition, a robust response that persists beyond the stress task period. In contrast, women only showed transient suppression of the LOrF/IFC during the performance of stress tasks.
Table 1.
Brain regions with significant findings in regression analyses of CBF data, and in comparison between male and female stress responses
| Brain regions | MNI coordinates |
Z-Score | Cluster size | Activation/deactivation | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Male: CBF during Task vs Perceived Stress (Figure 2A) | ||||||
| RPFC | 42 | 52 | −2 | 2.83 | 15 | Activation |
| LOrF/IFC | −30 | 28 | −16 | 4.08 | 129 | Deactivation |
| Male: CBF at Baseline vs Perceived Stress (Figure 2B) | ||||||
| RPFC | 24 | 56 | 6 | 3.39 | 290 | Activation |
| Rln | 54 | 14 | 4 | 3.19 | 121 | Activation |
| LOrF/IFC | −30 | 32 | −14 | 3.82 | 77 | Deactivation |
| Male: CBF at Baseline vs AUC cortisol (Figure 2C) | ||||||
| RPFC | 44 | 48 | 14 | 3.57 | 367 | Activation |
| LOrF/IFC | −36 | 22 | −16 | 3.43 | 139 | Deactivation |
| Female: CBF during Task vs Perceived Stress (Figures 3A and 4A) | ||||||
| LSt | −14 | 4 | 8 | 2.86 | 69 | Activation |
| RSt | 14 | −4 | 0 | 3.12 | 190 | Activation |
| LIn/Pu | −34 | −10 | 2 | 3.94 | 952 | Activation |
| Rln | 36 | −20 | 8 | 4.10 | 361 | Activation |
| LOrF/IFC | −24 | 36 | −12 | 3.36 | 59 | Deactivation |
| Female: CBF at Baseline vs Perceived Stress (Figures 3B and 4B) | ||||||
| Rin | 38 | 8 | 8 | 3.10 | 54 | Activation |
| ACC | 8 | 20 | 32 | 3.44 | 894 | Activation |
| DACC | 8 | 20 | 32 | 3.44 | 894 | Activation |
| PCC | 12 | −28 | 18 | 3.18 | 140 | Activation |
| Female: CBF at Baseline vs AUC cortisol (Figures 3C and 4C) | ||||||
| LTh | −6 | −10 | 18 | 2.89 | 43 | Activation |
| dACC | 14 | 24 | 26 | 3.01 | 26 | Activation |
| LIFC | −48 | 26 | −20 | 2.85 | 28 | Deactivation |
| Male > Female during Task (Figure 5A) | ||||||
| RPFC | 40 | 58 | −6 | 3.96 | 737 | |
| RPC/AG | 42 | −64 | 50 | 3.34 | 686 | |
| Female > Male at Baseline (Figure 5B) | ||||||
| LOrF | −20 | 42 | −14 | 3.87 | 187 | |
| dACC | 2 | 18 | 26 | 3.07 | 82 | |
| Lin | −38 | −18 | 14 | 2.91 | 58 | |
| LPC/SMG | −58 | −30 | 58 | 3.63 | 228 | |
Fig. 2.
Axial sections of regression analysis results performed in the male and female group respectively, showing consistent RPFC activation in all the three analyses performed in males. These analyses use the CBF change during stress tasks (high stress – low stress task) (A) and the CBF change at baseline (Baseline 2–1) (B) as the dependent variable, and the change in perceived stress from the low to high stress task as the independent variable. Additional analyses use the CBF change at baseline (C) as the dependent variable, and AUC measures of salivary cortisol as the independent variable. Data are thresholded at P < 0.005 (uncorrected) and activation cluster size of >15 voxels (2 × 2 × 2 mm3). The significance level of differences between the correlation coefficients in males and females is represented by ‘cc P’ values. Scatter plots of corresponding CBF changes in RPFC ROI (indicated by white circles) as a function of perceived stress or AUC measures of salivary cortisol are displayed. See Table 1 for coordinates of activated clusters for Figures 2–5.
Fig. 3.
