Abstract
Recently, DNA rearrangements in the cystic fibrosis transmembrane conductance regulator (CFTR) gene have been described with increasing frequency. These large DNA rearrangements are not detected using conventional methods of DNA sequencing, single-strand conformational polymorphism, or denaturing high-performance liquid chromatography. We and others have described methods to detect such rearrangements in the CFTR gene. With one exception, all rearrangements reported thus far are single or multiple exon deletions, whereas only one report has described a large duplication. We describe here the detection and characterization of a novel large duplication in the CFTR gene. This duplication, referred to as gIVS6a + 415_IVS10 + 2987Dup26817bp, was detected in a classic CF female patient whose other mutation was ΔF508. The duplication was inherited paternally. The duplication encompassed exons 6b to 10 and occurred on the IVS8-11TG/IVS8-7T/G1540 haplotype. This large duplication is predicted to result in the production of a truncated CFTR protein lacking the terminal part of NBD1 domain and beyond and thus can be considered a null allele. The combination of the ΔF508 and gIVS6a + 415_IVS10 + 2987Dup26817bp mutation probably causes the severe CF phenotype in this patient. We designed a simple polymerase chain reaction test to detect the duplication, and we further detected the same duplication from another independent laboratory. The duplication breakpoint is identical in all three patients, suggesting a likely founder mutation.
Cystic fibrosis (CF) is an autosomal recessive disease with an incidence of approximately 1 in 2000 to 4000 Caucasians with European ancestry, including Ashkenazi Jews.1 Classic CF is characterized by an elevated sweat chloride test, chronic obstructive pulmonary disease, pancreatic exocrine deficiency with malabsorption and malnutrition, and congenital bilateral absence of the vas deferens (CBAVD) leading to male infertility.1 The majority of mutations in the CFTR gene reported in the CFTR mutation database (http://www.genet.sickkids.on.ca/cftr/Home.html) are single nucleotide polymorphisms and small bp insertions or deletions (in/dels). The most common mutation in Caucasians, accounting for up to 70% of mutated alleles, is a 3-bp deletion in exon 10 known as ΔF508.
The preponderance of these single nucleotide polymorphisms and small in/dels type of mutations could be a result of their actual frequency in CF patients. On the other hand, it could be a reflection of the limitations of current detection methodologies for identifying novel CF mutations. DNA sequencing, the primary method used for mutation identification, single-strand conformational polymorphism, or denaturing high-performance liquid chromatography does not detect large DNA rearrangements. Using an in-house-developed semiquantitative fluorescent polymerase chain reaction (PCR) assay, we discovered 10 cases of large exonic deletions in patients with CF for whom DNA sequencing failed to identify a second mutated allele.2,3 Others using similar methods identified large DNA rearrangements in the CFTR gene as well.4,5 We also identified a deletion in a CBAVD patient.6 The availability of easier screening methods for these types of mutations might shed more light on the frequency and type of these kinds of mutations.
Most large DNA rearrangements identified thus far are large deletions encompassing one or more exons.4,5,7,8,9,10,11,12,13,14 Few duplications have been identified thus far, with one being fully reported, a duplication of exons 4 to 8 in a classic CF patient from France,5 but the duplication breakpoint for that mutation was not identified. This report describes the detection of a novel large duplication in the CFTR gene. This duplication encompasses exons 6b to 10 and was detected in the paternal DNA as well. In addition, we describe the full identification of the breakpoint of the duplication and the characterization of its haplotype and devise a simple PCR test for its detection.
Materials and Methods
Case Description
The patient is a 19-year-old Caucasian female with a diagnosis of classic CF. She was born with meconium ileus and has an elevated sweat chloride test result of 110 mmol/L, pulmonary disease, and liver cirrhosis. She had a similarly affected sister who is deceased. Previous testing had revealed the heterozygous presence of ΔF508 that was maternally inherited. A sample was submitted for extensive sequencing in an attempt to discover the second mutation responsible for her disease. DNA sequencing analysis failed to identify the second mutation. After consent was provided from the ordering physician, we analyzed the sample for rearrangements. This resulted in the identification of a duplication of exons 6b to 10 in this patient. After identification of the duplication in the proband, her father provided DNA for confirmatory testing. The mutation was deposited in the CFTR mutation database (http://www.genet.sickkids.on.ca/cftr/Home.html).
