Abstract
The rapid spread of human immunodeficiency virus (HIV) worldwide makes it a high priority to develop an effective vaccine. Since live attenuated or inactivated HIV are not likely to be approved as a vaccine due to safety concerns, HIV virus like particles (VLPs) offer an attractive alternative because they are safe due to the lack of a viral genome. Although HIV VLPs have been shown to induce humoral and cellular immune responses, it is important to understand the mechanisms by which they induce such responses and to improve their immunogenicity. We generated HIV VLPs, and VLPs containing Flt3 ligand (FL), a dendritic cell growth factor, to target VLPs to dendritic cells, and investigated the roles of these VLPs in the initiation of adaptive immune responses in vitro and in vivo. We found that HIV-1 VLPs induced maturation of dendritic cells and monocyte/macrophage populations in vitro and in vivo, with enhanced expression of maturation markers and cytokines. Dendritic cells pulsed with VLPs induced activation of splenocytes resulting in increased production of cytokines. VLPs containing FL were found to increase dendritic cells and monocyte/macrophage populations in the spleen when administered to mice. Administration of VLPs induced acute activation of multiple types of cells including T and B cells as indicated by enhanced expression of the early activation marker CD69 and down-regulation of the homing receptor CD62L. VLPs containing FL were an effective form of antigen in activating immune cells via dendritic cells, and immunization with HIV VLPs containing FL resulted in enhanced T helper type 2-like immune responses.
Keywords: HIV, Virus-like particle, Flt3 ligand, Immune cells
Introduction
Virus-like particles (VLPs) are an attractive and effective type of vaccine that includes immunologically relevant structural proteins of viruses. VLPs are safer than live replicating vectored vaccines because of their lack of infectivity. Previously both neutralizing antibodies and cellular immune responses were shown to be induced by various insect cell-produced VLPs, including HIV VLPs (Buonaguro et al., 2002; Buonaguro et al., 2005; Deml et al., 1997; Notka et al., 1999), human papillomavirus (HPV) VLPs (Harro et al., 2001), and hepatitis type C virus VLPs (Jeong et al., 2004).
Dendritic cells (DCs) are very efficient antigen presenting cells involved in priming naïve CD4+ and CD8+ T cells, thus inducing primary immune responses and permitting establishment of immunological memory. Located at strategic sites in the body where pathogens enter the organism, DCs capture and process antigens, migrate to lymphoid organs (the spleen and lymph nodes), and display antigens at the cell surface in the form of peptides associated with MHC II, which stimulate CD4 T helper (Th) cells. For induction of CD8 T cells, MHC I-associated peptides are derived from endogenously synthesized proteins as well as from exogenous antigens (Bachmann et al., 1996; Reimann and Schirmbeck, 1999). Another signal necessary to prime immune responses is the expression of co-stimulatory molecules on antigen presenting cells such as B7 family proteins (CD80, CD86) and CD40 that are recognized by CD28 and CD40 ligand on T cells. When activated, DCs up-regulate MHC I and II molecules and the costimulatory molecules CD80 and CD86, and increase the secretion of cytokines thereby facilitating the priming of naïve CD4+ T-helper and CD8+ cytotoxic T cells.
The feasibility of using DCs as a vaccine target is limited due to their extremely small number in peripheral tissues and in blood. Flt3 (fms-like tyrosine kinase receptor) ligand (FL) is a hematopoietic growth factor that results in expansion of hematopoietic progenitors in the bone marrow and spleen, and potent mobilization of progenitor cells into the circulation. Examination of the tissues in FL-treated mice has indicated that FL has the unique ability to expand the number of both lymphoid-derived (CD8α+) and myeloid-derived (CD8α−), and Langerhans cell-derived DC subsets in several sites including the spleen, lymph nodes, and Peyer’s patches, as well as in the circulation, lungs and liver (Maraskovsky et al., 1997; Pulendran et al., 1997).
VLPs are considered to be relatively less effective immunogens in inducing cellular immune responses as compared to live attenuated viruses or replicating viral vector-mediated vaccines due to their non-replicating property. Therefore, it is highly desirable to develop approaches to enhance the immunogenicity of VLPs, and targeting VLP antigens to DCs could be an approach to enhance their immunogenicity. In this study, we investigated the initial responses of immune cells to VLPs in vitro and in vivo, the potential of targeting HIV VLPs to DCs by incorporation of FL into VLPs resulting in enhancement of their immunogenicity, and the effects of VLPs on activating DC populations and other immune cells. These studies offer insight on understanding the onset of immune responses against particulate VLP antigens and explore novel approaches to develop effective vaccines based on VLPs.
Results
Production of HIV VLPs containing FL
A membrane-anchored form of human FL was constructed to determine whether it could be incorporated into VLPs. Using a similar approach, we recently demonstrated that membrane anchored-form of the soluble cytokine GM-CSF could be incorporated into VLPs (Skountzou et al., 2007). The human FL-encoding gene was modified to contain the sequences coding for the HIV transmembrane (TM) with partial extracellular and cytoplasmic tail domains and a signal peptide from tissue plasminogen activator (Fig. 1A). HIV gp160 is a full-length HIV-1 Env protein (Fig. 1A). Sf9 insect cells co-infected with rBVs expressing HIV-1 Gag, Env or FL released VLPs containing HIV Env (V-HIV; Gag + Env) or VLPs containing FL (V-HIV-FL; Gag+Env+human FL) in the culture supernatants. The presence of HIV Env, Gag, and human FL proteins in the purified VLPs was shown by Western blots (Fig. 1B and 1C).
Fig. 1.
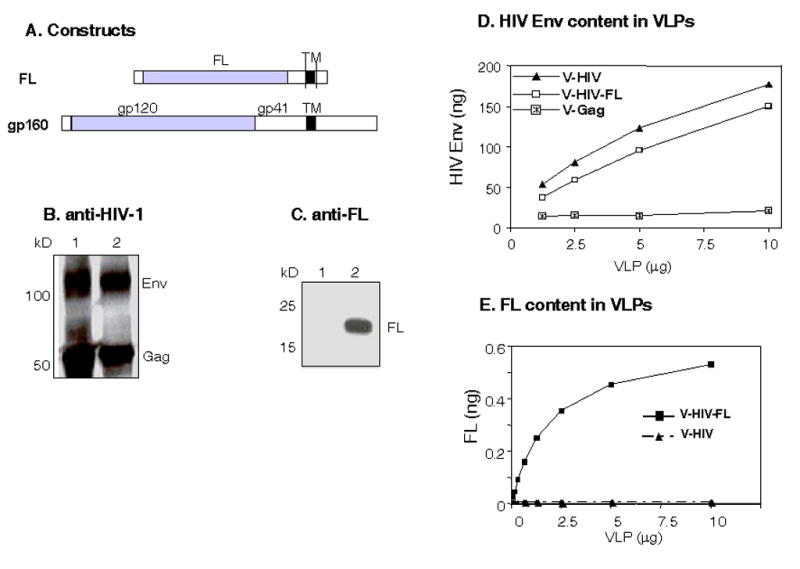
DNA constructs and characterization of HIV VLPs. (A) Schematic diagram of DNA constructs. The FL is composed of a TPA signal peptide, extracellar domain of FL (158 aa), and HIV-1 Env TM (aa 675–703) with some extracellular (aa 661–674) and truncated cytoplasmic tail (aa 704–721) domains. Gp160 is a full-length of HIV-1 ConB Env (863 aa). All these constructs were used to develop rBVs expressing FL, HIV-1 Gag, or Env gp160. (B) Western blot analysis of sucrose gradient purified VLPs probed with patient sera. 1: HIV VLPs (Gag + Env gp160), 2: VLPs containing FL (Gag + Env gp160 + FL). (C) Western blot analysis of sucrose gradient purified VLPs containing FL probed with goat anti-FL antibody. HIV-1 and VLPs containing FL were run on 12% gels and probed with anti FL antibody. 1: HIV VLP (Gag + Env gp160), 2: VLPs containing FL (Gag + Env gp160 + FL). (D) Quantitative estimation of Env in VLPs and FL-containing VLPs. ELISA plates were coated with sheep anti-HIV-1 gp120 antibody in bicarbonate buffer (5μg/ml). VLPs were treated with RIPA buffer (10μg/5 μl) and added to the ELISA plates. The amount of HIV Env was quantitatively determined using rabbit anti-HIV-1 polyclonal sera. HIV-1 gp120 MN was obtained from the NIH AIDS Research and Reference Reagent Program, and used as a standard. V-Gag: HIV-1 Env-negative VLPs (Gag), V-HIV: HIV-1 VLPs (Gag+Env), V-HIV-FL: HIV VLPs containing FL (Gag+Env+FL). (E) Quantitative estimation of FL in VLPs. VLPs containing FL were treated with RIPA buffer (10 μg /5μl) and were added to the ELISA plate coated with monoclonal antibody against human FL. The amount of human FL was determined using a standard curve of the purified FL protein.
