Abstract
Coenzyme Q (Q) functions in the mitochondrial respiratory chain and serves as a lipophilic antioxidant. There is increasing interest in the use of Q as a nutritional supplement. Although the physiological significance of Q is extensively investigated in eukaryotes, ranging from yeast to human, the eukaryotic Q biosynthesis pathway is best characterized in the budding yeast Saccharomyces cerevisiae. At least ten genes (COQ1-COQ10) have been shown to be required for Q biosynthesis and function in respiration. This review highlights recent knowledge about the endogenous synthesis of Q in eukaryotes, with emphasis on S. cerevisiae as a model system.
Keywords: Coenzyme Q, Mitochondria, Eukaryotes
1. Overview of Coenzyme Q Biosynthesis
Cells generally rely on de novo synthesis for their supply of Q. Current knowledge about the Q biosynthetic pathway in eukaryotes is mostly derived from characterization of accumulating intermediates in Q-deficient mutant strains of Saccharomyces cerevisiae, reviewed by (Jonassen and Clarke, 2001, Meganathan, 1996, Turunen et al., 2004). Q biosynthesis starts with formation of a hydroxybenzoic acid head group and a lipophilic polyisoprenoid tail (Olson and Rudney, 1983, Pennock and Threlfall, 1983). The aromatic precursor of the benzoquinone ring is 4-hydroxybenzoic acid (4-HB) derived from tyrosine, an essential amino acid in mammals. In yeast, 4-HB can also be synthesized from chorismate via the shikimate pathway (Goewert, 1980). The building blocks for the synthesis of the polyisoprenyl chain are provided by dimethylallyl diphosphate and isoprenyl diphosphate. In yeast and mammals, these five-carbon precursors are derived from acetyl-coenzyme A via the mevalonate pathway (Grunler et al., 1994).
The putative eukaryotic Q biosynthetic pathway is shown in Figure 1. First, the polyisoprenoid tail is assembled by polyprenyl diphosphate synthase, which is responsible for determining the number of isoprene units (designated as n). Next, polyprenyl diphosphate: 4-HB transferase catalyzes the formation of covalent linkage between the benzoquinone head group and the tail, producing the 4-hydroxy-3-polyprenyl benzoic acid intermediate. The order of subsequent reactions presented in Figure 1 is speculative, as only a few of the diagnostic intermediates of the blocked steps have been recovered in yeast mutant strains. Proposed modifications of the aromatic ring start with hydroxylation, followed by O-methylation, and decarboxylation to form the 6-methoxy-2-polyprenyl phenol intermediate. Afterward, two additional hydroxylations, one C-methylation, and one O-methylation step are necessary to generate the fully substituted hydroquinone.
Fig. 1. Proposed Q biosynthetic pathway in eukaryotes.
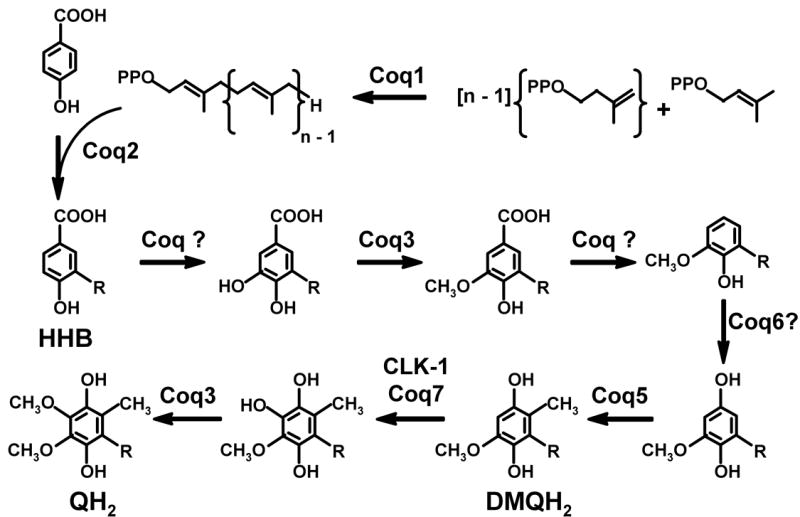
The length of the polyisoprenoid chain of Q, designated by n, varies depending on the species; n = 6 in S. cerevisiae, 9 in C. elegans, and 10 in H. sapiens. In S. cerevisiae, there are nine identified Coq proteins necessary for the synthesis of QH2 from dimethylallyl diphosphate and isopentenyl diphosphate precursors. The enzymatic functions of Coq4, Coq6, Coq8 and Coq9 polypeptides have yet to be characterized. Molecular oxygen and AdoMet are proposed donors for the hydroxy and methyl group, respectively (Olson and Rudney, 1983). CLK-1 is the C. elegans Coq7 homologue.
So far, nine complementation groups of Q-deficient yeast mutants (COQ1 through COQ9) have been identified (Tzagoloff and Dieckmann, 1990, Johnson et al., 2005). Mammalian homologues of the yeast COQ genes have been identified via sequence homology. Human homologues of Coq2, Coq3, and Coq7 proteins were demonstrated to functionally complement the corresponding yeast null mutants (Forsgren et al., 2004, Jonassen and Clarke, 2000, Vajo, 1999), further indicating that the yeast Q biosynthesis pathway is conserved in humans. The yeast coq mutants are non-respiring (unable to grow on non-fermentable carbon sources such as ethanol and glycerol) and petite (forming smaller colonies than wild-type cells when grown on glucose, a fermentable sugar) (Tzagoloff et al., 1975a, Tzagoloff et al., 1975b). The hallmark feature of these mutants is that defective NADH-cytochrome c reductase and succinate-cytochrome c reductase activities in isolated mitochondria of each coq mutant strain can be restored to near wild-type level by addition of Q2 (Tzagoloff et al., 1975b, Johnson et al., 2005). Addition of exogenous Q6 to coq mutants cultured in liquid media with vigorous aeration also restores respiration (Jonassen et al., 1998, Do et al., 2001). Recently, a novel yeast coq mutant with defects in respiration and Q-dependent oxidation of NADH and succinate has been identified (Barros et al., 2005). However, unlike the other Q-deficient coq mutants (coq1-coq9), the coq10 mutant has nearly normal levels of Q6, indicating that this protein is not required for Q biosynthesis. Instead, the Coq10 polypeptide may function as a Q-binding chaperone, required for the proper function of Q in respiratory electron transport. The evidence for this proposal is discussed in section three.
While Coq1, Coq2, Coq3, Coq5, Coq6, and Coq7 proteins have known or proposed enzymatic functions in Q biosynthesis (Jonassen and Clarke, 2001, Gin et al., 2003) (Figure 1), it is not clear whether the other Coq proteins also possess enzymatic activities. Coq1 through Coq9 polypeptides localize to the mitochondria (Belogrudov et al., 2001, Gin and Clarke, 2005, Gin et al., 2003, Hsu et al., 1996, Leuenberger et al., 1999, Jonassen et al., 1998, Do et al., 2001, Johnson et al., 2005, Dibrov et al., 1997). In vitro mitochondria import were investigated for seven of the yeast Coq polypeptides and demonstrated to be dependent on a mitochondrial membrane potential (Jonassen and Clarke, 2001). Following is a brief discussion about function and submitochondrial localization of the nine Coq proteins, required for Q biosynthesis in eukaryotes (summarized in Table 1). A model incorporating genetic and physical evidence for a yeast Q biosynthetic multi-subunit complex is shown in Figure 2.
Table 1.
Characteristics of the nine S. cerevisiae Coq proteins required for Q biosynthesis
| Yeast protein | Human homolog | mature M.W (kDa)a | Localized in vitro import | to mitochondria by: submitochondrial fractionation | Component of Q biosynthetic complex | Complementation of yeast mutants by human homologs |
|---|---|---|---|---|---|---|
| Coq1 | hDPS1/hDLP1* | 53 | Unknown | peripheral i.m. matrix side | ? | ? |
| Coq2 | Coq2 | 30 | + | integral i.m. matrix side | yes | yes |
| Coq3 | Coq3 | 33 | + | peripheral i.m. matrix side | yes | yes |
| Coq4 | NP_057119** | 36 | + | peripheral i.m. matrix side | yes | ? |
| Coq5 | CAI46073*** | 31 | + | peripheral i.m. matrix side | yes | ? |
| Coq6 | NP_872282*** | 51 | + | peripheral i.m. matrix side | yes | ? |
| Coq7/Cat5 | Clk-1/Coq7 | 23 | + | peripheral i.m. matrix side | yes | yes |
| Coq8/Abc1 | Adck-1—Adck5 | 53 | + | peripheral i.m. matrix side | ? | ? |
| Coq9 | AAH54340# | 25 | Unknown | peripheral i.m. matrix side | yes | ? |
via SDS-PAGE migration.
