Abstract
Background
The frequency, pattern, and correlates of neurocognitive impairment in older patients with bipolar disorder have received little study. We examined neurocognitive abilities in middle-aged and older adults with bipolar disorder to groups with schizophrenia or normal subjects, as well as the relation of neurocognition to clinical characteristics.
Method
We administered a battery of neurocognitive and clinical measures to older (45–85 years) outpatients with bipolar disorder (n=67), schizophrenia (n=150), and normal comparison subjects (n=85). Within the bipolar group, we assessed the association between neurocognitive performance and psychiatric symptoms, quality of life, and medication status.
Results
The group with bipolar disorder differed on nearly all neuropsychological tests compared to normal subjects, with medium effect sizes. Bipolar patients as impaired as those with schizophrenia on half of the tests administered, and performed better on the remaining tests, with small effect sizes. Neurocognitive deficits in bipolar disorder group related to lower quality of life, but not to psychiatric symptom severity or duration of illness.
Limitations
Samples were outpatients with mild-moderate symptoms, and findings may not generalize to acutely ill populations. We lacked data on illness history to examine the cumulative impact of psychopathology.
Conclusions
Among clinically stable middle-aged and older outpatients, bipolar disorder was associated with substantial neurocognitive impairment, with a pattern that was somewhat distinct from that found in schizophrenia. Deficits in the bipolar group were not related to severity or duration of psychiatric symptoms, but were related to quality of life. Bipolar disorder often involve disabling and enduring cognitive impairments in older outpatients.
Keywords: Bipolar disorder, neuropsychology, memory, aging, schizophrenia, depression
The limited research on neurocognitive deficits in bipolar disorder (BD) suggests that impairments are less severe and less diffuse than those which typify schizophrenia (SC) (Altshuler et al., 2004, Dickerson et al., 2004, Goldberg, 1999, Krabbendam et al., 2005). However, longer or more severe illness courses may relate to greater cognitive deficits in BD (Bearden et al., 2001, Robinson and Ferrier, 2006), raising questions about whether, and in what areas, neurocognitive functioning in BD and SC remain distinct in later life. There have been few investigations of BD patients, and no studies in older patients using comprehensive neuropsychological batteries nor comparisons across older outpatients with BD, SC, and normal comparison subjects (NCs). In light of the growing number of BD patients entering older age (Jeste et al., 1999a), research on the magnitude, pattern, and correlates of neurocognitive impairment in later-life BD is needed to understand the course of this illness over the lifespan.
Although the degree of cognitive impairment varies across studies depending on the clinical state of the patients and methods used to assess cognition, the most robust deficits reported in BD have been in the domains of executive functioning and verbal memory, whereas vocabulary, phonemic fluency, and psychomotor speed appear to be less impacted (Bearden et al., 2001, Robinson et al., 2006). Importantly, reports of neurocognitive deficits among euthymic patients suggest that impairment is not purely secondary to acute psychiatric symptoms. A recent meta-analysis of 31 studies concluded that younger adults with BD performed better than patients with SC in most domains tested (Krabbendam et al., 2005). Therefore, several investigators have concluded that the neuropsychological performance of patients with BD is intermediate between that of patients with SC and NCs, with a less diffuse pattern of impairment (Goldberg, 1999, Altshuler et al., 2004, Krabbendam et al., 2005).
Relatively little is known about the neuropsychological performance of older outpatients with BD, as the majority of available clinical data on older adults with BD derive from small (n<30) retrospective studies of inpatients with few data from standardized instruments or from age-matched comparison groups (Burt et al., 2000, Gildengers et al., 2004, Savard et al., 1980, Harvey et al., 1997). However, these findings suggest that neuropsychological deficits might be more pronounced among older relative to younger BD patients (Savard et al., 1980, Burt et al., 2000). Among mixed-age samples with BD, longer and more severe illness courses related to greater cognitive impairment (Bearden et al., 2001, Robinson and Ferrier, 2006).
The goals of this study were to investigate the magnitude, specificity, and clinical correlates of neurocognitive functioning among middle-aged and older outpatients with BD. We administered measures of neuropsychological functioning, psychopathology, and quality of life to community-dwelling older adults with BD, SC, and NCs. Based on studies of the neuropsychological performance of younger adults, we hypothesized that middle-aged and older BD patients would demonstrate intermediary cognitive abilities between NCs and patients with SC (i.e., SC<BD<NCs) using both raw and norm-referenced scores, and that, among BD patients, the greatest impairment would be observed on measures of executive functioning and verbal memory. We also examined the relationship of demographic, psychopathologic, functional, and medication status variables (e.g., duration of illness, severity of depressive, manic, and psychotic symptoms, history of substance use disorder, quality of life, and current medications) to cognitive performance in the BD group. Among BD patients, we hypothesized that neurocognitive impairment would relate to longer duration of illness, more severe symptoms, and worse quality of life.
