Abstract
Evidence suggests that dopamine (DA) agonists improve cognition after traumatic brain injury (TBI). Methylphenidate (MPH) is a DA agonist that blocks the dopamine transporter (DAT). Moreover, female sex hormones modulate DAT expression. Therefore, the purpose of this study was to evaluate how MPH affects behavioral performance in male and female rats. Under anesthesia, rats underwent either controlled cortical impact (CCI) or sham injury operations. Beginning post-operative day one, rats received daily intraperitoneal injections of MPH (5mg/kg) or saline. Beam balance (BB) and beam walking (BW) were assessed on postoperative days 1–5. Exploratory behavior was assessed using an open field free choice novelty (FCN) task on day 13. Spatial memory was assessed with a Morris water maze (MWM) task on days 14–20. Multivariate analyses showed TBI females performed better than TBI males on both motor tasks (P<0.05 both comparisons), and MPH improved BB performance for both male and female injury groups (P=0.05) compared to their respective vehicle treated injury groups. Multivariate analysis showed that MPH enhanced MWM performance (spatial learning and retention) after TBI. Significant improvements were noted in injured males treated with MPH compared to their vehicle control (P<0.05) with respect to improvements in memory acquisition and retention. Further, injured females treated with MPH had faster swimming speeds than all other groups (P<0.05 all comparisons), and MPH increased activity in TBI females but not males in the FCN task (P<0.05). These results suggest that MPH is beneficial after TBI. However there are gender specific drug differences in behavioral performance and sensitivity to treatment with MPH that may have implications for treatment efficacy and dosing clinically after TBI.
Keywords: Traumatic Brain Injury, Gender, Methylphenidate, Spatial Learning, Behavioral Performance
1.0 INTRODUCTION
An estimated 5.3 million Americans currently live with disabilities resulting from traumatic brain injury (TBI) [33]. Although females comprise approximately 25% of the population with TBI, relatively few studies exist evaluating how gender and sex hormones affect pathophysiology, recovery, and response to therapeutic or pharmacological intervention [30].
Altered neurotransmission is one of many sequelae associated with TBI. Previous work suggests ongoing regional alterations in tissue catecholaminergic concentrations after experimental TBI [19]. Studies from our laboratory have demonstrated chronic alterations in frontal cortex dopamine (DA) system proteins after experimental TBI, including increased tyrosine hydroxylase [43], a protein critical in the synthesis of DA, and decreased DA transporter (DAT), a protein critical for reuptake of DA into presynaptic terminals [44]. Additional work suggests that striatal DAT levels are decreased in males after TBI [38]. One possible theory for these observations is that the protein changes outlined are compensatory in nature to help restore neurotransmission after injury. However in vivo electrochemical monitoring after CCI suggests that striatal DA neurotransmission remains impaired [38]. Moreover, several behavioral studies suggest functional hypodopaminergia after experimental TBI with controlled cortical impact (CCI) by reporting that daily administration of DA receptor agonists including methylphenidate (MPH) [14], bromocriptine [13], and amantadine [6] improve spatial learning in the Morris Water Maze (MWM).
Evidence that nigro-striatal-cortical system function is impaired in humans following TBI is largely based on reports that DA agonists, including the DAT inhibitor MPH, can be beneficial in attenuating cognitive deficits. MPH has been shown in some small studies to affect verbal fluency [9], attention [17], and information processing speed [41,42] in patients with TBI. Based on these and other studies, treatment guidelines have now been outlined for MPH in treating attention and processing speed and well as for memory deficits and general cognitive dysfunction [40]. One small study also suggests that MPH treatment improves rate of functional recovery [22]. Physiological DA system dysfunction has been reported in humans where striatal DAT binding was decreased in patients after severe TBI [7].
A growing body of literature with experimental models of CNS injury suggests that female sex hormones provide acute neuroprotection against multiple aspects of the secondary insult [28]. Clinically, gender has also been shown to be an important determinant of cerebrospinal (CSF) levels of markers of excitotoxicity, ischemia, and oxidative stress [34]. Sex differences in brain structure and function of multiple neurotransmitter systems are also mediated by gonadal hormones [18]. Estrogens exert a significant influence over DA system regulation through genomic and nongenomic mechanisms [11]. DAT binding site density is reportedly higher in females than males in animal studies and can fluctuate with the estrous cycle [20]. DAT binding density with single-photon emission tomography imaging (SPECT) scanning has also been demonstrated clinically to be higher in females than males and is independent of age [16]. These findings corroborate additional findings from animal studies that show both increased striatal DAT density and increased DAT mRNA levels in DA cell bodies of the SN in females compared to males [25]. Moreover, sex hormones also affect behavioral responses to drugs such as amphetamine and cocaine. [3,4,21]
Despite the known influences of female sex hormones on TBI pathophysiology and modulation of DA systems, no studies exist evaluating how gender influences the efficacy of DA agonists in promoting recovery. Therefore, the primary purpose of this study was to evaluate the effectiveness of MPH in improving recovery after CCI injury in both male and female rats and whether or not differences occur between gender groups with respect to effectiveness and behavioral sensitivity with daily treatment.
2.0 MATERIALS AND METHODS
2.1 Animals
All the procedures for this study were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee, in accordance with the standards of the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals. Adult male (n=48) and cycling female (n=70) Sprague-Dawley rats (Hilltop laboratories, Scottsdale PA) were used. Females were an average of 79.8±4.6 days old at the time of injury and weighed between 241–292 grams. Males were an average of 59.14±3.17 days old and weighed between 302–369 grams. These age and weight ranges were chosen in order to have comparable body weights with minimal age differences between genders. All animals were housed with a 12-hour light/dark cycle and provided food ad libitum. Although the role of daily MPH treatment on TBI groups was of prime interest, eight treatment groups were evaluated in this study: male injury vehicle (MIA), male sham vehicle (MSA), male injury MPH (MIB), male sham MPH (MSB), female injury vehicle (FIA), female sham vehicle (FSA), female injury MPH (FIB), female sham MPH (FSB).
2.2 Estrous Cycle Staging
Vaginal cytologies were completed each morning for approximately one week prior to CCI or sham surgery in order to ensure that females were cycling. The morning of surgery, cycle status was classified as proestrous (high hormone state) or nonproestrous (low hormone state). Estrous categories for animals were classified based on cytological characteristics classically representative of each of the stages of the estrous cycle [29]. Despite previous work by our group suggesting that estrous stage at the time of injury does not significantly impact behavioral performance after CCI [39], we attempted to have comparable numbers of proestrous and nonproestrous females across each injury and treatment category for this study (47 females non-proestrous vs. 23 females proestrous).
