Abstract
Although lumbar radicular pain is the most common chronic neuropathic pain syndrome, there have been few randomized studies of drug treatments. We compared the efficacy of morphine (15–90 mg), nortriptyline (25 –100 mg), their combination, and a benztropine “active placebo” (0.25-1 mg) in patients with chronic sciatica. Each period consisted of 5 weeks of dose escalation, 2 weeks of maintenance at the highest tolerated doses, and 2 weeks of dose tapering. The primary outcome was the mean daily leg pain score on a 0–10 scale during the maintenance period. Secondary outcomes included a 6-point ordinal global pain relief scale, the Beck Depression Inventory (BDI), the Oswestry Back Pain Disability Index (ODI) and the SF-36. In the 28 out of 61 patients who completed the study, none of the treatments produced significant reductions in average leg pain or other leg or back pain scores. Pain reduction, relative to placebo treatment was 14% for nortriptyline (95% CI= [−2%, 30%]), 7% for morphine (95% CI= [−8%, 22%]), and 7% for the combination treatment (95% CI= [−4%, 18%]). Mean doses were: nortriptyline alone, 84 +/− 24.44 (SD)mg/day; morphine alone, 62 +/−29mg/day; and combination, morphine, 49 +/−27 mg/day plus nortriptyline, 55 mg+/− 33.18 mg/day. Over half of the study completers reported some adverse effect with morphine, nortriptyline or their combination. Within the limitations of the modest sample size and high dropout rate, these results suggest that nortriptyline, morphine and their combination may have limited effectiveness in the treatment of chronic sciatica.
INTRODUCTION
Recent systematic reviews (Dworkin et al. 2003a; Finnerup et al. 2005) recommend a similar treatment algorithm for any neuropathic pain syndrome, regardless of etiology, with the drugs of first choice being tricyclic antidepressants, gabapentin or pregabalin, and opioids. However, these papers acknowledge the limitation that most of their conclusions are drawn from randomized trials in patients with diabetic neuropathy and postherpetic neuralgia. Extrapolation to neuropathic pain of other etiologies is unproven.
Lumbar radiculopathy caused by injury or irritation to lumbar nerve roots as a result of disc disease is the most common type of neuropathic pain syndrome, with a point prevalence of 4.5% in individuals over the age of 30 (Heliovaara et al. 1987). Surprisingly, there are virtually no repeated dose analgesic trials in this neuropathic pain condition. Two studies which included small subgroups of patients with lumbar radiculopathy suggested possible pain-reducing effects of nortriptyline in 5 patients (Atkinson et al. 1998) or fentanyl in 25 patients (Dellemijn et al. 1998). In a crossover study of 29 patients (Khoromi et al. 2005) topiramate was only marginally effective in treating chronic sciatica. In a large unpublished pharmaceutical industry study, pregabalin (Remmers et al. 2000) did not reduce chronic lumbar radicular pain, in contrast to similarly designed studies of pregabalin in diabetic neuropathy (Lesser et al. 2004; Richter et al. 2005) and postherpetic neuralgia (Dworkin et al. 2003b; van Seventer et al. 2006).
In view of the negative results in the pregabalin study, we chose to investigate the other two drug classes recommended as first choices for neuropathic pain (Dworkin et al. 2003) (Finnerup et al. 2005), tricyclic antidepressants (TCAs) and opioids. TCAs that inhibit both norepinephrine and serotonin reuptake inhibitors may be more effective than more selective reuptake blockers (Max et al. 1992) (Sindrup et al. 2003). Nortriptyline was selected instead of the more commonly studied amitriptyline based on a head-to-head comparison (Watson et al. l998) showing that nortriptyline, which may be better tolerated, reduced postherpetic neuralgia as well as amitriptyline. We included a combination of nortriptyline with an opioid based on the hypothesis that attacking multiple pain mechanisms simultaneously will improve the therapeutic ratio (Gilron and Max 2005). The design was similar to the Gilron et al. study (2005), which showed that the combination of gabapentin and morphine surpassed either drug alone when each treatment was titrated to maximum tolerated side effects (Gilron et al. 2005). We conducted a four-period crossover study of MS Contin, a long acting form of morphine, nortriptyline, their combination and placebo in patients with lumbar radicular pain. We predicted that both individual drugs would relieve pain compared to placebo, and that the combination would be even more effective.
METHODS AND MATERIALS
Study Population
This study was conducted at the NIH Clinical Center. The protocol was approved by the NIDCR Institutional Review Board. Patients were recruited between February 2001 and March 2004 through local newspaper advertisements. After screening by telephone and with a clinical visit, eligible patients signed an informed consent form.
Inclusion criteria
-
Evidence of lumbar radiculopathy, including pain in one or both buttocks or legs for 3 months or greater for at least 5 days a week and at least one of the following features on the side corresponding to leg pain:
Sharp and shooting pain below the knee;
Pain evoked by straight leg raising to 60 degrees or less;
Decreased or absent ankle reflex;
Weakness of muscles below the knee.
Sensory loss in L5/S1 distribution;
Electromyographic evidence for L4, L5, or S1 root denervation;
Imaging (MRI, CT/myelogram) evidence of nerve root compression in the lower lumbar region;
Average leg pain of at least 4/10 for the past month on a numerical scale of 0 to 10 where 0 represents no pain and 10 represents the worst possible pain(Jensen et al. 1986);
Willingness to refrain from making changes in non-study medications taken for sciatica, and
Age between 18 and 65 years at the start of the study.
The principal investigator (S.K) performed the clinical examinations. Sensory testing included responses to pinprick, light touch, vibration and joint movement.
Exclusion criteria
Serious medical illness involving other organ systems such as unstable angina disease, advanced diabetes, and cancer.