METHOD
Participants
Participants were 67 community-dwelling outpatients with BD, 150 outpatients with SC, and 85 NCs between ages 45 and 80 years. The present report is based on analyses of data collected as part of several studies in the NIMH-funded Advanced Center for Interventions and Services Research at the University of California, San Diego (UCSD) between 1992 and 2006. Patients were recruited to participate from a number of settings, including UCSD outpatient psychiatry clinics, community clinics, the San Diego Veterans Administration Healthcare System, and board and care homes in the San Diego area. NCs were volunteers recruited through local advertisements. Some data from the SC patients and NCs have been previously reported (Heaton et al., 2001, Jeste et al., 1999b, Gladsjo et al., 2004). The Institutional Review Board of UCSD approved the studies and the data analyses. All participants provided a written informed consent prior to participating in the research.
The participants were screened with a medical history questionnaire and with laboratory and physical examinations to exclude the following: 1) history of major neurological disorders or head trauma resulting in loss of consciousness for more than 30 minutes, 2) DSM-III-R (Association, 1987) or DSM-IV (Association, 1994) diagnosis of dementia, 3) current systemic medical disease requiring inpatient treatment, or 4) diagnosis of substance abuse or dependence within the past 6 months. Diagnoses were made with administration of the Structured Clinical Interview for the DSM-III-R(Association, 1987) and DSM-IV(Association, 1994) by trained post-doctoral fellows, and confirmed during a subsequent consensus meeting attended by at least one board-certified geriatric psychiatrist.
BD patients were not required to be euthymic to be in this study unlike some previous reports (Altshuler et al., 2004, Gildengers et al., 2004, Robinson et al., 2006), as the aim of our investigation was to examine correlates of neuropsychological performance in a representative sample of community-dwelling BD patients. All of the participants were clinically stable enough to complete the evaluations. According to DSM-IV criteria, 14 BD participants were in full or partial remission, 16 were in a depressive episode, 15 in a manic episode, 11 mixed episode, and 9 were not otherwise specified. Two participants had BD II disorder, and the remaining 65 were BD I. A total of 36% had concurrent psychotic features. Current medication data were not available on three participants. A total of 31% were taking lithium, 15% were taking valproic acid, and 11% were taking an anticonvulsant. In addition, 55% were taking an antipsychotic medication (39% were on a typical and 16% on an atypical antipsychotic). A total of 58% were taking lithium, valproic acid, or carbamazepine as well as an antipsychotic medication, and 23% were taking none of these medications. In the SC group, 81% were taking antipsychotic medications (61% typical, 16% atypical, and 4% both atypical and typical). The proportion of the BD and SC patients taking atypical antipsychotic medications was relatively small, because some of the data were collected prior to the widespread usage of atypical antipsychotics.
Neuropsychological Measures
Trained research assistants administered a comprehensive neuropsychological battery, reflecting a broad range of neuropsychological abilities, to all of the patients and NCs. Most of these tests are widely used in both clinical neuropsychological assessment and research on cognitive abilities in both patient groups (Krabbendam et al., 2005). We compared groups on raw scores for individual neuropsychological tests, to aid in comparability with previous studies, and used normative data to calculate and compare demographically corrected T-scores. The most recent and appropriate normative data were used (Heaton et al., 2004, Heaton et al., 1991, Heaton et al., 1993) to correct for age, gender, education, and when available, ethnicity (only Heaton et al. 2004; Expanded (Heaton et al., 2004) norms are adjusted for African American ethnicity; the Caucasian norms were applied to Caucasian and non-African American groups). T-scores were averaged to form a Global Functioning Score and six composite ability areas (described below). These composite ability areas were derived from a confirmatory factor analysis of older persons with psychotic disorders and NCs (Gladsjo et al., 2004).
Global Cognitive Functioning (14 Measures): Verbal/Crystallized Knowledge: (2 measures) WAIS-R Vocabulary subtest (Wechsler, 1981); and Boston Naming Test (Total correct)(Kaplan et al., 1983); Attention/Working Memory (2 measures): WAIS-R Digit Span subtest (Wechsler, 1981); and Digit Vigilance (Time) (Lewis and Rennick, 1979); Verbal Memory (2 measures): Story Memory Test (Learning Score and Delayed Recall) (Heaton et al., 1991); and California Verbal Learning Test (CVLT) (Trials 1 through 5; Long delay free recall) (Delis et al., 1987); Visual Memory (1 measure): Figure Memory Test (Learning Score and Delayed Recall) (Heaton et al., 1991); Speed of Information Processing (5 measures): WAIS-R Digit Symbol-Coding subtest, Trailmaking Test Parts A and B (Time) (Heaton et al., 1991); Letter Fluency (Total correct) (Heaton et al., 1991); and Grooved Pegboard (Time in non-dominant hand) (Matthews and Klove, 1964); Reasoning/Problem Solving (2 Measures): WAIS-R Block Design; and Wisconsin Card Sorting Test (Preservative Errors) (Heaton et al., 1993).
For the Global Cognitive Functioning score we required 7 of 14 measures, and all but the Speed of Information Processing ability area, we required that participants have one or more tests available in the domain to be used in the analyses. For the Speed of Information Processing ability area, we required three of five measures.
Finally, we also calculated a mean Deficit Score to estimate the proportion of each group with global cognitive impairment. Deficit scores range from 0 (T>40) to 5 (T<20). Studies with diverse clinical populations indicate that a mean Deficit Score of 0.5 provides a reasonable discrimination between impaired and unimpaired performance (Heaton et al., 1994); we calculated the percentage of subjects with Deficits Scores greater than 0.5.
