Abstract
Impaired methylation due to accumulation of S-adenosylhomocysteine (SAH) may contribute to the pathophysiology of cobalamin deficient anemia. We assayed serum S-adenosylmethionine (SAM), SAH, total homocysteine (tHcy), and methylmalonic acid (MMA) in 15 subjects with cobalamin deficient megaloblastic anemia and compared results to 19 subjects with anemia/pancytopenia due to other causes. Cobalamin deficient subjects had a median hematocrit of 20% and mean cell volume of 111.7 fL. The median serum cobalamin was 37 pg/mL, MMA 3030 nmol/L and tHcy 62.0 umol/L. SAH was elevated in 13 of 15 subjects (median value 42 nmol/L) and the median SAM was normal (103 nmol/L) but SAM/SAH ratio was low, 2.5. The SAH was higher and SAM/SAH ratio lower in cobalamin deficient subjects as compared to those with other anemias after excluding 4 patients with renal insufficiency. SAM concentrations were not low in cobalamin deficiency. Cobalamin injections corrected anemia, MMA, tHcy, SAM/SAH ratio and SAH. Some hematologic variables were inversely correlated with SAH and cobalamin but not tHcy or MMA. In conclusion, serum SAH is elevated in cobalamin deficient subjects with megaloblastic anemia and corrects with parenteral cobalamin therapy.
Keywords: homocysteine, methylmalonic acid, folate, vitamin B12
Introduction
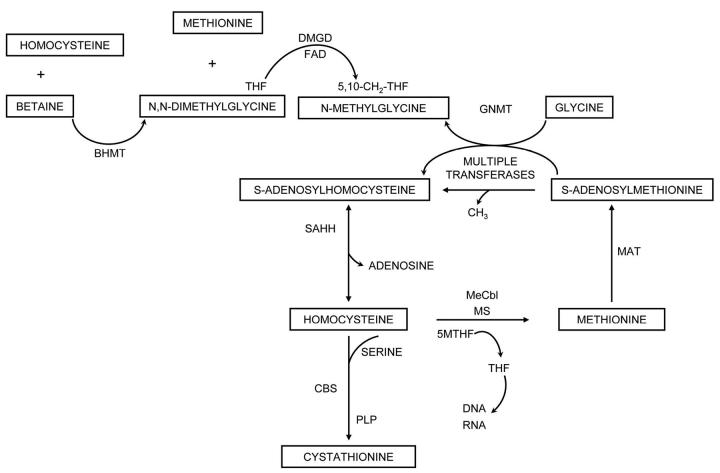
Severe megaloblastic anemia due to cobalamin (vitamin B12) or folate deficiency is uncommon yet important to recognize since other causes of macrocytic pancytopenia rarely have curative therapy. Assays of the cobalamin dependent metabolites are useful adjuncts to serum vitamin levels in diagnosing deficiency 1, 2 since virtually every patient with cobalamin deficient megaloblastic anemia has elevated serum and/or urine methylmalonic acid (MMA) and total homocysteine (tHcy) 1, 2. One coenzyme form, methyl-cobalamin, is a cofactor for methionine synthase, which is shown in Figure 1. Homocysteine is methylated to methionine, as N5-methyltetrahydrofolate is demethylated to form tetrahydrofolate, the precursor of cofactors for the synthesis of thymidine and purines as well as other reactions of one-carbon metabolism 4. Methionine is a precursor of S-adenosylmethionine (SAM), which is used in many SAM dependent methylations important in the synthesis of creatine, phospholipids, neurotransmitters and for DNA and RNA methylation reactions 5. S-adenosylhomocysteine (SAH) is the resulting product and can be cleaved by SAH hydrolase to form homocysteine. Homocysteine can also be condensed with serine by cystathionine beta synthase. The flow of methionine metabolism is regulated by the availability of SAM, because SAM is an activator of cystathionine beta synthase 6. SAM also inhibits methylenetetrahydrofolate reductase, (MTHFR) the enzyme that converts 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate 6. A SAM dependent methylation of glycine removes excess methyl groups and is inhibited by 5-methyltetrahydrofolate 7. Homocysteine is also methylated by betaine homocysteine methyltransferase with products of methionine and N, N-dimethylglycine. Both N, N-dimethylglycine and N-methylglycine are cleared using tetrahydrofolate as a cofactor 5. Previous investigations of subjects with severe cobalamin deficiency largely due to pernicious anemia have shown that tHcy and MMA are invariably elevated 1, 2, cystathionine is usually elevated, 2, 8 and N, N-dimethylglycine is frequently elevated 2, 9, whereas methionine and N-methylglycine concentrations are generally maintained 2, 9. In megaloblastic anemia due to folate deficiency, tHcy, 1, 2 cystathionine 2, 8, N, N-dimethylglycine, and N-methylglycine 2, 9 are usually elevated. MMA is in the reference range in folate deficiency.
Figure 1.
The pathways of methionine metabolism are shown. The following enzymes and vitamins are abbreviated as follows: methionine synthase (MS), methionine adenosyltransferase (MAT), glycine N-methyltransferase (GNMT), betaine homocysteine methyltransferase (BHMT), S-adenosylhomocysteine hydrolase (SAHH), cystathionine beta synthase (CBS), pyridoxal phosphate (PLP), tetrahydrofolate (THF), methyl-cobalamin, me-cobalamin, flavin adenosine dinucleotide (FAD)
Studies in animals with nitrous oxide induced cobalamin inactivation have suggested that SAH concentrations in tissues are increased and SAM concentrations may be decreased with an altered SAM/SAH ratio 10. Such abnormalities could be of pathophysiologic importance since SAH is an inhibitor of most methylation reactions. It is also possible that a deficiency of SAM could result in deficient methylation of critical compounds and cause some of the abnormalities seen in cobalamin or folate deficiency11. We recruited subjects with severe cobalamin deficient megaloblastic anemia and measured serum SAM and SAH13 as well as the other vitamin related metabolites both before and, in a subgroup, after treatment with cobalamin.