Prostatic disease requiring usage of urological medications
Pregnancy or lactation
History of depression requiring treatment with antidepressants within the 6 months preceding study participation or a score of 20 or greater on the Beck Depression Inventory(Williams and Richardson 1993) at the screening visit
History of narcotic or alcohol abuse
Narrow angle glaucoma
Seizure disorder
Fibromyalgia (Wolfe et al. 1990)
Pain of greater intensity in any other location than the low back or leg
Polyneuropathy and peripheral vascular disease associated with symptoms of numbness, or burning pain in the lower extremities
Allergy to morphine, nortriptyline or benztropine
Evidence for multisomatoform disorder as assessed by a 15 item questionnaire, the PHQ-15 (Kroenke et al. 1998))
Unwillingness to be tapered off of opioids and then maintained drug free for two weeks prior to randomization to study medications for participants on maintenance opioid regimen at the time of study enrollment
Imaging and laboratory evaluation
Patients submitted an MRI of the lumbosacral spine taken within a year prior to study enrollment or this was performed upon study entry. Two neuroradiologists blinded to the patients’ symptoms reviewed the films, commenting on degenerative disc or joint disease and other findings contributing to low back pain or radiculopathy according to definitions and classifications proposed by the American Society of Neuroradiology (Fardon and Milette 2001) . Patients were classified as lateral recess syndrome (LRS), neural foramen stenosis (NFS), canal stenosis (CS), or their combination if their clinical findings and MRI offered a consistent anatomical explanation of their root symptoms resulting from compression of roots due to degeneration of joint, facet and or disc. Patients with no visible root compression were classified as degenerative disc disease and/or degenerative joint disease ((DDD/ DJD).
Laboratory evaluation included a complete blood count with differential, sedimentation rate, antinuclear antibody titer, and rheumatoid factor to exclude inflammatory arthritis, cancer, and spinal infection.
Study Design
This was a single-center four-period, crossover, randomized trial comparing four treatments: sustained-release morphine, (MS Contin; Purdue Pharma, Stamford, CT); nortriptyline, (Dinamite Di Pharma, SPA, Milano, Italy); a combination of morphine and nortriptyline; and active placebo, benztropine (Sidmak Labs, East Hanover, NJ). Benztropine has no known effect on neuropathic pain but causes side effects such as dry mouth and mild constipation that mimic those of morphine and nortriptyline, which may therefore produce more effective blinding than inert placebo (Moulin et al. 1996; Watson et al. 2003).
Randomization was performed by the NIH Pharmaceutical Development Service. Patients were assigned by random numbers within blocks of four to one of four treatment sequences specified by a Latin square. During the MS Contin treatment period, each blue pill contained MS Contin 15 mg and each pink pill contained inert placecbo. During the nortriptyline treatment period, each blue pill contained inert placebo and each pink pill contained 25 mg of nortriptyline and. During the combination treatment period, each blue pill contained MS Contin 15 mg and each pink pill contained 25 mg of nortriptyline. During the placebo treatment period, each blue pill contained 0.25 mg of benztropine and each pink pill contained inert placebo. We considered the inert placebo to be sufficient in the treatment periods that included nortriptyline or morphine alone, because the active medication produced side effects.
Blue capsules were taken in the morning and at bedtime, and pink capsules were administered at bedtime. The maximum daily doses during both single drug and combination treatment periods were 90 mg for morphine, and 100 mg for nortriptyline. During week 1, patients were started on morphine 15 mg at bedtime or one morphine placebo capsule and if the patient tolerated this dose well, the dose was increased to one capsule twice daily on the fourth day of treatment. Over the next four weeks the morphine dose was increased by one 15 mg tablet per week at the beginning of each week. Patients were then maintained on the highest tolerated dose for two weeks before starting a taper. Nortriptyline was started at a 25 mg dose at bedtime at the beginning of the second week and this dose was increased by one 25 mg capsule at the beginning of the subsequent 3 weeks as tolerated. Doses of benztropine ranged from 0.25 – 1 mg/day.
All medications were tapered over a 10 day period and the patients were drug free for another 4 days prior to starting the next period. The patients were maintained at the maximum tolerated doses of blue and pink capsules for two weeks during each period if this was acceptable to the patient.
If patient’s pain level dropped to below 4 on the scale of 0 to 10 then the patient was asked to wait until his or her pain level increased to 4 or greater before starting the next treatment period. Patients also were asked to refrain from making changes in their analgesic medication regimen, or taking any opioids, SSRIs and tricylic medications outside of the protocol during the study. Anti-inflammatory medications and acetaminophen were allowed as rescue medications.
Patients were given a bottle of tablets consisting of docusate sodium 50 mg + sennosides 8.6 mg and were instructed to start taking two tablets at bedtime if they did not have a bowel movement two days after starting a new regimen or after a dose change, increasing the dose as needed to a maximum of 6 tablets per day.
Outcome Assessment
Each day at bedtime, patients rated their average back, leg and overall pain (leg + back), and worst back, leg and overall pain over the last 24 hours using 0–10 numerical scales (Jensen et al. 1986). For people who had pain in both legs, pain on the worse side was recorded. During the patients’ follow up visit to the Clinical Center at the end of each period, the study nurses collected the pain diaries and questionnaires. They then entered this data into a database from which all study outcomes were derived.
For the primary outcome we chose the comparison of mean scores for average leg pain during the two weeks of maintenance on morphine, nortriptyline, and their combination as compared to placebo. We chose leg pain over back pain because we were testing the principle that nortriptyline, morphine, and their combination relieve nerve root pain, and leg pain is more likely than back pain to reflect nerve root pathology.
Other outcome measures were: (1) Global pain relief (GPR) , (leg and back pain combined) using a categorical pain scale rating overall pain outcome as worse, no relief, slight, moderate, a lot, and complete relief; (2) the Oswestry Low Back Pain Disability questionnaire (Fairbank et al. 1980) (3) the Beck Depression Inventory (Williams and Richardson 1993) and (4) the 36-item Short Form of Health Survey (SF-36), a general health status instrument that measures the social, mental and emotional dimensions of health and illness (Ware 2000).