Also known as PDSS1/PDSS2.
NCBI Blastp score > 150.
Blastp score > 200.
Blastp score > 50
Fig. 2. A model of the mitochondrial Q biosynthetic protein complex in S. cerevisiae.
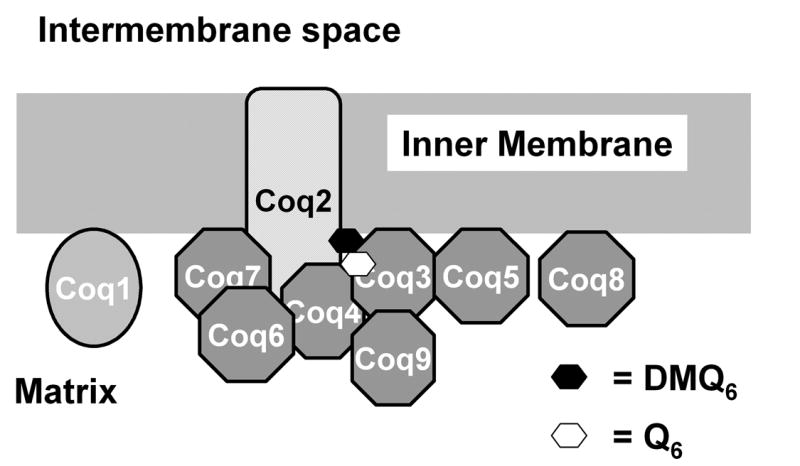
The putative complex contains six Coq polypeptides which are peripherally associated with the inner mitochondrial membrane (dark grey octagons) and a spanning integral membrane Coq protein (hatched rectangle). Proposed lipid components of the multi-subunit complexes include DMQ6 (black hexagon) and the final product Q6 (white hexagon). The stoichiometry of the components has yet to be determined.
Coq1
Formation of the trans-polyprenyl diphosphate synthase tail in S. cerevisiae is catalyzed by the polypeptide encoded by the COQ1 gene (Ashby and Edwards, 1990), which is responsible for determining the species-specific tail length of Q (Okada et al., 1996). The amino acid sequences of Coq1 protein and related isoprenyl diphosphate synthases from different eukaryotes contain seven highly conserved motifs (Wang and Ohnuma, 2000). Interestingly, expression of Coq1 homologues from a variety of organisms can restore Q biosynthesis and respiration in yeast coq1 null mutants via production of Q isoforms with distinct number of isoprene units (Okada et al., 1998, Okada et al., 1997). The Coq1 ortholog from the fission yeast Schizosaccharomyces pombe (Dps1) fails to complement the S. cerevisiae coq1 null mutant (Suzuki et al., 1997). However, polyprenyl diphosphate synthases of fission yeast, mouse, and human are each heterotetramers of two protein subunits, PDSS1 and PDSS2 (Saiki et al., 2005, Saiki et al., 2003), while Coq1 from S. cerevisiae and the plant Arabidopsis thaliana (Jun et al., 2004) function as homo-oligomers. Expression of both subunits of the trans-polyprenyl diphosphate synthase of S. pombe, mouse or human restores production of the polyisoprene diphosphate and production of Q in complementation assays (Saiki et al., 2005, Saiki et al., 2003). Submitochondrial fractionation studies demonstrated that the S. cerevisiae Coq1 protein is peripherally associated with the inner mitochondrial membrane on the matrix side (Gin and Clarke, 2005).
Coq2
The 4-HB polyprenyltransferase is a key enzyme catalyzing the attachment of the polyisoprenoid side chain to the 4-HB ring, generating the first membrane bound Q intermediate, 4-hydroxy-3-polyprenylbenzoic acid. The S. cerevisiae and Homo sapiens genes encoding this enzyme are called COQ2 (Ashby et al., 1992, Forsgren et al., 2004). Ortholog/homologues of Coq2 protein have also been isolated and characterized in other eukaryotes including S. pombe (Uchida et al., 2000), A. thaliana (Okada et al., 2004), and rice (Ohara et al., 2006). In vitro assays in isolated rat liver demonstrated that the polyprenyl diphosphate:4-HB activity is present mainly in mitochondria (Momose and Rudney, 1972). Polyprenyltransferases involved in Q biosynthesis generally display a lack of specificity for the chain length of the isoprenyl diphosphate substrate (Meganathan, 2001, Gin and Clarke, 2005, Ashby et al., 1992, Okada et al., 2004); however, the specificity was shown to be influenced by Mg2+ concentration in whole yeast extracts (Ashby et al., 1992).
Analysis of the predicted amino acid sequence of the S. cerevisiae Coq2 protein revealed two conserved putative substrate binding domains found in a family of polyprenyltransferases, six potential membrane spanning domains, and a typical mitochondrial targeting sequence (Ashby et al., 1992). In vitro import studies demonstrated that the polypeptide is imported and fully processed within the mitochondria (Leuenberger et al., 1999). Recently, submitochondrial fractionation analysis for the Coq2 protein has been carried out (Tran, U.C., Gulmezian, M., Santos-Ocaña, C., Saiki, R., Navas, P., and Clarke, C. F., manuscript in preparation). Coq2 protein behaves as an integral membrane protein associated to the inner mitochondrial membrane, facing the matrix side.
Coq3
Two O-methylation steps in the Q biosynthetic pathway are apparently catalyzed by the same enzyme encoded by COQ3 gene, as demonstrated by in vitro assays with synthetic farnesylated analogs (Poon et al., 1999, Shepherd et al., 1996). The COQ3 gene was originally identified in S. cerevisiae (Clarke et al., 1991) by its ability to restore Q biosynthesis, and hence respiration, in a coq3 mutant named C39 (Tzagoloff et al., 1975a, Sippel et al., 1983). Homologues of the COQ3 gene in rat, A. thaliana, and human were subsequently isolated via functional complementation of yeast coq3 null mutants (Marbois et al., 1994, Avelange-Macherel and Joyard, 1998, Jonassen and Clarke, 2000). The amino acid sequences of the proteins encoded by these COQ3 homologues all contain four regions that are conserved in a large family of methyltransferase enzymes utilizing S-adenosylmethionine (SAM or AdoMet) as the methyl donor (Kagan and Clarke, 1994, Niewmierzycka and Clarke, 1999, Katz et al., 2003) and required a divalent cation (Turunen et al., 2004, Jonassen and Clarke, 2001).
Like most of the other Coq polypeptides, the yeast Coq3 protein also contains a typical mitochondrial targeting sequence at the N-terminus. In vitro assays (Hsu et al., 1996) and subcellular localization (Poon et al., 1999) studies showed that the Coq3 preprotein was imported and processed to the mature form in the mitochondria, in a membrane-potential-dependent manner. Further submitochondrial fractionation demonstrated that it is a peripheral protein associated to the matrix side of the inner mitochondrial membrane (Poon et al., 1999). As indicated by the functional complementation and in vitro assays mentioned earlier, it is apparent that the Coq3 O- methyltransferase has broad substrate specificity. This type of promiscuous substrate recognition is a characteristic shared with catechol-O-methyltransferase (COMT), which has numerous physiological substrates including the biosynthetic precursors of dopamine and certain steroids and neurotransmitters (Vidgren et al., 1999). However, the amino acid sequence of COMT fails to show any homology with the yeast Coq3 polypeptide, outside of the conserved methyltransferase motifs (Turunen et al., 2004, Jonassen and Clarke, 2001).