The initial preliminary report of this duplication occurred at the annual meeting of the Cystic Fibrosis Foundation in 2005 (Hantash et al, Abstracts Ann Mtg, NACFC, 2005). Subsequently, at the annual meeting of the American College of Medical Genetics (ACMG 2006), a presentation from another laboratory (Ambry Genetics) described the identification of additional patient specimens that apparently had a duplication of exons 6b to 10 detected by a commercially available multiplex ligation probe amplification kit. Collaboration with the two laboratories resulted in the testing of these unrelated patients in our laboratory. Sample 1 was from a Caucasian patient with a high sweat chloride test (101 mmol/L performed twice) with Pseudomonas infection and pancreatic insufficiency. DNA sample 2 was from a blood spot from a newborn with elevated sweat chloride (112 and 84 mmol/L). Both patients harbored ΔF508. A third patient, 11 months old with Caucasian/Hispanic ethnicity, also harbored a duplication of exons 6b to 10 and R553X. The patient failed to thrive and had high sweat chloride test results (98 and 99 mmol/L). The DNA of that patient was not obtained for further testing.
DNA Extraction, DNA Sequencing, and Semiquantitative Fluorescent PCR
DNA was extracted using an automated Qiagen robotic workstation per manufacturer’s instruction (Qiagen, Valencia, CA). DNA sequencing was performed as described previously.3 For DNA sequencing, the samples were analyzed on the ABI 3730 system (Applied Biosystems, Foster City, CA), and sequences were compared with the wild-type CFTR nucleotide sequence using Seqscape software (Applied Biosystems). Semiquantitative fluorescent (SQF) PCR was performed as previously described.3 In brief, a single-tube multiplex reaction amplifies fragments representing the promoter and the 27 CFTR exons. Fragments are separated on an ABI 3100 system (Applied Biosystems), and data are analyzed using GeneMapper software (Applied Biosystems). Primers used for amplification of junction fragment and final sequencing were IVS10F3 (5′-TGTAAAACGACGGCCAGTCCTCAATGGTTATTTATATGGCATGC-3′) and IVS6aR3 (5′-CAGGAAACAGCTATGACCTCACTCATATTAGTTATTCTGTAACACAAAGTAAC-3′). DNA amplification was performed using the Expand long-template PCR system (Roche Applied Science, Indianapolis, IN) for amplification of junction fragment. Primers3 at a final concentration of 0.2 μmol/L were included with buffer 2 (MgCl2, dNTPs, Polymerase mix, and DNA, in a total volume of 15 μl). PCR was performed on an MJ200 cycler (Bio-Rad, Waltham, MA). The reactions were incubated at 94°C for 2 minutes, followed by 36 cycles of denaturing at 94°C for 45 seconds, annealing at 57°C for 70 seconds, and extension at 68°C for 3 minutes with an additional 5 seconds/cycle. Reactions were then incubated at 68°C for 8 minutes, followed by incubation at 4°C until reactions were removed from the cyclers. A product of calculated size of 3011 was detected and sequenced as described previously.3 The breakpoint junction was 241 bp from the 3′-end of IVS6aR primer, which facilitated its identification.
A different gap PCR was designed for rapid analysis of samples suspected of harboring the duplication using primers Dup6b10upF (5′-TGTAAAACGACGGCCAGTCAGCATAAGATCCTGAAGGTTTG-3′) and Dup6b10upR (5′-CAGGAAACAGCTATGACCAACACAAAGTAACTAAGGCTCTGGT-3′). The amplified product (459 bp) can be used for detecting the specific junction fragment and to perform DNA sequencing verification.