NNT and NNH
We used both global pain relief scores of moderate or better pain relief and a lot or better pain relief for each drug as response criteria to calculate the number needed to treat (NNT). NNT, the reciprocal of the absolute risk reduction, is an easily understood estimate of the effect of treatment and represents the therapeutic effort required to realize a specific clinical benefit (Irvine 2004). NNT = 1/ ((proportion of patients in the treatment group with GPR score meeting the response criterion) - (the proportion of patients in the placebo group with the GPR score meeting the response criterion)) (Irvine 2004)) Number needed to harm (NNH), was calculated by the same method based upon the number of patients who dropped out of each arm due to side effects. Because we pre-specified a single primary outcome measure, we considered all other statistical tests to be exploratory, and did not correct for multiple comparisons except for the multiple SF-36 subscales.
Blinding
The NIH Pharmaceutical Development Service was in charge of drug randomization. Patients and research staff were blinded to the randomization order. During the patients’ follow up visit to the Clinical Center at the end of each period, both the patients and the study nurses were asked to fill a blinding questionnaire. Patients were asked which of the four treatments they had just taken, how certain they were of their guess (not at all, somewhat and very), and what they based their guess on: side effects or pain relief.
Sample Size
We estimated the sample size to be 28 patients for alpha = 0.05 and beta = 0.20 using the sample size formula for a crossover study (Colton 1974). We used 2.9 as the standard deviation for the difference between drug and placebo based on a drug trial of opioids in patients with chronic low back pain (Schnitzer et al. 2000). We set the effect size to 1.6 on a scale of 0 to 10. This corresponds to approximately 30% pain reduction, which many patients with neuropathic pain consider to be a clinically meaningful difference (Farrar et al. 2001).
Adverse Effects
Adverse effects were elicited during twice weekly phone calls made by the study nurses throughout the study and they were rated as mild, moderate or severe. Patients who remained in pain were asked whether they could tolerate dose increases. For mild adverse effects, the patient’s dose was not increased further until the adverse effect resolved. Study medications were decreased to the previously tolerated doses whenever the patient developed an adverse effect of moderate intensity. For severe side effects the study medications were stopped or tapered based on patient and P.I. agreement.
Analysis
The preplanned analysis of the primary outcome was to compare patients’ mean scores for pain while taking the maximal tolerated dose of the assigned drug during week 4 and 5 across all treatments. The efficacy analysis included only patients who completed two or more treatment periods. Patients who dropped out during the first or second treatment periods were considered to be missing. Linear mixed models were set for the 28 study completers to assess whether there were any differences between the analysis of the pain outcomes between study participants with analyzable data vs. study completers. Patients who received at least one dose of any study medication were included in the analysis of adverse effects.
A linear mixed model in which “treatment”, “sequence”, and “treatment period” were the fixed effects and the patient (nested in the sequence of treatment periods) was the random effect was fitted with each of the pain outcome scores and all continuous secondary outcome scores. For all pain scores each model was adjusted for the baseline pain score and for the secondary outcomes, the model was adjusted for the baseline scores as well. In these models, “sequence” represents carry over effect and “treatment period” represents period effect (Hills and Armitage 1979; Milliken 1984). According to this method, the global difference among all treatments was first tested in the model. Only when this test was significant at the 0.05 level were pairwise comparisons made. Secondary continuous outcome measures were analyzed in a similar fashion. If “period” and/or “sequence” were significant at a p<0.05 in any particular model, then a different model was set where the data from only the first treatment was used.
An intent-to-treat analysis that included patients with either zero or one evaluable treatment was not done because of the crossover design and the haphazard pattern in missing scores from diaries for some study participants.
RESULTS
Subjects
Out of 1428 phone responders, 1362 had either back pain alone, pain location and quality that were typical of myofascial pain in the lower extremities (localized stiffness and dull aching pain in the buttock, thighs or legs), pain of lesser duration or intensity than required by the study, or were not interested in a drug treatment study. Sixty one of the sixty six respondents who qualified for the study based on the phone screening and were willing to participate in a drug trial met the inclusion and exclusion criteria.
Of 61 patients that underwent screening (figure 1), 6 declined to participate in the study prior to randomization to study medications. Nine patients dropped out during or after treatment with morphine alone. (Five withdrew because of side effects, 3 moved away and 1 withdrew for personal reasons); 3 dropped out during or after treatment with nortriptyline (2 withdrew because of side effects and 1 due to an unrelated medical problem); 6 patients dropped out during or after combination treatment (4 due to side effects and 2 for personal reasons); 9 dropped out during or after treatment with placebo (1 due to side effects, 3 because of lack of pain relief, 3 moved away, 1 due to an unrelated surgery, and 1 was excluded because of noncompliance). Examination of dropouts according to sequence shows that 9 patients dropped out of the morphine-placebo-nortriptyline-combination sequence; 7 patients dropped out of the placebo-combination-morphine-nortriptyline sequence; 4 patients dropped out of the combination-nortriptyline-placebo-morphine sequence; 6 dropped out of the nortriptyline-morphine-combination-placebo sequence. None of the patients who opted to participate in the study were on opioids during the two months prior to study entry.
Figure 1.