Clinical Evaluation
Participants were administered the following clinical rating scales: Hollingshead highest occupational functioning rating (Hollingshead and Redlich, 1958), Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1967), Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962) and Positive And Negative Syndrome Scale (PANSS) (Kay et al., 1988). We also extracted a four-item mania-like “excitement” subscale from the PANSS (items measuring uncooperativeness, poor impulse control, excitement, and hostility), which has previously been shown to have high concordance (Lindenmayer et al., 2004) with the Young Mania Rating Scale (Young et al., 1978). Health-related quality of life was assessed with the Quality of Well-Being (QWB) scale (Kaplan et al., 1998). The research assistants who administered and scored these rating scales were kept blind regarding the participants’ diagnoses (to the extent possible) and their neuropsychological performance.
Statistical Analysis
Distributions for each variable were checked for normality, and transformations were conducted when needed to meet assumptions for parametric analyses. One-way analysis of variance (ANOVA) was performed on the demographic and clinical variables. A MANOVA and subsequent ANOVAs were conducted on neuropsychological variables. When omnibus ANOVA tests were significant, pair-wise comparisons between groups were conducted with Tukey’s Honestly Significant Difference tests. To indicate the magnitude of differences between groups, effect sizes (Cohen’s d) were computed for pair-wise differences between the BD, NC, and SC groups. Due to identified differences in age and education between groups, we repeated all the comparisons on raw score data controlling for age and education. We did not control for age and education in comparisons of neuropsychological ability areas since these variables were already corrected for age and education via normative data (Heaton et al., 2004, Heaton et al., 1993). Finally, within the BD group, we examined the relationship of select demographic, psychopathologic, and quality of life variables to Global Cognitive Functioning and the six neuropsychological ability areas (T-Scores) with Pearson correlations. To account for the multiple comparisons, we set the alpha at 0.01. All the tests were two-tailed.
RESULTS
Demographic and Clinical Data Comparisons
The NC group was older than the BD and the SC groups by an average of about seven years, and had a higher proportion of women (Table 1). Educational and occupational attainment were higher in the NC and BD groups compared to the SC group. The two patient groups had similar ages of illness onset, number of medical conditions, and rates of past comorbid substance use disorder. Both patient groups were experiencing mild levels of depressive symptoms, general psychopathology, and psychotic symptoms, whereas the NC group reported experiencing no or minimal symptoms. There were no significant differences between patient groups on the HAM-D, but patients with SC had slightly higher ratings on the BPRS and the PANSS.
Table 1.
Comparisons between Normal Comparison Subjects (NCs) and Patients with Bipolar Disorder (BD) and Schizophrenia (SC)
| Normal Comparison Subjects (NCs) N=85 Mean (SD) | Bipolar Disorder Group (BD) N=67 Mean (SD) | Schizophrenia Group (SC) N=150 Mean (SD) | F(df) or X2 (df) | p-value | Pair-wise differences1 | |
|---|---|---|---|---|---|---|
| Age (years) | 64.2 (9.8) | 57.6 (9.1) | 57.4 (9.1) | F(2,326)=18.3 | <0.001 | SC=BD<NC |
| Education (years) | 13.1 (2.2) | 13.9 (2.9) | 12.5 (2.5) | F(2,299)=6.3 | 0.003 | SC<NC |
| Hollingshead Highest Occupation | 3.3 (1.0) | 3.0 (1.1) | 3.8 (1.0) | F(2,288)=15.0 | <0.001 | SC<NC=BD |
| Gender (% Female) | 58.8% | 28.4% | 33.3% | X2(2)=19.2 | 0.001 | BD=SC<NC |
| Ethnicity (% Caucasian) | 84.7% | 88.1% | 88.7% | X2 (2)=0.72 | 0.697 | N/A |
| Living Situation (% Independent) | 100% | 79.7% | 65.4% | X2 (6)=39.6 | <0.001 | SC=BD<NC |
| Marital Status (% Currently Married) | 52.9% | 29.9% | 14.8% | X2 (10)=60.8 | <0.001 | SC<BD<NC |
| % with History of Substance Abuse | 8.2% | 28.4% | 23.3% | X2 (4)=11.2 | 0.009 | SC=BD<NC |
| Number of Medical Diagnoses | 2.0 (1.9) | 1.8 (1.6) | 1.6 (1.6) | F(2, 326)=1.8 | 0.164 | N/A |
| Age of Onset of Illness (years) | N/A | 31.0 (12.6) | 31.0 (13.9) | F(1,198)=0.001 | 0.976 | N/A |
| HAM-D | 3.6 (4.0) | 10.7 (7.2) | 9.6 (5.6) | F(2,263)=27.5 | <0.001 | NC<BD=SC |
| BPRS | 22.6 (4.4) | 30.3 (7.4) | 33.8 (8.5) | F(2,277)=55.1 | <0.001 | NC<BD<SC |
| PANSS Positive Syndrome Scale | 9.1 (2.8) | 13.0 (4.9) | 16.5 (6.0) | F(2,200)=39.1 | <0.001 | NC<BD<SC |
| PANSS Negative Syndrome Scale | 8.3 (1.7) | 12.1 (4.2) | 15.2 (6.2) | F(2,200)=35.4 | <0.001 | NC<BD<SC |
| QWB Score | 0.68 (0.10) | 0.55 (0.09) | 0.54 (0.10) | F(2,234)=49.7 | <0.001 | SC=BD<NC |
HAM-D: Hamilton Depression Rating Scale; BPRS: Brief Psychiatric Rating Scale; PANSS: Positive and Negative Syndrome Scale; QWB: Quality of Well-Being Scale
Post-Hoc Tests were conducted with Tukey’s Honestly Significant Difference tests
Raw Neuropsychological Test Score Comparisons
The MANOVA comparing neuropsychological variables was significant [F(34,262)=2.6, p<0.001], with significant ANOVA differences on all neuropsychological measures (Table 2). In pair-wise tests, BD participants had significantly worse performance than the NCs on all measures except for the WAIS-R Vocabulary, Story Memory Learning, and Boston Naming Test. Across measures in Table 2, the mean effect size (Cohen’s d) for difference between BD and NC groups was 0.48 (sd=0.19; median=0.51. BD participants had significantly better scores than the SC sample on approximately half of the measures; the mean effect size for difference between these two groups was 0.29 (sd=0.15; median=0.31). The mean effect size for difference between SC and NCs groups was 0.74 (sd=0.22). ANOVAs were repeated controlling for age and education, and the results were nearly identical, except that pair-wise differences between BD and SC groups were no longer significant on the WAIS-R Vocabulary and Boston Naming Test.