Axial sections of regression analysis results performed in the male and female group, respectively, showing consistent deactivation of LOrF/IFC in all the three analyses performed in males. The analyses are the same as shown in Figure 2. Scatter plots of corresponding CBF changes in LOrF/IFC ROI (indicated by white circles) as a function of perceived stress or AUC measures of salivary cortisol are displayed.
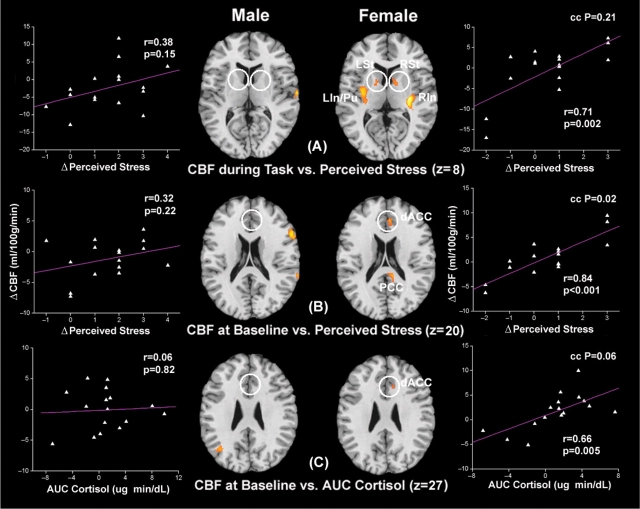
We then examined the limbic system along with closely interconnected brain regions including hippocampus, insula and cingulate cortex. During the performance of stress tasks, CBF increases in the left insula/putamen (LIn/Pu), right insula (RIn) and bilateral ventral striatum (LSt and RSt), including caudate and globus pallidus, were correlated with subjective stress ratings only in the female group. In contrast, the male group did not exhibit any stress related brain activation in the limbic regions during stress tasks (Figure 4A). After completion of stress tasks, persistent activation in the ACC, posterior cingulate cortex (PCC) and RIn were associated with heightened stress level during tasks in the female group. Particularly, in dorsal ACC, the correlation coefficient of CBF with perceived stress was significantly higher in females than males (P = 0.02). In the male group, persistent CBF elevation was observed only in the RIn in stressed subjects (Figure 2B). These results indicate that the female stress response is primarily associated with limbic activation of the ventral striatum, putamen and insula during stress tasks, and the ACC and PCC persisting beyond the task period.
Fig. 4.
Axial sections of regression analysis results performed in the male and female group, respectively, showing limbic and cingulate activation only in females. The analyses are the same as shown in Figure 2. Scatter plots of corresponding CBF changes in ventral striatum and dACC ROIs (indicated by white circles) as a function of perceived stress or AUC measures of salivary cortisol are displayed.
Since women experienced increased cognitive demand relative to men during stress tasks, there exists the concern that our observation may reflect gender differences in performing arithmetic tasks rather than stress reactivity. We therefore repeated the above regression analyses, while including subjective ratings of effort/difficulty as a covariate in conjunction with perceived stress. Behaviorally, subjective ratings of stress and effort/difficulty were not correlated (R = 0.07, P = 0.80). Including effort/difficulty as a covariate along with stress in regression analyses of CBF data did not affect the reported results on gender differences in brain activation associated with perceived stress (Supplementary Figure 2).
Conjunction of stress networks in men and women
Conjunction analyses were carried out to detect the overlap between the neural networks associated with perceived stress in the male and female group. At the significance level of P < 0.005, there was no overlapping brain activation between the two gender groups. At P < 0.01, the conjunction analysis only revealed one common brain region during the performance of stress tasks—CBF reduction in the LOrF/IFC, as well as one common region after completion of stress tasks—persistent CBF elevation in the RIn/Pu (Supplementary Figure 3A). When the significance level was relaxed to uncorrected P < 0.05, the conjunction analysis started to reveal more overlapping brain regions between the male and female group, including common activation of LSt, left insula/superior temporal cortex (LIn/STC) and right globus pallidus/thalamus (RGP/Th) as well as LOrF deactivation during the performance of stress tasks. The conjunction analysis also revealed common persistent activation of RPFC, RIn and ACC at baseline after task completion in both genders (Supplementary Figure 3B). Nevertheless, the acute RPFC response was still uniquely associated with male subjects even with P < 0.05.