To characterize HIV VLPs and VLPs containing FL in detail, it is important to determine quantitatively the amounts of protein incorporated into VLPs. The HIV Env protein in detergent-lysed VLP was captured onto an ELISA plate coated with HIV Env-specific antibody, and the amount of captured HIV Env was determined using HIV Env-specific polyclonal anti-sera (Fig 1D). HIV-1 Env reactivity was not found in the Gag VLPs (V-Gag) without HIV-1 Env used as a negative control. Similarly, antibodies specific to FL were used to determine FL incorporated into VLPs. HIV-1 VLPs without FL were used as a negative control (Fig. 1E). The HIV Env protein was found to be present at levels of 1–1.5% of total VLP protein and the level of FL incorporated into VLPs was found to be 0.05% of total protein content. The molar ratio of trimeric HIV Env: FL incorporated into VLPs was estimated as approximately 2:1.
Effects of VLPs on DC maturation and cytokine production in vitro
Injection of mice with a plasmid DNA encoding human FL was recently shown to expand DC populations (Nayak, Sailaja, and Jabbar, 2006; Sailaja et al., 2003). We found that this approach could provide a useful tool to expand and isolate DCs, and to study DC populations in vitro. The in vivo expanded DCs by FL-encoding DNA were used in this study (Fig. 2A). Unstimulated DCs showed only background levels of expression of activation markers CD40, CD80, and CD86 similar to those observed by isotype control antibody staining. Incubation of DCs with VLPs resulted in increases in the levels of mean fluorescence intensity (MFI) of DCs expressing CD40, even at higher levels than those observed with LPS stimulation (Figs. 2B and 2C). More prominent increases were also observed in CD80 and CD86 expression on DCs after incubation with VLPs as compared with CD40. Interestingly, HIV VLPs increased the expression of CD86 on DCs to higher levels than those observed upon treatment with Env-negative Gag VLPs. The effects of VLPs on activating DC populations were observed with as low as 0.1 μg/ml concentration of VLPs. VLPs containing FL showed similar increases in activation marker expression as compared with VLPs without FL (data not shown).
Fig. 2.
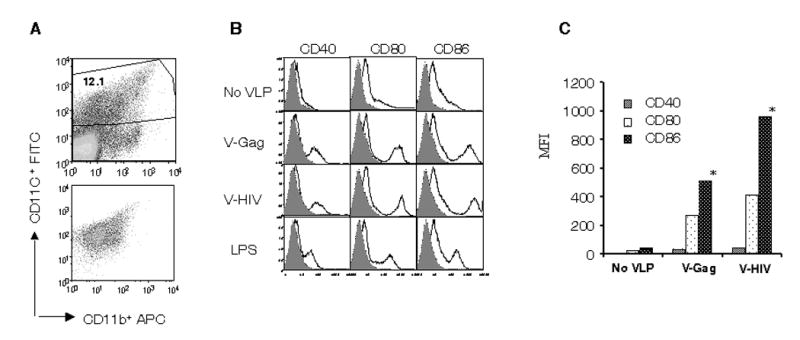
VLPs induce in vitro maturation of DCs. (A) Gating of CD11c+ DC populations. DCs expanded in vivo by injection of mice with the FL plasmid DNA were purified on CD11C+ magnetic beads. (B) Flow cytometry profile of DC activation markers of purified DC populations. DCs were obtained from spleens of mice that received the FL plasmid DNA, purified as in A, and DCs (1X106/ml) were incubated with VLPs (10μg/ml) for overnight. Stimulated DCs were characterized for DC maturation in vitro after staining the cells with antibodies against surface markers CD40, CD80, and CD86. C) Mean fluorescent intensity (MFI) for DC activation markers CD40, CD80 and CD86 induced by various VLPs was derived from the panel B and presented. These data are representative from three independent experiments.
To determine the type of responses elicited by DCs stimulated with VLPs, the pattern of cytokine production was determined. At concentrations over 1 μg of VLPs or after longer incubation with VLPs (48 hrs), DCs were stimulated to produce IL-12 (Fig. 3A). HIV VLPs stimulated DCs to secrete IL-12 at higher levels than Env-negative Gag VLPs (Fig. 3A). In contrast to IL-12, a low concentration (0.1 μg) of VLPs stimulated DCs to secrete IL-6, and HIV VLPs induced DCs to secrete significantly more IL-6 than Gag-VLPs (Fig. 3B). Levels of cytokines IL-6 and IL-12 produced by DCs stimulated with HIV VLPs were comparable to those induced by LPS stimulation (Fig. 3). Cytokines IFN-γ, IL-10 and TNF-α were also measured, but found to be below the detection levels (data not shown). We did not observe any significant differences in activation marker expression and cytokine production between VLPs with and without FL (data not shown). In summary, these results indicate that HIV VLPs can induce DC maturation and stimulate them to secrete specific cytokines, and that HIV VLPs were found to be more effective in stimulating DCs than Gag-VLPs.
Fig. 3. VLPs stimulate DCs to secrete cytokines.
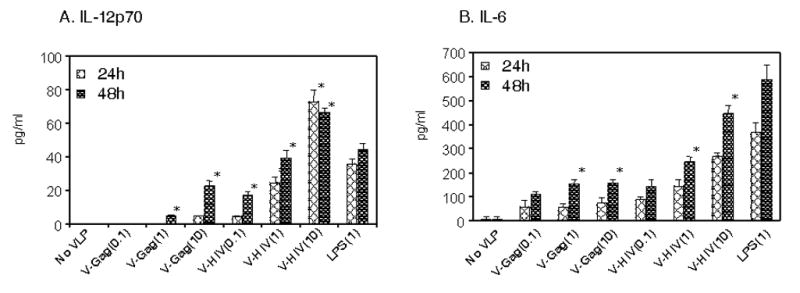
A) Cytokine IL-12 produced by VLP-activated DCs. B) Cytokine IL-6 produced by VLP-activated DCs. Purified DCs (1X106/ml) were incubated with three different concentrations of VLPs for 24 and 48 hrs, culture supernatants were collected and used for the determination of cytokines. Representative data are presented from five independent experiments, and the values represented are mean value of duplicate wells used in each assay. Numbers in the parentheses indicate the amount of VLPs in μg. V-Gag: VLPs with Gag alone, V-HIV: VLPs with Gag+Env, LPS: Lipopolysaccharide.