Methods
The patients were recruited from the Leonor Hospital in Sorocaba City, Sao Paulo, Brazil between March and May 2001. Patients were eligible if they had macrocytic red blood cells, anemia, and/or other cytopenias. The routine testing for hematologic abnormalities was performed in Brazil and included the determination of the hemoglobin, hematocrit, mean corpuscular volume (MCV), white blood count, absolute neutrophil count, and platelet count with a STK coulter counter. Serum cobalamin was measured by IMx System, Abbott Laboratories and serum and red blood cell folate by ionic capture methodology, Abbott Laboratories. Routine serum chemistries were determined by the clinical laboratory. Blood was also collected for methionine metabolites. Whole blood was allowed to clot on ice and then centrifuged to separate serum and stored frozen at -80° C. These samples were sent to the laboratory in Denver, Colorado by overnight express on dry ice and were still frozen on arrival. MMA, tHcy, cystathionine, 2-methylcitric acid, methionine, N, N-dimethylglycine and N-methylglycine were determined by stable isotope dilution, capillary gas chromatography/mass spectrometry as previously described 8, 9, 12. The reference ranges had been previously determined on 60 blood donors in the United States age 18-65 and were MMA 73-271 nmol/L, tHcy 5.4-13.9 umol/L, cystathionine 44-342 nmol/L, 2-methylcitric acid 60-228 nmol/L, methionine 13-45 umol/L, N, N-dimethylglycine 1.4-5.3 umol/L, and N-methylglycine 0.6-2.7 umol/L 8, 9, 12. Cobalamin deficiency was defined as serum cobalamin < 350 pg/ml and serum MMA > 271 nmol/L and MMA > 2-methylcitric acid value 12. Pure folate deficiency was defined as serum folate < 5 ng/ml and tHcy > 13.9 umol/L and MMA ≤ 271 nmol/L.
SAM and SAH were quantitated by stable isotope dilution, liquid chromatography/mass spectrometry13. In 48 normal subjects from the United States, reference range ± 2 SD for SAM was 71-168 and SAH 8-26 nmol/L and SAM/SAH ratio 4.4-12.4. Subjects were genotyped for the thermolabile variant of MTHFR C677T by PCR-RFLP14. The common variant at C677T was designated as follows: CC wild type, CT, heterozygous and TT, thermolabile homozygous variant.
The treatment of the subjects with various diagnoses followed the usual clinical practice at Leonor Hospital. Empiric folic acid therapy in varying doses had been given to seven subjects for one week to one year prior to being seen at Leonor Hospital and prior to the blood collections for vitamin and related metabolites. Those determined to be cobalamin deficient received cyanocobalamin injections. There were ten cobalamin deficient subjects who returned to the hospital after treatment and had repeat determinations of the blood counts and metabolites. Patients with iron deficiency anemia or other anemias had appropriate treatment. The protocol was approved by the institutional review board in Brazil and at the University of Colorado Health Sciences Center.
The various variables were analyzed with SPSS based software version 10.0, SPSS, Inc. (Chicago). Differences in means for continuous variables across 2 categories were evaluated with the T-test and Levene’s test for equality of variances. A p value < 0.05 was considered significant. Paired t-tests were used to compare pre and post treatment variables. The Spearman’s Rho correlation coefficient was calculated between variables.
Results
There were thirty-seven subjects with macrocytosis, anemia, neutropenia, or thrombocytopenia who were evaluated prospectively during the time period. There were 13 whites, 5 blacks, 17 mixed race, 1 asian, and one unknown race. There were fifteen who met our diagnosis of cobalamin deficiency because MMA was elevated and serum cobalamin was < 350 pg/ml. There was 1 subject who met our diagnosis of folate deficiency because of serum folate < 5 ng/ml with tHcy > 13.9 umol/L. Two other subjects probably had impaired folate status. In one, serum folate was low, tHcy was 13.9 umol/L but cystathionine was clearly elevated to 521 nmol/L. The other subject had a borderline serum folate of 5.0 ng/ml but tHcy was 19.8 umol/L. All three with impaired folate status also had low serum cobalamin although MMA ranged only from 183-255 nmol/L, thus they did not meet our cobalamin deficiency definition. There were eighteen subjects with anemia and 1 with isolated neutropenia who were also evaluated and did not meet the criteria for cobalamin deficiency. These 19 subjects are referred to as the group with Other Anemia.
Metabolites in Cobalamin Deficient Megaloblastic Anemia
Table 1 shows individual data from the 15 cobalamin deficient subjects arranged in descending order of elevation of serum MMA.
Table 1.