Table 2.
Comparison of Raw Scores on Individual Neuropsychological Tests among Subject Groups
| Test Scores | Normal Comparison Subjects (NCs) N=85 Mean (SD) | Bipolar Disorder Group (BD) N=67 Mean (SD) | Schizophrenia Group (SC) N=150 Mean (SD) | F(df) | p-value | Pair-wise Differences | Effect Sizes (Cohen’s d) | ||
|---|---|---|---|---|---|---|---|---|---|
| NC-BD | BD-SC | NC-SC | |||||||
| WAIS-R Vocabulary | 11.0 (2.5) | 10.9 (2.4) | 9.6 (3.0) | F(2,257)=8.0 | <.001 | S<B=NC | 0.04 | 0.48 | 0.51 |
| Boston Naming Test | 54.1 (5.4) | 53.0 (5.4) | 50.3 (8.2) | F(2,291)=9.0 | <.001 | S<B=NC | 0.20 | 0.39 | 0.55 |
| WAIS-R Digit Span | 9.8 (2.7) | 8.6 (2.6) | 7.8 (2.5) | F(2,292)=14.9 | <.001 | S=B<NC | 0.45 | 0.31 | 0.45 |
| Digit Vigilance (Seconds) | 439.9 (122.3) | 554.4 (291.4) | 598.0 (257.6) | F(2,293)=12.3 | <.001 | S=B<NC | 0.51 | 0.16 | 0.78 |
| CVLT Trial 1 to 5 Total | 48.5 (12.6) | 42.3 (12.5) | 36.9 (13.5) | F(2,296)=21.9 | <.001 | S<B<NC | 0.49 | 0.42 | 0.89 |
| Story Memory Learning | 10.0 (4.9) | 8.7 (4.5) | 6.8 (4.8) | F(2,264)=11.7 | <.001 | S<B=NC | 0.28 | 0.41 | 0.66 |
| CVLT Long Delay Free | 10.0 (3.6) | 8.4 (3.3) | 6.8 (3.9) | F(2,296)=20.4 | <.001 | S<B<NC | 0.46 | 0.44 | 0.85 |
| Story Memory Delay | 15.8 (3.0) | 14.6 (3.4) | 13.3 (5.1) | F(2,270)=9.4 | <.001 | S<NC | 0.37 | 0.30 | 0.60 |
| Figure Memory Delay | 14.2 (2.3) | 12.4 (4.1) | 11.3 (4.7) | F(2,278)=11.9 | <.001 | S=B<NC | 0.54 | 0.25 | 0.78 |
| Figure Memory Learning | 6.8 (4.6) | 4.7 (3.5) | 4.2 (3.6) | F(2,278)= 13.8 | <.001 | S=B<NC | 0.51 | 0.14 | 0.63 |
| WAIS-R Digit Symbol | 7.8 (2.3) | 5.9 (1.9) | 5.0 (1.9) | F(2,291)=46.9 | <.001 | S<B<NC | 0.90 | 0.47 | 1.33 |
| Trails A (Seconds) | 33.8 (13.1) | 44.6 (20.1) | 54.7 (31.0) | F(2,297)=19.5 | <.001 | S<B<NC | 0.64 | 0.39 | 0.88 |
| Trails B (Seconds) | 92.5 (45.1) | 126.7 (75.3) | 162.5 (108.5) | F(2,286)=17.3 | <.001 | S<B<NC | 0.58 | 0.38 | 0.84 |
| Grooved Pegs (Seconds) | 88.7 (21.3) | 120.4 (63.6) | 127.9 (58.5) | F(2,293)=155 | <.001 | S=B<NC | 0.67 | 0.12 | 0.89 |
| Letter Fluency (FAS) | 39.2 (12.0) | 32.1 (12.6) | 29.2 (12.0) | F(2,296)=18.6 | <.001 | S=B<NC | 0.58 | 0.24 | 0.83 |
| Wisconsin Card Sorting Test | 20.6 (15.0) | 28.0 (18.6) | 31.1 (21.6) | F(2,267)=7.4 | .001 | S<NC | 0.44 | 0.15 | 0.54 |
| WAIS-R Block Design | 8.7 (2.6) | 7.3 (2.5) | 7.4 (2.7) | F(2,255)=7.5 | .001 | S=B<NC | 0.55 | −0.04 | 0.49 |
Note: Large effect sizes (≥0.80) are given in bold; CVLT: California Verbal Learning Test;
Tukey’s Honestly Significant Difference tests
Demographically-Corrected Neuropsychological Global and Ability Area Comparisons
All the ANOVAs comparing T-scores for Global Cognitive Functioning and the six ability areas across groups were significant (Figure 1). Pair-wise comparisons indicated greater deficits in the BD group than the NC group on all ability areas. The BD group perfromed better than the SC group on four of the six ability areas, whereas no differences were observed in Reasoning/Problem-solving and Visual Memory ability areas. Finally, the percentage of BD patients with Global Cognitive Impairment (i.e., Deficit Score≥0.5) was 56%, compared to 65% of the SC group and 12% of the NC group.