Coq4
Similar to the Coq3 protein encoding gene, S. cerevisiae COQ4 gene was cloned via a functional complementation of a Q-deficient coq4 mutant harboring the E266K point mutation (C9-E1 or coq4-1) (Belogrudov et al., 2001). Growth on a non-fermentable carbon source (which requires respiration) caused up-regulation of COQ4 mRNA steady state levels, consistent with its role in Q biosynthesis. However, the enzymatic function of Coq4 protein, a peripheral protein associated with the inner mitochondrial membrane on the matrix side (Belogrudov et al., 2001) has been a mystery. While it is appealing to speculate that Coq4 protein may serve as a hydroxylase or a carboxylase in the yet-to-be-characterized steps (designated “Coq?” in Figure 1), the amino acid sequence of Coq4 does not share significant homology with protein domains or motifs with known enzymatic activity. Interestingly, steady state levels of Coq3 and Coq7 proteins, which are diminished in coq4 null mutants, are at wild-type levels in the coq4-1 point mutant (Belogrudov et al., 2001). This result, taken together with recent work demonstrating that the native Coq4 polypeptide co-migrates with Coq3, Coq6, and Coq7 proteins as a high molecular mass complex (Marbois et al., 2005, Tran et al., 2006), indicates that the Coq4 protein has a structural role in the putative polypeptide Q biosynthetic complex (further discussed below).
Coq5
2-methoxy-6-polyprenyl-1,4-benzoquinone methyltransferase catalyzes the only C-methylation step in the Q biosynthetic pathway, generating the 2-methoxy-5-methyl-6-polyprenyl-1,4-benzoquinone intermediate. In S. cerevisiae, the gene encoding this C-methyl transferase is designated COQ5. The COQ5 gene was isolated from a yeast genomic DNA library based on its ability to restore respiratory proficiency in two different coq5 point mutants, coq5-1 (Dibrov et al., 1997) and coq5-2 (Barkovich et al., 1997). Analysis of the COQ5 promoter region identified consensus binding sites for Gcr1, Mig1, Rtg1/2/3, and Hap2/3/4 transcription factors (Hagerman et al., 2002, Hagerman and Willis, 2002), which regulate gene expression in response to energy sources. Results of Northern blot and Western blot analyses clearly demonstrated COQ5 expression is up-regulated with glycerol and oleic acid treatments, compared to dextrose, with the highest induction observed during growth on oleic acid (Hagerman et al., 2002, Hagerman and Willis, 2002). The COQ5 open reading frame harbors four sequence motifs present in a large family of AdoMet-dependent methyltransferase enzymes (Katz et al., 2003). In vitro C-methyltransferase assays with the farnesylated analogs of the corresponding intermediates confirmed that Coq5 polypeptide is required for conversion of 2-methoxy-6-polyprenyl-1,4-benzoquinone to 2-methoxy-5-methyl-6-polyprenyl-1,4-benzoquinone in Q biosynthesis (Baba et al., 2004, Barkovich et al., 1997). These enzyme assays further demonstrated that the length of the polyisoprenoid tail does not play a crucial role in substrate recognition of Coq5 protein. Inclusion of NADH is essential for optimal enzymatic activity and is most likely required to convert the quinone to the hydroquinone, generating a nucleophile for the C-methyl transfer.
Submitochondrial fractionation analyes demonstrated the Coq5 protein is peripherally associated with the inner mitochondria membrane on the matrix side (Baba et al., 2004). Interestingly, the coq5-2 and coq5-5 point mutants maintained normal levels of Coq3, Coq4, and Coq5 polypeptides, while levels of these proteins were greatly diminished in each of the other coq5 mutants (Baba et al., 2004). These point mutants are the only coq5 mutants rescued by expression of Escherichia coli ubiE, a homolog of COQ5 (Lee et al., 1997). Taken together, these results indicate that Coq5 protein is essential for the stability and activity of at least two other Coq polypeptides, and provide genetic evidence for a complex of Coq polypeptides in yeast Q biosynthesis.
Coq6
Functional complementation of a yeast mutant from the G63 (coq6-1) complementation group (Tzagoloff and Dieckmann, 1990) resulted in the isolation of the COQ6 gene (Gin et al., 2003). In contrast to an earlier report (Fiori et al., 2000), COQ6 is a non-essential gene for viability but is required for growth on non-fermentable carbon sources (Gin et al., 2003). The Coq6 protein is a mitochondrial protein, which is imported in a membrane-potential-dependent manner and peripherally associated with the matrix side of the inner membrane (Gin et al., 2003). S. cerevisiae Coq6 protein and its homologues in H. sapiens, mouse, and C. elegans each contains three conserved regions (Gin et al., 2003): an ADP-binding fingerprint (Wierenga et al., 1986), a motif with a putative dual function in FAD/NAD(P)H binding (Eppink et al., 1997), and a consensus sequence that binds to the ribityl moiety of FAD (Eggink et al., 1990). These conserved regions are common features of a large family of FAD-binding-aromatic hydroxylases (Palfey et al., 1995). Consequently, Coq6 protein has been considered as a putative flavin-dependent monooxygenase responsible for adding the hydroxy group to 4-hydroxy-3-polyprenyl benzoic acid and/or 6-methoxy-2-polyprenyl phenol, two uncharacterized hydroxylation steps in Q biosynthesis.
Coq7
Yeast COQ7/CAT5 gene was independently isolated and characterized as required for Q synthesis (Marbois and Clarke, 1996, Tzagoloff and Dieckmann, 1990) and involved in carbon catabolite repression/de-repression (Proft et al., 1995). Catabolite repression/de-repression is a global system that regulates transcription of gluconeogenic genes, alternative sugar metabolism, and respiration (Gancedo, 1998). However, the catabolite-regulation defect in coq7 mutants was later demonstrated to be a secondary effect of respiration deficiency and could be rescued by the addition of exogenous Q6 (Jonassen et al., 1998), implicating direct involvement of COQ7 in Q biosynthesis. Moreover, expression of COQ7 homologues from C. elegans (Ewbank et al., 1997), rat (Jonassen et al., 1996), or human (Vajo, 1999) were shown to rescue the yeast coq7 null mutant for growth on non-fermentable carbon sources, indicating functional conservation across species.
Coq7 protein was shown to be required for the hydroxylation of 5-demethoxyubiquinol (DMQH2) (Marbois and Clarke, 1996). Interestingly, G65D coq7 point mutant was found to accumulate DMQ6, as well as the earlier intermediate 3-hexaprenyl-4-hydroxybenzoic acid (HHB), though the coq7 null mutant produced only HHB (Marbois and Clarke, 1996). Similarly, yeast mutants expressing Coq7 protein with the missense mutation (E194K) produced DMQ6, while DMQ6 was not detected in strains harboring a coq7-nonsense mutation, where the carboxyl-terminal half of the protein is missing (Padilla et al., 2004). These results suggest that Coq7 protein is either involved in one or more mono-oxygenase steps or serves as an essential component of the putative multi-subunit enzyme complex. Biochemical function of Coq7 protein as a hydroxylase was further supported by the determination that it belongs to a family of di-iron binding oxidases containing a conserved sequence motif for the iron ligands, EXXH (Stenmark et al., 2001). Coq7 homologues from Pseudomonas aeroginosa, Thiobacillus ferroxidans, C. elegans restored Q biosynthesis in an E.coli ubiF mutant (Adachi et al., 2003, Stenmark et al., 2001). E. coli UbiF, a flavin-dependent DMQ hydroxylase that shares no homology to Coq7 protein, has been shown to functionally complement both the coq7 null mutant and the DMQ6-producing coq7E194K mutant, with better efficiency in the latter (Tran et al., 2006). Collectively, these findings indicate that yeast Coq7 protein functions in the hydroxylation of DMQ. Moreover, steady state levels of the Coq3, Coq4, and Coq6 polypeptides were higher in the coq7E194K mutant than in the null mutant, suggesting that Coq7 protein and DMQ6 serve to stabilize other Coq polypeptides.
Recent submitochondria fractionation studies (Tran, U.C., Gulmezian, M., Santos-Ocaña, C., Saiki, R., Navas, P., and Clarke, C. F., manuscript in preparation) demonstrate that yeast Coq7 protein, like its homologues in mice (Jiang et al., 2001), is peripherally associated to the inner membrane on the matrix side. However, earlier studies have modeled Coq7 as an interfacial inner mitochondrial membrane protein (Stenmark et al., 2001, Berthold and Stenmark, 2003). Interfacial membrane proteins, such as prostaglandin synthase (Picot et al., 1994) and squalene cyclase (Wendt et al., 1999), are embedded in the membrane via interaction with only one leaflet of the bilayer. Unlike the Coq7 polypeptide, proteins classified as interfacial (based on X-ray crystal structures), including prostaglandin synthase, squalene cyclase, fatty acid amide hydrolase, and microsomal cytochrome P450, each behaved as integral proteins in biochemical assays (Bracey et al., 2004). The true nature of the Coq7 protein-membrane association awaits a structure determination for yeast Coq7p or one of its homologues.