VLP-loaded DCs induce lymphocyte proliferation and cytokine production
To investigate functional activity of VLP-loaded DCs as antigen presenting cells, purified DCs were pulsed with VLPs and then used to determine whether the VLP-loaded DCs could stimulate naïve splenocytes. The total splenocytes, purified CD4+, or CD8+ T cells from naïve mice were incubated with VLP-loaded DCs for 7 days, and [3H]-thymidine incorporation was used as an indicator for lymphocyte proliferation. Purified DCs without exposure to VLPs (medium control) showed only background levels of spleen cell proliferation, whereas DCs loaded with Gag VLPs or HIV VLPs induced proliferation of spleen cells at significant levels (Fig. 4). When proliferation of purified naïve CD4+ and CD8+ T cells was determined after incubation with VLP-loaded DCs, we observed that CD4+ T cells proliferated at significantly higher levels compared to CD8+ T cells. In contrast, LPS stimulated both CD4+ and CD8+ T cells. This may be due to the fact that purified populations of CD4+ T cells have a higher number of CD4+ T cells than the unfractionated whole spleen cell preparations. DCs exposed to VLPs containing FL were found to be more effective in stimulating lymphocytes, particularly in proliferating CD4+ T cells (Fig. 4) (p=0.011 between V-HIV and V-HIV-FL). Thus, these results indicate that VLP-loaded DCs preferentially stimulate naïve CD4+ T lymphocytes rather than naive CD8+ T cells.
Fig. 4.
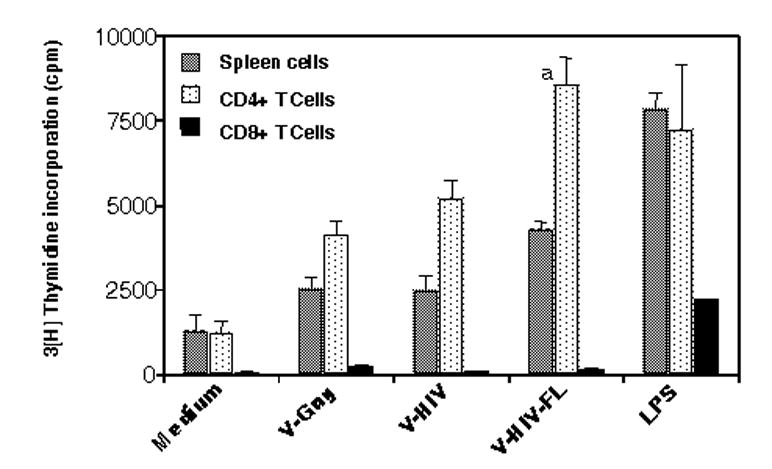
VLP-loaded DCs induce lymphocyte proliferation. Cell proliferation of splenocytes stimulated by VLP-loaded DCs. Total spleen cells, CD4+ and CD8+ T cells (1x105) were incubated with VLP-activated DCs (1x104) in 200 μl media. After 6 days of incubation, cells were pulsed with [3H]-thymidine and incorporated radioactivity was measured as an indicator of cell proliferation. Geometric mean values are shown from triplicate wells and representative results were from three independent experiments. Difference in CD4+ proliferation between V-HIV and V-HIV-FL is indicated, a: P=0.011.
The culture supernatants after incubation with VLP-loaded DCs for 7 days were used to quantify the secreted cytokines using ELISA. Incubation of splenocytes with purified DCs without VLP stimulation (medium controls) did not induce cytokine production (Fig. 5). Purified CD4+ T lymphocytes were prominently stimulated by VLP-loaded DCs to produce these cytokines at higher levels than unfractionated splenocytes (Fig. 5A, B). This indicates that the enriched CD4+ T cells in the purified CD4+ splenocytes are responsible for producing IL-5 and IL-10 cytokines, which is consistent with the results of proliferation (Fig. 4). The FL containing VLP-loaded DCs resulted in production of the highest levels of IL-10 and IL-5 cytokines particularly from CD4+ T cells. In contrast to IL-10 and IL-5, splenocytes stimulated by VLP-loaded DCs secreted IFN-γ at higher levels than fractionated CD4+ or CD8+ T lymphocytes and there were no significant differences between DC populations exposed to V-HIV and V-HIV-FL (Fig. 5C). In summary, the results suggest that VLPs can be an attractive antigenic form for uptake by DCs and that DCs pulsed with VLPs can effectively induce naïve immune cells to proliferate and to secrete cytokines, consistent with their role as antigen presenting cells.
Fig. 5.
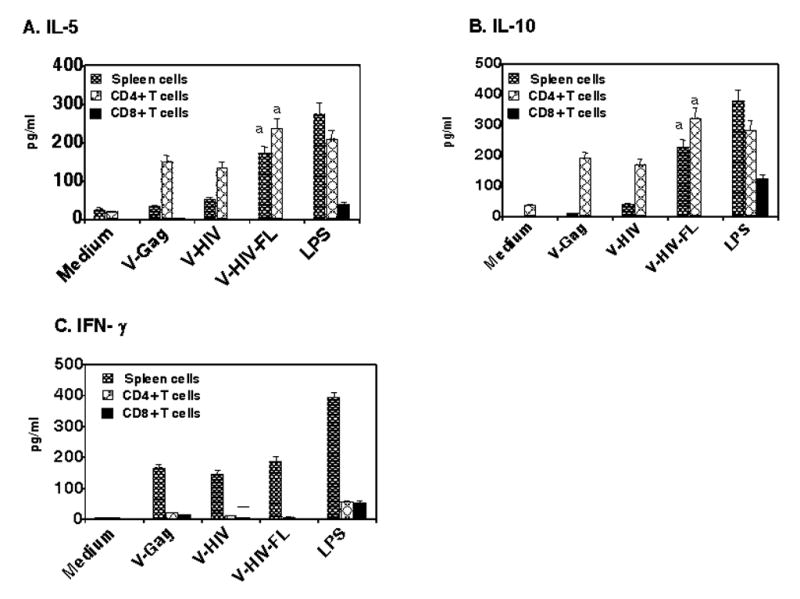
VLP-loaded DCs stimulate lymphocyte to secrete cytokine production in vitro. The experiments were set up similar to cell proliferation assay (see Fig. 4 legend) for total naïve spleen cells and CD4+ and CD8+ cells. The culture supernatants were used to determine the concentration of cytokines using ELISA. A) IL-5, B) IL-10, and C) IFN-γ. The values represented are mean value of duplicate wells used in the assay and representative data are presented from two independent experiments. Medium, DCs without VLP incubation; V-Gag, DCs loaded with Gag VLPs; V-HIV, DCs loaded with HIV VLPs; V-HIV-FL, DCs loaded with HIV VLPs containing FL; LPS, DCs loaded with LPS. All values in DCs stimulated with VLPs were significantly higher than DCs without VLPs (medium control), and V-HIV-FL values that showed statistical significance were marked (a:P<0.005).