Figure 1. T-Scores in Individual Ability Areas among Normal Comparison Subjects (NCS), Bipolar Disorder (BD), and Schizophrenia (SC) Groups.
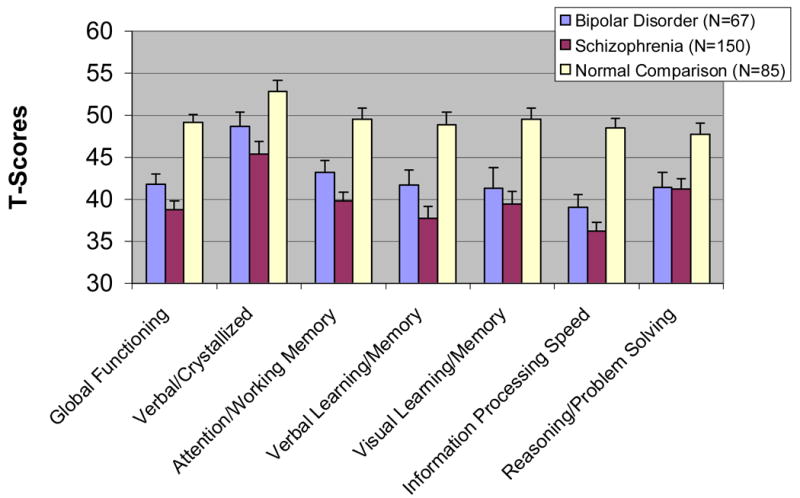
T-Scores have a mean of 50 and a Standard Deviation of 10. Error bars represent the 90% confidence interval of the standard error of the mean. Results of ANOVAs and Tukey Pairwise tests comparing groups were Global Cognitive Functioning: F(2,253)=53.1,p<0.001;SC<BD<NC; SC=BD<NC; Verbal/Crystallized Knowledge: F(2,291)=18.1, p<0.001; SC<BD<NC; Attention/Working Memory: F(2,299)=40.1,p<0.001 SC<BD<NC; Verbal Learning and Memory: F(2,298)=35.7,p<0.001;SC<BD<NC Visual Learning and Memory: F(2,279)=25.7,p<0.001; SC=BD<NC; Speed of Information Processing: F(2,299)=75.9,p<0.001;SC<BD<NC; Reasoning/Problem Solving: F(2,276) = 17.6, p <0.001; SC=BD<NC
Relationships of Neurocognitive Ability Areas with Demographics, Psychopathology, Quality of Life, and Medication Status in the BD Group
There were no significant correlations between age and Global Cognitive Functioning (r=−0.118, p-value=0.362) or any of the six ability area scores, indicating that age did not relate to impairment in performance beyond that corrected by published norms. No correlations were significant (at alpha < 0.01) between Global Cognitive Functioning and education (r=0.043; p-value=0.738), duration of illness (r=−0.077; p-value=0.545), HAM-D (r=−0.020; p-value=0.679), BPRS (r=−0.060; p-value=0.691), PANSS negative (r=−0.086; p-value=0.646, PANSS positive (r=−0.114; p-value=0.542 or PANSS ‘excitement’ subscale scores (r=0.046; p-value=0.804). Only one significant correlation emerged among individual ability areas (between Attention/Working Memory and Education, r=0.407; p-value=0.001). In contrast, quality of life (QWB scores) was positively correlated with Global Cognitive Functioning (r=0.439, p-value=0.439). Finally, no difference in Global Cognitive Functioning was observed between BD patients with versus without histories of substance use disorders.
Patients taking lithium had worse mean Global Cognitive Functioning scores than those not taking lithium (T-score=38.6, sd=6.7 vs. T-score=43.3, sd=5.3 respectively; F(1,62)=9.0, p=0.004). Similarly, patients taking antipsychotic medications were more impaired than those not taking antipsychotic medications (T-score=40.3, sd=6.5 vs. T-score=43.7, sd=4.8 respectively; F(1,62)=5.9, p=0.017). There was no significant difference in Global Cognitive Functioning between those taking typical versus atypical antipsychotic medications. BD patients not taking lithium and/or antipsychotic medications still performed significantly worse than the NCs on Global Cognitive Functioning. Too few BD patients were taking other medications to examine their relationship to cognitive functioning.