Neural pathways associated with salivary cortisol in men and women
The earlier results were based on regression of brain responses with subjective stress experience, which may differ between men and women. For instance, it has been reported that females may have a lower threshold for perceived stress compared to males since puberty (Hampel and Petermann, 2006). We therefore performed a third regression analysis to detect associations between baseline CBF variations (Baseline 2–1) and AUC measures of salivary cortisol—a physiological index of overall stress elevation caused by undergoing the experimental stress paradigm. Again, in the male subjects, we found that baseline CBF increase in the RPFC and CBF reduction in the LOrF/IFC were correlated with AUC measures of salivary cortisol (Figures 2C and 3C). In contrast, significant cortisol related CBF increases were observed in the dorsal ACC (dACC) and left thalamus (LTh) only in the female but not the male group (Figures 2C and 4C). Females also showed cortisol related CBF reduction in the left IFC (LIFC), but at a much weaker significance level compared to the LOrF/IFC suppression observed in males (Figure 3C). A subsequent conjunction analysis with a lowered threshold of uncorrected P < 0.05 revealed only one common cortisol related brain activation in dACC in both males and females (no significant overlap at P < 0.01). These additional analyses relying on a physiological parameter—salivary cortisol—are consistent with our findings based on behavioral assessments of stress.
Neural pathways associated with perceived anxiety in men and women
Although we demonstrated that differences in subjective feelings of effort/difficulty did not contribute to the observed gender-specific brain activation pattern under stress, there remains the concern that females may feel more threatened by the arithmetic task than males, thereby eliciting greater limbic activation. In terms of behavioral measures of anxiety, neither gender nor the interaction of gender and experimental condition showed a significant effect. Regression analyses of CBF data with perceived anxiety revealed primary limbic activation in both genders. During the performance of stress tasks, anxiety was associated with CBF elevation in the left amygdala/insula/putamen and RIn/Pu in both male and female groups. Perceived anxiety also elicited CBF increase in LSt and RSt only in female subjects, as well as CBF reduction in left IFC only in male subjects (Supplementary Figure 4). During the baseline conditions, men showed persistent LIn/Pu activation, whereas women showed persistent activation in RIn/Pu, dACC and LSt that were associated with changes in reported anxiety level during stress tasks. In these analyses, targeting neural pathways mediating subjective feelings of anxiety, prefrontal activity was largely missing in the male group. This observation suggests that the gender-specific central stress response in our study cannot be (solely) attributed to potential differences in negative emotions such as perceived anxiety.
Comparison of average stress responses between men and women
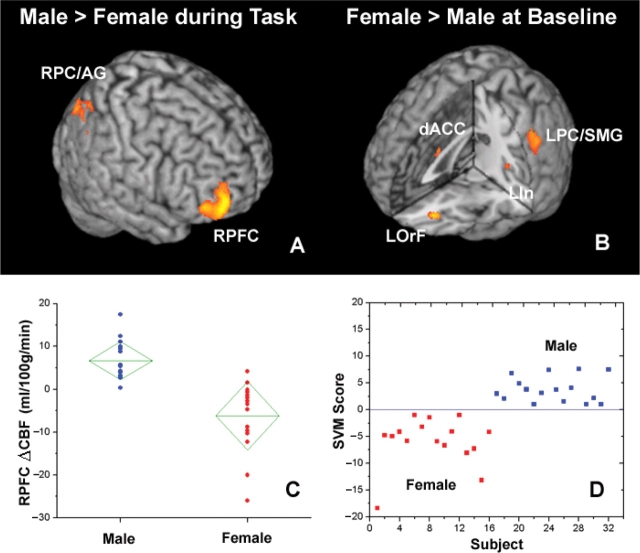
To address whether the average brain activation pattern under stress differs between men and women, we compared the mean acute (High–Low stress task) and persistent (Baseline 2–1) CBF responses to stress between the male and female group, using a regression analysis including gender as the independent variable (Figure 5A) (i.e. unpaired t-test between male and female groups). During the stress tasks, men showed predominantly greater CBF augmentations than women in the right hemisphere including the RPFC and right parietal cortex/angular gyrus (RPC/AG), whereas women only showed greater activation in PCC compared to men. The greater acute RPFC activation in the male group was the most significant finding (peak Z = 3.96). This activation survived the small volume corrected threshold (P = 0.04) using the right frontal lobe as the search volume. When perceived stress was also included as a covariate along with gender in the regression analysis, this gender effect in the RPFC was still significant (Supplementary Figure 5). Based on estimation of effect size using analysis of variance (ANOVA), gender and perceived stress accounted for 51.5% (P < 0.001) and 49.8% (P = 0.015) of the total variance of RPFC CBF changes during stress tasks, respectively.