2.3 Controlled Cortical Impact Device
The CCI injury device, described in detail by Dixon et al. [5], consists of a small (1.975 cm) bore, double-acting stroke-constrained pneumatic cylinder with a 5.0 cm stroke. The cylinder is rigidly mounted on a crossbar in an angled position. The lower rod end has an impact tip with a diameter of 6 mm, and the upper rod end is attached to the core of a linear velocity displacement transducer (LVDT) (Schaevitz Model 500 HR, Macrosensors, Howard A. Schaevitz Inc., Pennsauken NJ). The impactor tip is pneumatically driven at a pre-programmed velocity, depth, and duration of tissue deformation. The velocity of the cylinder is controlled by gas pressure and measured directly by the LVDT.
2.4 Surgical Procedures
During the surgical procedures, all animals received gas anesthesia initially using 4% isoflurane and a 2:1 N2O/O2 followed by 1–1.5% isoflurane. After anesthesia, each rat was secured in a stereotactic frame and a craniotomy was made between lambda and bregma, exposing the dura over the right parietal cortex. Cortical injury was performed with 2.7mm tissue deformation and an impact velocity of 4m/s. Sham animals underwent the same procedures, with the exception of the impact. Core body temperature was maintained at 37±0.5°C during surgery. Post surgical righting reflexes were monitored after completion of surgical procedures and removal from anesthesia to determine the extent of neurological injury [5].
2.5 Drug Treatment
Methylphenidate Hydrochloride (MPH) (Sigma Aldrich Inc. St. Louis, MO) was administered via intraperitoneal (i.p.) injection at a dose of 5 mg/kg. MPH was prepared by dissolving it in normal saline solution (5 mg/ml). Daily dosing with methylphenidate or saline (0.9% saline, equivalent volume) began one day after injury and continued through the duration of behavioral testing (20 days after injury). The reported half-life of MPH is approximately 2 hours [15], and animals were dosed each day approximately 15 minutes prior to beginning each behavioral task or habituation session described below.
2.6 Motor Function Testing
Gross vestibular motor function was assessed using a balance beam task [39]. For this task, each rat was given three daily trials to balance on a narrow (1.5 cm width) wooden beam. For the first five days after surgery, the latency that each animal remained on the beam, up to a maximum of 60 seconds, was recorded. The daily beam balance latency for each rat was calculated as the average of the three trials.
The beam-walking task was utilized to evaluate fine motor coordination and function using a paradigm [39] adapted from that first reported by Feeney et al. [8]. Two days prior to surgery, animals were trained to escape a loud noise by traversing a narrow beam (2.7 cm width) and entering a darkened goal box. Rats were pre-assessed on the beam-walking task on the day of surgery to establish their baseline performance. At pre-surgery and for the first five days after TBI or sham surgery, rats were given three daily trials and a maximum time of 60 seconds per trial to traverse the beam. The daily beam walking latency for each rat was calculated as the average of the three trials.
2.7 Open Field Novelty Task
Exploratory behavior and activity was evaluated using an open field free choice novelty task (FCN) beginning post-operative day nine adapted from that reported by Rebec et.al. [24]. Previous reports have shown this task to be sensitive to stimulant induced changes in behavior and activity [23]. On days 9–12 (first four days of testing), each rat was randomly assigned to be habituated for 30 minutes in one of two compartments of a (71 cm X 48 cm X 46 cm) enclosed chamber, separated by a partition. One compartment of the chamber was painted dark green and the other painted dark blue. Unpublished data from our group with this task using both a light and a dark colored compartment in the chamber indicated a color bias for animals to spend more time in the dark chamber during novelty testing, regardless of injury status or the chamber in which they were habituated. Different shapes were placed on the walls of each compartment, and marbles were placed in the novel compartment to encourage exploratory behavior on day 13. On post-operative day 13, rats were placed in the habituation compartment for 30 minutes, and afterward the partition was removed allowing access into the novel compartment. Animals were then observed continuously for 15 minutes. The number of active behaviors displayed by each animal (grooming, rearing, repetitive head movements) was recorded for the 15 minute interval. In addition, latency to enter the novel environment, number of entries between compartments, and time spent in the novel environment was recorded. The FCN testing apparatus and objects used were disinfected between each animal evaluation.
2.8 Morris Water Maze Testing
Cognitive testing was assessed using the spatial memory paradigm of the MWM beginning 14 days after surgery [10]. This task has been used in the past to detect improvements in spatial learning with chronic interventions after CCI [13, 14, 37]. Each animal underwent five days of acquisition trials. On each of these days, rats received four daily trials to find a hidden platform consistently placed in one quadrant of the pool. Animals began each trial in a different cardinal position of the maze and utilized constant spatial cues to find the platform. A maximum latency of 120 seconds to find the platform was allotted for each trial, and there was a four-minute rest period between trials. Rats not finding the platform in the allotted time were manually guided to it and placed on the platform. All rats were allowed to remain on the platform for 30 seconds at the conclusion of each trial. The daily latency for each rat during acquisition trials was calculated as the average of the four trials. On day 19, the hidden platform was removed, and each rat was subjected to two (60 second) probe trials with a four minute intertrial interval. Since first trial performance may affect performance on the second trial, data from the first trial was used for analysis. Swimming speed was measured on day 19. Swim speed was assessed using a video analysis system (Chromotrack; San Diego Instruments, San Diego CA)
2.9 Statistical Approach
The data are presented as mean ± standard error of the mean (SEM). Differences between groups with respect to return of acute neurological reflexes post surgery were analyzed using Student’s t-test. The data obtained from the motor and MWM behavioral tasks utilizing repeated measures were analyzed using mixed effects multivariate regression modeling. This approach was chosen to account for the effects of time, in conjunction with gender, treatment, injury status, and other task specific covariates on performance. Multivariate linear regression was utilized to assess the impact of injury, gender, and drug treatment on swim speed and probe trial performance in the MWM as well as several aspects of exploratory behavior in the FCN task (percent time spent in the novel environment, total activity, and number of entries between compartments). In each case, all two way interactions between independent variables were explored for their effect on the outcome. Only significant interactions for each model are reported. Appropriate linear contrasts were used for selected pair-wise comparisons to further explore the main effects of MPH treatment and significant interactions with MPH on the outcome. Female rats in each estrous category of each injury and treatment group were combined for final analysis after preliminary analysis revealed no significant differences in behavioral performance between estrous groups in each category. Adjusted mean comparisons derived from the multivariate models as well as individual group raw means are presented ± SEM. SAS (Cary NC) was used for all regression analyses, and a p-value of ≤ 0.05 was considered statistically significant.