Coq8
The COQ8 gene was initially identified as ABC1 (Activity of bc1 complex) for its ability to partially suppress, in multicopy, the cytochrome b translation defect due to the cbs2-223 mutation in the CBS2 gene (Bousquet et al., 1991). CBS2 is a yeast nuclear gene encoding a translational activator of cytochrome b (Rodel, 1986). It was observed that inactivation of ABC1 resulted in respiratory defect and absence of NADH-cytochrome c reductase activity (Bousquet et al., 1991); a phenotype similar to that of Q-deficient strains (Tzagoloff and Dieckmann, 1990). It was subsequently shown that the respiratory complexes II, III, and IV of the abc1 null mutant were thermo-sensitive and addition of exogenous Q could partially compensate for the respiratory deficiency (Brasseur et al., 1997). These results led to a hypothesis that the ABC1 gene product may function as a chaperone that is essential for the proper conformation and activity of the bc1 and its neighboring complexes (Brasseur et al., 1997). However, Do et al (Do et al., 2001) demonstrated that the COQ8 gene, required for Q biosynthesis (Poon et al., 1997), is the same as the ABC1 gene and provided data indicating that Q-deficiency is exclusively responsible for the pleiotropic defects of abc1/coq8 mutants. Moreover, a neighboring tRNATRP gene located downstream of COQ8/ABC1 gene was demonstrated to account for the suppression of the cbs2-223, a UGA nonsense mutation (Hsieh et al., 2004). Although its biochemical function in Q biosynthesis is currently unknown, Coq8/Abc1 protein has been classified as putative protein kinase based on the identification of four kinase conserved motifs in its amino acid sequence (Leonard et al., 1998).
Coq9
The COQ9 gene was recently identified and characterized as a new gene that, when mutated, results in a Q-deficient phenotype, in a similar manner to other COQ genes (Johnson et al., 2005). However, the function of Coq9 protein in Q biosynthesis is not yet known. The amino acid sequence of Coq9 protein contains a distinct conserved domain present in the COG5590 protein family (Marchler-Bauer et al., 2005). While COQ9 homologues are well-represented in genomes of eukaryotes and alpha-proteobacteria, Coq9 protein has no homology to proteins with known function. Intriguingly, multicopy expression of the COQ8 gene was shown to restore respiration in a specific coq9 point mutant (coq9-1; E151STOP nonsense mutation). Although a small amount of Coq9 polypeptide was detected in the coq9-1 nonsense mutant strain, levels were not elevated by the multicopy suppression mediated by the COQ8 gene (Johnson et al., 2005, Hsieh et al., 2007). Consequently, the mechanism responsible for the multi-copy COQ8 suppression of coq9-1 is unknown.
Based on the mobility in the SDS-PAGE, the molecular mass of Coq9 protein is about 25 kDa (Hsieh et al., 2007), slightly smaller than the predicted precursor (29.9 kDa) (Johnson et al., 2005), and is consistent with the removal of a putative mitochondrial targeting sequence. However, the native size of Coq9 protein estimated from its sedimentation on sucrose gradients is about three times larger, indicating that the protein is either a homo-oligomer or in a complex with other proteins (Johnson et al., 2005). Potential partners in such a complex are Coq3 and Coq5 polypeptides, which were shown to co-sediment with the Coq9 protein. Recently, submitochondrial localization analysis has demonstrated that Coq9 protein is a peripheral membrane protein associated with the matrix side of the mitochondrial inner membrane (Hsieh et al., 2007).
2. S. cerevisiae Q Biosynthesis Requires a Multiple-enzyme Complex or Complexes
There are many well-characterized mitochondrial respiratory protein complexes in yeast, for example, cytochrome oxidase, ATP synthase, and the cytochrome bc1 complexes. In these systems, the absence or mutation in one component results in proteolytic degradation, instability, or inactivation of the remaining subunits (Glerum et al., 1997, Tzagoloff et al., 1994). Multi-subunit enzyme complexes allow channeling of labile/reactive intermediates, enhance catalytic efficiency, and provide a mechanism for coordinative regulation of components. This seems to be the case in Q biosynthesis as well.
Previous studies have provided numerous lines of genetic evidence for a Q biosynthetic complex and for interdependent relationship among Coq polypeptides. Each of the null coq3 to coq9 mutants predominantly accumulates the same earlier intermediate HHB, the product of Coq2p, instead of the corresponding diagnostic intermediate (Poon et al., 1995, Poon et al., 1997, Johnson et al., 2005). Steady state levels of Coq3, Coq4, Coq6, Coq7, and Coq9 polypeptides are significantly decreased in mitochondria isolated from any of the other coq null mutants (Hsu et al., 2000, Baba et al., 2004, Belogrudov et al., 2001, Gin and Clarke, 2005, Tran et al., 2006, Hsieh et al., 2007). In addition, a null mutation in any of the COQ genes led to decreased Coq3 O-methyl transferase activity, although COQ3 RNA levels were not affected (Hsu et al., 2000). These phenotypes were not due to a lack of respiration, because other mutants with defects in the respiratory complexes such as atp2 and cor1 null mutants retained wild-type levels of O-methyl transferase activity. Although Coq1 polypeptide levels remain unchanged in any of the other coq null mutants, the protein itself and/or its lipid product appears to be essential for stabilization of Coq3, Coq4, Coq6, Coq7, and Coq9 proteins (Gin and Clarke, 2005, Hsieh et al., 2007). It has been demonstrated that phenotypes of certain coq point mutants dramatically differ from the respective null mutants. For example, the coq7E194K point mutant but not the coq7 null mutant was rescued by low copy expression of E. coli ubiF (Tran et al., 2006). Similarly, the coq5-2 and coq5-5 mutants, which have normal levels of Coq3, Coq4, and Coq5 polypeptides, are the only two coq5 mutants significantly rescued by expression of E. coli ubiE, a homolog of COQ5 gene (Baba et al., 2004). Moreover, the coq4-1 (E226K) mutant maintained wild-type levels of Coq3 and Coq7 polypeptides, which were greatly diminished in the null coq4 mutants (Belogrudov et al., 2001). This data renders support for the proposed structural/regulatory role of Coq4 protein in a multi-protein complex in Q biosynthesis. In such a model, the complete absence of Coq4p results in instability of several of the other Coq polypeptides, while certain amino acid substitution mutations in the Coq4 protein serve to stabilize the Coq polypeptides. Taken together, these results are consistent with the involvement of the Coq polypeptides and/or the Q-intermediates formed by these proteins in a multi-subunit complex or complexes. Postulated lipid components of the Q biosynthetic complex may also include the final product Q6 because the addition of exogenous Q was shown to stabilize steady state levels of Coq3 and Coq4 polypeptides in the null coq7 mutant (Tran et al., 2006).
Recent biochemical analyses provide physical evidence for the model of Q biosynthetic complex. Size exclusion chromatography (gel filtration) coupled with O-methyltransferase assays of the supernatant from digitonin-solubilized mitochondria demonstrated that Coq3, Coq4, Coq6, and Coq7 polypeptides co-elute as a high molecular mass complex with the Coq3 O-methyltransferase activity (Marbois et al., 2005, Tran et al., 2006). Further analysis of the representative gel-filtration fractions with mass spectrometry indicated that the DMQ6 intermediate, the substrate of Coq7 protein, is also associated with the complex (Marbois et al., 2005). Recent gel filtration analysis using newly generated specific antibodies showed that Coq2 protein co-elutes with Coq4 and Coq7 polypeptides (Tran, U.C., Gulmezian, M., Santos-Ocaña, C., Saiki, R., Navas, P., and Clarke, C. F., manuscript in preparation), and Coq9 polypeptide co-elutes with Coq3, Coq4, Coq5, and Coq8 proteins (Hsieh, E. J. and Clarke, C. F., unpublished data). Additionally, two dimensional Blue native gel electrophoresis (BN-PAGE/ SDS-PAGE) analyses of the supernatant from digitonin-solubilized mitochondria yield data indicating that Coq2, Coq3, Coq4, Coq7, and Coq9 polypeptides co-migrate as a high molecular mass complex or complexes (Tran et al., 2006) (Tran, U.C., Gulmezian, M., Santos-Ocaña, C., Saiki, R., Navas, P., and Clarke, C. F., manuscript in preparation) (Hsieh et al., 2007). Co- precipitation of biotinylated-Coq3 protein with Coq4 identified a physical interaction between Coq3 and Coq4 polypeptides (Marbois et al., 2005). Moreover, Coq9-HA (hemagglutinin antigen) fusion protein was recently demonstrated to physically interact with Coq4, Coq5, Coq6, and Coq7 polypeptide via co- precipitation. All together, these results support the existence of a multi-subunit-Q-biosynthetic complex or complexes consisting of the Coq polypeptides and some of the corresponding lipid Q-intermediates.