Fig. 5.
Three-dimensional rendering of the results comparing the mean acute (High–Low stress task) and lasting (Baseline 2–1) CBF responses between the male and female groups. The greater right-sided male activation during tasks (A) and greater left-sided female activation at baseline (B) are shown. Also shown are diamond plot of changes in RPFC CBF from the low to high stress task (C) (93.8% separation), and scatter plot of the SVM scores for classification of female and male stress responses based on CBF changes in 4 ROIs of RPFC, LOrF, dACC and LIn (D) (100% separation).
In contrast to the acute stress responses, during the post- vs pre-stress baseline conditions, women showed much greater CBF elevations than men primarily in the left hemisphere, including the LOrF, left insula (LIn), dorsal ACC and left parietal cortex/supramarginal gyrus (LPC/SMG) (Figure 5B), whereas men only showed greater activation in the right thalamus compared to women. Taken together, the group comparison results and the regression analyses carried out independently in the male and female groups suggest that the RPFC activation provides a unique biomarker of the acute stress response in men. In contrast, females show greater persistent activation of the dorsal ACC and LIn, and less suppression of the LOrF after task completion compared to men.
Classification of stress responses in men and women
We further employed a SVM based linear classification approach to differentiate the female and male stress response. As shown in Figure 5C, the CBF changes in the RPFC from the low to high stress task provided a relatively clean separation between the male and female groups, which yielded an accuracy of 93.8% (two errors in 32 subjects) for SVM classification based on just a single ROI of the RPFC. We then sequentially included corresponding CBF changes in stress-related brain regions demonstrating gender differences into the SVM classification, including the LOrF, dorsal ACC and LIn. We were able to achieve a perfect (100%) separation of the male and female group, when all 4 ROIs were included (Figure 5D). The RPFC was the most important factor in the SVM classifier, with a weighting factor of 51.5%.
DISCUSSION
The results of this study support a gender-specific neural activation model underlying the central stress response, featuring asymmetric prefrontal activity in males and primarily limbic activation in females. Regression analyses were primarily employed to probe convergent brain activation patterns associated with both behavioral and physiological stress responses in the male and female group, respectively. This data analysis approach largely controlled confounding factors due to increased cognitive demand and effort experienced by the female group. Further, performance data showed no significant difference between the two genders in our experiment. Gender difference in neural activation was also observed during baseline conditions without any cognitive task, further controlling potential confounding effects due to cognitive differences between males and females in carrying out the arithmetic tasks. In the following sessions, we discuss the present findings within the broad context of several existing theories regarding gender differences in stress reactivity.
‘Fight-or-flight’ vs ‘Tend-and-befriend’
A noticeable theory based on neuroendocrine and behavioral evidence posits that stress responses may be characterized by ‘fight-or-flight’ in men and ‘tend-and-befriend’ in women (Taylor et al., 2000). Evolutionarily, males have to confront a stressor—such as a predator—either by overcoming or fleeing it. Females respond to stress by nurturing offspring and affiliating with social groups that maximize the survival of the species in times of adversity. Whereas the physiological stress response typically involves activation of the sympathetic nervous system and the HPA axis in both genders, the female stress response may specifically build on attachment–caregiving processes (especially those mediated by oxytocin) that buffer the sympathetic and HPA arousal.