3.0 RESULTS
3.1 Acute Neurological Reflexes
There were no significant differences in righting reflexes among injured groups with respect to either gender or treatment, indicating that severity of injury was equivalent (injured vehicle females 388.9±14.1 seconds, injured MPH females 363.6±12.1 seconds, injured vehicle males 364.6±11.2 seconds, injured MPH males 363.8±7.6 seconds; p>0.05 for all comparisons).
3.2 Motor Performance
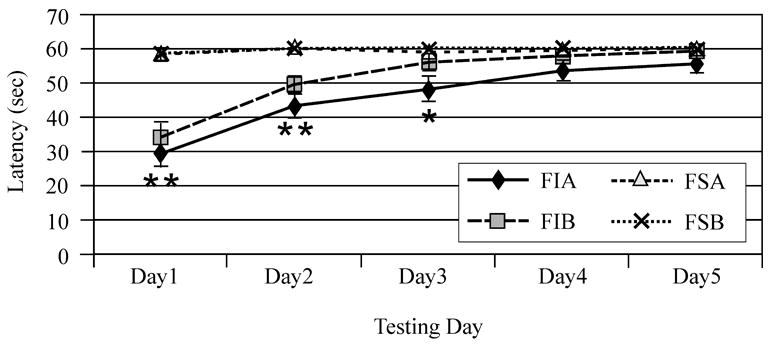
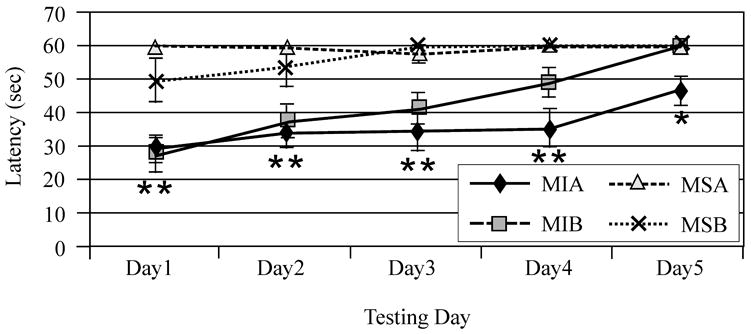
Mixed effects multivariate regression modeling revealed a significant injury (F-value 66.44; P<0.0001) day (F-value 27.28; P<0.0001), and injury*day (F-value 17.66; P<0.0001) effect for beam balance performance. All injury groups performed worse than sham groups but did improve their performance over time. Additionally, the multivariate model showed a significant gender effect (F-value 7.40; P=0.008) and gender*injury interaction (F-value 4.33; P=0.04) with injured females, out performing injured males on this task. Interestingly, treatment with MPH improved beam balance for both the injured male and female groups as reflected by a significant treatment*injury interaction noted in the multivariate model (F-value 3.89; P=0.05) (Figure 1A). Pair-wise comparisons showed that TBI females treated with MPH had improved recovery such that this group was not significantly different from sham by day three post injury, while vehicle treated TBI females performed similarly to sham females by day four of testing. In comparison, pair-wise analysis of beam balance performance showed that injured males treated with MPH were not significantly different from sham by day five of testing while vehicle treated injured males continued to have deficits compared to sham groups on day five of testing (Figure 1B/C). Mixed effects multivariate analysis also revealed a significant injury (F value 181.56; P<0.0001), day (F value 73.67; P<0.0001), and group*day (F value 54.37; P<0.0001) effect in beam walking performance. All TBI groups performed worse than sham groups, but did improve their performance over time (Figures 2A/B). Treatment with MPH (F-value 1.24; P=0.27) and pre-surgery beam walking latencies (F-value 0.35; P=0.55) did not significantly impact beam-walking function for experimental groups. Female gender was a significant factor in multivariate analysis with decreased latency to traverse the beam, even after accounting for pre-injury assessment latencies, for both injury and sham groups (F value 5.17; P=0.02). Further, multivariate analysis suggests a significant gender*day interaction (F value 3.36, P=0.01), where return to baseline profiles across days, particularly for the injured groups, differed significantly across days for males and females (Figure 2C).
Figure 1.
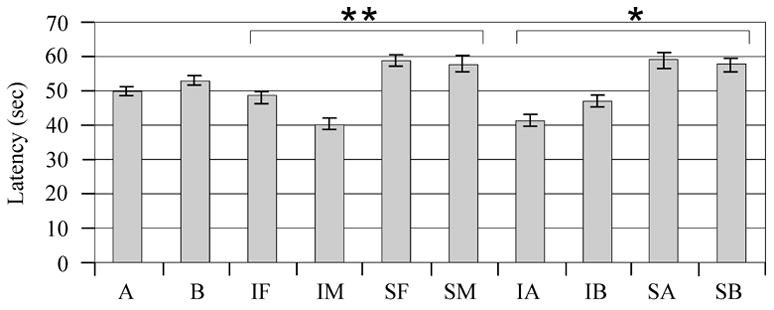
Beam Balance Latency performance on days 1–5 after injury. A) adjusted mean latencies to remain on the beam across testing days. There was a significant interaction between injury status and gender (**P=0.04) and injury status and treatment (*P=0.05). B) Raw group mean beam balance latencies for females on each testing day. **P<0.05 Injury vehicle and Injury MPH group vs. sham. *P<0.05 Injury vehicle vs. sham. C) Raw group mean beam balance latencies for males on each testing day. **P<0.05 Injury vehicle and Injury MPH group vs. sham. *P<0.05 Injury vehicle vs. sham. F=Female, M=Male, I=Injury, S=Sham, A=Vehicle, B=MPH.
Figure 2.