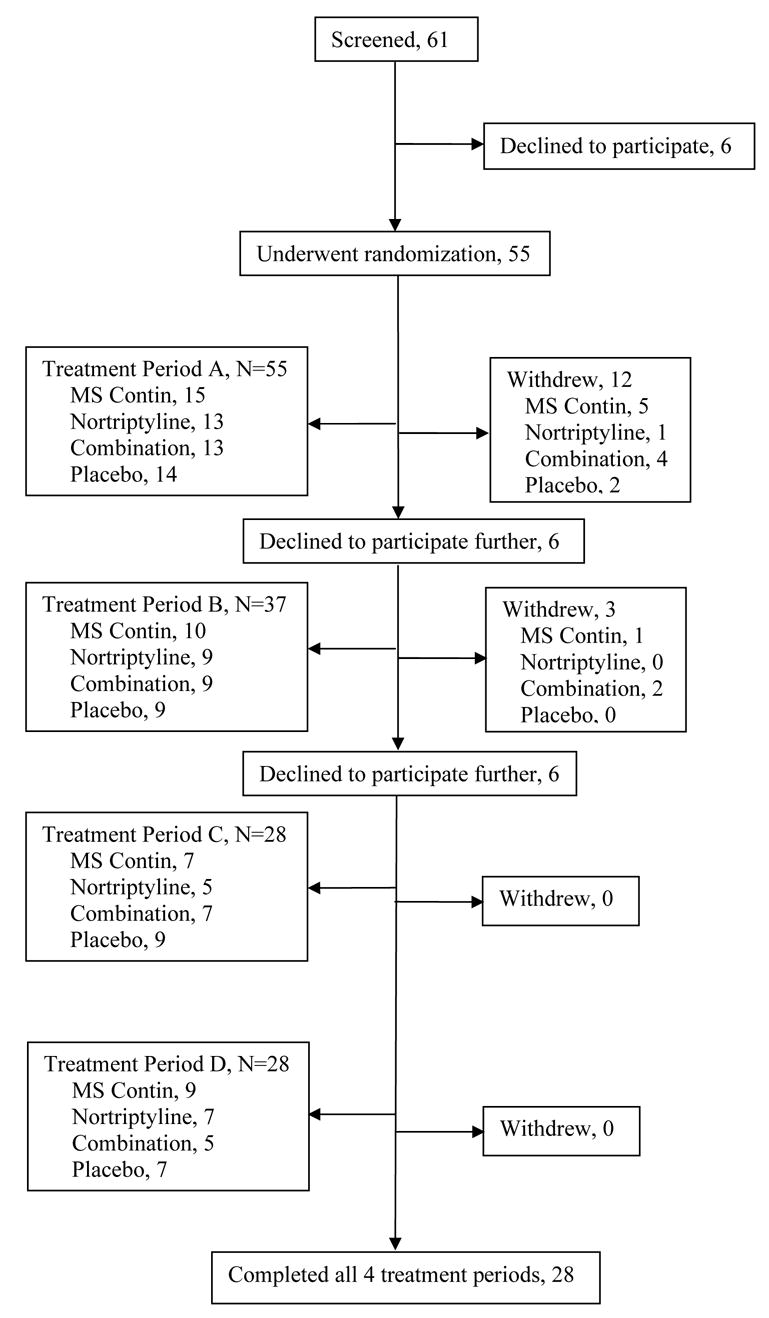
Study Algorithm
Twenty-eight patients completed the trial (Figure 1). One patient violated the protocol by taking opioids in addition to study medications during the second period of the study. He was therefore was excluded from the trial at that time and his scores were not included in the analysis There were no differences among the demographic and baseline characteristics of patients who underwent randomization, study completers and patients who completed at least two periods (Table 1). None of the patients were involved in litigation for compensation or disability for their pain at the time of screening and during the course of the study. None of the patients who participated in the study met critieria for multisomatoform disorder on the PHQ-15.
Table 1.
Demographics
| All Participants | 28 Study Completers | |
|---|---|---|
| Sex (F/M) | 25/30 | 14/14 |
| Age (years) | ||
| Median | 53 | 52.5 |
| Range | 19–65 | 30–64 |
| Work Status | ||
| (Non-retired/retired/unemployed or on disability) | 42/6/6 | 23/2/3 |
| Pain Duration (years) | ||
| Median | 5 | 5 |
| Range | 0.3–37 | 0.3–37 |
| Diagnosis | ||
| L4/L5 radiculopathy | 5 | 2 |
| L5/S1 radiculopathy | 40 | 22 |
| L4/S1 radiculopathy | 1 | 0 |
| L4/L5/S1 radiculopathy | 5 | 3 |
| L5 radiculopathy | 1 | 0 |
| S1 radiculopathy | 3 | 1 |
| MRI Diagnosis | ||
| Neural foraminal stenosis | 5 | 2 |
| Canal stenosis | 16 | 8 |
| Lateral recess syndrome | 6 | 2 |
| DJD/DDD | 18 | 11 |
| NFS, CS | 3 | 1 |
| LRS, CS | 3 | 2 |
| NFS, LRS | 1 | 1 |
| LRS, CS, NFS | 2 | 1 |
| Not Diagnosed | 1 | 0 |
| Prior Analgesic Medications | ||
| Non steroidal anti-inflammatory drugs | 44 | 22 |
| Opioids | 18 | 8 |
| Anticonvulsants | 6 | 3 |
| Antidepressants | 4 | 1 |
| Muscle relaxants | 11 | 3 |
| Other | 26 | 15 |
Mean doses were: nortriptyline alone, 84 +/− 24.44 (SD)mg/day; morphine alone, 62 +/−29mg/day; and combination, morphine, 49 +/−27 mg/day plus nortriptyline, 55 mg+/− 33.18 mg/day. None of the patients were taking quinidine, SSRIs or other significant inhibitors or inducers of cytochrome P450 2D6 isozymes, the key metabolizers of nortriptyline.
Effect of Treatments
There were no significant differences among the four treatments for average leg pain, the primary study outcome, either for patients who completed at least two treatments or for the 28 study completers. For the 28 study completers, percent reductions in average leg pain during the last two weeks of maintenance treatment were 7% for morphine (95% CI= [−8%, 22%]), 14% for nortriptyline (95% CI= [−2%, 30%]), and 7% for the combination treatment (95% CI= [−4%, 18%]) as compared to placebo for the 28 study completers(Table 2). Treatment effects on the other pain outcome scores for the 28 study completers were also not significant (Table 2).
Table 2.