Under stressful situations, the ‘fight-or-flight’ response invokes resources that increase focus, alertness and fear, while inhibiting appetitive goals to cope with the threat or challenge (McEwen, 2000; Sapolsky, 2000). Consistent with this model, in our prior study the RPFC—an important part of both the negative emotion and vigilance systems—is activated in response to stress, while the LOrF, which is associated with positive emotion and hedonic goals, is suppressed by stress (Wang et al., 2005a). That RPFC activation and LOrF deactivation with stress was predominately observed in the male brain is consistent with the idea that stress responses in men may be primarily characterized as ‘fight-or-flight’. Our neuroimaging findings agree with the general trend of greater acute HPA and autonomic responses in males compared to females using performance stress paradigms (Kajantie and Phillips, 2006). The parallel changes in prefrontal CBF and salivary cortisol suggest that cortisol may mediate the effect of HPA arousal on target brain regions of a male brain (Carrasco and Van de Kar, 2003; Charney, 2004), although this hypothesis needs to be tested experimentally.
In contrast, the female stress response primarily involves the limbic system including ventral striatum, putamen, insula and cingulate cortex. In particular, the ventral striatum including caudate and globus pallidus are critical substrates of the reward system that possess rich receptors for oxytocin, vasopressin, dopamine and endorphin (McClure et al., 2004). The striatum activation along with cingulate cortex, insula and putamen have been reported in previous fMRI studies on social attachment such as maternal and romantic love (Bartels and Zeki, 2000, 2004; Leibenluft et al., 2004), although there are also inconsistencies between our findings and previous studies (Gobbini et al., 2004; Nitschke et al., 2004). Nonetheless, the observed limbic activation to stress in female subjects is more consistent with a ‘tend-and-befriend’ rather than a ‘fight-or-flight’ model. Within this theoretical framework, the observed ventral striatum activation might indicate an intrinsic neurobiological mechanism of the female brain to activate the reward system under stress, thereby down-regulating the ‘fight-or-flight’ response. As a result, both behavioral and CBF data showed a relatively blunt acute stress response between the low and high stress tasks in female subjects in our study. However, an alternative explanation would be that women were more stressed by the low stress task compared to men.
It is worth noting that considerable inconsistencies exist between the ‘tend-and-befriend’ model and our empirical data. Ventral striatum activation has been implicated in numerous processes, and is not a unique marker for involvement of the reward system (Poldrack, 2006). In our study, striatum CBF in female subjects was also associated with changes in negative emotion—perceived anxiety. The isolated fMRI environment is hostile to the formation of social attachment under stress. The ‘tend-and-befriend’ theory would also predict a differential endocrine response (e.g. cortisol and oxytocin) between the two genders, which was not observed in the present study. Similarly, the present finding of greater prefrontal and limbic activation in males and females, respectively should not be implicated with the sex stereotype in lay culture for the ‘emotional women’ and ‘rational men’. As suggested by several studies, the gender difference in emotionality per se may be an ill-posed question (Fischer, 1993; Barrett et al., 1998).
Compromised cortisol feedback, ruminative thinking and depression
Recent endocrine studies on the interaction between the reproductive system and the HPA axis demonstrate that females (compared to males) show resistance to negative feedback of both cortisol in the fast-feedback paradigm and dexamethasone in the standard delayed-feedback paradigm (Young and Altemus, 2004). In other words, female sex hormones, while attenuating the sympathoadrenal and HPA responsiveness, could lead to sluggish cortisol feedback on the brain and less or delayed containment of the stress response. Compromised cortisol feedback effects on HPA arousal in females has been proposed as a major neurobiological pathway mediating the tendency of women to develop depression (Young and Altemus, 2004). Compared to the male group, we observed fewer correlations between regional CBF and cortisol variations in the female subjects (only in dACC). This observation is in line with the theory of compromised cortisol feedback effects on stress responses in females. We hypothesize that persistent limbic activation following stress without adequate cortisol containment may be a potential neurobiological precipitant to depression in women. However, the causal role of cortisol in CBF responses to stress remains to be tested experimentally.