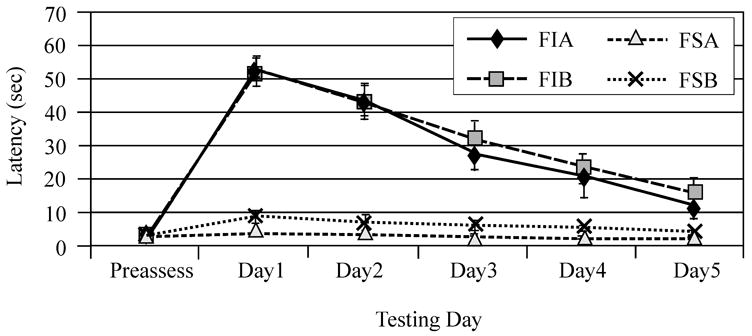
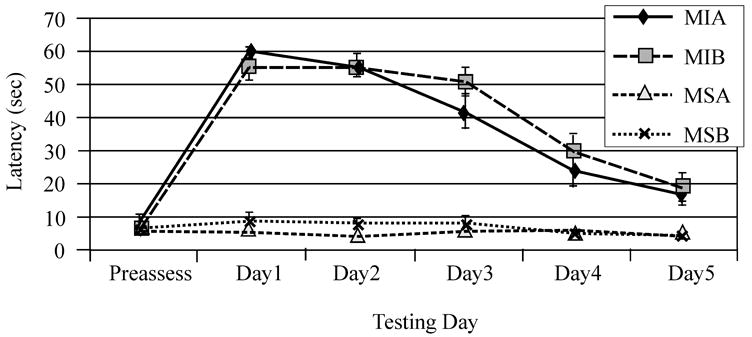

Beam walking performance pre-injury and days 1–5 after injury. A) Raw group mean beam walking latencies for females on each testing day. B) Raw group mean beam walking latencies for males on each testing day. C) Adjusted mean latencies to traverse the beam across testing days. There was a significant interaction between gender and testing day for latency to traverse the beam (*P=0.01). F=Female, M=Male, I=Injury, S=Sham, A=Vehicle, B=MPH
3.3 Open Field Novelty Task
Injury status, gender, and drug treatment did not significantly affect percent time spent in the novel environment in multivariate regression analysis. Additionally, there were no significant main effects in multivariate analysis or pair wise comparisons for latency to enter the novel environment.
Multivariate analysis showed that injury status (F value 0.01; P=0.90) was not associated with number of entries between compartments. However, treatment with MPH (F value 14.15; P=0.008) appeared to increase the number of entries between compartments (Figure 3A). Additionally, gender (F value 0.04; P=0.85) was not significantly associated with number of entries between compartments, and there were no interactions between these variables that significantly affected this endpoint. Pair-wise comparisons show that MPH significantly increased the number of entries for injured females and sham males (P<0.05 all comparisons) (Figure 3B), suggesting that MPH treatment in these two groups primarily contributed to the main effect of MPH treatment on locomotion. Although injury status was not significant (F value 0.07; P=0.78), multivariate analysis showed that MPH (F value 7.47; P<0.001) and gender (F value 4.0; P<0.05) were associated with an increased number of active behaviors. Females and MPH treated groups had higher activity levels (Figure 4A). Pair-wise comparisons suggest that MPH significantly increased the number of active behaviors only for injured females (P<0.05), however trends were noted for MPH related increases in activity for all groups except injured males (Figure 4B). As such, injured females seemed to show a larger influence than injured males on the main effect of MPH treatment with activity in the FCN task.
Figure 3.
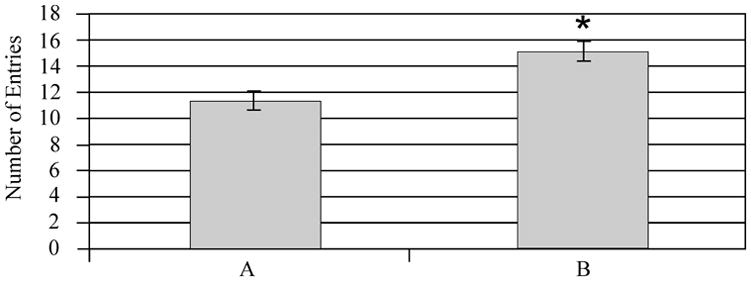
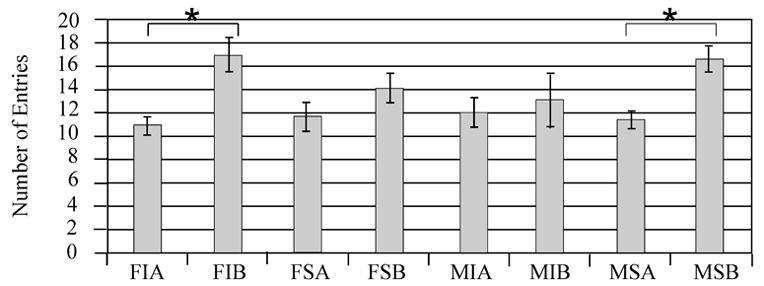
The Open Field Free Choice Novelty task: the number of crossings between familiar and novel environments. A) Adjusted mean comparison of number of crossing for MPH vs. vehicle treated animals *P=0.003 MPH vs. Vehicle. B) Individual group raw means of number of crossings. Injured females treated with MPH had the highest number of crossings. Pair-wise comparisons show that they had significantly higher numbers of crossings compared to injured females treated with vehicle (*P<0.05). Sham males treated with MPH also had significantly higher number of crossings compared to sham males treated with vehicle (*P<0.05). F=Female, M=Male, I=Injury, S=Sham, A=Vehicle, B=MPH.
Figure 4.

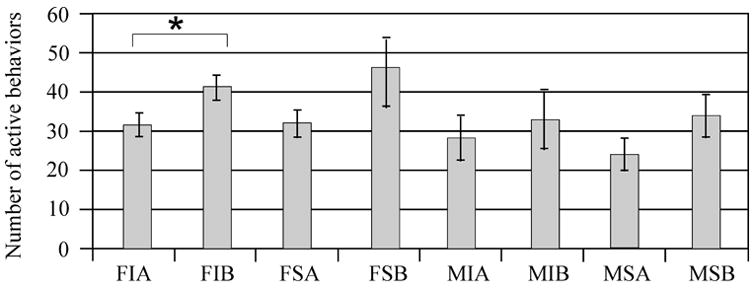
The Open Field Free Choice Novelty task: active behaviors. A) Adjusted mean comparison of the number of active behaviors for gender groups and MPH treated groups. *P<0.01 MPH vs. vehicle. **P<0.05 male vs. female. B) Individual group raw means of number of active behaviors. The numbers of active behaviors were highest for MPH treated injured and sham females. Pair-wise comparisons show that injured females treated with MPH had significantly greater number of active behaviors compared to their injured vehicle comparison group (*P<0.05). F=Female, M=Male, I=Injury, S=Sham, A=Vehicle, B=MPH
3.4 Morris Water Maze Performance
Mixed effects multivariate regression analysis showed a significant effect of injury status (F value 172.09; P<0.0001) and testing day (F value 65.37; P<0.0001) on latency to find the hidden platform with MWM testing. Gender (F value 0.71; P=0.40), swimming speed measurements obtained during the probe trial (F value 0.04; P=0.84), and MPH treatment (F value 0.44; P=0.51), as a single variable did not significantly impact MWM latencies in multivariate analysis. However, there was a significant injury group*treatment interaction (F-value 5.16; P=0.02), where MPH treated shams perform somewhat worse across testing days compared to vehicle treated shams and MPH treated injury groups perform somewhat better than vehicle treated groups (Figure 5A). Importantly, multivariate analysis also suggests a significant treatment*testing day interaction (F value 3.37; P<0.01) where MPH treated groups appear to have a larger decrease in latencies to find the hidden platform across days compared to vehicle treated groups. Adjusted means for each testing day in Figure 5B shows that MPH groups had slightly longer latencies, compared to vehicle groups, to find the hidden platform on the first day of testing, but they were performing slightly better than vehicle groups by the last day of testing.
