Abstract
Our objective is to determine whether unilateral atropine as amblyopia therapy leads to an asymmetric change in refractive error compared to patching. Patients were enrolled in a clinical trial in which atropine 1% solution or occlusion with an adhesive patch was administered daily to the sound eye of children 3 to < 7 years of age for a period of at least 6 months to a maximum of 2 years. Refractive error at entry and at 2 years was determined with cycloplegic retinoscopy for 282 of 419 patients enrolled. The baseline mean refractive error was +3.13 Diopters (D) in eyes randomized to atropine and +2.58D in eyes randomized to patch. The mean change in refractive error of the sound eye was +0.10D in the atropine group (N=134) and +0.08D in the patching group (N=148). Patients also were subdivided into those treated with atropine only (n=41) and patching only (n=64). The mean change for the sound eye was -0.21 D for the patients receiving only atropine and -0.06 D for the patients receiving only patching. Unilateral atropine applied to the sound eye compared to occlusion was not associated with any adverse effect on refractive error following up to 2 years of treatment.
Introduction
The effect of amblyopia therapy on refractive error has not been well studied. If there is an effect on refractive error development of the sound eye, treatment could result in unequal changes in the eyes of children with amblyopia. The two most commonly used treatments for amblyopia are topical atropine sulfate 1% and patching of the sound eye. Atropine is used specifically for patients with hypermetropia and amblyopia.
Atropine, combined with bifocal spectacles, has been used to slow the progression of childhood myopia. 1-4. The slowing of myopic progression has been thought to be due in part to the inhibition of accommodation by atropine. This mechanism has been challenged by the finding that a similar reduction of myopia progression by the relatively selective M1 muscarinic receptor blocker, pirenzepine, which does not block accommodation. 5, 6 No matter the precise mechanism, if the effect of atropine slowing myopic refractive error change were to occur in the hypermetropic child with amblyopia, there would be a relative persistence of hypermetropia in the sound eye.7, 8
An additional concern is that we were treating hypermetropic children with atropine, so these children would have experienced image defocus at near and in some cases at distance. Image defocus has been shown to induce a myopic shift.9,10 If this myopic shift were to happen in the sound eye during the treatment of amblyopia it would result in the loss of hypermetropia in the sound eye.
The purpose of this study was to assess whether unilateral atropine when used for the treatment of amblyopia in hypermetropic patients in a clinical trial produced changes in the spherical equivalent refractive error of the sound eye different from those seen with unilateral occlusion therapy.
Methods
The study was supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health. The protocol and informed consent forms were approved by each site’s institutional review board and complied with the Health Insurance Portability and Accountability Act of 1996. The parent or guardian provided written informed consent. 11, 12 Study oversight was provided by an independent data and safety monitoring committee.
Children 3 to less than 7 years of age were enrolled in a randomized controlled treatment trial of moderate amblyopia (20/40 to 20/100). Eligible patients could have no more than 0.50 D of myopia at enrollment so that there would be blur at near with atropine. Refractive error was measured at baseline with a cycloplegic refraction performed with topical cyclopentolate 1% solution. The protocol specified a cycloplegic refraction within the 6 months prior to the 2-year outcome measurement of visual acuity. For the first 6 months of treatment, patients remained on their randomized treatment of daily atropine 1% solution or patching (initially a minimum of 6 hours) of the sound eye. Between 6 months and 2 years treatment was at investigator discretion and was not always continuous. These data have been reported previously.12
The change in spherical equivalent refractive error for each eye measured under cycloplegia was calculated. The patients were analyzed by initial randomized treatment assignment. Because some children changed treatment during their study participation, we also evaluated two additional subgroups who received only atropine or only patching and who were not on treatment at the time of their outcome refraction. This secondary analysis of refractive error change was felt to be reasonable as it is unlikely that continuing or discontinuing a particular treatment was based on changes in refractive error.
Results
The 2-year refraction was completed in 282 (67%) of 419 patients in the clinical trial within the preset window from 44 sites. These children are comparable to those not completing the refraction in terms of baseline characteristics including age, race, gender, cause of amblyopia (anisometropia, strabismic, or combined), visual acuity in the amblyopic and sound eyes, intereye acuity difference, and refractive error.
