Abstract
Many recent studies and several reviews have highlighted the potential clinical applications of experimental pain testing (e.g., for predicting post-surgical pain, treatment responsiveness, etc.). However, the implementation of quantitative sensory testing of pain sensitivity on a broad scale is limited by requirements of time, equipment, and expertise, and their associated costs. One reasonable question is whether one can obtain, via self-report, a valid index of an individual’s pain sensitivity and pain tolerance. We analyzed data from a large number of subjects (n= 505) who had undergone standardized thermal pain testing, and found that while higher self-reported pain sensitivity was associated with higher scores on a measure of anxiety, no relationship was observed between subjects’ self-report of pain sensitivity and subjects’ actual pain threshold or tolerance. These findings suggest that circumventing psychophysical pain testing by assessing individuals’ self-reported pain sensitivity is unlikely to be a useful strategy.
Keywords: pain sensitivity, threshold, tolerance, self-report
Introduction
The large magnitude of individual differences in the perception of pain has been recognized for centuries (Fillingim 2005;Bonica 1991). This broad inter-individual variability in experimental pain responses such as pain thresholds, or ratings of standardized noxious stimuli, is temporally stable and approximately normally distributed in the general population (Edwards 2005). Numerous studies have revealed that psychophysical pain responses (i.e., ratings of calibrated noxious stimuli) closely parallel the magnitude of brain activation within the pain neuromatrix (Coghill et al. 2003), reflect genetic as well as environmental influences (Diatchenko et al. 2005;Zubieta et al. 2003), and are correlated with an individual’s clinical pain report (Edwards et al. 2005). Several recent reports have also indicated that sensitivity to painful heat stimuli (e.g., heat pain thresholds) is prospectively predictive of higher levels of post-surgical pain (Granot et al. 2003;Pan et al. 2006), a greater probability of developing a clinical pain syndrome (in combination with other pain testing modalities) (Diatchenko et al. 2005), and poorer responses to treatment for chronic pain (Granot et al. 2004;Edwards et al. 2006). In general, heat pain threshold and tolerance are significantly and strongly correlated with pain threshold and tolerance assessed via other modalities such as pressure (Petzke et al. 2003;Hastie et al. 2005;Bhalang et al. 2005), indicating that these factors form part of a “pain sensitivity phenotype”. If, as these studies suggest, the laboratory-based assessment of individual differences in pain sensitivity has the potential to prospectively predict individual differences in acute clinical pain, chronic pain, and pain treatment response, then the ramifications and clinical applications of these findings are broad and quite important. However, performing sophisticated quantitative sensory testing requires time, expertise, and equipment which are not readily available in many clinical settings. It would be helpful to know whether simply asking individuals about their pain sensitivity provides information similar to that obtained from experimental pain testing. In the present set of brief, simple analyses, we assessed whether an individual’s normative self-report (i.e., compared to the rest of the population) of pain sensitivity was correlated with standardized measures of thermal pain threshold and tolerance. In addition, as a validity check, we also evaluated the relationship between self-reported pain sensitivity and general psychological distress in a sub-sample of subjects.
Materials and Methods
A total of 505 generally healthy participants, ranging in age from 17 to 70, were recruited for various studies from several university campuses and surrounding communities. Some of these thermal pain data have been included in prior publications (Edwards & Fillingim 2001;Campbell et al. 2005;Edwards et al. 2004). The majority of the sample (60.6%) was female, and most (63.4%) subjects indicated their race as ‘white’. Participants underwent standardized testing of heat pain threshold and tolerance, as in previous studies (Edwards et al. 2004). In brief, contact heat stimuli were delivered using a Medoc Thermal Sensory Analyzer (TSA-2001, Ramat Yishai, Israel) with a 9 cm2 thermode placed on the volar forearm, as in prior studies (Edwards & Fillingim 2001;Edwards et al. 2003). Heat pain threshold and tolerance were assessed four times each using an ascending method of limits paradigm with a rate of rise of .5°C/Sec; subjects were asked to press a button “when the sensations ”first become painful“, and when the sensations ”become intolerable“ (i.e., for threshold and tolerance measures, respectively). Between trials, the thermode was repositioned on the volar forearm. All subjects provided verbal and written informed consent, and all procedures were approved by an Institutional Review Board.