Laboratory and Clinical Variables in Fifteen Cobalamin Deficient Subjects
| Case No. | Age Yr. | Sex | MMA umol/L | tHcy nmol/L | SAM nmol/L | SAH nmol/L | SAM/SAH ratio | Meth umol/L | Cbl pg/ml | Folate* Use +/- | Hct % | Tx Hct† % | MCV FL | Tx MCV‡ FL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | 13900 | 197.8 | 93 | 58 | 1.6 | 9.2 | 26 | - | 18.3 | 50.5 | 130.3 | 89.3 |
| 2 | 77 | F | 12300 | 134.0 | 164 | 66 | 2.5 | 14.8 | 34 | - | 27.3 | 34.8 | 122.5 | 89.7 |
| 3 | 43 | F | 8730 | 76.3 | 234 | 59 | 4.0 | 14.4 | 0 | + | 11.2 | 38.6 | 119.2 | 89.2 |
| 4 | 77 | M | 6600 | 119.2 | 91 | 36 | 2.5 | 31.1 | 66 | - | 34.8 | 38.9 | 111.7 | 98.5 |
| 5 | 19 | F | 3160 | 70.6 | 65 | 26 | 2.5 | 13.8 | 91 | - | 33.9 | 104.1 | ||
| 6 | 58 | F | 3150 | 117.3 | 131 | 65 | 2.0 | 10.3 | 25 | + | 18.0 | 45.1 | 100.4 | 82.1 |
| 7 | 40 | M | 3100 | 107.0 | 144 | 47 | 3.1 | 12.9 | 20 | - | 18.5 | 47.5 | 116.9 | 86.8 |
| 8 | 38 | F | 3030 | 44.3 | 65 | 42 | 1.6 | 17.2 | 20 | + | 20.0 | 109.6 | ||
| 9 | 65 | M | 1880 | 56.8 | 104 | 29 | 3.6 | 21.2 | 89 | + | 24.6 | 34.5 | 56.1 | 63.4 |
| 10 | 56 | F | 1810 | 55.9 | 94 | 50 | 1.9 | 15.8 | 82 | + | 18.7 | 38.1 | 128.1 | 89.6 |
| 11 | 15 | F | 1550 | 62.0 | 96 | 42 | 2.3 | 13.4 | 0 | - | 15.7 | 113.2 | ||
| 12 | 29 | F | 1090 | 55.1 | 103 | 41 | 2.5 | 13.4 | 34 | + | 17.4 | 41.8 | 112.0 | 95.8 |
| 13 | 68 | M | 833 | 8.1 | 222 | 48 | 4.6 | 15.3 | 212 | + | 26.6 | 65.0 | ||
| 14 | 39 | F | 373 | 7.6 | 104 | 18 | 5.8 | 40.1 | 185 | - | 32.9 | 34.3 | 75.9 | 80.5 |
| 15 | 56 | M | 299 | 13.5 | 88 | 29 | 3.0 | 22.2 | 252 | - | 38.0 | 81.2 | ||
| Mean | 48.5 | 4120 | 75.0 | 120 | 44 | 2.9 | 17.7 | 77 | 23.7 | 103.0 | ||||
| (SD) | (19.2) | (4310) | (52.3) | (51) | (14) | (1.2) | (8.2) | (79) | (8.1) | (22.9) | ||||
| Median | 48 | 3030 | 62.0 | 103 | 42 | 2.5 | 14.8 | 34 | 20.0 | 111.7 | ||||
| Reference ranges | (73-271) | (5.4-13.9) | (71-168) | (8-26) | (4.4-12.4) | (13-45) | (>350) | (>36 F) | (80-100) | |||||
| (>40 M) | ||||||||||||||
Folate use for 1 week to one year prior to metabolite measurements
Tx Hct refers to Hct after parenteral cobalamin treatment
Tx MCV refers to MCV after parenteral cobalamin treatment
The megaloblastic anemia was frequently severe with median hemoglobin of 6.9 g/dL and hematocrit 20%. The MCV was > 100 fL in eleven of the 15 subjects and the median value was 111.7 fL. The median white blood count was only 3.3 × 109/L with a median absolute neutrophil count of 1.8 × 109/L (Table 2). Six of the subjects had an absolute neutrophil count < 1.5 × 109/L. The median platelet count was 171 × 109/L however seven subjects were < 120 × 109/L. The serum cobalamin values were markedly low with a median value of 34 pg/ml. The median serum MMA was 3030 nmol/L and was greater than 5000 nmol/L in four subjects and between 1000-5000 in eight subjects. Elevations in tHcy were also extreme with a median value of 62.0 umol/L. The values were greater than 100 umol/L in five of the 15 subjects and between 40-100 umol/L in seven subjects. Three subjects had tHcy ≤ 13.9 even though MMA was elevated. The SAH was elevated in fourteen of the 15 subjects and the median value was 42 nmol/L. SAH was at the upper level of the reference range (26 nmol/L) in one of the subjects. The median value for SAM was 103 nmol/L and only two subjects were low (< 71 nmol/L). The median value for cystathionine was 630 nmol/L and all but four individuals were elevated over our previously determined reference range (Table 2).
Table 2.