DISCUSSION
To our knowledge, this is the first comprehensive study of neuropsychological functioning in middle-aged and older outpatients with BD. As hypothesized, the group with BD performed worse than NCs (with medium effect sizes) on nearly all neuropsychological tests. However, in contrast to our hypothesis, impairment was distributed across nearly all ability areas assessed, and were unrelated to psychiatric symptom severity or duration of illness. As hypothesized, neurocognitive ability among BD patients predicted a significant proportion of variance in quality of life. The BD patients performed similarly to those with SC in half of the cognitive domains, and performed better on the remaining tests with small effect sizes. These results highlight cognitive impairment as a common and disabling aspect of late-life BD, but also suggest some differences in neurocognitive abilities between BD and SC in later life.
Our data suggested that over half (56%) of the BD patients had clinically significant overall cognitive impairment (defined as a Deficit Score≥0.5). Given the heterogeneity of BD and variance in methods used to assess cognitive abilities, comparisons across studies are somewhat difficult. Nevertheless, this finding is consistent with previous studies of late-life BD employing cognitive screening measures. Gildengers et al. (2004) found impairment in about half of a sample of 18 older BD inpatients and outpatients and Burt et al. (2000) (n=13) and Savard, Rey and Post (1980) (n=7) found significant (although not age-corrected) differences between younger and older inpatients with BD in verbal memory and problem solving.
Relative to previous studies directly comparing younger adults with BD, NCs, or SC (Altshuler et al., 2004, Krabbendam et al., 2005, Dickerson et al., 2004), our findings suggest broader impairment among older BD patients compared to NCs. Moreover, these differences between BD and NC groups were unrelated to educational and occupational attainments, which were both slightly higher in the BD group. It is further notable that occupational level had no association with cognitive performance in the BP patients, although this finding requires replication. Among younger adults, deficits in verbal memory and executive functioning have been identified in euthymic states, and are perhaps the most robust domains of impairment (Bearden et al., 2001, Krabbendam et al., 2005). In our outpatient sample, depressive or psychotic symptoms did not correlate with verbal memory or reasoning/problem solving scores, further suggesting that these deficits are not simply a byproduct of these symptoms. We also found evidence for speed of information processing deficits, with the largest effect sizes between BD and NC groups among all measures seen on the Trailmaking Test (Parts A and B), WAIS-R Digit Symbol, and Grooved Pegboard, and performance on these measures related to health-related quality of life. Cognitive slowing has been identified as a central and persistent component of late-life depression (Butters et al., 2004), which raises the question of whether late-life BD may involve similar neurocognitive underpinnings.
The neurocognitive distinctions between the BD and SC samples were more subtle than between the BD and NC groups, with smaller effect sizes than those observed in studies of younger adults. In a meta-analysis of 31 studies, Krabbendam et al. (2005) reported a mean effect size difference across cognitive measures of 0.49, which is approximately twice as large as that found in our sample. This relatively small level of distinction between older patients with BD and SC was also evident in a study of chronically institutionalized patients (Harvey et al., 1997). As predicted, our BD group performed better than patients with SC on measures of verbal skills/crystallized knowledge, information processing speed, and verbal memory. Therefore, our findings support the notion that BD patients are less cognitively impaired than patients with SC in later life. However, the overall gap in neurocognitive abilities between these disorders may be narrower in later life. There is evidence for stability in cognitive functioning among community-dwelling middle-aged and older adults with SC (Heaton et al., 2001), and longitudinal studies comparing these two disorders will be useful to determine if there are actual disease-related declines in BD. Possible cohort differences between younger and older persons with BD cannot be ruled out in cross-sectional comparisons.
Although we did not group BD patients into euthymic, depressed, and manic subgroups as in some previous reports (Martinez-Aran et al., 2004, Altshuler et al., 2004), it is doubtful that their neurocognitive deficits are solely attributable to concurrent depression or mania for two main reasons: 1) Our sample was comprised of outpatients who had, in general, mild levels of depressive, manic, and psychotic symptoms, and 2) None of the symptom measures showed any significant relationships with any domains of cognitive performance in the BD group, similar to the lack of association between manic symptoms and cognitive functioning reported among older adults with BD (Young et al., 2006). Thus, from a clinical standpoint, neuropsychological impairment should be considered an important part of the presentation of older adults with BD, and are at least somewhat independent from symptom severity.
Patients with BD taking lithium or antipsychotic medications (typical or atypical) had worse cognitive functioning, which corresponds to previous literature on the incidence of cognitive side effects of these medications in elderly BD patients (Young et al., 2004). However, we cannot determine from our data whether the relationship of these medications to cognition can be attributed to the medication itself or to other patient or provider-related factors, especially since a higher proportion of our sample was unmedicated than might be predicted. Nevertheless, these findings suggest that further research should investigate the incidence and severity of cognitive side effects of the medications commonly used to treat BD. Finally, similar to a previous study linking global cognitive functioning with community living skills among older BD patients (Martinez-Aran et al., 2004), we found that better attention/working memory, verbal memory, and information processing speed related to better health-related quality of life. Parallel to the growing recognition of the centrality of neurocognition to treatment of SC (Heinrichs, 2005), the extent to which these deficits in later-life BD is altered by medications should be examined.