Biological activity of FL in VLPs
Previous studies demonstrated that mice injected with 10 μg of purified human FL once a day for 7 to 10 consecutive days exhibited significantly increased CD11c+ DC populations in peripheral tissues (Maraskovsky et al., 1996; Pulendran et al., 1997). Using a similar protocol as described (Maraskovsky et al., 1996; Pulendran et al., 1997) to determine the effects of FL incorporated into VLPs (approximately equivalent to 25 ng of FL), groups of mice were intraperitoneally (i.p.) injected with VLPs or VLPs containing FL. Spleens collected after the day of last injection with VLPs were found to be significantly increased in size and in total splenocyte numbers compared to those in naïve mice (data not shown). Mice injected with VLPs containing FL showed higher levels of CD11c+ splenocyte cellularity than those injected with VLPs without FL, which was statistically significant (Fig. 6A,B). Also, FL incorporated into VLPs was found to increase bone marrow derived CD11c+ populations in vitro (data not shown). It is interesting to note that mice injected with VLPs containing FL also showed significant increases in CD11b+CD11c− monocyte/macrophage populations compared to mice that received VLPs without FL, which is more prominent than those of CD11c+ populations. Thus, the results indicate that FL incorporated into VLPs exhibits biological activity in expanding both CD11c+ and CD11b+ populations in vivo.
Fig. 6.
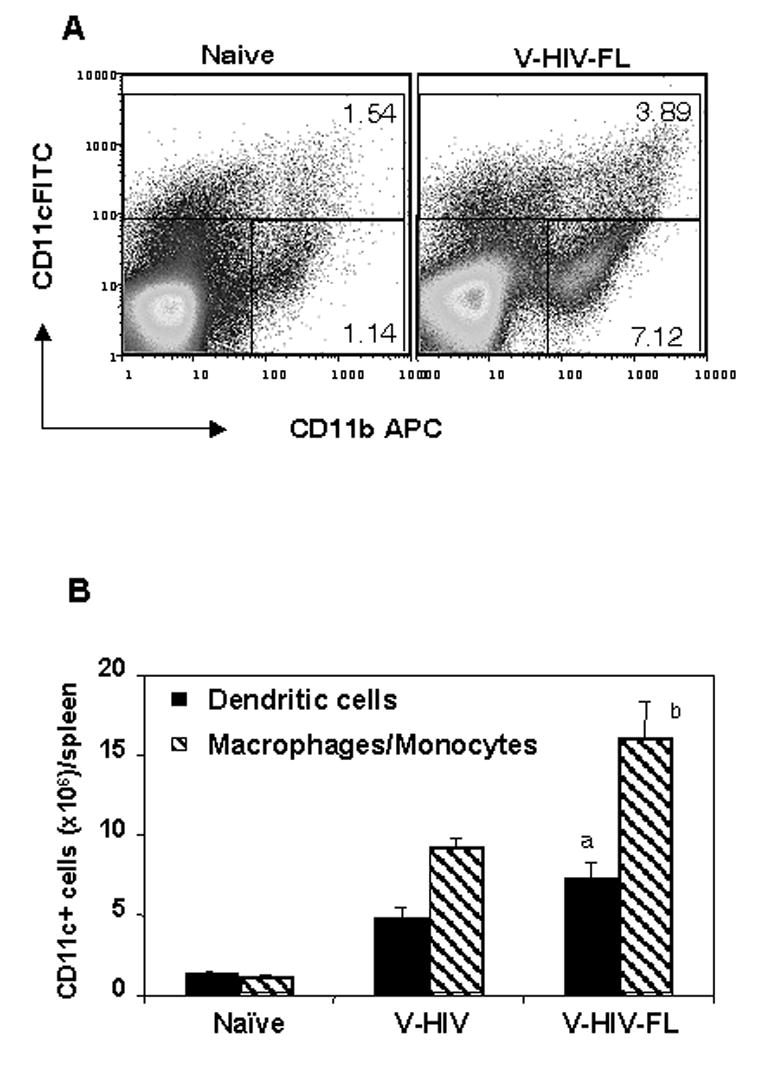
VLPs containing FL induced DCs and monocyte/macrophage expansion in vivo. A) Gating of CD11c+ (DCs) and CD11b+CD11c− (monocyte/macrophage) splenocytes. Mice (5 Balb/C mice per group) were injected (i.p.) with 50 μg of HIV VLPs (V-HIV) or HIV VLPs containing FL (V-HIV-FL) once a day for 9 consecutive days. On the tenth day spleens were collected from mice and analyzed. Spleens were processed for single cell suspension and total cell numbers were counted after staining them with trypan blue. One million cells were stained for mouse DC surface markers by anti mouse CD11b+APC and CD11c+ FITC antibodies, and 50,000 events were acquired in Excalibur and analyzed by FlowJo software. B) Cellularity of CD11c+ (DC) and CD11b+CD11c− (monocyte/macrophage) populations. The cellularity of CD11c+ and CD11b+CD11c− populations was obtained from the total spleen cell number with percentage of DCs calculated using Flow analysis from each mouse. The bar graph represents the mean of total DCs in spleen of each mouse and standard deviations are shown from five mice of each group. Statistical significances between VLPs and VLPs containing FL are shown as follows, a: P = 0.0068; b: P = 0.001. V-HIV: HIV VLPs (Gag+Env), V-HIV-FL: HIV VLPs containing FL (Gag+Env+FL).
VLPs induce activation of multiple cell types in vivo
For a vaccine to be effective, it should be capable of activating various immune cell types, including DCs, B and T cells. To determine whether VLPs can activate immune cells in vivo, mice were injected once with HIV VLPs, and cellular responses to VLP administration were assessed in splenocytes at day 3. VLP treatment induced significant changes in the activation status of multiple cell types in the spleen (Fig. 7). The activation markers CD80 and CD86 but not CD40 were found to be expressed at higher levels in CD11c+ DC populations in the VLP-administered mice compared to those in the naïve mice group (Fig. 7A). Since no significance differences in DC activation marker expression were found between VLPs with FL and without FL, it seems that FL may not play a significant role in activating DCs. This is consistent with in vitro observation as shown in Fig. 2. When CD11b+CD11c− monocyte/macrophage populations were gated and analyzed, mice injected with FL-containing VLPs showed moderately higher levels of CD40 and CD86 activation markers than those in mice that received VLPs lacking FL. (Fig. 7B).
Fig. 7.
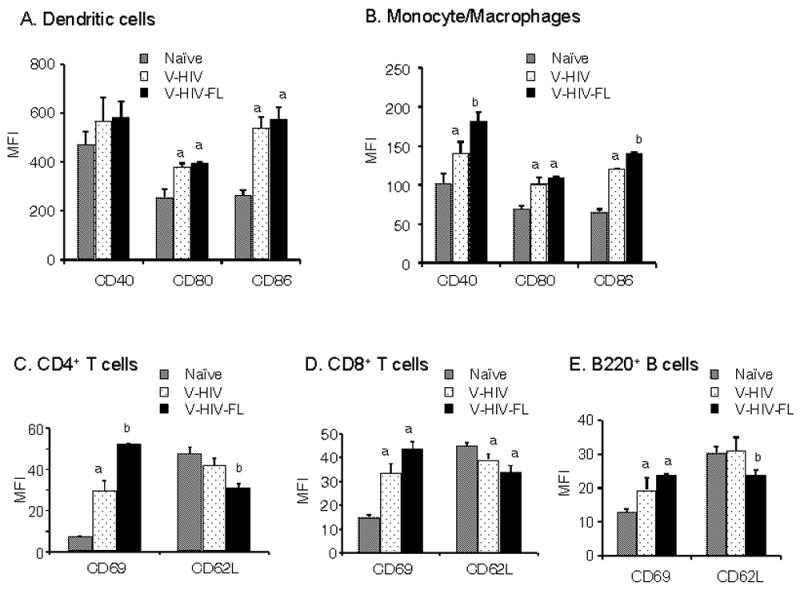
VLP induced activation of multiple cell types in vivo. Mice (5 Balb/C mice per group) were injected intraperitoneally with either VLPs or VLPs with FL (V-HIV-FL) (10μg /200μl) on day 1 and splenocytes were isolated from each mouse at day 3 post-injection. Splenocytes were stained with antibodies for DC surface and activation marker, T and B cell marker antibodies. The activation markers are represented as mean fluorescent intensity (MFI) of total gated cells. A) CD11c+ DC populations. B) CD11c−CD11b+ monocyte/macrophage populations. C) CD4+ T cells. D) CD8+ T cells. E) B220+ B cells. As determined by ANOVA tests, statistical differences are marked between naïve and V-HIV (or V-HIV-FL) groups (a, P <0.005), and between V- HIV and V- HIV -FL groups (b:P <0.05). V-HIV: HIV VLPs (Gag+Env), V-HIV-FL: HIV VLPs containing FL (Gag+Env+FL).