Figure 5.
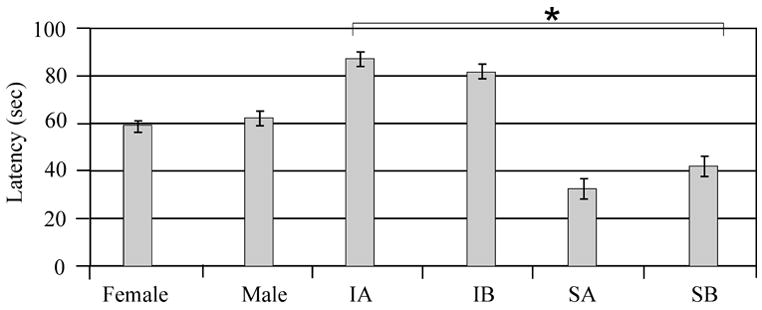

Morris Water Maze (MWM) performance beginning 14 days after injury. A) Adjusted mean comparisons of latencies to find the hidden platform across days. There was a significant interaction between injury and sham vs. treatment with MPH (*P<0.03). B) Adjusted mean comparisons of latencies to find the hidden platform on each testing day. There was a significant interaction between treatment with MPH and testing day (*P<0.001). F=Female, M=Male, I=Injury, S=Sham, A=Vehicle, B=MPH
Multivariate analysis showed a significant injury (F value 5.58; P<0.02), treatment (F value 6.14; P<0.02), and swimming speed (F value 3.96; P=0.05) association with learning. with sham animals and MPH treated animals having the largest change in platform latency between day 14 and day 18 (Figure 6a). In multivariate analysis, gender did not significantly impact change in platform latency (F value 0.14, P=0.70). Pair wise comparisons suggest that injury effects on latency change were significant for the male vehicle group, and latency change significantly improved with MPH treatment for injured males (p<0.05). Sham treated groups and injured females treated with MPH did not have a significant change in platform latency across days when compared to their respective vehicle groups. (Figure 6b).
Figure 6.
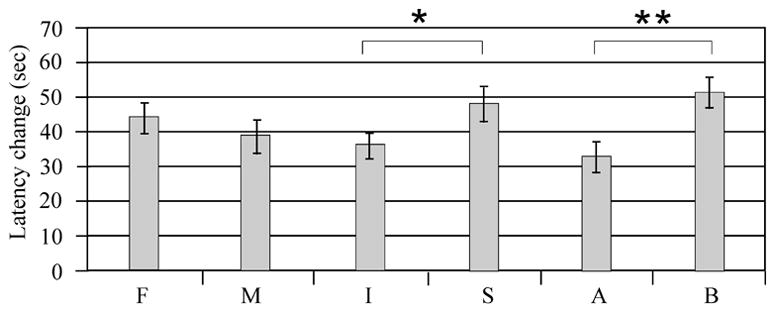
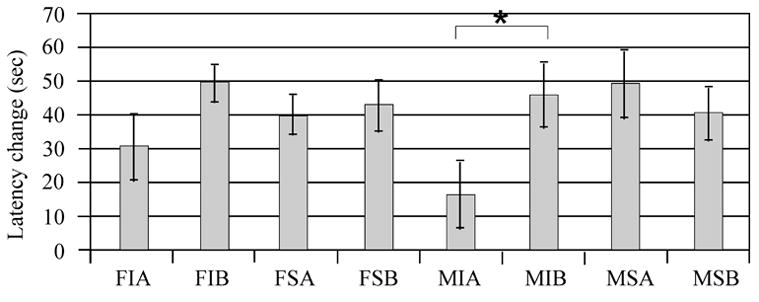
Latency differences in day 14-day vs. day 18 performance with time to find the hidden platform. A) Adjusted mean comparisons differences in latency to find the hidden platform day 14 vs. day 18. *P<0.02 injury vs. sham. **P<0.02 MPH vs. vehicle. B) Individual group raw means difference in latency to find hidden platform day 14 vs. day 18. Pair-wise comparisons suggest that injured males treated with MPH were the only group having a significant improvement in MWM performance (*P<0.05) compared to their respective vehicle group. F=Female, M=Male, I=Injury, S=Sham, A=Vehicle, B=MPH
Multivariate regression analysis for day 19 probe trial performance showed a significant difference in injury group performance (F value 14.76; P=0.0002), with injury groups spending significantly less time in the target quadrant than their respective sham groups. Additionally, there was a significant injury*treatment effect in multivariate analysis, with injury groups treated with MPH generally spending more time in the target quadrant compared to injured vehicle treated groups (F value 5.31; P=0.02) (Figure 7A). Swimming speed positively affected probe trial performance (F value 6.37; P=0.01), and gender did not significantly effect performance (F value 0.01; P=0.90). Pair wise comparisons suggest that time spent in the target quadrant was significantly increased with MPH treatment only for injured male group (p<0.05). Sham treated groups and injured females treated with MPH did not have a significant increase in time spent in the target quadrant when compared to their respective vehicle groups. (Figure 7B).
Figure 7.