Approximately 30-60 min prior to undergoing these thermal assessment procedures, subjects completed sets of questionnaires; while different questionnaires were used for different studies, the one questionnaire common to all of these participants was the Kohn Reactivity Scale, a general measure of responsivity or sensitivity to a variety of stimuli, which has been used in prior studies of experimental and clinical pain (McDermid et al. 1996;Campbell et al. 2005). A single question on this scale queries respondents specifically about pain; subjects rated their level of agreement, on a 5-point scale, with the following item: “Pain doesn’t bother me as much as it does most people”. Response choices ranged from ‘strongly disagree’ to ‘strongly agree’. We assessed the association, using analysis of covariance (ANCOVA), between responses to this item and heat pain threshold and tolerance after controlling for age, sex, and racial group. In addition, the simple Pearson correlations between this item and pain threshold and tolerance are presented.
Moreover, a subset of participants, n=268, completed the Anxiety subscale of the Profile of Mood States (POMS; Bi-Polar form), which is a 12-item measure of recent anxiety, tension, nervousness, etc., that shows strong correlations with other measures of negative affect (McNair et al. 1992). We examined the association between this subscale and the pain sensitivity item as a type of validity check.
Results
In this sample, mean heat pain threshold (mean= 43.1°C, SD= 4.0) and heat pain tolerance (mean= 47.5°C, SD=2.9) varied relatively widely across individuals, an observation that is nearly universal in psychophysical studies of pain (Edwards et al. 2005;Fillingim 2005). In terms of self-report on the questionnaire item “Pain doesn’t bother me as much as it does most people” (i.e., scored from 1-5), the distribution of scores approximated normality (see Table 1), and the modal response was “Neither agree nor disagree”. Simple correlations revealed that heat pain threshold was uncorrelated with self-reported pain sensitivity (r= .07, p> .10). Similarly, heat pain tolerance was minimally correlated with self-reported pain sensitivity (r=.10, p=.05). Findings were similar (i.e., non-significant correlations) when correlation coefficients were computed separately within the different sites of data collection.
Table 1.
Comparison of subjects as a function of self-reported pain sensitivity.
| Subject responses to the following item: “Pain doesn’t bother me as much as it does most people” | |||||
|---|---|---|---|---|---|
| Strongly Disagree (n= 28) | Disagree (n= 82) | Neither (n= 191) | Agree (n= 171) | Strongly Agree (n= 33) | |
| Age | 25.4 ± 13.8 | 29.7 ± 17.0 | 27.5 ± 14.7 | 30.6 ± 16.3 | 31.3 ± 17.1 |
| Sex (% Women) | 71.4 % | 69.5 % | 60.7 % | 54.4 % | 60.6 % |
| Race (% White) | 35.7 % | 59.8 % | 63.9 % | 69.0 % | 63.6 % |
The ANCOVA using heat pain threshold as the dependent variable revealed that female sex [F(1,497)= 25.2, p<.01], non-white race [F(1,497)= 12.4, p<.01], and younger age [F(1,497)= 5.9, p<.05] were associated with lower thresholds, as we have reported previously (Campbell et al. 2005;Edwards et al. 2004). Self-reported pain sensitivity was not related to heat pain threshold [F(4, 497)= 0.2, p= .96]. Similar findings were observed for the ANCOVA using heat pain tolerance as the dependent variable. Female sex and non-white race were again related to lower pain tolerance values (p’s< .01), though age was not associated with pain tolerance (p> .10). As with heat pain thresholds, self-reported pain sensitivity was unrelated to heat pain tolerance [F(4, 363)= 0.9, p= .47]. See Figure 1 for a depiction of these data.
Figure 1. Association of Self-Reported Pain Sensitivity with Heat Pain Threshold and Tolerance.

Note. Subjects were asked to rate their agreement with the following item: “Pain doesn’t bother me as much as it does most people”.
SD= ‘Strongly Disagree’, D= Disagree, N= ‘Neither Agree nor Disagree’, A= ‘Agree’, SA= ‘Strongly Agree’.