Additional Laboratory and Metabolic Variables in Fifteen Patients with Cobalamin Deficiency
| Variable | Mean (SD) | Median | Patient Rangea | Reference Rangeb |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 7.9 (2.7) | 6.9 | 4-12.6 | (12-17) |
| White blood count (x 109/L) | 4.6 (3.8) | 3.3 | 1.9-17.2 | (4.5-11.0) |
| Absolute neutrophil count (x 109/L) | 2.7 (3.4) | 1.8 | 0.6-14.7 | (3.0-5.8) |
| Platelets (x 109/L) | 215 (162) | 171 | 51-564 | (150-450) |
| Cystathionine (nmol/L) | 737 (371) | 630 | 306-1410 | (44-342) |
| 2-methylcitrate (nmol/L) | 841 (650) | 589 | 167-2537 | (60-228) |
| N-methylglycine (umol/L) | 1.5 (0.5) | 1.3 | 0.9-2.5 | (0.6-2.7) |
| N, N-dimethylglycine (umol/L) | 5.6 (2.5) | 5.5 | 3.1-13.0 | (1.4-5.3) |
| Ferritin (ug/L) | 227 (265) | 188 | 9-967 | (20-250) |
| Creatinine (mg/dL) | 0.8 (0.3) | 0.8 | 0.4-1.5 | (< 1.4) |
| BUN (mg/dL) | 29 (11) | 26 | 15-56 | (< 30) |
| Serum folate (ng/mL) | 13.7 (5.2) | 13.0 | 6.7-24 | (> 5.0) |
The actual range of values in the 15 cobalamin deficient sub ects is shown
Generally excepted normal values are shown except for the cobalamin dependent metabolites which were determined as in Methods
The median 2-methylcitric acid value (reference range, 60-228 nmol/L) was also increased to 589 nmol/L and was elevated in all but two individuals. The median methionine value was 14.8 umol/L, which was in the lower part of the reference range (13.1-45 umol/L) and three of the cobalamin deficient subjects were low. The median N, N-dimethylglycine of 5.5 umol/L was near the top of our previously determined reference range (1.4-5.3 umol/L) and was elevated above the reference range in eight of the 15 subjects.
There were seven cobalamin deficient subjects who received oral folic acid therapy prior to the phlebotomy for intervals of one week up to one year. The serum and RBC folate values were not significantly different in those pretreated with folate or not. The mean hematocrit and hemoglobin were lower 19.5 vs. 28.6% (p = 0.023) and 6.4 vs. 9.7 g/dL (p = 0.011) in those who were treated. N-methylglycine trended lower in those treated, 1.3 vs. 1.6 umol/L (p = 0.08). The other metabolites and variables were not significantly different.
The serum SAH was elevated in the one subject who met our diagnosis of folate deficiency. This subject also had macrocytic anemia, elevated tHcy, N, N-dimethylglycine and N-methylglycine also consistent with folate deficiency. The SAM/SAH ratio was low (2.8) in this subject and in another possible folate deficient subject even though SAH was normal in the latter.
There were four cobalamin deficient subjects (case no. 9, 13, 14, 15 on Table 1) who likely also had iron deficiency based on serum ferritin values of 9-17 ug/L in three of them and low MCV of 65.0, 75.9 and 81.2 fL. Ferritin was not available for the fourth subject, however MCV was 56.1fL in this subject. Cases 14 and 15 had only mild cobalamin deficient metabolic abnormalities but did meet our diagnosis of cobalamin deficiency. Cases nine and thirteen, however, had MMA values, which overlapped those of the other cobalamin deficient patients. Hemoglobin electrophoresis was performed on samples from 14 of the subjects and showed normal findings except a small increase in hemoglobin F in one and sickle trait in another subject. No subject appeared to have B-thalassaemia, however electrophoresis was not available for the subject with the severely depressed MCV of 56.1 fL. It therefore appeared that the low MCV in three of the subjects with cobalamin deficiency were probably related to low iron status rather than coexisting B-thalassemia.
Thermolabile Methylenetetrahydrofolate Reductase (MTHFR) and Cobalamin Deficiency
The genotype for MTHFR C677T polymorphisms was available for 14 of the cobalamin deficient patients. There were seven of each genotype, CC and CT. The serum cobalamin and the platelet count were significantly higher in those with the CT status. The serum SAH trended lower in those with the CT status. In the Other Anemia group there were ten CC, six CT, and one TT genotype. Hematocrit and hemoglobin were lower in those with the CT rather than the CC status. One of the possible folate deficient subjects had the TT variant.
Serum Metabolites in Cobalamin Deficient as Compared to Those with Other Causes of Anemia
The clinical variables and mean (SD) of the serum metabolites are shown in Table 3 for the cobalamin deficient patients as compared to the 19 with other apparent causes of anemia or cytopenias.
Table 3.
Mean (SD) Metabolites and Other Variables in Cobalamin Deficient Subjects vs. Those with Other Causes of Anemia
| Cobalamin deficient | Other Anemia | Other Anemia* Normal Renal Status | |||
|---|---|---|---|---|---|
| N=15 | N=19 | p† | N=15 | p‡ | |
| Hematocrit (%) | 23.7 (8.1) | 27.1 (7.2) | NS§ | 28.1 (6.6) | NS |
| MCV (fl) | 103.1 (22.9) | 87.9 (17.3) | 0.035 | 86 (18) | 0.029 |
| Cbl (pg/ml) | 77 (79) | 666 (512) | <0.001 | 580 (429) | <0.001 |
| MMA (nmol/L) | 4120 (4310) | 226 (166) | 0.004 | 225 (187) | 0.004 |
| tHcy (umol/L) | 75.0 (52.3) | 9.4 (4.0) | <0.001 | 8.6 (3.9) | <0.001 |
| SAM (nmol/L) | 120 (51) | 127 (62) | NS | 104 (40) | NS |
| SAH (nmol/L) | 44 (14) | 37 (29) | NS | 26 (16) | 0.004 |
| SAM/SAH ratio | 2.9 (1.2) | 4.1 (1.3) | 0.008 | 4.4 (1.2) | 0.002 |
The subjects with poor renal function (BUN ≥60) were excluded.
p value comparing cobalamin deficient vs Other Anemia by t test.
p value comparing cobalamin deficient vs Other Anemia without the 4 subjects with renal insufficiency.
NS refers to not significant.