The strengths of this study include a relatively large outpatient sample of older BD patients, use of two comparison groups that have been better characterized in previous literature, and administration of a comprehensive battery of neuropsychological tests and symptom measures to assess a range of cognitive and psychopathologic factors. However, these findings must be interpreted in light of several limitations of the study. Both patient samples were comprised of community-dwelling outpatients who were generally experiencing low levels of symptoms. Therefore, our findings may not generalize to hospitalized or acutely ill patients. In addition, the proportion of women in the sample was low and fewer patients had a history of substance abuse than is typically reported for BD samples, which may further limit the generalizability of the results. Some of our data predate the widespread usage of atypical antipsychotics, which may attenuate the differences between the SC and BD groups if atypical antipsychotics indeed enhance cognitive abilities, a point of some controversy. These data are cross-sectional; thus, future longitudinal research will be needed to examine the effects of aging on the development of cognitive deficits. We did not have available a commonly used standardized measure of mania such as the Young Mania Rating Scale (Young et al., 1978), and therefore, the effect of manic symptoms on neuropsychological performance in older adults deserves further study. However, we did not find evidence for a significant relationship with a mania-like ‘excitement’ subscale of the PANSS nor general psychopathology (i.e., BPRS scores), and thus, it is unlikely that acute and undetected manic symptoms greatly impacted performance in the BD group. Finally, we did not have detailed information about illness history, such as the number of previous manic or depressive episodes, nor reliable information on cumulative exposure to psychiatric medications. In previous studies, cumulative illness burden appeared to have a greater negative impact on cognitive functioning than duration of illness or concurrent symptoms (Bearden et al., 2001, Robinson and Ferrier, 2006) Again, a longitudinal investigation of cognitive functioning would help address these shortcomings.
In conclusion, we found neurocognitive impairments in a sample of community-dwelling middle-aged and older patients with BD that were more diffuse and also more similar in severity to those in patients with SC than has been reported in studies of younger adults with these diagnoses. Therefore, clinical care of BD patients who enter later life should take into account the presence of cognitive impairment, and future longitudinal research should examine the mechanisms related to the development of cognitive deficits in BD across the life span, so that preventive and rehabilitative interventions could be designed and implemented.
Acknowledgments
This work was supported, in part, by the National Institute of Mental Health grants MH66248, MH59101, MH64722, and by the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALTSHULER LL, VENTURA J, VAN GORP WG, GREEN MF, THEBERGE DC, MINTZ J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56:560–9. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington,DC: American Psychiatric Association; 1994. [Google Scholar]
- BEARDEN CE, HOFFMAN KM, CANNON TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–50. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151–3. [DOI] [PubMed] [Google Scholar]
- BURT T, PRUDIC J, PEYSER S, CLARK J, SACKEIM HA. Learning and memory in bipolar and unipolar major depression: effects of aging. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:246–53. [PubMed] [Google Scholar]
- BUTTERS MA, WHYTE EM, NEBES RD, BEGLEY AE, DEW MA, MULSANT BH, ZMUDA MD, BHALLA R, MELTZER CC, POLLOCK BG, REYNOLDS CF, 3RD, BECKER JT. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–95. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- DELIS DC, KRAMER JH, KAPLAN E, OBER BA. California Verbal Learning Test (CVLT) San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- DICKERSON F, BORONOW J, STALLINGS C, ORIGONI A, COLE S, YOLKEN RH. Cognitive functioning in schizophrenia and bipolar disorder: Comparison of performance on the Repeatable Battery for the Assessment of Neuropsychological Status. Psychiatry Res. 2004;129:45–53. doi: 10.1016/j.psychres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- GILDENGERS AG, BUTTERS MA, SELIGMAN K, MCSHEA M, MILLER MD, MULSANT BH, KUPFER DJ, REYNOLDS CF., 3RD Cognitive functioning in late-life bipolar disorder. Am J Psychiatry. 2004;161:736–8. doi: 10.1176/appi.ajp.161.4.736. [DOI] [PubMed] [Google Scholar]
- GLADSJO JA, MCADAMS LA, PALMER BW, MOORE DJ, JESTE DV, HEATON RK. A six-factor model of cognition in schizophrenia and related psychotic disorders: Relationships with clinical symptoms and functional capacity. Schizophr Bull. 2004;30:739–754. doi: 10.1093/oxfordjournals.schbul.a007127. [DOI] [PubMed] [Google Scholar]