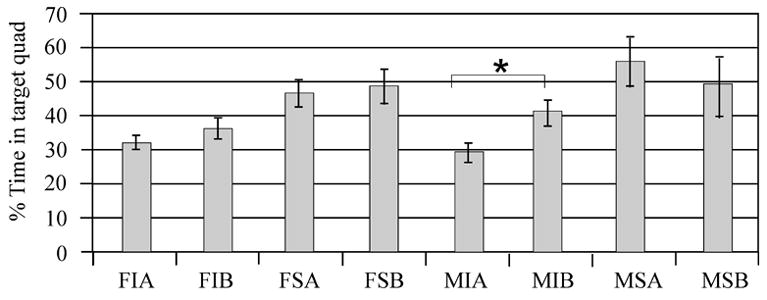
Probe trials performance day 19 measuring spent exploring the target quadrant. A) Adjusted mean comparisons of time spent in the target quadrant for injury groups, gender groups, and drug treatment groups. There was a significant effect of injury (p=0.0002) and an injury*MPH treatment (*P=0.02) on time spent exploring the target quadrant. B) Individual group raw means of time spent exploring the target quadrant. Pair-wise comparisons suggest that injured males treated with MPH were the only group having a significant improvement in time spent in the target quadrant (*P<0.05) compared to their respective vehicle group. F=Female, M=Male, I=Injury, S=Sham, A=Vehicle, B=MPH
Multivariate regression analysis showed a significant difference with injury group (F value 12.80; P<0.001), gender (F value 17.05; P<0.0001), and treatment with MPH (F value 6.89; P<0.001) on swimming speeds obtained during the probe trial. Injured groups, female groups, and MPH treated groups had faster swim speeds (Figure 8A). Pair-wise comparisons suggest that injured MPH treated females had significantly faster swim speeds than all other groups (P<0.05 all comparisons) (Figure 8B).
Figure 8.
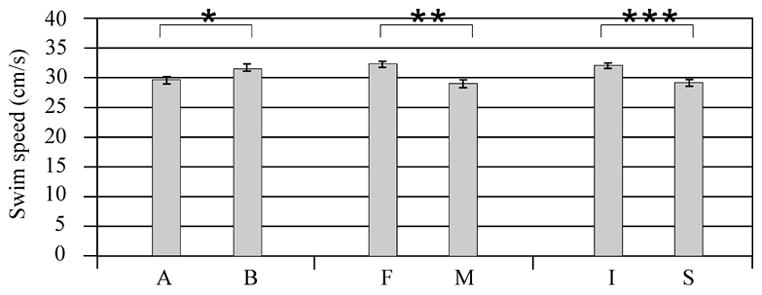

Swim speeds calculated during probe trial testing. A) Adjusted mean comparisons for injury groups, gender groups, and treatment groups. There was a significant effect of injury (***P<0.001), gender (**P<0.0001), and treatment (*P<0.001) on swimming speed performance. B) Individual group comparisons of swimming speed. Pair-wise comparisons show that injured females treated with MPH had higher swimming speeds compared to every other group (*P<0.05). F=Female, M=Male, I=Injury, S=Sham, A=Vehicle, B=MPH
4.0 DISCUSSION
TBI is a complex phenomenon disrupting function of multiple neurotransmitter systems. Identifying and evaluating promising treatment strategies that promote physical and cognitive recovery is of prime importance when caring for patients with TBI during and after rehabilitation. MPH is a common neurostimulant used by rehabilitation professionals in promoting recovery after TBI, but systematic evaluation of this drug on function and recovery in either experimental models or clinical populations is incomplete. To date, identifying possible gender differences in efficacy and dosing of this drug in the setting of TBI has not been addressed. In addition to a strong gender effect with injured females having better motor performance compared to injured males, the findings of this study show that daily treatment with MPH helps improve beam balance performance in both sexes. While MPH treatment had an overall beneficial effect on cognition for TBI groups, males appeared to derive a somewhat greater benefit than females with respect to MWM performance. MPH treatment at this dose also resulted in significant increases in locomotion and active behaviors in an open field for injured treated females as well as faster swim speeds in the MWM. Taken together, these data suggest that daily treatment with MPH was not as effective in improving cognitive recovery in injured females compared to injured males and that injured females were susceptible to more motor activation with this dose of MPH.
Results suggest that injured females performed better on the beam balance task than males. Despite adjusting for any possible differences in pre-injury beam walking performance, females also performed better than males on the beam walking task, with the primary differences in performance noted in the injured group. The presence of female sex hormones may be a key variable in mediating rate and extent of motor recovery between gender groups. For example, several studies have shown that progesterone is effective in decreasing cerebral edema early after injury [26, 27]. While estrous cycle stage at the time of injury may not be critically important for motor performance after CCI, we speculate that the presence of endogenous progesterone in females at the time of injury may be one factor contributing to better motor recovery for females by resolving pericontusional cerebral edema, particularly near primary and association motor cortex, early after injury.
Regardless of possible hormonally mediated neuroprotection on motor performance, treatment with MPH was associated with improvements in beam balance performance for both injured males and females. Injured males treated with MPH had similar beam balance performance as injured vehicle females. Since sham animals performed at or near ceiling levels with this task MPH did not measurably improve their beam balance ability. However, the data do show that MPH did not adversely affect sham beam balance performance. Reasons for the benefits of MPH on beam balance performance may be supported by previous work where treatment with either amphetamine [8, 31] or MPH [12] enhanced locomotor quality, including hind-limb foot-slip analysis, with beam walking after cortical ablation in both rats and cats. However, treatment with MPH did not improve beam-walking latency in our study. Quality of beam walking during this task was not included in our study design, and therefore, subtle deficits in locomotor quality associated with balance and motor coordination early after injury, may have gone undetected.
Previous reports by our group show improvements in spatial learning with the chronic administration of MPH after moderate CCI [14]. The results of our study support previous studies by showing significant effects of MPH across all treated groups in latency to find the hidden platform over the course of acquisition trial testing and increases in time spent searching in the target quadrant for the platform during the probe trial for injured groups. However pairwise analysis suggests that, at this dose, injured females did not have the same magnitude of improvement as injured males.
Subtle differences in baseline MWM performance for injured male and female vehicle groups may, in part, contribute to these findings. For instance, injured vehicle treated females had a 31 second improvement in performance over the five days of acquisition trials, while injured vehicle males had only a 16 second improvement (figure 6). However, both injury groups treated with MPH had an almost 50 second improvement. So while both injured males and females treated with MPH had comparable improvements in MWM latencies, only males performed significantly better than their respective injured vehicle group. Probe trial testing provides additional support for a gender difference in MPH treatment efficacy with spatial learning after CCI. Both injured males and females showed some improvement in probe trial performance with MPH treatment. However, treated females only spent an additional 4% time in the target quadrant, while treated males spent and additional 11% time in this quadrant. Interestingly, multivariate analysis suggests that CCI animals had faster swimming speeds compared to sham despite longer latencies to find the hidden platform. Moreover, injured females treated with MPH had faster swim speeds than any other group, suggesting increased motor activation with MPH treatment compared to other treated groups. Despite enhanced swimming speeds, injured females treated with MPH did not significantly improve with acquisition or probe trials compared to injured vehicle suggesting that MPH did not significantly impact the cognitive components of this task (e.g. spatial mapping, searching strategy) for this group.