A proposed model for the Q biosynthetic complex in which Coq2 serves as an anchor to the inner mitochondrial membrane is depicted in Figure 2. In this figure, peripheral protein components of the complex are modeled in association with Coq2. However, the complex could also be anchored to the membrane via other uncharacterized polypeptides and/or lipid components of the inner membrane. Further studies are needed to determine the stoichiometry of the polypeptide and lipid components of the complex, elucidate the nature of the membrane association, identify other potential constituents, and examine the effects of coq mutations on the dynamic of the complex.
3. Regulation of Q Function and Biosynthesis in S. cerevisiae
3.1. A potential Q6 binding polypeptide-Coq10 protein
Complementation of a partially respiratory deficient mutant from the Genome Deletion Strain Collection identified COQ10, a new gene required for Q function in respiration (Barros et al., 2005). Similar to previously characterized coq mutants (coq1-coq9), the yeast coq10 mutant exhibits defective NADH-cytochrome c reductase and succinate-cytochrome c reductase activities, which can be restored to near wild-type level by addition of Q2. Unlike the other coq mutants, however, the coq10 mutant grows slowly on medium containing non-fermentable carbon sources (ethanol and glycerol) and produces near wild-type levels of Q6. Interestingly, the slow-growing phenotype on of the coq10 mutant on medium containing ethanol and glycerol was partially rescued by exogenous Q6 supplementation or multi-copy expression of the COQ2, COQ7, or COQ8 genes. This suggests that endogenous Q6 produced by the mutant is not as “functional” as that synthesized in the corresponding wild-type strain. Sequence analysis of yeast Coq10 protein, as well as its homologues in Caulobacter crescentus and other eukaryotes, identifies it as a member of the protein superfamily containing the START domain (Shen et al., 2005). The solution structure of the C. crescentus homolog of Coq10 identified a hydrophobic tunnel which in other START family members functions in binding cholesterol, polyketides, or phospholipids (Miller, 2007). Because polypeptides belonging to this superfamily have been shown to be involved in lipid binding and trafficking, it is likely that Coq10 protein may function in transport and/or directing newly synthesized Q to its correct location in the mitochondrial electron transport chain.
3.2. Effects of carbon catabolites on Q biosynthesis
In S. cerevisiae, Q levels are directly correlated to mitochondrial development and oxygen availability (Pennock and Threlfall, 1983). Biosynthesis of Q was found to be highest in aerobically grown cells and barely detectable in anaerobic cells (Lester and Crane, 1959). When yeasts were grown in aerobic batch cultures, the amount of Q varied depending on carbon catabolites (Gordon and Stewart, 1969). High glucose concentration inhibited Q biosynthesis to a higher degree than similar concentration of galactose, a non-repressing fermentable carbon source. As expected, Q production was greatly increased in media containing non-fermentable carbon source, when catabolite repression is at the minimum. Interestingly, supplementation with cAMP alleviates the inhibitory effect of glucose on Q biosynthesis at the enzymatic level (Sippel et al., 1983). Previous studies have demonstrated that mRNA levels of COQ3, COQ4, COQ5, and COQ7 genes were higher in yeasts grown in glycerol containing media than in cultures containing fermentable dextrose (Marbois and Clarke, 1996, Hagerman et al., 2002, Clarke et al., 1991, Belogrudov et al., 2001). The amount of Coq7 polypeptide was significantly increased by growth on media containing ethanol (Jonassen et al., 1998). To further understand the mechanism that underlies this carbon-catabolite regulatory control of Q biosynthesis, it is necessary to examine how growth in media containing different carbon sources affects Q6 content, steady state levels of Coq proteins, and the dynamic of the multi-subunit-Q-biosynthetic complex.
4. Perspectives
Coenzyme Q (ubiquinone or Q) is a prenylated benzoquinone lipid that is found in membranes throughout eukaryotic cells. The reversible redox chemistry of Q is responsible for its function in the respiratory electron transport chain of inner mitochondrial membranes and as a lipophilic antioxidant. Q is widely used as a dietary supplement and in a variety of clinical therapies, including treatment of several neuro-degenerative diseases (Ferrante et al., 2002, Grundman et al., 2002, Muller et al., 2003, Beal, 2004, Shults, 2005) and certain respiratory chain defects (Geromel et al., 2002).The studies reviewed in this article employed a combination of genetics, molecular biology, and biochemistry to delinate the eukaryotic biosynthetic pathway of Q, with S. cerevisiae as model system. Considering the nutritional and therapeutic aspects of Q, it is likely that characterization of Q biosynthesis and regulation will promote our understanding Q metabolism and its recent use in clinical therapies.
Acknowledgments
We thank our colleagues and collaborators for many stimulating discussions and Drs. Edward J. Hsieh, Melissa Gulmezian, Ryoichi Saiki, Peter Gin, and Beth Marbois for permission to quote from their unpublished results. The studies on Q biosynthesis and function in the authors' laboratory have been supported in part by National Institutions of Health Grant GM45952 and AG19777.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi A, Shinjyo N, Fujita D, Miyoshi H, Amino H, Watanabe Y, Kita K. Complementation of Escherichia coli ubiF mutation by Caenorhabditis elegans CLK-1, a product of the longevity gene of the nematode worm. FEBS Lett. 2003;543:174–8. doi: 10.1016/s0014-5793(03)00419-8. [DOI] [PubMed] [Google Scholar]
- Ashby MN, Edwards PA. Elucidation of the deficiency in two yeast coenzyme Q mutants. Characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J Biol Chem. 1990;265:13157–64. [PubMed] [Google Scholar]
- Ashby MN, Kutsunai SY, Ackerman S, Tzagoloff A, Edwards PA. COQ2 is a candidate for the structural gene encoding para-hydroxybenzoate:polyprenyltransferase. J Biol Chem. 1992;267:4128–36. [PubMed] [Google Scholar]
- Avelange-Macherel MH, Joyard J. Cloning and functional expression of AtCOQ3, the Arabidopsis homologue of the yeast COQ3 gene, encoding a methyltransferase from plant mitochondria involved in ubiquinone biosynthesis. Plant J. 1998;14:203–213. doi: 10.1046/j.1365-313x.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- Baba SW, Belogrudov GI, Lee JC, Lee PT, Strahan J, Shepherd JN, Clarke CF. Yeast coq5 C-methyltransferase is required for stability of other polypeptides involved in coenzyme q biosynthesis. J Biol Chem. 2004;279:10052–9. doi: 10.1074/jbc.M313712200. [DOI] [PubMed] [Google Scholar]
- Barkovich RJ, Shtanko A, Shepherd JA, Lee PT, Myles DC, Tzagoloff A, Clarke CF. Characterization of the COQ5 gene from Saccharomyces cerevisiae. Evidence for a C-methyltransferase in ubiquinone biosynthesis. J Biol Chem. 1997;272:9182–8. doi: 10.1074/jbc.272.14.9182. [DOI] [PubMed] [Google Scholar]