- GOLDBERG TE. Some fairly obvious distinctions between schizophrenia and bipolar disorder. Schizophr Res. 1999;39:127–32. doi: 10.1016/s0920-9964(99)00111-5. discussion 161–2. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. Development of a rating scale for primary depressive illness. British Journal of Psychiatry. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- HARVEY PD, POWCHIK P, PARRELLA M, WHITE L, DAVIDSON M. Symptom severity and cognitive impairment in chronically hospitalised geriatric patients with affective disorders. Br J Psychiatry. 1997;170:369–74. doi: 10.1192/bjp.170.4.369. [DOI] [PubMed] [Google Scholar]
- HEATON R, CHELUNEM G, TALLEY J, KAY G, CURTIS G. Wisconsin Card Sorting Test (WCST) Manual Revised and Expanded. Odessa, Florida: Psychological Assessment Resources; 1993. [Google Scholar]
- HEATON R, MILLER W, TAYLOR M, GRANT I. Revised comprehensive norms for an Expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African-American and Caucasian adults (HRB) Odessa, Fla: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- HEATON R, PAULSEN JS, MCADAMS LA, KUCK J, ZISOOK S, BRAFF D, HARRIS J, JESTE DV. Neuropsychological deficits in schizophrenics. Relationship to age, chronicity, and dementia. Arch Gen Psychiatry. 1994;51:469–76. doi: 10.1001/archpsyc.1994.03950060033003. [DOI] [PubMed] [Google Scholar]
- HEATON RK, GLADSJO JA, PALMER BW, KUCK J, MARCOTTE TD, JESTE DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- HEATON RW, GRANT I, MATTHEWS CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- HEINRICHS RW. The primacy of cognition in schizophrenia. American Psychologist. 2005;60:22–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- HOLLINGSHEAD AB, REDLICH FC. Social Class and Mental Illness. New York: John Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JESTE DV, ALEXOPOULOS GS, BARTELS SJ, CUMMINGS JL, GALLO JJ, GOTTLIEB GL, HALPAIN MC, PALMER BW, PATTERSON TL, REYNOLDS CF, 3RD, LEBOWITZ BD. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999a;56:848–53. doi: 10.1001/archpsyc.56.9.848. [DOI] [PubMed] [Google Scholar]
- JESTE DV, LACRO JP, BAILEY A, ROCKWELL E, HARRIS M, CALIGIURI MP. Lower incidence of tardive dyskenesia with risperidone compared with haloperidol in older patients. Journal of the American Geriatrics Society. 1999b:6. doi: 10.1111/j.1532-5415.1999.tb01595.x. [DOI] [PubMed] [Google Scholar]
- KAPLAN E, GOODGLASS H, WEINTRABU S. The Boston Namin Test. 2. Philadephia: Lea & Febiger; 1983. [Google Scholar]
- KAPLAN RM, GANIATS TG, SIEBER WJ, ANDERSON JP. The Quality of Well-Being Scale: critical similarities and differences with SF-36. Int J Qual Health Care. 1998;10:509–20. doi: 10.1093/intqhc/10.6.509. [DOI] [PubMed] [Google Scholar]
- KAY S, OPLER L, LINDENMAYER J. Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenia. Schizophrenia Bulletin. 1988;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- KRABBENDAM L, ARTS BM, VAN OSJ, ALEMAN A. Cognitive functioning in patients with schizophrenia and bipolar disorder: A quantitative review. Schizophr Res. 2005 September; doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- LEWIS RF, RENNICK PM. Manual for the Repeatable Cognitive-Perceptual-Motor Battery. Grosse Point Park, MI: Axon Publishing Co; 1979. [Google Scholar]
- LINDENMAYER JP, BROWN E, BAKER RW, SCHUH LM, SHAO L, TOHEN M, AHMED S, STAUFFER VL. An excitement subscale of the Positive and Negative Syndrome Scale. Schizophr Res. 2004;68:331–7. doi: 10.1016/S0920-9964(03)00087-2. [DOI] [PubMed] [Google Scholar]
- MARTINEZ-ARAN A, VIETA E, REINARES M, COLOM F, TORRENT C, SANCHEZ-MORENO J, BENABARRE A, GOIKOLEA JM, COMES M, SALAMERO M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–70. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C, KLOVE N. Instruction manual for the Adult Neuropsychology Test Battery. Madison: University of Wisconsin Medical School; 1964. [Google Scholar]
- OVERALL J, GORHAM D. The Brief Psychiatric Rating Scale. Psychological Reportsq. 1962;10:799–812. [Google Scholar]
- ROBINSON LJ, FERRIER IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8:103–16. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- ROBINSON LJ, THOMPSON JM, GALLAGHER P, GOSWAMI U, YOUNG AH, FERRIER IN, MOORE PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–15. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- SAVARD RJ, REY AC, POST RM. Halstead-Reitan Category Test in bipolar and unipolar affective disorders. Relationship to age and phase of illness. J Nerv Ment Dis. 1980;168:297–304. doi: 10.1097/00005053-198005000-00010. [DOI] [PubMed] [Google Scholar]
- WECHSLER D. Wechsler Adult Intelligence Scale - Revised. Cleveland, OH: Psychological Corporation; 1981. [Google Scholar]
- YOUNG RC, BIGGS J, ZIEGLER V, MEYER D. A rating scale for mania: Reliability, validity, and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- YOUNG RC, GYULAI L, MULSANT BH, FLINT A, BEYER JL, SHULMAN KI, REYNOLDS CF., 3RD Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12:342–57. doi: 10.1176/appi.ajgp.12.4.342. [DOI] [PubMed] [Google Scholar]
- YOUNG RC, MURPHY CF, HEO M, SCHULBERG HC, ALEXOPOULOS GS. Cognitive impairment in bipolar disorder in old age: literature review and findings in manic patients. J Affect Disord. 2006;92:125–31. doi: 10.1016/j.jad.2005.12.042. [DOI] [PubMed] [Google Scholar]