The activation status of T and B cells was also examined by analysis of the surface markers CD69 and CD62L (Rigby and Dailey, 2000; Vilanova et al., 1996). VLP administration induced significant increases in levels of the early activation marker CD69 on CD4+ and CD8+ T cells, as well as B220+ B cells compared to those in naïve mice (Fig. 7C, D, E). Administration of VLPs containing FL further enhanced expression of CD69 on these cells. Importantly, CD62L was significantly down-regulated on T and B cells in mice that received VLPs containing FL. These results indicate that HIV VLPs activate multiple cell types and that incorporation of FL into VLPs can further enhance these effects.
Incorporation of FL into HIV VLPs enhances immune responses to HIV Env
To investigate the immunogenicity of VLPs, mice were subcutaneously immunized with HIV VLPs or HIV VLPs containing FL (Fig. 8). HIV-1 Env specific serum antibody was determined in serum samples collected prior to immunization and at 12 days after priming and boost immunizations by ELISA. Immunization of mice with HIV VLPs showed moderate levels of HIV Env specific antibody, which is significantly higher than those with Env-negative Gag VLPs (V-Gag). Mice immunized with HIV VLPs containing FL (V-HIV-FL) showed 2-fold increase in levels of HIV Env specific antibodies compared to those in HIV VLPs (V-HIV), which was statistically significant. Since DCs exposed to the VLPs containing FL stimulated CD4+ cells to secrete cytokines, we have determined serum isotype antibodies specific to HIV Env. Mice immunized with VLPs containing FL induced the highest levels of IgG1 among other isotypes (Fig. 8B), which is an indicator of T helper type 2 responses. IgG2a isotype levels were similar to that of VLP-immunized mice which showed a balanced level of IgG1 and IgG2a. This result may be explained by the fact that DCs exposed to the VLPs containing FL stimulated CD4+ T cells to secrete IL-5 and IL-10, which are potential T helper type 2 cytokines (Fig. 5). These results indicate that FL incorporation into HIV VLPs can increase their immunogenicity.
Fig. 8.
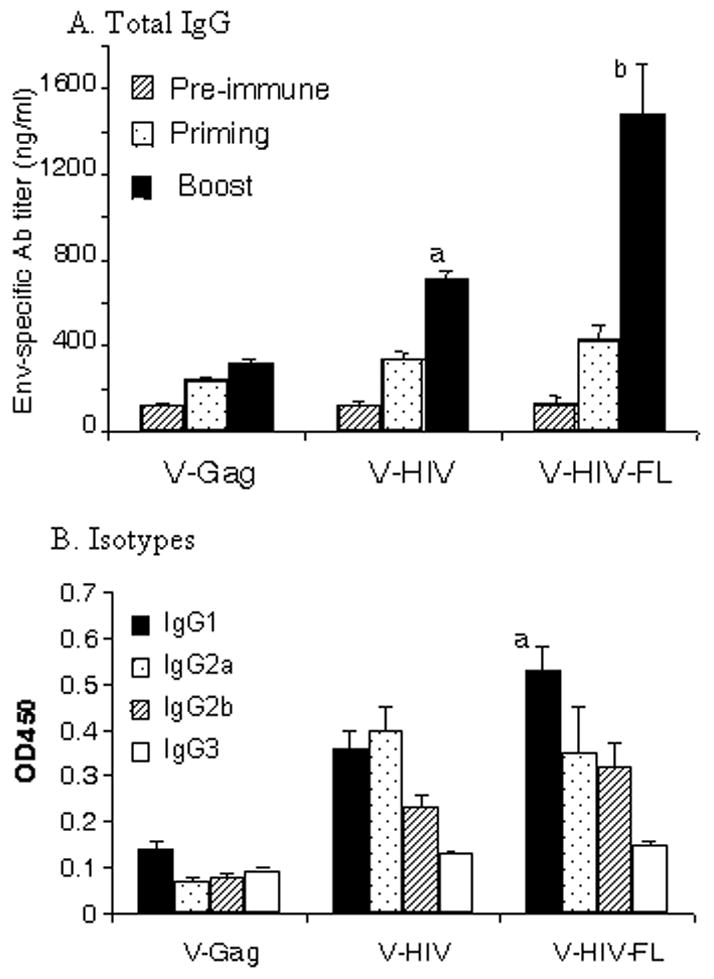
Immunogenicity of HIV VLPs. A) Total IgG specific to HIV Env. Mice were subcutaneously immunized (5 Balb/C mice per group) with VLPs or HIV VLPs containing FL (at week 0 and 4) to assess the immune responses. At 12 days after each immunization, blood samples were collected and then used to determine HIV-1 Env specific antibody responses by ELISA as described in materials and methods. Individual serum samples were serially diluted and were run in duplicates. Purified mouse IgG was used as a standard to calculate Env specific antibody IgG concentrations. ANOVA tests confirmed the following statistical differences between V-Gag and V-HIV groups (a:P =0.0071), and between V- HIV and V- HIV -FL (b:P =0.024). B) Isotype antibodies specific to HIV Env. Serum samples after the first boost were diluted to 200X, added to the ELISA plate coated with HIV Env, and levels of isotypes were determined using the HRP-conjugated isotype specific antibodies. Statistical difference in IgG1 is marked between between V- HIV and V- HIV -FL (a:P =0.02).
Discussion
Although DC maturation and types of immune responses stimulated by model antigens have been studied in depth and well-characterized (Agrawal et al., 2003; Bauer et al., 2001; Pulendran, 2005), the mechanisms by which enveloped VLPs stimulate immune cells are unknown. In this study, we investigated the events of immune cells in response to HIV VLPs and VLPs containing FL. HIV VLPs were found to activate DCs in vitro resulting in enhanced expression of maturation markers (CD40, CD80, CD86) and cytokines (IL-6, IL-12p70). In addition, VLP-loaded DCs induced splenocytes to proliferate and to secrete cytokines (IL-5, IL-10, INF-γ). Levels of in vitro stimulation of DCs by VLPs and activation of naïve splenocytes by VLP-loaded DCs were equivalent or higher than those induced by LPS. VLPs containing FL were more effective in activating splenocytes via DCs. In vivo administration of VLPs induced acute activation of multiple types of cells including DCs, monocyte/macrophages, T and B cells. Incorporation of FL into VLPs resulted in enhancement of VLP-induced activation of T and B immune cells in vivo as indicated by increased expression of the early activation marker CD69 and down-regulation of homing receptor CD62L. Finally, the results demonstrated that incorporation of FL into VLPs increased their immunogenicity.