- Barros MH, Johnson A, Gin P, Marbois BN, Clarke CF, Tzagoloff A. The Saccharomyces cerevisiae COQ10 gene encodes a START domain protein required for function of coenzyme Q in respiration. J Biol Chem. 2005;280:42627–35. doi: 10.1074/jbc.M510768200. Epub 2005 Oct 17. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36:381–6. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- Belogrudov GI, Lee PT, Jonassen T, Hsu AY, Gin P, Clarke CF. Yeast COQ4 encodes a mitochondrial protein required for coenzyme Q synthesis. Arch Biochem Biophys. 2001;392:48–58. doi: 10.1006/abbi.2001.2448. [DOI] [PubMed] [Google Scholar]
- Berthold DA, Stenmark P. Membrane-bound diiron carboxylate proteins. Annu Rev Plant Biol. 2003;54:497–517. doi: 10.1146/annurev.arplant.54.031902.134915. [DOI] [PubMed] [Google Scholar]
- Bousquet I, Dujardin G, Slonimski PP. ABC1, a novel yeast nuclear gene has a dual function in mitochondria: it suppresses a cytochrome b mRNA translation defect and is essential for the electron transfer in the bc 1 complex. EMBO J. 1991;10:2023–2031. doi: 10.1002/j.1460-2075.1991.tb07732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracey MH, Cravatt BF, Stevens RC. Structural commonalities among integral membrane enzymes. FEBS Lett. 2004;567:159–65. doi: 10.1016/j.febslet.2004.04.084. [DOI] [PubMed] [Google Scholar]
- Brasseur G, Tron G, Dujardin G, Slonimski PP, Brivet-Chevillotte P. The nuclear ABC1 gene is essential for the correct conformation and functioning of the cytochrome bc1 complex and the neighbouring complexes II and IV in the mitochondrial respiratory chain. Eur J Biochem. 1997;246:103–11. doi: 10.1111/j.1432-1033.1997.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- Clarke CF, Williams W, Teruya JH. Ubiquinone biosynthesis in Saccharomyces cerevisiae. Isolation and sequence of COQ3, the 3,4-dihydroxy-5-hexaprenylbenzoate methyltransferase gene. J Biol Chem. 1991;266:16636–44. [PubMed] [Google Scholar]
- Dibrov E, Robinson KM, Lemire BD. The COQ5 gene encodes a yeast mitochondrial protein necessary for ubiquinone biosynthesis and the assembly of the respiratory chain. J Biol Chem. 1997;272:9175–81. doi: 10.1074/jbc.272.14.9175. [DOI] [PubMed] [Google Scholar]
- Do TQ, Hsu AY, Jonassen T, Lee PT, Clarke CF. A defect in coenzyme Q biosynthesis is responsible for the respiratory deficiency in Saccharomyces cerevisiae abc1 mutants. J Biol Chem. 2001;276:18161–8. doi: 10.1074/jbc.M100952200. Epub 2001 Mar 9. [DOI] [PubMed] [Google Scholar]
- Eggink G, Engel H, Vriend G, Terpstra P, Witholt B. Rubredoxin reductase of Pseudomonas oleovorans. Structural relationship to other flavoprotein oxidoreductases based on one NAD and two FAD fingerprints. J Mol Biol. 1990;212:135–42. doi: 10.1016/0022-2836(90)90310-I. [DOI] [PubMed] [Google Scholar]
- Eppink MH, Schreuder HA, Van Berkel WJ. Identification of a novel conserved sequence motif in flavoprotein hydroxylases with a putative dual function in FAD/NAD(P)H binding. Protein Sci. 1997;6:2454–8. doi: 10.1002/pro.5560061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank JJ, Barnes TM, Lakowski B, Lussier M, Bussey H, Hekimi S. Structural and functional conservation of the Caenorhabditis elegans timing gene clk-1. Science. 1997;275:980–3. doi: 10.1126/science.275.5302.980. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, Beal MF. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J Neurosci. 2002;22:1592–9. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori A, Bianchi MM, Fabiani L, Falcone C, Francisci S, Palleschi C, Solimando N, Uccelletti D, Frontali L. Disruption of six novel genes from chromosome VII of Saccharomyces cerevisiae reveals one essential gene and one gene which affects the growth rate. Yeast. 2000;16:377–86. doi: 10.1002/1097-0061(20000315)16:4<377::AID-YEA537>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Forsgren M, Attersand A, Lake S, Grunler J, Swiezewska E, Dallner G, Climent I. Isolation and functional expression of human COQ2, a gene encoding a polyprenyl transferase involved in the synthesis of CoQ. Biochem J. 2004;382:519–26. doi: 10.1042/BJ20040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–61. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geromel V, Darin N, Chretien D, Benit P, Delonlay P, Rotig A, Munnich A, Rustin P. Coenzyme Q(10) and idebenone in the therapy of respiratory chain diseases: rationale and comparative benefits. Mol Genet Metab. 2002;77:21–30. doi: 10.1016/s1096-7192(02)00145-2. [DOI] [PubMed] [Google Scholar]
- Gin P, Clarke CF. Genetic evidence for a multi-subunit complex in coenzyme Q biosynthesis in yeast and the role of the Coq1 hexaprenyl diphosphate synthase. J Biol Chem. 2005;280:2676–81. doi: 10.1074/jbc.M411527200. Epub 2004 Nov 17. [DOI] [PubMed] [Google Scholar]
- Gin P, Hsu AY, Rothman SC, Jonassen T, Lee PT, Tzagoloff A, Clarke CF. The Saccharomyces cerevisiae COQ6 gene encodes a mitochondrial flavin-dependent monooxygenase required for coenzyme Q biosynthesis. J Biol Chem. 2003;278:25308–16. doi: 10.1074/jbc.M303234200. [DOI] [PubMed] [Google Scholar]
- Glerum DM, Muroff I, Jin C, Tzagoloff A. COX15 codes for a mitochondrial protein essential for the assembly of yeast cytochrome oxidase. J Biol Chem. 1997;272:19088–94. doi: 10.1074/jbc.272.30.19088. [DOI] [PubMed] [Google Scholar]
- Goewert RR. Studies on the Biosynthesis of Ubiquinone. St. Louis, MO: Saint Louis University; 1980. [Google Scholar]
- Gordon PA, Stewart PR. Ubiquinone formation in wild-type and petite yeast: the effect of catabolite repression. Biochim Biophys Acta. 1969;177:358–60. doi: 10.1016/0304-4165(69)90150-0. [DOI] [PubMed] [Google Scholar]
- Grundman M, Grundman M, Delaney P. Antioxidant strategies for Alzheimer's disease. Proc Nutr Soc. 2002;61:191–202. doi: 10.1079/PNS2002146. [DOI] [PubMed] [Google Scholar]
- Grunler J, Ericsson J, Dallner G. Branch-point reactions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins. Biochim Biophys Acta. 1994;1212:259–77. doi: 10.1016/0005-2760(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Hagerman RA, Trotter PJ, Willis RA. The regulation of COQ5 gene expression by energy source. Free Radic Res. 2002;36:485–90. doi: 10.1080/10715760290021360. [DOI] [PubMed] [Google Scholar]
- Hagerman RA, Willis RA. The yeast gene COQ5 is differentially regulated by Mig1p, Rtg3p and Hap2p. Biochim Biophys Acta. 2002;1578:51–8. doi: 10.1016/s0167-4781(02)00496-7. [DOI] [PubMed] [Google Scholar]
- Hsieh EJ, Dinoso JB, Clarke CF. A tRNA(TRP) gene mediates the suppression of cbs2-223 previously attributed to ABC1/COQ8. Biochem Biophys Res Commun. 2004;317:648–653. doi: 10.1016/j.bbrc.2004.03.096. [DOI] [PubMed] [Google Scholar]
- Hsieh EJ, Gin P, Gulmezian M, Tran UC, Saiki R, Marbois BN, Clarke CF. Saccharomyces cerevisiae Coq9 polypeptide is a subunit of the mitochondrial coenzyme Q biosynthetic complex. Arch Biochem Biophys. 2007 doi: 10.1016/j.abb.2007.02.016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AY, Do TQ, Lee PT, Clarke CF. Genetic evidence for a multi-subunit complex in the O-methyltransferase steps of coenzyme Q biosynthesis. Biochim Biophys Acta. 2000;1484:287–97. doi: 10.1016/s1388-1981(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Hsu AY, Poon WW, Shepherd JA, Myles DC, Clarke CF. Complementation of coq3 mutant yeast by mitochondrial targeting of the Escherichia coli UbiG polypeptide: evidence that UbiG catalyzes both O-methylation steps in ubiquinone biosynthesis. Biochemistry. 1996;35:9797–806. doi: 10.1021/bi9602932. [DOI] [PubMed] [Google Scholar]