HPTH= Heat pain Threshold; HPTO= Heat Pain Tolerance
Data are presented as estimated means with standard deviations
Validity Check
The mean score on the POMS Anxiety subscale was 23.8 ± 6.9, well within the normal range for this non-clinical sample (McNair et al. 1992). As a validity check, we examined the association between negative affect and perceived pain sensitivity (see Figure 2); the ANCOVA revealed that less self-reported pain sensitivity was related to lower anxiety scores [F(4,260)= 3.1, p=.01], as was older age (p< .01).
Figure 2. Association of Self-Reported Pain Sensitivity with Anxiety.
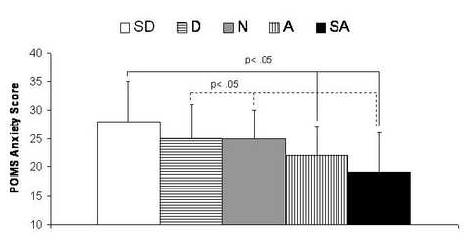
Note. Subjects were asked to rate their agreement with the following item: “Pain doesn’t bother me as much as it does most people”.
SD= ‘Strongly Disagree’, D= Disagree, N= ‘Neither Agree nor Disagree’, A= ‘Agree’, SA= ‘Strongly Agree’, POMS= Profile of Mood States.
Data are presented as estimated means with standard deviations. Results of post-hoc Least Significant Difference tests are included.
Discussion
The present results suggest that self-reported sensitivity to pain is unrelated to laboratory-derived measures of heat pain threshold and tolerance, which explained ≤ 1% of the variability in self-report of sensitivity to pain (i.e., R2 for correlations was .005 for heat pain threshold, and .01 for heat pain tolerance). While these analyses are limited to healthy participants undergoing a single experimental pain testing modality, the rather large sample size utilized for this laboratory pain study increases confidence in the findings. Moreover, the clinical relevance of heat pain responses has been demonstrated in recent reports indicating that sensitivity to painful heat stimuli (e.g., heat pain thresholds) differs across sex (Riley et al. 1998) and ethnicity (Edwards & Fillingim 1999), differentiates chronic pain patients from controls (Kleinbohl et al. 1999;Carli et al. 2002), reflects alterations in brain activity in the pain neuromatrix in inter- and intra-individual analyses (Coghill et al. 2003), is prospectively predictive of higher levels of post-surgical pain (Granot et al. 2003;Pan et al. 2006), and predicts poorer responses to treatment for chronic pain (Granot et al. 2004;Edwards et al. 2006). Thus, heat pain threshold and tolerance appear to capture a global “pain sensitivity” construct which has broad and important clinical correlates, but which is not associated with individuals’ self-report of pain sensitivity.
The outcome of this study should not be surprising in light of other data on discrepancies between self-report and other types of measurement. For example, it is well-known that individuals’ self-report of behaviors such as medication usage (Wang et al. 2004) and completion of diaries (Stone et al. 2003) corresponds less than perfectly with more objective indices of their actions. Indeed, even self-report of physical attributes such as height and weight show consistent inaccuracies (Doll & Fairburn 1998). At least one previous experimental pain study has reported findings similar to the present results; indices of self-perceived pain sensitivity and pain endurance were not significantly correlated with ratings of suprathreshold noxious thermal stimuli, although a measure of gender role perceptions of willingness to report pain (e.g., report of how willing a typical man and typical woman are to report pain) was associated with heat pain responses (Robinson et al. 2004).