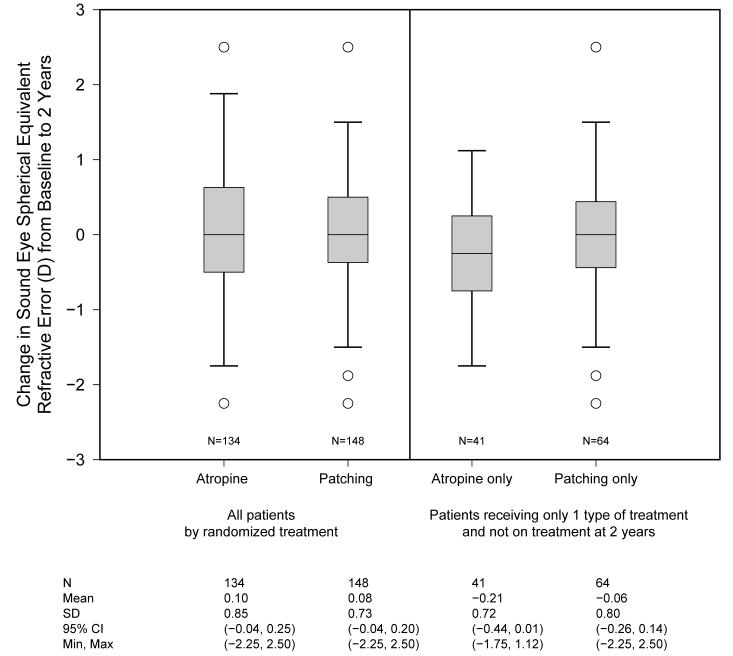
The mean spherical equivalent refractive error of the sound eyes in the atropine and patching groups at baseline was 3.13 D (range: 0 - 10.50 D) and 2.58 D (range: 0-8.00 D) respectively. The mean change in the spherical equivalent of the sound eyes was similar for patients randomized to atropine (+0.10 D; 95% CI = -0.04, 0.25 D) and patching (+0.08 D; 95% CI = -0.04, 0.20 D), with an estimated difference between treatment groups of 0.02D (95% CI= -0.20, 0.17 D) (Figure). The mean change also was similar for the atropine only group (-0.21 D; 95% CI = -0.44, 0.01 D) and the patching only group (-0.06 D; 95% CI = -0.26, 0.14 D), with an estimated difference of -0.16D (95% CI= -0.46, 0.15D). Only 17% of patients in the atropine group and 11% in the atropine group had a change in sound eye refractive error that was more than 1 D (Fisher’s exact test P=0.23).
Figure.
Boxplots of the change in sound eye spherical equivalent refractive error in Diopters from baseline to 2 years by amblyopia treatment. The box encloses the 25th to 75th percentile of the data; the line through the box is the median. The whiskers show the range of the data, excluding statistical outliers. The circles represent individual patients who are statistical outliers.
Discussion
A concern about the use of prolonged unilateral cycloplegia for treatment of amblyopia is the potential for producing unequal effects on the development of refractive error. We found that when topical atropine was used to treat moderate amblyopia in children 3 to <7 years of age for up to 2 years, there was no difference in effect on refractive error of the sound eye associated with unilateral atropine administration when compared to patching.
There are important limitations of our data. First, because patients were treated at the investigator’s discretion after 6 months, only select subgroups of patients were treated only with atropine or patching. Second, we do not have data on the actual number of days treated between 6 and 24 months. Third, there is no control group of untreated sound eyes to which we could compare the two treatment groups to confirm that treatment has no adverse effect. However, this latter limitation does not affect the treatment group comparison, which was the primary goal of this report.
The lack of a difference during the treatment of amblyopia can not be considered an assessment of the effectiveness of the atropine for the prevention of myopia as our patients were hypermetropic and they did not receive atropine treatment in the dosage or for the duration typically suggested for myopia. 1, 3
We conclude that unilateral atropine compared to occlusion for the treatment of amblyopia for up to two years does not suggest an adverse effect on the progression of refractive error.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chiang MF, Kouzis A, Pointer RW, Repka MX. Treatment of childhood myopia with atropine eyedrops and bifocal spectacles. Binocul Vis Strabismus Q. 2001;16:209–15. [PubMed] [Google Scholar]
- 2.Bedrossian RH. The effect of atropine on myopia. Ophthalmology. 1979;86:713–7. doi: 10.1016/s0161-6420(79)35455-0. [DOI] [PubMed] [Google Scholar]
- 3.Brodstein RS, Brodstein DE, Olson RJ, Hunt SC, Williams RR. The treatment of myopia with atropine and bifocals: a long-term prospective study. Ophthalmology. 1984;91:1373–9. doi: 10.1016/s0161-6420(84)34138-0. [DOI] [PubMed] [Google Scholar]
- 4.Shih Y, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthal Scand. 2001;79:233–236. doi: 10.1034/j.1600-0420.2001.790304.x. [DOI] [PubMed] [Google Scholar]
- 5.Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS, Asian Pirenzepine Study Group One-Year Multicenter, Double-Masked, Placebo-Controlled, Parallel Safety and Efficacy Study of 2% Pirenzepine Ophthalmic Gel in Children with Myopia. Ophthalmology. 2005;112:84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Siatkowski RM, Cotter S, Miller JM, et al. Safety and Efficacy of 2% Pirenzepine Ophthalmic Gel in Children With Myopia. Arch ophthalmol. 2004;122:1667–1674. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 7.Norton T. Animal models of myopia: learning how vision controls the size of the eye. ILAR Journal. 1999;40:59–79. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 8.Gwiazda J, Hyman L, Norton T, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Inves Ophthal Vis Sci. 2004;45:2143–2151. doi: 10.1167/iovs.03-1306. [DOI] [PubMed] [Google Scholar]
- 9.Robb RM. Refractive errors associated with hemangiomas of the eyelids and orbit in infancy. Am J Ophthalmol. 1977;83:52–8. doi: 10.1016/0002-9394(77)90191-x. [DOI] [PubMed] [Google Scholar]
- 10.Raviola E, Wiesel TN. An animal model of myopia. The New England Journal of Medicine. 1985;312:1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- 11.Pediatric Eye Disease Investigator Group A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002;120:268–78. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 12.Pediatric Eye Disease Investigator Group Two-year follow up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2005;123:149–57. doi: 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]