- Jiang N, Levavasseur F, Mccright B, Shoubridge EA, Hekimi S. Mouse CLK-1 is imported into mitochondria by an unusual process that requires a leader sequence but no membrane potential. J Biol Chem. 2001;276:29218–25. doi: 10.1074/jbc.M103686200. Epub 2001 May 31. [DOI] [PubMed] [Google Scholar]
- Johnson A, Gin P, Marbois BN, Hsieh EJ, Wu M, Barros MH, Clarke CF, Tzagoloff A. COQ9, a new gene required for the biosynthesis of coenzyme Q in Saccharomyces cerevisiae. J Biol Chem. 2005;280:31397–404. doi: 10.1074/jbc.M503277200. Epub 2005 Jul 18. [DOI] [PubMed] [Google Scholar]
- Jonassen T, Clarke CF. Isolation and functional expression of human COQ3, a gene encoding a methyltransferase required for ubiquinone biosynthesis. J Biol Chem. 2000;275:12381–12387. doi: 10.1074/jbc.275.17.12381. [DOI] [PubMed] [Google Scholar]
- Jonassen T, Clarke CF. Genetic Analysis of Coenzyme Q Biosynthesis. In: Kagan VE, Quinn PJ, editors. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton, FL: CRC PRESS; 2001. [Google Scholar]
- Jonassen T, Marbois BN, Kim L, Chin A, Xia YR, Lusis AJ, Clarke CF. Isolation and sequencing of the rat Coq7 gene and the mapping of mouse Coq7 to chromosome 7. Arch Biochem Biophys. 1996;330:285–289. doi: 10.1006/abbi.1996.0255. [DOI] [PubMed] [Google Scholar]
- Jonassen T, Proft M, Randez-Gil F, Schultz JR, Marbois BN, Entian KD, Clarke CF. Yeast Clk-1 homologue (Coq7/Cat5) is a mitochondrial protein in coenzyme Q synthesis. J Biol Chem. 1998;273:3351–3357. doi: 10.1074/jbc.273.6.3351. [DOI] [PubMed] [Google Scholar]
- Jun L, Saiki R, Tatsumi K, Nakagawa T, Kawamukai M. Identification and subcellular localization of two solanesyl diphosphate synthases from Arabidopsis thaliana. Plant Cell Physiol. 2004;45:1882–8. doi: 10.1093/pcp/pch211. [DOI] [PubMed] [Google Scholar]
- Kagan RM, Clarke S. Widespread occurrence of three sequence motifs in diverse S-adenosylmethionine-dependent methyltransferases suggests a common structure for these enzymes. Arch Biochem Biophys. 1994;310:417–427. doi: 10.1006/abbi.1994.1187. [DOI] [PubMed] [Google Scholar]
- Katz JE, Dlakic M, Clarke S. Automated identification of putative methyltransferases from genomic open reading frames. Mol Cell Proteomics. 2003;2:525–40. doi: 10.1074/mcp.M300037-MCP200. Epub 2003 Jul 18. [DOI] [PubMed] [Google Scholar]
- Lee PT, Hsu AY, Ha HT, Clarke CF. A C-methyltransferase involved in both ubiquinone and menaquinone biosynthesis: isolation and identification of the Escherichia coli ubiE gene. J Bacteriol. 1997;179:1748–1754. doi: 10.1128/jb.179.5.1748-1754.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Aravind L, Koonin EV. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 1998;8:1038–1047. doi: 10.1101/gr.8.10.1038. [DOI] [PubMed] [Google Scholar]
- Lester RL, Crane FL. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959;234:2169–75. [PubMed] [Google Scholar]
- Leuenberger D, Bally NA, Schatz G, Koehler CM. Different import pathways through the mitochondrial intermembrane space for inner membrane proteins. Embo J. 1999;18:4816–22. doi: 10.1093/emboj/18.17.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbois B, Gin P, Faull KF, Poon WW, Lee PT, Strahan J, Shepherd JN, Clarke CF. Coq3 and Coq4 define a polypeptide complex in yeast mitochondria for the biosynthesis of coenzyme Q. J Biol Chem. 2005;280:20231–8. doi: 10.1074/jbc.M501315200. Epub 2005 Mar 25. [DOI] [PubMed] [Google Scholar]
- Marbois BN, Clarke CF. The COQ7 gene encodes a protein in Saccharomyces cerevisiae necessary for ubiquinone biosynthesis. J Biol Chem. 1996;271:2995–3004. doi: 10.1074/jbc.271.6.2995. [DOI] [PubMed] [Google Scholar]
- Marbois BN, Hsu A, Pillai R, Colicelli J, Clarke CF. Cloning of a rat cDNA encoding dihydroxypolyprenylbenzoate methyltransferase by functional complementation of a Saccharomyces cerevisiae mutant deficient in ubiquinone biosynthesis. Gene. 1994;138:213–217. doi: 10.1016/0378-1119(94)90810-9. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Cherukuri PF, Deweese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Liebert CA, Liu C, Lu F, Marchler GH, Mullokandov M, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Yamashita RA, Yin JJ, Zhang D, Bryant SH. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 2005;33:D192–6. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganathan G. Biosynthesis of the isoprenoid quinones menaquinone (vitamin K2) and ubiquinone (coenzyme Q) In: Neidhardt FC, Curtiss R, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella, Cellular and Molecular Biology. Washington D. C.: American Society for Microbiology; 1996. [Google Scholar]
- Meganathan R. Ubiquinone biosynthesis in microorganisms. FEMS Microbiol Lett. 2001;203:131–139. doi: 10.1111/j.1574-6968.2001.tb10831.x. [DOI] [PubMed] [Google Scholar]
- Miller WL. StAR search--what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol. 2007;21:589–601. doi: 10.1210/me.2006-0303. [DOI] [PubMed] [Google Scholar]
- Momose K, Rudney H. 3-Polyprenyl-4-hydroxybenzoate synthesis in the inner membrane of mitochondria from p-hydroxybenzoate and isopentenylpyrophosphate. A demonstration of isoprenoid synthesis in rat liver mitochondria. J Biol Chem. 1972;247:3930–40. [PubMed] [Google Scholar]
- Muller T, Buttner T, Gholipour AF, Kuhn W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson's disease. Neurosci Lett. 2003;341:201–4. doi: 10.1016/s0304-3940(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Niewmierzycka A, Clarke S. S-Adenosylmethionine-dependent methylation in Saccharomyces cerevisiae. Identification of a novel protein arginine methyltransferase. J Biol Chem. 1999;274:814–824. doi: 10.1074/jbc.274.2.814. [DOI] [PubMed] [Google Scholar]
- Ohara K, Yamamoto K, Hamamoto M, Sasaki K, Yazaki K. Functional Characterization of OsPPT1, Which Encodes p-hydroxybenzoate Polyprenyltransferase Involved in Ubiquinone Biosynthesis in Oryza sativa. Plant Cell Physiol. 2006;24:24. doi: 10.1093/pcp/pcj025. [DOI] [PubMed] [Google Scholar]
- Okada K, Kainou T, Matsuda H, Kawamukai M. Biological significance of the side chain length of ubiquinone in Saccharomyces cerevisiae. FEBS Lett. 1998;431:241–4. doi: 10.1016/s0014-5793(98)00753-4. [DOI] [PubMed] [Google Scholar]
- Okada K, Kamiya Y, Zhu X, Suzuki K, Tanaka K, Nakagawa T, Matsuda H, Kawamukai M. Cloning of the sdsA gene encoding solanesyl diphosphate synthase from Rhodobacter capsulatus and its functional expression in Escherichia coli and Saccharomyces cerevisiae. J Bacteriol. 1997;179:5992–8. doi: 10.1128/jb.179.19.5992-5998.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ohara K, Yazaki K, Nozaki K, Uchida N, Kawamukai M, Nojiri H, Yamane H. The AtPPT1 gene encoding 4-hydroxybenzoate polyprenyl diphosphate transferase in ubiquinone biosynthesis is required for embryo development in Arabidopsis thaliana. Plant Mol Biol. 2004;55:567–77. doi: 10.1007/s11103-004-1298-4. [DOI] [PubMed] [Google Scholar]
- Okada K, Suzuki K, Kamiya Y, Zhu X, Fujisaki S, Nishimura Y, Nishino T, Nakagawa T, Kawamukai M, Matsuda H. Polyprenyl diphosphate synthase essentially defines the length of the side chain of ubiquinone. Biochim Biophys Acta. 1996;1302:217–23. doi: 10.1016/0005-2760(96)00064-1. [DOI] [PubMed] [Google Scholar]