Pain Scores in 28 Study Completers
| Average Leg* | Average Back | Average Overall | Worst Leg | Worst Back | Worst Overall | |
|---|---|---|---|---|---|---|
| Baseline | 4.9+/−2.43 | 4.5+/−2.4 | 5.0+/−2.25 | 5.7+/−2.48 | 5.3+/−2.44 | 5.7+/−2.2 |
| p value§ | 0.2 | 0.3 | 0.2 | 0.2 | 0.3 | 0.06 |
| Placebo | 3.7+/−2.7 | 3.8+/−2.5 | 3.9+/−2.4 | 4.6+/−2.8 | 4.4+/−2.6 | 4.8+/−2.5 |
| Morphine | 3.4+/−2.8 | 3.4+/−2.5 | 3.8+/−2.5 | 4.5+/−3.1 | 4.2+/−3 | 4.5+/−2.8 |
| Pain Reduction | 0.3 | 0.2 | 0.04 | 0.04 | 0.03 | 0.2 |
| below placebo | ||||||
| % Reduced | 7% | 5% | 1% | 1% | 1% | 4% |
| 95% CI | [−8%, 22%] | [−5%, 14%] | [−16%, 18%] | [−18%, 20%] | [−15%, 16%] | [−14%, 21%] |
| Nortriptyline | 3.0+/−2.7 | 2.9+/−2.4 | 3.2+/−2.4 | 3.8+/−3 | 3.8+/−2.9 | 3.8+/−2.9 |
| Pain Reduction | 0.5 | 0.4 | 0.5 | 0.6 | 0.4 | 0.6 |
| Below Placebo | ||||||
| % Reduced | 14% | 10% | 14% | 13% | 9% | 13% |
| 95% CI | [−2%, 30%] | [−4%, 25%] | [−2%, 30%] | [−5%, 31%] | [−5%, 23%] | [−3%, 30%] |
| Combination | 3.4+/−2.5 | 3.2+/−2.4 | 3.4+/−2.5 | 3.8+/−2.4 | 4.0+/−2.6 | 4.0+/−2.4 |
| Com-Pl see above | 0.3 | 0.2 | 0.4 | 0.6 | 0.2 | 0.7 |
| % Reduced | 7% | 7% | 11% | 12% | 6% | 15% |
| 95% CI | [−4%, 18%] | [−5%, 19%] | [2%, 20%] | [−1%, 26%] | [−8%, 20%] | [3%, 27%] |
Average leg pain was the primary outcome measure.
These p values were derived from the mixed linear model for the “treatment” variable fitted for the 6 pain outcome score categories whereas the 95% CIs were approximated from the paired t tests comparing each treatment to placebo.
Abbreviations: Mor-P: Morphine- Placebo; Nor-Pl: Nortriptyline- Placebo; Comb-Pl: Combination-Placebo.3
Among patients who completed at least two periods, treatment was significant only in the model for worst overall pain (p=0.02). A Bonferroni procedure was used to adjust for the 6 comparisons made within each pair of drug treatments. Adjusted pairwise comparisons showed that only the combination treatment was significantly better than placebo (p=0.04). The analysis of the first period data only for average back pain showed that treatment was significant in the overall model (p=0.005). After adjusting p-values in a similar fashion for multiple testing, pairwise comparisons showed that nortriptyline was significantly better than placebo (p=0.01) and morphine alone (p=0.02).
The primary analysis for the 28 study completers showed no significant period or carry over effect between any two possible sequences of treatment. Among the 34 patients who completed at least two periods, there were no significant period or carryover effects between any two possible sequences of treatments for any of the pain scores except for average back pain where period effect was significant (p= 0.04).
The number of patients reporting moderate pain relief or better on the Global Pain Relief questionnaires for, morphine, nortriptyline, the combination treatment and placebo were 13 (42%), 12 (40%), 18 (67%), and 11 (37%) respectively. Wilcoxon Rank Tests were associated with p values of 0.46 for morphine, 1 for nortriptyline and <0.0001 for the combination treatment as compared to placebo respectively. P values for the comparison of morphine to nortriptyline (p=0.27), morphine to the combination treatment (p=0.061), and nortriptyline to the combination treatment (p=0.093) were not significant.
Secondary Outcomes
“Sequence” was not significant for any of the SF-36 outcome scores in the linear mixed models using scores for the patients who completed at least two periods. Significant period effect was found for the reported health transition category of the SF-36 (p=0.045); “treatment” in the modified model for this category where only data from the first period treatment was used did not reach statistical significance. In the analysis for the 28 study completers, there was a period effect (p=0.04) for one category of the SF-36, the role physical and for the category social function there was a carry over effect (p=0.02). Treatment in the modified models with the scores from the first treatment only was not significant for either category. For patients who completed at least two periods, “treatment” was not statistically significant in the linear mixed models fitted for either the Beck Depression Inventory (p=0.14) (Table 4) or the Oswestry Disability Index (p= 0.2) (Table 4). For the 28 study completers treatment was not significant in the models fitted for the Beck Depression Inventory (p=0.15) and the Oswestry Disability Index (p=0.082) either.
Table 4.
Mean Scores on the Beck Depression Inventory (BDI) and the Oswestry Disability Index (ODI):
| Baseline | Morphine | Nortriptyline | Combination | Placebo | |
|---|---|---|---|---|---|
| Mean Score on BDI | 8 | 9.6 | 7.3 | 6 | 9 |
| Standard Deviation | 6.7 | 8.5 | 7.1 | 5 | 8.5 |
| Mean Score on ODI | 30 | 25.7 | 27.5 | 27.4 | 30.5 |
| Standard Deviation | 15.0 | 16.5 | 16.7 | 15.40 | 15.9 |
Analysis in all patients with analyzable data for the SF-36 health survey (Table 5) showed that treatment was not associated with statistical significance for any of the categories except for: (1) The “Emotional Role” subscale (overall model’s p = 0.04), for which after adjusting the p value with a Bonferroni procedure only the combination treatment was associated with higher scores compared to placebo (p=0.03); And (2)The “Mental Health” subscale, for which treatment was significant at 0.04 in the overall model but the adjusted p values for pairwise comparisons using the Bonferroni procedure did not reach statistical significance.