The mean values are also shown for 15 Other Anemia subjects after excluding the 4 subjects who had renal insufficiency, as defined by BUN ≥ 60 mg/dL. These 4 had blood urea nitrogen (BUN) values ranging from 60-85 mg/dl and serum creatinine of 0.9, 1.6, 1.8 and 2.8 mg/dl. Only two cobalamin deficient subjects had evidence for mild renal insufficiency with serum creatinine 1.5 mg/dl in one and BUN of 56 mg/dl in another.
The MCV, MMA, and tHcy were lower in the Other Anemia subjects as compared to those with cobalamin deficiency, as expected (Table 3). The serum SAM was not different in the two groups. The SAH was not significantly lower in the total group of subjects with Other Anemia because of the elevated values in the 4 subjects with renal failure whose values ranged from 56-124 nmol/L. When these 4 subjects were removed from the analysis, then the mean SAH was significantly higher in the cobalamin deficient subjects 44 vs. 26 nmol/L, p = 0.004. Additional analysis of SAH values excluding the two cobalamin deficient subjects with borderline renal insufficiency and the four “Other Anemia” subjects was also significant, (p < 0.005, data not shown). Anemia may have been related to renal failure with erythropoietin deficiency in these four Other Anemia subjects, although it was not investigated further. The SAM/SAH ratio was also significantly lower in cobalamin deficient as compared to the subjects with Other Anemia. Serum MMA was normal in all of the subjects in the Other Anemias group except one who had a value of 869 nmol/L with a simultaneous serum cobalamin concentration of 454 pg/ml and thus did not meet our diagnosis for cobalamin deficiency. He had undergone gastric surgery, had normal serum creatinine, and appeared to have iron deficiency also with a MCV of 56.7 fL and ferritin 29 ug/dL. Since his cobalamin value was over our predetermined cut-point, we could not include him in the cobalamin deficient group but it is extremely likely that cobalamin deficiency was the cause of his elevated MMA value. The tHcy was ≥ 13.9 umol/L in three Other Anemia subjects however serum folate was not decreased and the MMA and serum cobalamin values did not meet our criteria for deficiency. One of these three subjects had macrocytic anemia, one normocytic and one microcytic.
Microcytic anemia likely due to iron deficiency was present in eight of the subjects with Other Anemia, of whom five had low serum ferritin ranging from 2-15 ug/L, one, 29 ug/L and 2 had unknown values. There were six subjects with Other Anemia with pancytopenia and macrocytosis who most likely had bone marrow failure syndromes. One of these subjects was diagnosed 3 months later as acute leukemia. Two of those with pancytopenia also had elevated BUN and creatinine levels.
There were six subjects with Other Anemia who received folic acid treatment prior to phlebotomy for metabolite levels. The serum and red blood cell folate were significantly higher 12.1 vs. 7.9 ng/ml (p = 0.018) and 1490 vs. 448 ng/ml (p = 0.025) in those treated, and the tHcy was significantly lower, 5.8 vs. 11.2 umol/L (p = 0.001). The serum SAH trended lower in those treated, 21 vs. 42 nmol/L (p = 0.079). Hematologic variables were not different.
Correlations Between Variables in the Cobalamin Deficient Subjects
Correlations were determined between variables for the entire group of cobalamin deficient patients (N=15) and for some variables in the subgroup of cobalamin deficient patients excluding those with apparent co-existing iron deficiency (N=11). For all 15 subjects, age was correlated with creatinine as expected, Spearman’s Rho (0.600, p=0.018) but not to other metabolites.
SAH was correlated with SAM (0.523, p=0.045), methylcitric acid (0.533, p=0.041), cystathionine (0.623, p=0.013), tHcy (0.544, p=0.036), MMA (0.535, p=0.040) and inversely with methionine (-0.552, p=0.033), cobalamin (-0.535, p=0.040) and N-methylglycine (-0.607, p=0.017). SAM was only correlated with SAM/SAH ratio (0.560, p=0.030). THcy, MMA, cystathionine and methylcitric acid were all mutually correlated (p <0.001). Methionine was directly correlated with cobalamin (0.581, p=0.023) and indirectly with tHcy (-0.542, p=0.037) and cystathionine (-0.468, p=0.078). The white blood cell count was directly correlated with cobalamin (0.513, p=0.050).
Correlations between metabolic variables and the other hematologic parameters were done on the 11 subjects thought to have “pure” cobalamin deficiency. The creatinine was directly correlated with hematocrit (0.608, p=0.047) and hemoglobin (0.637, p=0.035). Hematocrit was correlated with cobalamin (0.700, p=0.016), trended with methionine (0.551, p=0.079) and inversely trended with SAM (-0.556, p= 0.076) with similar findings for hemoglobin. The white blood cell count and absolute neutrophil count had a strong inverse correlations with SAH (-0.683, p=0.021) and (-0.672, p=0.023) respectively. The correlation of SAH with hematocrit was not significant (-0.337, p=0.311).
The hemoglobin and hematocrit trended inversely with SAH (-0.478, p=0.099) and (-0.506, p=0.078) when the correlations were adjusted for serum ferritin in the entire group of 15 cobalamin deficient patients. MCV became inversely correlated with SAM/SAH ratio in the same analysis (-0.657, p=0.015).
Response of Treatment in Cobalamin Deficient Megaloblastic Anemia
There were 10 patients who were available for investigation after treatment with cyanocobalamin injections. The individual and mean pre and post treatment values are shown in Tables 1 and 4 demonstrating an excellent correction of hematologic abnormalities in most cases. There were dramatic increases in mean hematocrit; 82%, white blood cells, 77%, absolute neutrophil count, 115% and the MCV fell by 20%. One subject with combined iron and cobalamin deficiency remained severely microcytic. Mean tHcy fell 12-fold and MMA fell 34-fold. Cystathionine and methylcitric acid values were corrected.