A somewhat related cognitive style more common in women than men that increases the risk for depression is ruminative thinking—repetitively and passively focusing on symptoms of distress and their possible causes and consequences (Butler and Nolen-Hoeksema, 1994). In the present study, the lasting stress response in females was mainly characterized by persistent activation of the ACC and PCC. The ACC is a primary region involved in attentional processing of emotion, self-assessment of the mental state, empathy and social exclusion as suggested by recent studies (Davis et al., 1997; Davidson and Irwin, 1999; Frith and Frith, 1999; Eisenberger et al., 2003; Singer et al., 2004). Functional alternations in PCC have also been reported in posttraumatic stress disorders (Nutt and Malizia, 2004). The persistent cingulate activation observed following stress in women might reflect a greater degree of emotional ‘rewinding’ (melancholy thinking) or reflection of own emotional traits in the females compared to males after completion of stress tasks, which is consistent with the tendency for ruminative thinking in women (Papadakis et al., 2006).
Relationship between prefrontal vs limbic activation
The association between negative and positive emotions and right and left-sided prefrontal activation respectively was originally based on electrophysiologic findings (Davidson and Irwin, 1999; Davidson et al., 2000). However, a laterality effect associated with emotional valence has not been conclusively supported by hemodynamic based neuroimaging studies (Wager et al., 2003). It is possible that perfusion fMRI, with its capability for visualizing sustained behavioral states and orbitofrontal CBF, may advance research in this respect. High levels of right-sided prefrontal activation have also been linked with negative affective style and suppressed immune function (Davidson et al., 2000; Rosenkranz et al., 2003). The observed CBF increase in RPFC, along with CBF reduction in LOrF, may provide a plausible neural mechanism underlying negative health consequences including hypertension, aggression, substance abuse and immune suppression often seen in men.
Emerging evidence suggests that the RPFC plays a major role in regulating negative emotions, especially in moderating and inhibiting dACC and amygdala hyperactivities associated with negative affect (Beauregard et al., 2001; Eisenberger et al., 2003; Kalisch et al., 2006; Lieberman et al., 2006). This hypothesis has several implications for the present study. Without the potential buffering/modulation effect of the RPFC, the persistent dACC activation following stress observed in female subjects might predispose women to mood disorders and depression. The lack of correlation between amygdala activity and stress in our study is somewhat surprising. However, with a relaxed threshold of P < 0.05, we observed both acute and lasting amygdala hyperactivity in female but not male subjects in regression analyses of CBF data with perceived stress (Supplementary Figure 6). Hippocampal CBF was positively correlated with perceived stress during the performance of stress tasks in the female group, while hippocampal CBF was negatively associated with perceived stress in the male group. The findings of RPFC and opposite limbic activation in the male and female groups, respectively merit further study, which may also address the reciprocal relationship between the left and right prefrontal cortex, as well as the RPFC with cingulate, amygdala and hippocampal neural activities using connectivity analyses.
The spatial localization of RPFC in our study matches closely with the rostrolateral prefrontal cortex implicated in self generation of higher order rules and strategies to support performance of complex cognitive tasks (Dagher et al., 1999; Christoff et al., 2003). The broad role of RPFC in higher order executive function, cognitive control, emotional regulation and attentional processes suggest that it may be a critical neural substrate mediating adaptation and coping under stress.