The in vitro and in vivo effects of HIV VLPs on immune cells have not been previously investigated. Injection of FL-encoding DNA resulted in a large expansion of CD11c+ DC populations, which was found to be useful for the study of VLP effects on DCs. Characterization of these populations indicates that FL-DNA expanded CD11c+ populations are in an immature state as determined by activation markers CD40, CD80, and CD86. This is consistent with the results that FL incorporated into VLPs did not result in significant differences in DC activation marker expression levels in vivo as shown in Fig. 7A and in vitro (data not shown). These results suggest that the activation of DCs observed in this study is mainly due to the particulate nature of VLPs. FL incorporated into VLPs may play a role for inducing DCs for better antigen presentation as indicated by activation of T and B cells. Incubation of the DCs with VLPs induced maturation as shown by expression of these activation markers even at higher levels than those induced by LPS, which suggests that VLPs can be a strong stimulator for DCs, an important antigen presenting cell. It is interesting to note that HIV Env-containing VLPs were more effective in inducing maturation of DC populations than Env-negative Gag VLPs, indicating possible additional interactions between HIV Env on VLPs and an unknown receptor(s) expressed on DCs.
The types of cytokines produced by DCs play a critical role in determining the resulting adaptive immune responses. We have found that HIV VLPs are efficient in stimulating DCs to secrete Th1 type cytokine IL-12p70. A 70-kDa heterodimeric cytokine IL-12 is the key factor that drives the differentiation of naïve T lymphocytes to Th1 cells (Pulendran, 2004). It is an interesting finding in this study that DCs exposed to VLPs secreted IL-6 at high levels. This is consistent with previous studies demonstrating that murine DCs exposed to HPV VLPs (Lenz et al., 2001) or human blood DCs to HIV-1 (Ghanekar et al., 1996) produced IL-6 at higher levels than other cytokines. IL-6 is a multifunctional cytokine involved in immunity and hemopoiesis, and IL-6 was demonstrated to skew the differentiation of monocytes to macrophages (Chomarat et al., 2000), which may explain our result that HIV VLPs containing FL showed more significant increases in the CD11b+ monocyte/macrophage populations than CD11c+ DCs.
DCs are capable of not only producing cytokines but also stimulating naïve immune cells as potent antigen presenting cells. We found that DCs exposed to HIV VLPs matured to fully functional antigen presenting cells capable of inducing naïve splenocytes to proliferate and to secrete cytokines. VLP-loaded DCs preferentially activated naïve CD4+ T cells as determined by proliferation and production of cytokines IL-10 and IL-5, at levels comparable to those induced by LPS stimulation. In mice, IL-10 has been considered to be both a regulatory cytokine as well as a Th2 inducing cytokine (Laouini et al., 2003). The pattern of cytokine secretion from naïve splenocytes stimulated by DCs pulsed with HIV VLPs seems to induce Th2 type responses without much CD8+ T cell activation. This is consistent with a previous study that DCs are prone to prime alloreactive CD4+ T lymphocytes toward Th 2 type cytokines (IL-5, IL-10) in the absence of CD8+ T cell activation, which is independent of IFN-γ production (Foucras et al., 2000). We observed that IFN-γ was produced by whole splenocytes from naïve mice but not by CD4+ or CD8+ T cells after stimulation with DCs loaded with HIV VLPs, indicating that other cell types or cross talk between CD4 and CD8 T cells in naïve splenocytes might be responsible for IFN-γ production when incubated with VLP-loaded DCs.
We observed that IFN-γ was produced by whole splenocytes but not by CD4+ or CD8+ T cells after stimulation with DCs loaded with HIV VLPs, indicating that non- CD4/8 T cells in naïve splenocytes were responsible for IFN-γ production when incubated with VLP-loaded DCs. A likely candidate cell type to be involved would be the natural killer (NK) cell. Although the main function of DCs is the priming of naïve T cells, DCs have also been demonstrated to affect the functions of NK cells. Human DCs were found to be capable of stimulating NK cells resulting in IFN-γ secretion (Fernandez et al., 1999). Thus, the reciprocal interactions between DCs and NK cells induced during microbial infections promote rapid innate immune responses as well as favor the generation of appropriate adaptive responses against pathogens. However, further studies are needed to determine whether VLP-loaded DCs can activate NK cells to secrete IFN-γ.
Interestingly, we found that CD11b+ monocyte/macrophage populations in spleens were significantly increased by HIV VLP treatment and that FL-containing VLPs further increased these populations. We also observed that a single dose of VLPs induced significant changes in the activation status of DCs and monocyte/macrophages as indicated by increased expression of activation markers CD80 and CD86, which implies that VLP antigens are effective stimulators for activation of DC and macrophages in vivo. These changes in DC/macrophage activation status induced by VLPs are likely to be efficient at alerting adaptive immune responses resulting in activation of T and B cells. Analysis of splenocytes after VLP administration showed that significant enhancement in expression of the early activation marker CD69 was observed in multiple cell types of the adaptive immune system including CD4+ and CD8+ T, and B cells. The level of CD69 was significantly higher in mice that received VLPs containing FL than that in mice that received VLPs without FL. After successful antigen encounter, the responding lymphocytes down-regulate CD62L, the lymph node homing receptor (Rigby and Dailey, 2000). Consistent with these previous studies, analysis of splenocytes from mice that received FL-containing VLPs showed a significant down-regulation in CD62L expression on CD4+ T and CD8+ T cells and B cells, indicating that these lymphocytes encountered antigens as a result of VLP administration.
In summary the present study has shown, for the first time, that HIV VLPs and VLPs containing FL can expand DCs and monocyte/ macrophage populations. HIV VLPs induced the early maturation of DC populations resulting in activation of multiple cell types both in vitro and in vivo. DCs pulsed with VLPs induce activation of CD4+ T cells preferentially, and VLPs containing FL were found to be a more effective antigenic form for activating immune cells via DCs. Finally, FL-VLPs were found to be a more effective immunogen in enhancing immune responses to VLPs when mice were immunized. The results obtained in this study provide insight in understanding VLP-induced early events of immune responses.
Materials and Methods
Cells and DNA constructs
Spodoptera frugiperda Sf9 cells were maintained in suspension in serum free SF900II medium (GIBCO-BRL) at 27°C. Codon optimized consensus B (ConB) sequences for Gag and Env genes of HIV-1 were described (Kothe et al., 2006) and obtained from Dr. Beatrice Hahn (University of Alabama at Birmingham). ConB genes encoding HIV-1 Gag and Env were cloned into the pc/pS1 transfer vector under the hybrid capsid-polyhedrin promoter, and recombinant baculoviruses (rBV) expressing HIV-1 ConB Gag or Env were constructed as described previously (Yamshchikov et al., 1995; Yao et al., 2000). A plasmid pNGVL3-hFLex containing a gene encoding human FL with a signal peptide of tissue plasminogen activator (TPA) was kindly provided by Dr. Abdul M. Jabbar (Emory Vaccine Center). For incorporation of FL into HIV VLPs, the extracellular domains of the human FL gene were PCR-amplified using the forward primer, 5′-CAGTCCCCCGGGTCGACGCCGCCACCATG-3′ and the reverse primer, 5′-CAGAGGGATATCCGGGGCTGT CGGGGC-3′. The PCR product of the TPA-FL encoding gene was digested with EcoRV and in-frame ligated to the transmembrane (TM) domain with a small portion of the extracellular domain and truncated cytoplasmic tail of HIV Env (aa 661–721), and cloned into the baculovirus transfer vector pc/pS1, which was subsequently used to generate an rBV expressing human FL in a membrane-anchored form.