Table 4.
Mean (SD) Values Pre and Post Cobalamin Treatment in Ten Cobalamin Deficient Patients
| Pre | Post | p* | |
|---|---|---|---|
| Hematocrit (%) | 22.2 (7.5) | 40.4 (5.7) | 0.001 |
| Hemoglobin (g/dL) | 7.5 (2.5) | 14.0 (2.2) | < 0.001 |
| MCV (fl) | 107.3 (23.8) | 86.5 (9.7) | 0.004 |
| White blood cells (x 109/L) | 3.4 (0.9) | 6.0 (3.4) | 0.025 |
| Absolute neutrophil count (x 109/L) | 1.7 (0.6) | 3.6 (2.4) | 0.023 |
| Platelets (x 109/L) | 165 (128) | 219 (66) | 0.092 |
| THcy (umol/L) | 92.7 (53.6) | 7.7 (3.2) | 0.001 |
| MMA (nmol/L) | 5300 (4860) | 154 (63) | 0.009 |
| SAH (nmol/L) | 47 (16) | 25 (11) | 0.001 |
| SAM (nmol/L) | 126 (45) | 114 (41) | 0.563 |
| SAM/SAH (ratio) | 3.0 (1.3) | 4.9 (1.3) | 0.006 |
| Cystathionine (nmol/L) | 886 (363) | 265 (169) | < 0.001 |
| Methylcitric acid (nmol/L) | 1050 (708) | 145 (79) | 0.002 |
| Methionine (umol/L) | 18.3 (9.9) | 23.2 (8.8) | 0.200 |
| N-methylglycine (umol/L) | 1.6 (0.5) | 1.6 (0.6) | 0.747 |
| N, N-dimethylglycine (umol/L) | 5.4 (1.4) | 5.2 (2.5) | 0.766 |
p value for paired samples t test is shown.
The mean SAH decreased by nearly 50%, and SAM was unchanged. The SAM/SAH ratio however, was increased significantly. SAH remained elevated at 51 nmol/L post treatment in one subject who had elevated BUN. The SAM/SAH ratio improved in this subject, 2.5 to 4.0, because the SAM increased from 164 to 203 nmol/L post treatment. In contrast to serum SAH, the tHcy fell from 134 to 7.6 umol/L and MMA decreased from 12,300 to 139 nmol/L in this subject. Like the SAH value, cystathionine remained elevated at 610 nmol/L and methylcitric acid was also slightly over the reference range at 232 nmol/L, the latter three metabolites very sensitive to impaired renal status. The MCV declined from 122 to 89.7 fl over the same interval confirming a hematologic response to cobalamin therapy.
Only one subject still had elevated tHcy post treatment, (14.6 umol/L), although the value had decreased from the extreme value of 197.8 umol/L. A different subject had a modest elevation in MMA of 303 nmol/L post treatment.
The MCV was decreased to less than 100 fL in every case and remained quite low in a microcytic subject at 63.4 fL. Four of the subjects remained mildly anemic including the 2 with the lowest MCV values who probably had coexisting iron deficiency. The white blood cell count and absolute neutrophil count remained low in the one subject with the elevated SAH post treatment mentioned above. One patient with a clear response of metabolites, MCV and hematocrit post treatment, continued to be thrombocytopenic post treatment.
Discussion
We have prospectively assayed serum SAH and SAM/SAH ratio for the first time in patients with well documented cobalamin deficient megaloblastic anemia and determined that SAH is elevated, SAM/SAH ratio is depressed and that these abnormalities correct after parenteral cobalamin treatment. Many of the subjects had severe anemia accompanied by very low serum cobalamin values and often extreme elevations of tHcy and MMA. Therefore, this cohort was suitable for testing the hypothesis that SAM deficiency or altered methylation ratio occurs in cobalamin deficient megaloblastic anemia. Another strength of our investigation was that the samples were carefully collected, stored and shipped frozen and assayed promptly so that artifactual increases in SAH and decreases in SAM, which we have previously described,13 did not occur. Although we found that SAH was elevated approximately twice normal in cobalamin deficiency, our surprising finding was that serum SAM was not decreased, and did not change significantly in the ten subjects who also had values obtained post-treatment. In fact, inspection of Table 1 shows that some of the subjects had SAM values which were high or high normal. We also found a surprising inverse relationship between hemoglobin and SAM. An important hypothesis explaining pathophysiology of cobalamin or folate deficiency has been the depletion of SAM, which is a crucial substrate for the methylation of DNA, neurotransmitters, formation of creatine, phospholipids and many other important reactions 11. We are not able to provide data to substantiate this hypothesis. Other investigators recently found that SAM was not low in cobalamin deficient subjects15. The archived samples in that report were not suitable for SAH assay so SAM/SAH ratio was not reported 15.
In contrast, high SAH concentrations may be important in the pathophysiology of megaloblastic anemia since SAH correlated inversely with white blood count and hematocrit (after adjustment for ferritin) in our cobalamin deficient cohort, in contrast to MMA and tHcy. The elevation of SAH in the subjects could be just a marker for the level of inhibition of methionine synthase in cobalamin deficiency with a resulting decrease in production of methionine and an accumulation of homocysteine. SAH hydrolase actually favors the synthesis of SAH and only the removal of homocysteine and adenosine keeps the reaction going in the catabolic direction under usual physiologic conditions 6. The fact that tHcy was not a predictor of the hematologic variables suggests that the build up of SAH is detrimental, independent of its relationship with tHcy. Since SAH is an inhibitor of many methyltransferases it may be having other detrimental effects 17. For example, we found a direct relationship between the serum creatinine and hematocrit, which was unexpected. It is well known that renal insufficiency causes anemia, which could have caused an inverse relationship. However, since this group of cobalamin deficient subjects had normal renal function, lower serum creatinine could reflect decreased creatine synthesis. Approximately 75% of daily SAM-dependent methylations are utilized in the formation of creatine from guanidinoacetic acid 5. The positive relationship between hematocrit and creatinine in this study could be interpreted as showing that in severe cobalamin deficiency, the inhibition of creatine formation by SAH is physiologically important.