Table 5.
SF-36
| Baseline | MS | Nortriptyline | Combination | Placebo | |
|---|---|---|---|---|---|
| Physical Functioning | |||||
| Mean | 48 | 56 | 64 | 59 | 51.3 |
| St. Dev | 26 | 27 | 27 | 27 | 25.8 |
| Social Functioning | |||||
| Mean | 63 | 69 | 78 | 76 | 67 |
| St. Dev | 30 | 28 | 28 | 26 | 31 |
| Role Physical | |||||
| Mean | 41 | 53 | 60 | 55 | 54 |
| St. Dev | 46 | 42 | 43 | 41 | 46 |
| Role Emotional* | |||||
| Mean | 81 | 69 | 72 | 83† | 63† |
| St. Dev | 33 | 42 | 42 | 34 | 43 |
| Body Pain | |||||
| Mean | 38 | 48 | 56 | 50 | 44 |
| St. Dev | 18 | 26 | 23 | 23 | 20 |
| Mental Health** | |||||
| Mean | 74 | 68 | 79 | 76 | 69 |
| St. Dev | 16 | 21 | 16 | 16 | 24 |
| Vitality | |||||
| Mean | 50 | 47 | 57 | 53 | 51 |
| St. Dev | 21 | 24 | 20 | 22 | 28 |
| General Health | |||||
| Mean | 68 | 61 | 67 | 66 | 61 |
| St. Dev | 20 | 23 | 21 | 20 | 23 |
| Reported Health Transition | |||||
| Mean | 3 | 3 | 3 | 3 | 3 |
| St. Dev | 1 | 1 | 1 | 0.9 | 0.9 |
p value significant at 0.04 for overall model
Emotional Role subscale was significantly better with the combination treatment as compared to placebo treatment after correction for multiple comparisons (p=0.03)
p value significant at 0.04 for the overall model but none of the pair-wise comparisons were significant after Bonferroni correction for multiple comparisons
Blinding
By chance alone, an observer would identify each of four treatments correctly an average of 25% of the time. On the blinding questionnaires, the numbers of correct guesses by patients were 9 (32%) for morphine treatment periods, 9 (32%) for nortriptyline, 10 (37%) for the combination treatment, and 11 (39%) for placebo. The frequencies of nurses’ correct guesses were 7 (35%) for morphine, 5 (17%) for nortriptyline, 4 (25%) for the combination treatment, and 9 (50%) for placebo. A percent larger than 25% for any treatment suggests that the patients or nurses were not optimally blinded during that treatment.
Adverse effects and NNH
At the maximal tolerated doses, the most frequent adverse effects reported by the study completers were constipation, dry mouth, drowsiness, and fatigue (Table 7). During the morphine treatment two patients withdrew because of sedation, one because of nausea, vomiting and severe constipation, one because of a rash, and severe dry mouth, while during the nortriptyline treatment two withdrew because of sedation. During the combination treatment two patients withdrew because of sedation, one because of nausea and vomiting, and one because of a rash whereas during the placebo treatment one patient withdrew because of sedation. NNHs were 10, 30, and 11 for morphine, nortriptyline and the combination treatment respectively.
DISCUSSION
To our surprise, this trial showed that based on our primary outcome score, average leg pain, chronic lumbar radicular pain did not respond well to either a tricyclic antidepressant or an opioid in doses that have been effective in many studies of painful diabetic neuropathy and postherpetic neuralgia (Kishore-Kumar et al. 1990; Max et al. 1992), (Watson et al. 1982; Max et al. 1988) ,, (Watson et al. 1998) (Dellemijn and Vanneste 1997; Dellemijn et al. 1998), (Rowbotham et al. 2003), (Rowbotham et al. 1991), (Raja et al. 2002; Gilron et al. 2005), (Watson and Babul 1998; Gimbel et al. 2003; Watson et al. 2003). A morphine-nortriptyline combination was also ineffective. The rank ordering of the small trends towards pain relief varied among the pain measures, but morphine was the least effective on all measures, reducing leg and back pain by 1–7% compared to placebo (Table 2). These results suggest that responses of neuropathic pain to various drugs may differ depending upon the etiology of neuropathic lesion (Sindrup and Jensen 1999; Sang et al. 2002; Finnerup et al. 2005).
We cannot rule out the possibility that the lack of statistical significance for most treatment effects was due to chance effect. For example, the 95% confidence interval for the effect of nortriptyline alone includes a maximum pain reduction of 30% relative to placebo, equivalent to the more impressive results reported in diabetic neuropathy pain(Max et al. 1992) and postherpetic neuralgia (Watson et al. 1998). The modest sample size of about 30 patients per treatment accounts for this uncertainty, but this is a limitation of any single-investigator study. Isolated results approached statistical or clinical significance, such as the very small p value for the global pain relief scores for the combination (Table 3), 9-14% reductions in the numerical pain scores for nortriptyline (Table 2), and the significant p value associated with the comparison of the combination treatment vs. placebo for worst overall pain in the analysis of the 34 patients who had completed two or more treatments. However the entire pattern of data does not recommend any of the treatments. No conclusion can be drawn from the significant p value associated with the combination treatment as compared to the placebo treatment for average back pain using “the first period only” scores either given the very small number of patients assigned to each of the 4 subgroups during the first treatment period. Although the global pain relief scores for the combination treatment were significantly better than placebo (Table 3), this was probably a chance effect because the other pain scores do not confirm this.
Table 3.