Gender differences in task strategy
Activation of RPFC and right parietal regions has been associated with various cognitive control tasks, including working memory, response selection and task switching, as well as inhibitory functions (Miller and Cohen, 2001; Aron et al., 2004). Ventral striatum along with several limbic regions have also been involved in learning in addition to tasks related to reward, motivation and emotion (Poldrack and Rodriguez, 2004). The different computational roles subserved by these brain regions may contribute to the observed gender differences in central stress responses. Although somewhat controlled in the regression analyses, this possibility (e.g. inhibiting incorrect responses in males and updating task strategies in females) cannot be completely ruled out, especially in the direct comparison of average stress responses between men and women. Nonetheless, recent studies suggest that there exists a higher degree of similarities between men and women in terms of capabilities in mathematics and science than the stereotyped difference (Hyde and Linn, 2006).
Alternative possibilities include the potential difference in stress coping strategies between men and women. Different neural pathways may be recruited across the spectrum of low to high stress (e.g. females may experience a high stress level, thereby recruiting greater limbic activation). Multiple stress tasks across several cognitive domains would be preferable to show convincing evidence regarding gender-specific neural correlates of psychological stress in future studies.
Other considerations
Our conjunction analyses revealed a small degree of overlap between the male and female stress pathways with a group-level threshold of P < 0.01. When the threshold was relaxed to P < 0.05, we observed a greater degree of overlap showing the involvement of limbic regions in males and poststress RPFC activation in females. Nevertheless, the RPFC response during stress tasks was still only observed in male subjects, supporting the notion that RPFC activation is a unique marker of the acute stress response in men. These findings suggest both qualitative and quantitative gender differences in brain activation to stress, with males and females sharing similar underlying neural processes, which are activated at different intensities in each gender. This hypothesis is also partly supported by the largely insignificant gender differences in correlation coefficients derived from ROI data.
In the present study, while the subjects’ physiological responses did not differ across genders, the psychological measures and neuroimaging data clearly showed sex related differences. The inconsistencies between physiological and psychological stress responses have been documented in previous studies (Kudielka and Kirschbaum, 2005). Our results suggest that many gender differences in stress reactivity may not be revealed by existing techniques relying primarily on assay of neuroendocrine data. Combining neuroimaging, behavioral and physiologic approaches could provide improved power in probing the neurobiological basis of psychological stress.
The present study has a few limitations. The menstrual phase of the female participants was not controlled. The observed female stress response can be considered as representing brain activation patterns averaged across the menstrual cycle. The stress tasks had to be performed in a fixed order to avoid emotional ‘carry-over’ effects, which raises time-related issues such as fatigue. Further, not all cognitive components could be well balanced between the high and low stress tasks. For instance, the infrequent (every 2 min) verbal harassment was absent during the counting backward condition. Due to time limitation, the poststress baseline condition was not long enough for cortisol to return to baseline. Because only part of the subjects had performance data, the relationship between behavioral performance and brain activation to stress will be addressed in future studies.
In summary, our neuroimaging data revealed systematic gender differences in the neural response to mild to moderate stress elicited by mental arithmetic tasks. The greater limbic activation (especially the ventral striatum) in females may be tentatively linked to the ‘tend-and-befriend’ stress model as opposed to the ‘fight-or-flight’ response in males. The acute RPFC response in males and persistent ACC activation in females adhere with the hypothesis that RPFC may regulate ACC and limbic hyperactivity. Variations in cortisol were associated with the asymmetric prefrontal activity in males, whereas the female limbic activation showed a lower degree of correlations with cortisol, supporting compromised cortisol feedback effects on the female stress response. Prolonged limbic activation to stress, without adequate RPFC regulation and containment by cortisol, might mediate the high propensity of women to depression. Given the sensitivity of stress responses to specific context and intensity, we are cautious to generalize the current finding to different types of stress. Nevertheless, our study may represent an important initial step in uncovering the neurobiological basis underlying the contrasting health consequences of psychosocial stress in men and women.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank Dr Heather Collins for assay of the salivary cortisol samples, which was supported by RIA/Biomarkers Core of the Diabetes Research Center DK 19525. This research was supported by a University of Pennsylvania Comprehensive Neuroscience Center pilot grant, NSF grant BCS0224007, NIH grants NS045839 and MH072576.
Footnotes
Conflict of Interest
None declared.