We have previously found that methionine was not low in subjects with severe cobalamin deficiency,2 and the data in this cohort confirms that finding for the entire group. We previously did find lower methionine and SAM in pregnant Brazilian women who had low cobalamin status, although a diet deficient in animal protein probably was causal of both conditions 16. The serum methionine in the Brazilian subjects studied here and previously 16 appeared to be somewhat lower than a normal range derived in the United States, which may reflect differences in the quantity of dietary animal protein consumed. It is likely that methionine concentrations, however, can be maintained in the cobalamin deficient subject by both dietary intake of protein and by hepatic synthesis of methionine by betaine homocysteine methyltransferase (BHMT) 6. The high N, N-dimethylglycine values in these deficient subjects suggests that BHMT synthesis of methionine was high. Since serum and presumably hepatic methionine concentrations are maintained in cobalamin deficiency, it is not surprising that the serum SAM concentrations were maintained. Unexplained, however, is that a number of the cobalamin deficient subjects in this investigation actually had elevated concentrations of SAM. The inverse correlation of hematocrit and SAM was also unexpected. Investigations of hepatic methionine adenosyltransferase activity in cobalamin deficiency would be interesting, although not feasible in human subjects.
Even though most subjects had normal SAM concentrations, the elevation in SAH resulted in a decreased SAM/SAH ratio, which corrected after treatment. The elevated SAH and altered SAM/SAH ratio could be important because SAH is a potent product inhibitor of most SAM dependent methyltransferases. There are at least 39 mammalian SAM dependent methyltransferases involved in reactions of DNA, RNA, lipid, small molecule, and protein methyl transfer reactions 17. The activity of an erythrocyte repair protein L-isoaspartate (D-aspartate) O-methyltransferase has been studied in patients with renal failure and found to be inhibited by the build up of SAH in such patients 18. It would be of interest to study this enzyme in subjects with severe cobalamin deficient megaloblastic anemia.
One of the most striking features of cobalamin and folate deficient megaloblastic anemia is the death of the immature red cell precursors in the bone marrow prior to release, which has been termed “ineffective erythropoiesis 19.” It appears that many of the erythrocyte precursors undergo intramedullary apoptosis, which has been postulated to be due to the inability of the cells to replicate and repair DNA due to the lack of thymidylate synthesis 19. It is also possible that accumulation of the methylation inhibitor SAH could contribute to apoptosis. In one report DNA fragmentation was more severe with treatment using a SAH hydrolase inhibitor combined with extra homocysteine 20. Experimental evidence is also accumulating that apoptosis of erythroid precursors in experimental folate deficiency is due to DNA damage resulting from deficient thymidylate and purine synthesis 21.
We were not able to demonstrate an impact of the common thermolabile mutation of MTHFR in our small population. There were only three out of the 34 individuals who were homozygous for the TT polymorphism. The racial mix of the studied population was probably responsible for the low frequency since we studied many persons with African ancestry, who are known to have a low prevalence 22.
One limitation of our study is that we assayed serum SAM and SAH instead of tissue levels, and our previous research has shown that the concentration of these compounds are highly dependent on renal status 13. This is an inherent problem of performing clinical research since it is extremely unlikely that hepatic concentrations of these compounds will be measured in humans with cobalamin deficiency. A strength of our investigation is that most of the subjects had clear unequivocal evidence of severe cobalamin deficient megaloblastic anemia. The subjects who received empiric folate therapy also had severe megaloblastic anemia and can be said to have “pure” cobalamin deficiency, since folate status had been previously corrected. The dramatic rise in hematocrit, fall in MCV, and normalization of MMA and tHcy in the subjects available for follow-up further confirmed the specificity of the diagnosis. Another strength of our investigation is that renal function was normal in most of the subjects so that elevations of SAM and SAH due to impaired clearance of these metabolites was less of a diagnostic problem. Furthermore, our conditions of sample preparation and storage likely minimized antifactual increases in SAH since prolonged storage and room temperature incubations increase SAH and decrease SAM 13. Since these subjects did not have low serum SAM, it can be concluded that serum SAM is not decreased in severe cobalamin deficiency. It is possible that liver SAM concentrations could have been decreased but this will be difficult to study in humans. Although SAH is elevated, the increase in SAH and the decrease in the methylation ratio is modest compared to the increases in tHcy and MMA seen in severe cobalamin deficiency. It seems likely that serum SAH assays will have limited diagnostic utility for cobalamin deficiency for that reason. Elevated SAH has been found in renal insufficiency in past investigations 13, 23 and was seen in our investigation. One subject with clear normalization of MMA and tHcy after cobalamin treatment had elevated SAH post treatment likely related to renal insufficiency. It appears that SAH is more likely to be elevated in renal insufficiency than MMA or tHcy, thus again may be a less specific diagnostic test for cobalamin deficiency. Our studies provide background for future investigations of perturbations of SAM dependent methyltransferases in megaloblastic anemia and possibly also in renal failure associated anemia.