There was a significant injury status*treatment interaction noted with MWM acquisition trials and probe trial performance, where MPH treated sham animals performed somewhat worse than vehicle treated sham animals. Although reasons for this are unclear, it may be that, in sham animals with an otherwise normal dopaminergic environment, chronic MPH treatment at this dose slightly impairs spatial mapping abilities, searching strategy, or both.
Dopamine systems are implicated in both novelty and reward behaviors. For example, Rebec [24] reports significant increases in accumbal and prefrontal DA levels as rats enter a novel environment. Since experimental TBI results in altered frontal cortex DA systems and novelty seeking may be related to the motivated state needed for new learning, we anticipated that cognitive abilities associated with exploratory behavior in a new environment would be diminished after TBI. Previous reports have also shown this task to be sensitive to stimulant induced changes in behavior and activity [23], and it was further anticipated that treatment with DA agonists would increase activity and exploratory behavior with the FCN task. The results of this study indicate that injury did not have an inherent effect on general activity levels or with time spent in the novel environment 13 days after injury. However, the task was sensitive in detecting a drug induced increase in locomotor activity and in discerning a gender and drug main effect, with significant increases in active behaviors observed in injured treated females compared to injured vehicle controls. Given that deficits observed with some tasks after CCI are transient and resolve over time, perhaps implementing this task earlier after injury might result in identifying inherent TBI mediated exploratory behavior deficits.
Estrogens have been shown not only to play an important role in regulating homeostatic function in DA pathways, but also to affect sensitivity to drugs such as amphetamine and cocaine [3, 4, 21]. Amphetamine stimulated release of DA is enhanced with estrogen and affected by stage of estrous cycle, while ovariectomy attenuates striatal DA release with amphetamine [2]. Sensitization to cocaine and increased locomotor behaviors have also been shown in conjunction with increased striatal DA release in estrogen treated rats [21]. Based on this literature, increased active behaviors for females with MPH in the FCN task, taken together with increased swim speeds for MPH treated injured females, suggest an increased behavioral sensitivity to the drug at this dose compared to males. The increased motor activation with treatment may have blunted the potential for MPH mediated cognitive improvements for injured females with spatial learning in the MWM task.
Injured males treated with MPH had no change in active behaviors. Further, they displayed significant improvements with the MWM task, indicating that daily treatment with this dose of MPH results in enhanced cognition without noticeable motor enhancement. Work by our group indicates that there are significant decreases in frontal cortex and striatal DAT levels in males chronically after injury that are not present in females [35]. Additionally, DAT concentrations are increased physiologically in females compared to males [25]. Lower DAT concentrations physiologically and post injury for males compared to females may modulate whether MPH treatment for a particular group in this study resulted primarily in motor activation or cognitive improvement. The effects of gender, injury, and MPH treatment on DAT concentration and DA neurotransmission should be a point of future study. We suspect that lower dosing of MPH for injured females may be required to augment learning and memory pathways without inducing stereotypical behaviors that may interfere with attention and learning processes. Future studies should also focus on a dose-response evaluation of MPH in improving motor and cognitive performance for both males and females in order to determine optimal dosing for each group.
While this initial study is the first, to our knowledge, to evaluate gender differences in DA agonist efficacy in an experimental model of TBI, some potential study limitations should be considered when interpreting these findings. Females were not all injured in the same phase of estrous, but rather, were injured in either proestrous or a nonproestrous stage of their cycle. We chose this approach due to its relevance in the clinical setting, where hormonal status at injury is mixed. However, post-injury righting reflexes and behavioral data were similar between estrous groups, allowing us to combine these groups for final analysis. Additional work from our group suggests that, among females, neither estrous stage nor estrogen/progesterone level at the time injury substantially impacts injury severity or behavioral performance. However, gender differences in motor tasks were noted, suggesting that while specific hormone levels at the time of injury may not significantly impact these tasks, the presence or absence of ovarian hormones in female versus male may influence motor task performance in the first five days after CCI [39]. We did not perform a dose response curve to determine the optimal dosing level to maximize improvements in behavioral performance for males and females. Future studies should evaluate this issue as well as provide a more comprehensive analysis of locomotor quality with beam-walking performance. Additionally, the effects of MPH on recovery were only evaluated while animals were receiving the treatment. Future work could also utilize dosing regimes where any lasting or residual effects of the treatment on recovery, once it has been removed, are studied. The gender differences in motor performance and in response to MPH treatment are thought to be secondary to hormonal influences on neuroprotection from secondary injury and on DA system modulation. However, without future studies including the evaluation of ovariectomized females and hormone replacement studies, this conclusion remains somewhat speculative.
The results of this study may have important implications for study design of pre-clinical and clinical trials in TBI. Other studies have shown gender differences in response to treatment with environmental enrichment and hypothermia, after experimental TBI [32,37]. Clinically, important gender differences have also been noted with cerebral spinal fluid markers of secondary injury including oxidative stress, excitoxicity and ischemia [34] as well as the responsiveness of these biomarkers to treatment with hypothermia [1,36]. Often pre-clinical trials do not include appropriate investigation of potential gender differences in treatment efficacy, a practice that may be detrimental to identifying potentially useful acute or chronic interventions in a mixed clinical population [30].
CONCLUSIONS
In summary, daily treatment with 5 mg/kg MPH improves motor function for both males and females after moderate CCI and improves spatial learning more effectively in males. Analysis of behavioral activity in a novelty task paradigm, in addition to MWM swim speed data, indicate a possible increase in drug sensitivity for injured females versus males at the dose studied. These findings may have implications for treatment efficacy and dosing in the clinical setting. Future work should focus on dose-response curves for optimal treatment efficacy in males and females and determining the role of sex hormones in DA system modulation post-TBI.