Information regarding one’s own pain sensitivity, relative to the general population, would presumably be available to individuals by observation and comparison of their responses and other’s responses to common painful events such as injections, mild injuries, etc. However, individuals’ self-perceived pain sensitivity does not appear to be related to laboratory-derived measures of responses to standardized noxious stimuli. Interestingly, our data suggest that such perceptions of pain sensitivity are not random; those who reported more pain sensitivity tended to be more anxious than those who described themselves as less pain-sensitive, indicating that self-perceived pain sensitivity is likely influenced by negative affect. It would certainly be of interest to determine what other factors drive individuals’ formation of these self-impressions of pain sensitivity, and also to determine whether self-reported pain sensitivity is correlated with important prospective endpoints such as the development of clinical pain conditions. Unfortunately, this study is somewhat limited by the single-item measure of perceived sensitivity to pain; in future work, it will be necessary to employ a multi-item scale in order to definitively address these issues. Other limitations of the present study include the possibility of inter-observer differences across study centers, and the utilization of only one pain induction modality (i.e., noxious heat). Indeed, it is certainly possible that asking more specific questions about sensitivity to types of pain (e.g., cold pain, visceral pain) and then measuring a wider variety of pain responses (e.g., pressure pain threshold, ratings of pain due to hypertonic saline injection) would produce a closer correspondence between self-reported pain sensitivity and psychophysical pain responses to particular noxious stimuli. However, based on these initial findings, it appears that the clinical applications of quantitative sensory testing of pain sensitivity, such as predicting the intensity of post-operative pain or evaluating risk for the development of clinical pain syndromes, are unlikely to be substantially usurped by self-report measures of sensitivity to pain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhalang K, Sigurdsson A, Slade GD, Maixner W. Associations among four modalities of experimental pain in women. J.Pain. 2005;6:604–611. doi: 10.1016/j.jpain.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Bonica JJ. History of pain concepts and pain therapy. Mt.Sinai J.Med. 1991;58:191–202. [PubMed] [Google Scholar]
- Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Carli G, Suman AL, Biasi G, Marcolongo R. Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. Pain. 2002;100:259–269. doi: 10.1016/S0304-3959(02)00297-X. [DOI] [PubMed] [Google Scholar]
- Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc.Natl.Acad.Sci.U.S.A. 2003;100:8538–8542. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum.Mol.Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Doll HA, Fairburn CG. Heightened accuracy of self-reported weight in bulimia nervosa: a useful cognitive “distortion”. Int.J.Eat.Disord. 1998;24:267–273. doi: 10.1002/(sici)1098-108x(199811)24:3<267::aid-eat4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005;65:437–443. doi: 10.1212/01.wnl.0000171862.17301.84. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosomatic Medicine. 1999;61:346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J.Gerontol.A Biol.Sci.Med.Sci. 2001;56:M180–M185. doi: 10.1093/gerona/56.3.m180. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111:335–341. doi: 10.1016/j.pain.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Haythornthwaite JA, Tella P, Max MB, Raja SN. Basal heat pain thresholds predict opioid analgesia in patients with post-herpetic neuralgia. Anesthesiology. 2006 doi: 10.1097/00000542-200606000-00020. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114:315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Fillingim RB. Individual differences in pain responses. Curr.Rheumatol.Rep. 2005;7:342–347. doi: 10.1007/s11926-005-0018-7. [DOI] [PubMed] [Google Scholar]
- Granot M, Lowenstein L, Yarnitsky D, Tamir A, Zimmer EZ. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–1426. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- Granot M, Zimmer EZ, Friedman M, Lowenstein L, Yarnitsky D. Association between quantitative sensory testing, treatment choice, and subsequent pain reduction in vulvar vestibulitis syndrome. J.Pain. 2004;5:226–232. doi: 10.1016/j.jpain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hastie BA, Riley JL, III, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Kleinbohl D, Holzl R, Moltner A, Rommel C, Weber C, Osswald PM. Psychophysical measures of sensitization to tonic heat discriminate chronic pain patients. Pain. 1999;81:35–43. doi: 10.1016/s0304-3959(98)00266-8. [DOI] [PubMed] [Google Scholar]
- McDermid AJ, Rollman GB, McCain GA. Generalized hypervigilance in fibromyalgia: evidence of perceptual amplification. Pain. 1996;66:133–144. doi: 10.1016/0304-3959(96)03059-x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. POMS manual profile of mood states. EdiTS/Educational and Industrial Testing Service; San Diego: 1992. [Google Scholar]
- Pan PH, Coghill R, Houle TT, Seid MH, Lindel WM, Parker RL, Washburn SA, Harris L, Eisenach JC. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology. 2006;104:417–425. doi: 10.1097/00000542-200603000-00007. [DOI] [PubMed] [Google Scholar]
- Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J.Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin.Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Wang PS, Benner JS, Glynn RJ, Winkelmayer WC, Mogun H, Avorn J. How well do patients report noncompliance with antihypertensive medications?: a comparison of self-report versus filled prescriptions. Pharmacoepidemiol.Drug Saf. 2004;13:11–19. doi: 10.1002/pds.819. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]