REFERENCES
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Robin L, Pietromonaco PR, Eyssell KM. Are women the ‘more emotional’ sex? evidence from emotional experiences in social context. Cognition & Emotion. 1998;12(4):555–78. [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–34. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock H. In Social Statistics. NY: McGraw-Hill; 1972. pp. 406–7. [Google Scholar]
- Butler LD, Nolen-Hoeksema S. Gender differences in response to depressed mood in a college sample. In Sex Roles. 1994;30:331–46. [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. European Journal of Pharmacology. 2003;463:235–72. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Charney DS. Pscyhobiological mechanisms of resilience and vulnerability: implicationis for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behavioral Neuroscience. 2003;117:1161–8. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Archives of General Psychiatry. 2000;57(1):21–7. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain. 1999;122(Pt 10):1973–87. doi: 10.1093/brain/122.10.1973. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Science. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. Journal of Neurophysiology. 1997;77:3370–80. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J. Technical aspects and utilities of fMRI using BOLD and ASL. Clinical Neurophysiology. 2002;113:621–34. doi: 10.1016/s1388-2457(02)00038-x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fischer AH. Sex differences in emotionality: fact or stereotype? Feminism & Psychology. 1993;3(3):303–18. [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology. 2007 Mar 21; doi: 10.1038/sj.npp.1301371. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25:661–7. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds - a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Leibenluft E, Santiago N, Haxby JV. Social and emotional attachment in the neural representation of faces. Neuroimage. 2004;22(4):1628–35. doi: 10.1016/j.neuroimage.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25:9309–16. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14:233–8. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hampel P, Petermann F. Perceived stress, coping, and adjustment in adolescents. Journal of Adolescent Health. 2006;38:409–15. doi: 10.1016/j.jadohealth.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Holden C. Sex and the suffering brain. Science. 2005;308:1574. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Linn MC. Gender similarities in mathematics and science. Science. 2006;314(5799):599–600. doi: 10.1126/science.1132154. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. Journal of Cognitive Neuroscience. 2006;18:1266–76. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny S, Ye FQ, Birn R, Braun AR. Comparison of continuous overt speech fMRI using BOLD and arterial spin labeling. Human Brain Mapping. 2005;24(3):173–83. doi: 10.1002/hbm.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Whyte J, Wang J, Rao H, Tang K, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: quantification of tonic CBF changes during sustained attention and working memory tasks. NeuroImage. 2006;31:376–85. doi: 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69(1):113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56(4):225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity to affective stimuli. Psychological Science. 2006 doi: 10.1111/j.1467-9280.2007.01916.x. In press. [DOI] [PubMed] [Google Scholar]
- Lundberg U. Stress hormones in health and illness: the roles of work and gender. Psychoneuroendocrinology. 2005;30:1017–21. doi: 10.1016/j.psyneuen.2005.03.014. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–8. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Research. 2000;886:172–89. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21(2):583–92. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL. Structural and functional brain changes in posttraumatic stress disorder. Journal of Clinical Psychiatry. 2004;65:11–7. [PubMed] [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30(1):80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Papadakis AA, Prince RP, Jones NP, Strauman TJ. Self-regulation, rumination, and vulnerability to depression in adolescent girls. Development and Psychopathology. 2006;18(3):815–29. doi: 10.1017/s0954579406060408. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Rodriguez P. How do memory systems interact? Evidence from human classification learning. Neurobiology of Learning and Memory. 2004;82(3):324–32. doi: 10.1016/j.nlm.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proceedings of the National Academy of Science USA; 2003. pp. 11148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress hormones: good and bad. Neurobiology of Disease. 2000;7:540–2. doi: 10.1006/nbdi.2000.0350. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–27. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–29. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Vapnik VN. The nature of statistical learning theory. 2nd. New York: Springer-Verlag; 1999. [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19(3):513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Science USA; 2005a. pp. 17804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude modulated continuous arterial spin labeling perfusion MR with single coil at 3T-feasibility study. Radiology. 2005b;235:218–28. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Annual NewYork Academy of Science. 2004;1021:124–33. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]