Acknowledgments
The authors would like to thank Dr. Robin Gandley, Magee Women’s Research Institute, for her technical expertise and guidance with estrous cycle staging and Bryan Bolinger for behavioral testing. This work was supported by NIH R03HD41399 and K08HD40833.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayir H, Marion DW, Kagan VE. Marked gender effect of lipid peroxidation after traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- 2.Becker JB. Gender differences in Dopaminergic function in striatum and accumbens. Pharmacol Biochem Behav. 1999;64(4):803–802. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 3.Becker JB, Molenda H, Hummer DH. Gender differences in behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann NY Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64(1):53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 5.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 6.Dixon CE, Kraus MF, Kline AE, Ma X, Yan HQ, Griffith RG, Wolfson BM, Marion DW. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor Neurol Neurosci. 1999;14(4):285–294. [PubMed] [Google Scholar]
- 7.Donnemiller E, Brenneis C, Wissel J, Scherfler C, Poewe W, Riccabona G, Wenning GK. Impaired Dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur J Nucl Med. 2000;27(9):1410–1414. doi: 10.1007/s002590000308. [DOI] [PubMed] [Google Scholar]
- 8.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 9.Gualtieri CT, Evans RW. Stimulant treatment for the neurobehavioural sequelae of traumatic brain injury. Brain Inj. 1988;2(4):273–290. doi: 10.3109/02699058809150898. [DOI] [PubMed] [Google Scholar]
- 10.Hamm RJ, Dixon CE, Dbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9(1):11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 11.Kelly SJ, Ostrowski NL, Wilson MA. Gender differences in brain and behavior: hormonal and neural bases. Pharmacol Biochem Behav. 1999;64(4):655–664. doi: 10.1016/s0091-3057(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 12.Kline AE, Chen MJ, Tso-Olivas DY, Feeney DM. Methylphenidate treatment following ablation-induced hemiplegia in rat: experience during drug action alters effects on recovery of function. Pharmacol Biochem Behav. 1994;48(3):773–779. doi: 10.1016/0091-3057(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 13.Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19(4):415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 14.Kline AE, Yan HW, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci Lett. 2000;280(3):163–166. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn CM, Schanberg SM. Metabolism of amphetamine after acute and chronic administration to the rat. J Pharmacol Exp Ther. 1978;207:544–554. [PubMed] [Google Scholar]
- 16.Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with (123I) FP-CIT SPECT in Healthy volunteers. Eur J Nucl Med. 2007;27(7):867–869. doi: 10.1007/s002590000279. [DOI] [PubMed] [Google Scholar]
- 17.Mahalick DM, Carmel PW, Greenberg JP, Molofsky W, Brown JA, Heary RF, Marks D, Zampella E, Hodosh R, von der Schmidt E. Psychopharmacologic treatment of acquired attention disorders in children with brain injury. Pediatr Neurosurg. 1998;29(3):121–126. doi: 10.1159/000028705. [DOI] [PubMed] [Google Scholar]
- 18.McEwan B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994;63(4):1426–1433. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- 20.Morissette M, Di Paolo T. Sex and estrous cycle variations of rat Striatal dopamine uptake sites. Neuroendocrinology. 1993;58(1):16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- 21.Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated Striatal (3H) Dopamine release. Brain Res. 1991;566(1–2):255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- 22.Plenger PM, Dixon CE, Castillo RM, Frankowski RF, Yablon SA, Levin HS. Subacute methylphenidate treatment for moderate to moderately sever traumatic brain injury: a preliminary double-blind placebo-controlled study. Arch Phys Med Rehabil. 1996;77(6):536–540. doi: 10.1016/s0003-9993(96)90291-9. [DOI] [PubMed] [Google Scholar]
- 23.Rebec GV, Bashore TR. Critical issues in assessing the behavioral effects of amphetamine. Neurosci Biobehav Rev. 1984;8:153–159. doi: 10.1016/0149-7634(84)90030-7. [DOI] [PubMed] [Google Scholar]
- 24.Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76(3):707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- 25.Rivest R, Falardeau P, Di P. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res. 1995;692(1–2):269–272. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- 26.Roof RL, Duvdevani R, Stein DG. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restor Neurol Neurosci. 1992;4:425–427. doi: 10.3233/RNN-1992-4608. [DOI] [PubMed] [Google Scholar]
- 27.Roof RL, Duvdevani R, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours still effective. Exp Neurol. 1996;138:246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- 28.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 29.Sharp PE, LaRegina MC. The Laboratory Rat. New York: CRC Press; 1998. [Google Scholar]
- 30.Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil. 2003;6(1):13–22. doi: 10.1080/1363849031000095279. [DOI] [PubMed] [Google Scholar]
- 31.Sutton RL, Hovda DA, Feeney DM. Amphetamine accelerates recovery of locomotor function following bilateral frontal cortex ablation in cats. Behav Neurosci. 1989;103(4):837–841. doi: 10.1037//0735-7044.103.4.837. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Bramlett HM, Dietrich WB. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184(2):1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- 33.Thurman DJ, Alverson C, Dunn KA, Guerrero JL, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;12(6):1027–1054. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Wagner AK, Bayir H, Ren D, Puccio A, Zafonte R, Kochanek P. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative stress after severe TBI: the impacts of gender, age, and hypothermia. J Neurotrauma. 2004;21(2):125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- 35.Wagner AK, Chen X, Kline AE, Zafonte RD, Dixon CE. Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exp Neurol. 2005;195(2):475–483. doi: 10.1016/j.expneurol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Wagner AK, Fabio A, Puccio AM, Hirschberg R, Li W, Zafonte RD, Marion DW. Gender associations with cerebrospinal fluid glutamate and lactate/pyruvate levels after severe traumatic brain injury. Crit Care Med. 2005;33(2):407–13. doi: 10.1097/01.ccm.0000153931.23488.dd. [DOI] [PubMed] [Google Scholar]
- 37.Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci Lett. 2002;334:165–168. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- 38.Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95(2):457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- 39.Wagner AK, Willard LA, Kline AE, Bolinger BD, Zafonte RD, Dixon CE. Evaluation of estrous cycle stage and gender on behavioral outcome after traumatic brain injury. Brain Res. 2004;998(1):113–121. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 40.Warden DL, Gordon B, McAllister TW, Silver JM, Barth JT, Drake A, Gentry T, Jagoda A, Katz DI, Kraus J, Labbate LA, Ryan LM, Sparling MB, Walters B, Whyte J, Zapata A, Zitnay G. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 1006;23(10):1468–501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 41Whyte J, Hart T, Schuster K, Fleming M, Polansky M, Coslett HB. Effects of methylphenidate on attentional function after traumatic brain injury.A randomized, placebo-controlled trial. Am J Phys Med Rehabil. 1997;76(6):440–450. doi: 10.1097/00002060-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Whyte J, Hart T, Vaccaro M, Grieb-Neff P, Risser A, Polansky M, Coslett HB. Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am J Phys Med Rehabil. 2004;83(6):401–20. doi: 10.1097/01.phm.0000128789.75375.d3. [DOI] [PubMed] [Google Scholar]
- 43.Yan HQ, Kline AE, Ma X, Pooghe-Peters EL, Marion DW, Dixon CE. Tyrosine hydroxylase. But not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. Neuroreport. 2001;12(11):2323–2327. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- 44.Yan HQ, Kline AE, Ma X, Li Y, Dixon CE. Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. Neuroreport. 2002;13(15):1899–1901. doi: 10.1097/00001756-200210280-00013. [DOI] [PubMed] [Google Scholar]