- Olson RE, Rudney H. Biosynthesis of ubiquinone. Vitam Horm. 1983;40:1–43. doi: 10.1016/s0083-6729(08)60431-8. [DOI] [PubMed] [Google Scholar]
- Padilla S, Jonassen T, Jimenez-Hidalgo MA, Fernandez-Ayala DJ, Lopez-Lluch G, Marbois B, Navas P, Clarke CF, Santos-Ocana C. Demethoxy-Q, an intermediate of coenzyme Q biosynthesis, fails to support respiration in Saccharomyces cerevisiae and lacks antioxidant activity. J Biol Chem. 2004;279:25995–6004. doi: 10.1074/jbc.M400001200. Epub 2004 Apr 12. [DOI] [PubMed] [Google Scholar]
- Palfey BA, Ballou DP, Massey V. In: Active Oxygen in Biochemistry. Valentine JS, Foote CS, Greenberg A, Liebman JF, editors. Glasgow, UK: Blackie Academic and Professional Press; 1995. [Google Scholar]
- Pennock JF, Threlfall DR. Biosynthesis of Ubiquinone and Related Compounds. In: Porter JW, Spurgeon SL, editors. Biosynthesis of Isoprenoid Compounds. New York: John Wiley & Sons; 1983. [Google Scholar]
- Picot D, Loll PJ, Garavito RM. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994;367:243–9. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- Poon WW, Barkovich RJ, Hsu AY, Frankel A, Lee PT, Shepherd JN, Myles DC, Clarke CF. Yeast and rat Coq3 and Escherichia coli UbiG polypeptides catalyze both O-methyltransferase steps in coenzyme Q biosynthesis. J Biol Chem. 1999;274:21665–72. doi: 10.1074/jbc.274.31.21665. [DOI] [PubMed] [Google Scholar]
- Poon WW, Do TQ, Marbois BN, Clarke CF. Sensitivity to treatment with polyunsaturated fatty acids is a general characteristic of the ubiquinone-deficient yeast coq mutants. Molec Aspects Med. 1997;18:s121–s127. doi: 10.1016/s0098-2997(97)00004-6. [DOI] [PubMed] [Google Scholar]
- Poon WW, Marbois BN, Faull KF, Clarke CF. 3-Hexaprenyl-4-hydroxybenzoic acid forms a predominant intermediate pool in ubiquinone biosynthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1995;320:305–14. doi: 10.1016/0003-9861(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Proft M, Kotter P, Hedges D, Bojunga N, Entian KD. CAT5, a new gene necessary for derepression of gluconeogenic enzymes in Saccharomyces cerevisiae. Embo J. 1995;14:6116–26. doi: 10.1002/j.1460-2075.1995.tb00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodel G. Two yeast nuclear genes, CBS1 and CBS2, are required for translation of mitochondrial transcripts bearing the 5′-untranslated COB leader. Curr Genet. 1986;11:41–5. doi: 10.1007/BF00389424. [DOI] [PubMed] [Google Scholar]
- Saiki R, Nagata A, Kainou T, Matsuda H, Kawamukai M. Characterization of solanesyl and decaprenyl diphosphate synthases in mice and humans. Febs J. 2005;272:5606–22. doi: 10.1111/j.1742-4658.2005.04956.x. [DOI] [PubMed] [Google Scholar]
- Saiki R, Nagata A, Uchida N, Kainou T, Matsuda H, Kawamukai M. Fission yeast decaprenyl diphosphate synthase consists of Dps1 and the newly characterized Dlp1 protein in a novel heterotetrameric structure. Eur J Biochem. 2003;270:4113–21. doi: 10.1046/j.1432-1033.2003.03804.x. [DOI] [PubMed] [Google Scholar]
- Shen Y, Goldsmith-Fischman S, Atreya HS, Acton T, Ma L, Xiao R, Honig B, Montelione GT, Szyperski T. NMR structure of the 18 kDa protein CC1736 from Caulobacter crescentus identifies a member of the START domain superfamily and suggests residues mediating substrate specificity. Proteins. 2005;58:747–50. doi: 10.1002/prot.20365. [DOI] [PubMed] [Google Scholar]
- Shepherd JA, Poon WW, Myles DC, Clarke CF. The biosynthesis of ubiquinone: Synthesis and enzymatic modification of biosynthetic precursors. Tetrahedron Letters. 1996;37:2395–2398. [Google Scholar]
- Shults CW. Therapeutic role of coenzyme Q(10) in Parkinson's disease. Pharmacol Ther. 2005;107:120–30. doi: 10.1016/j.pharmthera.2005.02.002. Epub 2005 Apr 21. [DOI] [PubMed] [Google Scholar]
- Sippel CJ, Goewert RR, Slachman FN, Olson RE. The regulation of ubiquinone-6 biosynthesis by Saccharomyces cerevisiae. J Biol Chem. 1983;258:1057–61. [PubMed] [Google Scholar]
- Stenmark P, Grunler J, Mattsson J, Sindelar PJ, Nordlund P, Berthold DA. A new member of the family of di-iron carboxylate proteins. Coq7 (clk-1), a membrane-bound hydroxylase involved in ubiquinone biosynthesis. J Biol Chem. 2001;276:33297–300. doi: 10.1074/jbc.C100346200. Epub 2001 Jul 2. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Okada K, Kamiya Y, Zhu XF, Nakagawa T, Kawamukai M, Matsuda H. Analysis of the decaprenyl diphosphate synthase (dps) gene in fission yeast suggests a role of ubiquinone as an antioxidant. J Biochem (Tokyo) 1997;121:496–505. doi: 10.1093/oxfordjournals.jbchem.a021614. [DOI] [PubMed] [Google Scholar]
- Tran UC, Marbois B, Gin P, Gulmezian M, Jonassen T, Clarke CF. Complementation of Saccharomyces cerevisiae coq7 Mutants by Mitochondrial Targeting of the Escherichia coli UbiF Polypeptide: TWO FUNCTIONS OF YEAST COQ7 POLYPEPTIDE IN COENZYME Q BIOSYNTHESIS. J Biol Chem. 2006;281:16401–9. doi: 10.1074/jbc.M513267200. Epub 2006 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–99. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A, Akai A, Needleman RB. Assembly of the mitochondrial membrane system. Characterization of nuclear mutants of Saccharomyces cerevisiae with defects in mitochondrial ATPase and respiratory enzymes. J Biol Chem. 1975a;250:8228–35. [PubMed] [Google Scholar]
- Tzagoloff A, Akai A, Needleman RB, Zulch G. Assembly of the mitochondrial membrane system. Cytoplasmic mutants of Saccharomyces cerevisiae with lesions in enzymes of the respiratory chain and in the mitochondrial ATPase. J Biol Chem. 1975b;250:8236–42. [PubMed] [Google Scholar]
- Tzagoloff A, Dieckmann CL. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990;54:211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A, Yue J, Jang J, Paul MF. A new member of a family of ATPases is essential for assembly of mitochondrial respiratory chain and ATP synthetase complexes in Saccharomyces cerevisiae. J Biol Chem. 1994;269:26144–51. [PubMed] [Google Scholar]
- Uchida N, Suzuki K, Saiki R, Kainou T, Tanaka K, Matsuda H, Kawamukai M. Phenotypes of fission yeast defective in ubiquinone production due to disruption of the gene for p-hydroxybenzoate polyprenyl diphosphate transferase. J Bacteriol. 2000;182:6933–9. doi: 10.1128/jb.182.24.6933-6939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajo Z, King LM, Jonassen T, Wilkin DJ, Ho N, Munnich A, Clarke CF, Francomano CA. Conservation of the Caenorhabditis elegans timing gene clk-1 from yeast to human: a gene required for ubiquinone biosynthesis with potential implications for aging. Mamm Genome. 1999;10:1000–1004. doi: 10.1007/s003359901147. [DOI] [PubMed] [Google Scholar]
- Vidgren J, Ovaska M, Tenhunen J, Tilgmann C, Lotta T, Mannisto PT. In: S-Adenosylmethionine-dependent Methyltransferases:Structures and Functions. Cheng X, Blumenthal RM, editors. River Edge, NJ: World Scientific Publishing; 1999. [Google Scholar]
- Wang KC, Ohnuma SI. Isoprenyl diphosphate synthases. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2000;1529:33–48. doi: 10.1016/s1388-1981(00)00136-0. [DOI] [PubMed] [Google Scholar]
- Wendt KU, Lenhart A, Schulz GE. The structure of the membrane protein squalene-hopene cyclase at 2.0 A resolution. J Mol Biol. 1999;286:175–87. doi: 10.1006/jmbi.1998.2470. [DOI] [PubMed] [Google Scholar]
- Wierenga RK, Terpstra P, Hol WG. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187:101–7. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]