Production of HIV VLPs and VLPs containing FL
VLPs were produced from Sf9 insect cells co-infected with rBVs expressing Gag at an MOI (multiplicity of infection) of 2 and rBVs expressing HIV Env at an MOI of 4. Gag VLPs were produced in insect cells infected with rBV expressing Gag alone without co-infection with rBVs expressing HIV Env. HIV VLPs containing FL were produced by coinfecting Sf9 cells with rBVs expressing HIV Gag, HIV Env and FL at MOI ratios of 2:4:4 respectively. After 60 hrs of culture, VLPs in the supernatants were precipitated by adding polyethylene glycol 6000 (7%) and NaCl (2.3%), collected by centrifugation (2000 rpm), and purified by sucrose step gradients as described previously (Yao et al., 2000). Purified VLPs and VLPs containing FL were analyzed on 8% SDS–PAGE gels, Western blotted and probed with HIV patient sera. For analysis of FL, biotinylated goat anti-human FL (R&D systems) and HRP-conjugated streptavidin were used to probe the membrane blot. Endotoxin analysis using the Limulus Amebocyte Lysate test (BioWhittaker Inc, Walkersville, MD) indicated that VLP preparations were endotoxin-free.
Quantitative estimation of Env and FL on VLPs
ELISA plates were coated with sheep anti-HIV-1 gp120 antibody (5μg/ml) (CLINIQA, Fallbrook, CA) and monoclonal anti-human FL antibody (R&D systems) for determining concentrations of HIV Env and FL respectively. VLPs and VLPs containing FL were lysed and added to plates in serial dilutions. To determine the amounts of captured HIV Env and FL antigens, rabbit anti-HIV Env sera (1:1000) and biotinylated anti-human FL antibody (R&D systems) were added and incubated for 1h at 37°C. After incubation with appropriate HRP conjugated secondary antibodies or streptavidin, plates were developed with TMB ELISA substrate (PIERCE) and OD values were read at 450 nm. HIV-1 gp120 MN and human FL (R & D systems) were used as standards to calculate amount of gp120 and hFL incorporated in VLPs respectively.
DC expansion in vivo
In vivo expansion of DCs in BALB/c mice was carried out by injecting plasmid DNA that expresses the human FL extracellular domain as described by Sailaja et al. (2003). After 9 days, spleens were collected from mice that received FL DNA and single cell suspensions were prepared after treatments with type IV collagenase (Worthington) and lysis of red blood cells. Single cell suspensions were incubated with CD11c (N418) microbeads and CD11c+ DC cells were obtained by passing through magnetic columns according to the manufacturer’s instructions (Miltenyi Biotec Inc. Auburn, CA).
VLP-induced activation of DCs in vitro
To study the effects of VLPs on DC activation in vitro, DCs (1x106) were incubated with VLPs overnight. The cells were harvested, washed and stained for DC surface markers APC-conjugated anti-CD11b and FITC-conjugated anti-CD11c antibodies, and DC activation markers CD40, CD80, CD86 and MHCII coupled to PE for 20 min on ice, in the presence of an Fc receptor blocking antibody. Cells were then washed and fixed with 1.5% paraformaldehyde and acquired in FACS Excalibur. The results were analyzed using FlowJo software. To determine VLP-induced cytokine secretion in DCs in vitro, culture supernatants were used to quantify the cytokines secreted by DCs after incubation with VLPs for 24h or 48h at 37°C. The cytokines, IL-6, IL-10, TNF-α, IFN-γ and IL-12p70 were assayed using ELISA kits according to the manufacturer’s instructions (eBioscience).
In vitro functional assay of VLP-loaded DC
Mouse DCs purified as described above were incubated with VLPs or VLPs containing FL (10μg/ml) for 6 hrs. DCs loaded with VLPs were washed and counted. Spleens were isolated from naïve BALB/c mice (6 weeks old) and single cell suspensions were prepared. CD4+ and CD8+ cells were isolated from spleen cells using a CD4+ and CD8+ isolation kit according to the manufacturer’s instructions (Miltenyi Biotec Inc). Splenocytes, CD4+ and CD8+ T cells (1x105) were incubated in triplicates with VLP-loaded DCs (1x104) at a ratio of 1:10 at 37° C for 6 days. Cultures were pulsed with 1.0 μCi [3H]thymidine for 12 hrs and 3H-incorporation was measured by β-scintillation spectroscopy. The culture supernatants were assayed for cytokines IL-4, IL-5, IL-6, IL-10 and IFN-γ using cytokine ELISA kits according to the manufacturer’s instructions (eBioscience).
In vivo biological function assay of FL incorporated into VLP
VLPs or VLPs containing FL (50μg/200μl) were intraperitoneally (i.p.) injected into mice (5 /group) daily for 9 days as described previously for purified human FL (Maraskovsky et al., 1996; Pulendran et al., 1997). On the 10th day spleens were obtained from mice, single cell suspensions were prepared and analyzed for cellularity and DC expansion. 1x106 spleen cells were stained with mouse anti-CD11b coupled to APC, anti-CD11c coupled to FITC for 20 min on ice, in the presence of an Fc receptor blocking antibody. Cells were then washed, fixed with 1.5% paraformaldehyde, acquired using FACSCalibur, and analyzed by FlowJo software
VLP-induced activation of multiple cell types in vivo
VLPs and VLPs containing FL (10μg/200μl) were injected into mice (5/group) intraperitoneally (i.p.). On day 3, mice were sacrificed and spleens were isolated and single cell suspensions were prepared. The splenocytes were stained for DC surface markers using mouse anti -CD11c+ coupled to FITC, and anti-CD11b+coupled to APC, and DC activation markers anti CD40 or CD80 or CD86coupled to PE. One million cells were stained with antibodies for CD4, CD8 T cells and B cells with surface markers mouse anti -CD4 coupled to PE, anti- CD8 coupled to APC, anti-B220 coupled to PerCP and early activation markers anti -CD69 and anti- CD 62L coupled to FITC. After staining, the cells were acquired in Excalibur and analyzed by Flow Jo software as described above.
Analysis of immune responses after immunizations of mice
BALB/c mice (5/group) were subcutaneously immunized with 50 μg of VLPs or VLPs containing FL to assess the immune responses. At four weeks after priming, these mice were boosted similarly with VLPs or FL-containing VLPs. At 12 days after each immunization, blood samples were drawn from the retroorbital plexus and serum was separated. The serum was used to determine Env specific antibody titer by ELISA. HIV-ConB Env used for coating the ELISA plate was expressed in recombinant vaccinia virus and purified using lectin affinity column as described (Kang and Compans, 2003; Kang et al., 2003). After blocking, diluted mice serum before and after immunization was added to the HIV Env-coated plate. Purified mouse IgG was used as standards. The plates were developed using anti-mouse IgG, IgG1, IgG2a, IgG2b, and IgG3 coupled to HRP (Southern Biotechnology Associates. Inc) and TMB ELISA substrate (PIERCE) and absorbance was measured at 450 nm.
Statistical Analysis
The significance of differences between groups in DC expansion and multiple cell activation and proliferation was determined between two groups by Student’s t-test and an ANOVA test was performed for multiple comparisons. A value of P < 0.05 was considered significant.