Acknowledgements
We thank Dr. Marcelo G. Cliquet and Dr. Fátima C. Minari from Division of Hematology, Pontificia Universidade Católica de São Paulo for referring their patients, Bev Raab and Carla Ray for expert technical assistance and Theresa Martinez for secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contract grant sponsor, Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP in Brazil contract grant number Proc. 00/12467/0, contract grant sponsor, National Institute of Health, contract grant number AG-09834.
References
- 1.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96:239–246. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 2.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiencies. FASEB J. 1993;7:1344–1353. doi: 10.1096/fasebj.7.14.7901104. [DOI] [PubMed] [Google Scholar]
- 3.Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood. 1990;76:871–881. [PubMed] [Google Scholar]
- 4.Cook RJ. Folate metabolism. In: Carmel R, Jacobsen DW, editors. Homocysteine in Health and Disease. Cambridge University Press; Cambridge, United Kingdom: 2001. pp. 113–134. [Google Scholar]
- 5.Mudd SH, Levy HL, Kraus JP. Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. b. 8th ed. McGraw-Hill; New York: 2001. pp. 2007–2056. [Google Scholar]
- 6.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 7.Wagner C, Briggs WT, Cook RJ. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun. 1985;127:746–752. doi: 10.1016/s0006-291x(85)80006-1. [DOI] [PubMed] [Google Scholar]
- 8.Stabler SP, Lindenbaum J, Savage DG, Allen RH. Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood. 1993;81:3104–3113. [PubMed] [Google Scholar]
- 9.Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N-N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism. 1993;42:1448–460. doi: 10.1016/0026-0495(93)90198-w. [DOI] [PubMed] [Google Scholar]
- 10.McKeever MP, Molloy AM, Weir DG. Demonstration of hypomethylation of proteins in the brain of pigs (but not in rats) associated with chronic B12 inactivation. Clin Sci. 1995;88:471–477. doi: 10.1042/cs0880471. [DOI] [PubMed] [Google Scholar]
- 11.Scott JM, Dinn JJ, Wilson P, Weir DG. Pathogenesis of subacute combined degeneration: A result of methyl group deficiency. Lancet. 1981;2:334–337. doi: 10.1016/s0140-6736(81)90649-8. [DOI] [PubMed] [Google Scholar]
- 12.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Elevation of 2-methylcitric acid I and II levels in serum, urine and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993;42:978–988. doi: 10.1016/0026-0495(93)90010-l. [DOI] [PubMed] [Google Scholar]
- 13.Stabler SP, Allen RH. Quantification of serum and urinary S-adenosylmethionine and S-adenosylhomocysteine by stable-isotope-dilution liquid chromatography-mass spectrometry. Clin Chem. 2004;50:365–372. doi: 10.1373/clinchem.2003.026252. [DOI] [PubMed] [Google Scholar]
- 14.Frosst O, Blom HJ, Goyette P, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 15.Carmel R, Melnyk S, James SJ. Cobalamin deficiency with and without neurologic abnormalities: differences in homocysteine and methionine metabolism. Blood. 2003;101:3302–3308. doi: 10.1182/blood-2002-09-2746. [DOI] [PubMed] [Google Scholar]
- 16.Guerra-Shinohara EM, Morita OE, Peres S, et al. Low ratio of S-adenosylmethionine to S-adenosylhomocysteine is associated with vitamin deficiency in Brazilian pregnant women and newborns. Am J Clin Nutr. 2004;80:1312–1321. doi: 10.1093/ajcn/80.5.1312. [DOI] [PubMed] [Google Scholar]
- 17.Clarke S, Banfield K. S-adenosylmethionine-dependent methyltransferases. In: Carmel R, Jacobsen DW, editors. Homocysteine in Health and Disease. Cambridge University Press; Cambridge, United Kingdom: 2001. pp. 63–78. [Google Scholar]
- 18.Perna AF, Ingrosso D, Zappia V, et al. Enzymatic methyl esterification of erythrocyte membrane proteins is impaired in chronic renal failure. Evidence for high levels of the natural inhibitor S-adenosylhomocysteine. J Clin Invest. 1993;91:2497–2503. doi: 10.1172/JCI116485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickramasinghe SN. The wide spectrum and unresolved issues of megaloblastic anemia. Semin Hematol. 1999;36:3–18. [PubMed] [Google Scholar]
- 20.Rounds S, Yee WL, Dawicki DD, et al. Mechanism of extracellular ATP and adenosine induced apoptosis of cultured pulmonary artery endothelial cells. Am J Physiol. 1998;275:L379–388. doi: 10.1152/ajplung.1998.275.2.L379. [DOI] [PubMed] [Google Scholar]
- 21.Koury MJ, Price JO, Hicks GG. Apoptosis in megaloblastic anemia occurs during DNA synthesis by a p53-independent, nucleoside-reversible mechanism. Blood. 2000;96:3249–255. [PubMed] [Google Scholar]
- 22.Arruda VR, Siqueira LH, Goncalves MS, et al. Prevalence of the mutation C677 → T in the methylene tetrahydrofolate reductase gene among distinct ethnic groups in Brazil. Am J Med Genet. 1998;78:332–335. doi: 10.1002/(sici)1096-8628(19980724)78:4<332::aid-ajmg5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Loehrer FM, Angst CP, Brunner FP, Haefeli WE, Fowler B. Evidence for disturbed S-adenosylmethionine: S-adenosylhomocysteine ratio in patients with end stage renal failure-a cause for disturbed methylation reactions. Nephrol Dial Transplant. 1998;13:656–661. doi: 10.1093/ndt/13.3.656. [DOI] [PubMed] [Google Scholar]