Global Pain Relief Global Pain Relief
| MS Contin (N=32) | Nortriptyline (N=31) | Combination* (N=28) | Placebo | |
|---|---|---|---|---|
| (N=33) | ||||
| Worse pain | 2 | 4 | 0 | 1 |
| No pain relief | 8 | 9 | 5 | 11 |
| Slight pain relief | 9 | 6 | 5 | 10 |
| Moderate pain relief | 5 | 2 | 10 | 6 |
| A Lot of pain relief | 7 | 8 | 7 | 5 |
| Complete pain relief | 1 | 2 | 1 | 0 |
| NNT(Moderate or | 13 | 18 | 3 | |
| NA greater relief) | ||||
| NNT(A lot or complete NA relief) | 10 | 6 | 7 |
P-value for the Wilcoxon Rank test was significant (p < .0001) for the combination treatment as compared to placebo
However, drug companies developing new antidepressants that block reuptake of norepinephrine and serotonin might view our equivocal nortriptyline data as a “glass half full.” If the true pain reduction is similar to our estimate of 14% on the 0–10 scale, or an NNT of 6 for the criterion of “a lot” or “complete” relief, a larger study might establish the treatment as the only proven treatment for a large population of pain patients.
We must also consider the possibility that bias affected the result. The most worrisome issue is the high dropout rate. Twelve patients dropped out due to side effects and 15 patients dropped out due to unrelated reasons after randomization to study medications. Several considerations make us think that the dropouts were not likely to have caused a false negative result: (1) The proportion of drop outs from the 4 possible sequences were fairly balanced. Therefore it is unlikely that a bias would have been introduced against any particular treatment. (2) Patients who dropped out due to side effects may have been somewhat reluctant to do so if they were experiencing pain relief with any of the study drugs. Therefore, if they had completed the study, this might have led to even more negative results. (3) The modest trend towards unblinding of treatment identity would have tended to increase differences in scores between active drug and placebo and led to more positive results. In addition to the high dropout rate, recruitment of patients by newspaper advertisements may limit the generalizability of these results to any particular clinic population.
We recently participated in a study in which nortriptyline and morphine produced much more impressive pain reduction in postherpetic neuralgia, using similar treatment regimens and outcome measures (Raja et al., 2002). In this 76-patient crossover study, morphine or methadone, and nortriptyline or desipramine were effective in alleviating pain based both on mean pain reduction (=1.2 for nortriptyline vs. 0.7 for nortriptyline in our study, and = 2.2 for morphine vs. 0.35 for morphine in our study) and NNT scores (NNT= 2.7 for opioids vs. 11 for opioids in our study, and NNT= 4 for TCAs vs. 17 for nortriptyline in our study.
If replicated in additional groups of patients with chronic lumbar root pain, these data would suggest that features of the underlying pathophysiology renders them less responsive to opioids and tricyclics than patients with diabetic neuropathy and postherpetic neuralgia, at least in dosages that have been shown to be effective in the treatment of these other syndromes. This finding also helps explain the longstanding controversy among pain clinicians regarding whether neuropathic pain is “opioid resistant.” (Arner and Meyerson 1988) (Eisenberg et al. 2005). We would expect clinicians’ impressions about this point to vary widely, depending upon the types of neuropathic pain syndromes they treat.
In conclusion, our results suggest that opioids, tricyclic antidepressants, and their combination may be relatively ineffective in the treatment of lumbar radicular pain. However replication is needed because our sample size was small, dropout rates high, and newspaper-based recruitment may have drawn a treatment-resistant population. These results stand in contrast to findings in painful diabetic neuropathy and post-herpetic neuralgia where TCAs and opioids have been repeatedly shown to be effective. These differences could be due to distinctions in the underlying etiology of pain. Studies using other classes of medications are warranted in this vexing yet common clinical pain syndrome. Moreover, these results suggest that more research needs to be performed in animal models specific to spinal root pain (Lacroix-Fralish et al. 2006) in order to identify new analgesic targets for the pain caused by root lesions.
Table 6.
Adverse Effects for 28 Study Completers
| Symptom | Morphine (%) | Nortriptyline (%) | Combination (%) | Placebo (%) |
|---|---|---|---|---|
| Constipation | 64 | 25 | 71 | 7 |
| Dry Mouth | 21 | 36 | 29 | 21 |
| Headache | 14 | 7 | 14 | 14 |
| Drowsiness | 25 | 7 | 11 | 4 |
| Tired/Fatigue | 7 | 11 | 14 | 18 |
| Dizziness | 14 | 7 | 4 | 4 |
| Insomnia | 7 | 11 | 11 | 0 |
| Nausea | 7 | 0 | 4 | 0 |
| Difficulty urinating | 4 | 4 | 7 | 0 |
| Sexual Dysfunction | 11 | 0 | 4 | 0 |
| Abdominal pain | 4 | 4 | 7 | 0 |
| Weakness | 0 | 0 | 7 | 7 |
| Decreased appetite | 7 | 0 | 4 | 0 |
| Heartburn | 4 | 7 | 0 | 4 |
| Blurred Vision | 7 | 0 | 4 | 11 |
| Thirsty/Dehydrated | 0 | 7 | 0 | 0 |
| Weight Gain | 0 | 7 | 0 | 0 |
| ANY SIDE EFFECT | 93 | 68 | 89 | 50 |
Data are reported only for adverse effects with an incidence greater than 5 percent for any treatment.