Acknowledgments
S.K. was a recipient of an amfAR fellowship grant and this work was supported in part by amfAR award 70587-32-RF, and NIH/NIAID grants AI57015 (S.K.) and AI28147 (R.W.C.). We thank Dr. Beatrice Hahn for the codon optimized consensus B sequences for Gag and Env genes of HIV-1 and patient serum antibodies, Dr. Abdul M. Jabbar for the plasmid containing the human FL gene, Drs Anshu Agrawal and Stephanie Dillon for assistance with DC isolation, Bridget Love for technical assistance, Tanya Cassingham for assistance in preparing the manuscript, and NIH AIDS Research and Reference Reagent Program for providing the HIV Env MN protein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171(10):4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, Ricciardi-Castagnoli P. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26(11):2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- Bauer M, Redecke V, Ellwart JW, Scherer B, Kremer JP, Wagner H, Lipford GB. Bacterial CpG-DNA triggers activation and maturation of human CD11c-, CD123+ dendritic cells. J Immunol. 2001;166(8):5000–5007. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- Buonaguro L, Racioppi L, Tornesello ML, Arra C, Visciano ML, Biryahwaho B, Sempala SD, Giraldo G, Buonaguro FM. Induction of neutralizing antibodies and cytotoxic T lymphocytes in Balb/c mice immunized with virus-like particles presenting a gp120 molecule from a HIV-1 isolate of clade A. Antiviral Res. 2002;54(3):189–201. doi: 10.1016/s0166-3542(02)00004-9. [DOI] [PubMed] [Google Scholar]
- Buonaguro L, Visciano ML, Tornesello ML, Tagliamonte M, Biryahwaho B, Buonaguro FM. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J Virol. 2005;79(11):7059–67. doi: 10.1128/JVI.79.11.7059-7067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- Deml L, Schirmbeck R, Reimann J, Wolf H, Wagner R. Recombinant human immunodeficiency Pr55gag virus-like particles presenting chimeric envelope glycoproteins induce cytotoxic T-cells and neutralizing antibodies. Virology. 1997;235(1):26–39. doi: 10.1006/viro.1997.8668. [DOI] [PubMed] [Google Scholar]
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5(4):405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- Foucras G, Coudert JD, Coureau C, Guery JC. Dendritic cells prime in vivo alloreactive CD4 T lymphocytes toward type 2 cytokine- and TGF-beta-producing cells in the absence of CD8 T cell activation. J Immunol. 2000;165(9):4994–5003. doi: 10.4049/jimmunol.165.9.4994. [DOI] [PubMed] [Google Scholar]
- Ghanekar S, Zheng L, Logar A, Navratil J, Borowski L, Gupta P, Rinaldo C. Cytokine expression by human peripheral blood dendritic cells stimulated in vitro with HIV-1 and herpes simplex virus. J Immunol. 1996;157(9):4028–36. [PubMed] [Google Scholar]
- Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, Mast TC, Robinson R, Murphy BR, Karron RA, Dillner J, Schiller JT, Lowy DR. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93(4):284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- Jeong SH, Qiao M, Nascimbeni M, Hu Z, Rehermann B, Murthy K, Liang TJ. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J Virol. 2004;78(13):6995–7003. doi: 10.1128/JVI.78.13.6995-7003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Compans RW. Enhancement of mucosal immunization with virus-like particles of simian immunodeficiency virus. J Virol. 2003;77(6):3615–23. doi: 10.1128/JVI.77.6.3615-3623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Yao Q, Guo L, Compans RW. Mucosal immunization with virus-like particles of simian immunodeficiency virus conjugated with cholera toxin subunit B. J Virol. 2003;77(18):9823–30. doi: 10.1128/JVI.77.18.9823-9830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothe DL, Decker JM, Li Y, Weng Z, Bibollet-Ruche F, Zammit KP, Salazar MG, Chen Y, Salazar-Gonzalez JF, Moldoveanu Z, Mestecky J, Gao F, Haynes BF, Shaw GM, Muldoon M, Korber BT, Hahn BH. Antigenicity and immunogenicity of HIV-1 consensus subtype B envelope glycoproteins. Virology. 2006 doi: 10.1016/j.virol.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouini D, Alenius H, Bryce P, Oettgen H, Tsitsikov E, Geha RS. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J Clin Invest. 2003;112(7):1058–66. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166(9):5346–55. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184(5):1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E, Pulendran B, Brasel K, Teepe M, Roux ER, Shortman K, Lyman SD, McKenna HJ. Dramatic numerical increase of functionally mature dendritic cells in FLT3 ligand-treated mice. Adv Exp Med Biol. 1997;417:33–40. doi: 10.1007/978-1-4757-9966-8_6. [DOI] [PubMed] [Google Scholar]
- Nayak BP, Sailaja G, Jabbar AM. Augmenting the immunogenicity of DNA vaccines: Role of plasmid-encoded Flt-3 ligand, as a molecular adjuvant in genetic vaccination. Virology. 2006 doi: 10.1016/j.virol.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Notka F, Stahl-Hennig C, Dittmer U, Wolf H, Wagner R. Accelerated clearance of SHIV in rhesus monkeys by virus-like particle vaccines is dependent on induction of neutralizing antibodies. Vaccine. 1999;18(3–4):291–301. doi: 10.1016/s0264-410x(99)00200-5. [DOI] [PubMed] [Google Scholar]
- Pulendran B. Modulating TH1/TH2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol Res. 2004;29(1–3):187–96. doi: 10.1385/IR:29:1-3:187. [DOI] [PubMed] [Google Scholar]
- Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol. 2005;174(5):2457–65. doi: 10.4049/jimmunol.174.5.2457. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand- treated mice. J Immunol. 1997;159(5):2222–2231. [PubMed] [Google Scholar]
- Reimann J, Schirmbeck R. Alternative pathways for processing exogenous and endogenous antigens that can generate peptides for MHC class I-restricted presentation. Immunol Rev. 1999;172:131–152. doi: 10.1111/j.1600-065x.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Rigby S, Dailey MO. Traffic of L-selectin-negative T cells to sites of inflammation. Eur J Immunol. 2000;30(1):98–107. doi: 10.1002/1521-4141(200001)30:1<98::AID-IMMU98>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sailaja G, Husain S, Nayak BP, Jabbar AM. Long-term maintenance of gp120-specific immune responses by genetic vaccination with the HIV-1 envelope genes linked to the gene encoding Flt-3 ligand. J Immunol. 2003;170(5):2496–507. doi: 10.4049/jimmunol.170.5.2496. [DOI] [PubMed] [Google Scholar]
- Skountzou I, Quan FS, Gangadhara S, Ye L, Vzorov A, Selvaraj P, Jacob J, Compans RW, Kang SM. Incorporation of GPI-anchored GM-CSF or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J Virol. 2007 doi: 10.1128/JVI.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilanova M, Tavares D, Ferreira P, Oliveira L, Nobrega A, Appelberg R, Arala-Chaves M. Role of monocytes in the up-regulation of the early activation marker CD69 on B and T murine lymphocytes induced by microbial mitogens. Scand J Immunol. 1996;43(2):155–63. doi: 10.1046/j.1365-3083.1996.d01-25.x. [DOI] [PubMed] [Google Scholar]
- Yamshchikov GV, Ritter GD, Vey M, Compans RW. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology. 1995;214(1):50–58. doi: 10.1006/viro.1995.9955. [DOI] [PubMed] [Google Scholar]
- Yao Q, Kuhlmann FM, Eller R, Compans RW, Chen C. Production and characterization of simian--human immunodeficiency virus- like particles. AIDS Res Hum Retroviruses. 2000;16(3):227–236. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]