Acknowledgments
This study was supported by an intramural grant from the National Institute of Dental and Craniofacial Research. MS Contin placebo tablets were a gift from Purdue Pharma. We thank Dr. Judith Starling at NIH Pharmaceutical Development Services, the Clinical Center Radiology and Nursing Departments, Dr. Nathaniel Katz for his critique of the draft clinical protocol, and Dr. Bikash Mishra and Dr. Nancy Low for review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arner S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33(1):11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Atkinson JH, Slater MA, Williams RA, Zisook S, Patterson TL, Grant I, Wahlgren DR, Abramson I, Garfin SR. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76(3):287–296. doi: 10.1016/S0304-3959(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Colton T. Statistics in Medicine. Boston: Little Brown; 1974. [Google Scholar]
- Dellemijn PL, van Duijn H, Vanneste JA. Prolonged treatment with transdermal fentanyl in neuropathic pain. J Pain Symptom Manage. 1998;16(4):220–229. doi: 10.1016/s0885-3924(98)00070-0. [DOI] [PubMed] [Google Scholar]
- Dellemijn PL, Vanneste JA. Randomised double-blind active-placebo-controlled crossover trial of intravenous fentanyl in neuropathic pain. Lancet. 1997;349(9054):753–758. doi: 10.1016/S0140-6736(96)09024-1. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003a;60(11):1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Corbin AE, Young JP, Jr, Sharma U, LaMoreaux L, Bockbrader H, Garofalo EA, Poole RM. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003b;60(8):1274–1283. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. Jama. 2005;293(24):3043–3052. doi: 10.1001/jama.293.24.3043. [DOI] [PubMed] [Google Scholar]
- Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26(5):E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352(13):1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and future directions. Expert Rev Neurother. 2005;5(6):823–830. doi: 10.1586/14737175.5.6.823. [DOI] [PubMed] [Google Scholar]
- Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology. 2003;60(6):927–934. doi: 10.1212/01.wnl.0000057720.36503.2c. [DOI] [PubMed] [Google Scholar]
- Heliovaara M, Knekt P, Aromaa A. Incidence and risk factors of herniated lumbar intervertebral disc or sciatica leading to hospitalization. J Chronic Dis. 1987;40(3):251–258. doi: 10.1016/0021-9681(87)90161-5. [DOI] [PubMed] [Google Scholar]
- Hills M, Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmacol. 1979;8(1):7–20. doi: 10.1111/j.1365-2125.1979.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine EJ. Measurement and expression of risk: optimizing decision strategies. Am J Med. 2004;117(Suppl 5A):2S–7S. doi: 10.1016/j.amjmed.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Khoromi S, Patsalides A, Parada S, Salehi V, Meegan JM, Max MB. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6(12):829–836. doi: 10.1016/j.jpain.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kishore-Kumar R, Max MB, Schafer SC, Gaughan AM, Smoller B, Gracely RH, Dubner R. Desipramine relieves postherpetic neuralgia. Clin Pharmacol Ther. 1990;47(3):305–312. doi: 10.1038/clpt.1990.33. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, deGruy FV, 3rd, Swindle R. A symptom checklist to screen for somatoform disorders in primary care. Psychosomatics. 1998;39(3):263–272. doi: 10.1016/S0033-3182(98)71343-X. [DOI] [PubMed] [Google Scholar]
- Lacroix-Fralish ML, Tawfik VL, Tanga FY, Spratt KF, DeLeo JA. Differential spinal cord gene expression in rodent models of radicular and neuropathic pain. Anesthesiology. 2006;104(6):1283–1292. doi: 10.1097/00000542-200606000-00025. [DOI] [PubMed] [Google Scholar]
- Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104–2110. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250–1256. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- Max MB, Schafer SC, Culnane M, Smoller B, Dubner R, Gracely RH. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology. 1988;38(9):1427–1432. doi: 10.1212/wnl.38.9.1427. [DOI] [PubMed] [Google Scholar]
- Milliken G, Johnson DE. Analysis of Messy Data. New York: Van Nostrand Reinhold Co; 1984. [Google Scholar]
- Moulin DE, Iezzi A, Amireh R, Sharpe WK, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996;347(8995):143–147. doi: 10.1016/s0140-6736(96)90339-6. [DOI] [PubMed] [Google Scholar]
- Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, Royall RM, Max MB. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59(7):1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- Remmers AE, Sharma U, Lamoreaux L, Young JP, Moore J, Poole RM. Pregabalin treatment of patients with chronic low back pain. Proceedings of the 19th Annual Meeting of the American Pain Society 2000; Atlanta, Georgia. Abstract 660. [Google Scholar]
- Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Reisner-Keller LA, Fields HL. Both intravenous lidocaine and morphine reduce the pain of postherpetic neuralgia. Neurology. 1991;41(7):1024–1028. doi: 10.1212/wnl.41.7.1024. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348(13):1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- Sang CN, Booher S, Gilron I, Parada S, Max MB. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials. Anesthesiology. 2002;96(5):1053–1061. doi: 10.1097/00000542-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Schnitzer TJ, Gray WL, Paster RZ, Kamin M. Efficacy of tramadol in treatment of chronic low back pain. J Rheumatol. 2000;27(3):772–778. [PubMed] [Google Scholar]
- Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284–1289. doi: 10.1212/01.wnl.0000058749.49264.bd. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- van Seventer R, Feister HA, Young JP, Jr, Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006;22(2):375–384. doi: 10.1185/030079906x80404. [DOI] [PubMed] [Google Scholar]
- Ware JE., Jr SF-36 health survey update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology. 1998;50(6):1837–1841. doi: 10.1212/wnl.50.6.1837. [DOI] [PubMed] [Google Scholar]
- Watson CP, Evans RJ, Reed K, Merskey H, Goldsmith L, Warsh J. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32(6):671–673. doi: 10.1212/wnl.32.6.671. [DOI] [PubMed] [Google Scholar]
- Watson CP, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain. 2003;105(1–2):71–78. doi: 10.1016/s0304-3959(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Watson CP, Vernich L, Chipman M, Reed K. Nortriptyline versus amitriptyline in postherpetic neuralgia: a randomized trial. Neurology. 1998;51(4):1166–1171. doi: 10.1212/wnl.51.4.1166. [DOI] [PubMed] [Google Scholar]
- Williams AC, Richardson PH. What does the BDI measure in chronic pain? Pain. 1993;55(2):259–266. doi: 10.1016/0304-3959(93)90155-I. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]