To screen for the downstream junction fragment, a gap PCR was designed using primers Dup6b10dnF (5′-TGTAAAACGACGGCCAGTTGGCAATGGGGTTGGGAAGT-3′) and Dup6b10dnR (5′-CAGGAAACAGCTATGACCCTGCTCCTCACTATCACAGTCAGTGA-3′) to yield a calculated product size of 586 bp. The proband and the two samples from Ambry Genetics were screened for the detection of the upstream and downstream junction fragments as well.
Confirmation of Deletions Using the Multiplex Ligation Probe Amplification Kit
The presence of deletions was confirmed using a commercially available multiplex ligation probe amplification kit (MRC, Amsterdam, Holland). Samples were analyzed per the manufacturer’s protocol.
Results
Initial DNA Sequencing Results
DNA sequencing of the promoter and the 27 CFTR exons and flanking intronic sequences detected only the ΔF508 mutation. The patient was also heterozygous for M470V (A1540G) polymorphism in exon 10 and carried the IVS8-11TG/10TG and IVS8-7T/9T alleles. With permission of the ordering physician, we subjected the sample to SQF PCR analysis.
Detection of Duplication 6b-10 by SQF PCR
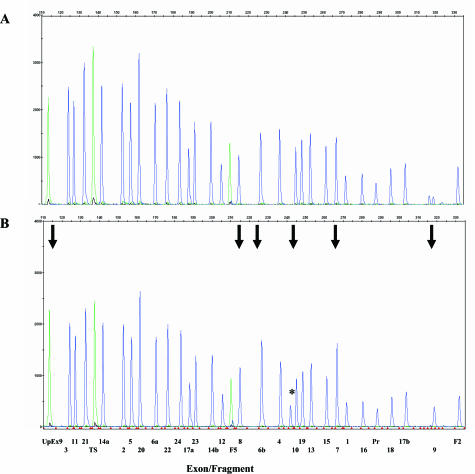
The test detects the presence of exon deletions and duplications using SQF PCR of fragments representing the promoter and all 27 CFTR exons. In this patient, signals from exons 6b to 9 showed an increase in the relative fluorescent units of their respective areas under the peaks (Figure 1). A fragment that represents a region in IVS8 upstream of exon 9 and serves as a confirmatory fragment for exon 9 changes in copy number also showed an increase in both the signal and area under the peak, confirming a duplication of exon 9. For exon 10, summing the areas under the wild-type and ΔF508 peaks demonstrated that exon 10 was also duplicated. Other fragments representing the other CFTR exons or the promoter did not appear to harbor any deletions or duplications. Therefore, it seems that the duplication includes exons 6b to 10. The duplication was confirmed using a multiplex ligation probe amplification kit (data not shown).
Figure 1.
Detection of duplication of exons 6b to 10 in the proband. A: DNA sample with no exon deletions or duplications; B: probands’ DNA. A and B: SQF PCR analysis of proband DNA for exon deletions or duplications. Arrows in B indicated duplicated exons. The duplicated fragments included 6b, 7, 8, UpEx 9 (a fragment in IVS8 upstream of exon 9), 9, and 10. The asterisk in the proband’s DNA indicate the detection of ΔF508 mutation, a faster migrating fragment related to exon 10.
Prior testing showed that the proband inherited the ΔF508 mutation from her mother; therefore, we expected that the duplication was inherited from the father. After detecting the duplication in the proband, the father submitted a blood sample for confirmatory testing. Using SQF PCR, we detected the same duplication in the paternal DNA, spanning the same region of exons 6b to 10. This also verifies that the duplication occurred in trans to the ΔF508 mutation in the proband. Because the ΔF508 mutation seems to be associated exclusively with the IVS8-10TG/IVS8-9T/A1540 haplotype,17,18 by extrapolation, the novel duplication is assumed to have occurred on the IVS8-11TG/IVS8-7T/G1540 haplotype.
Amplification of Duplication Junction
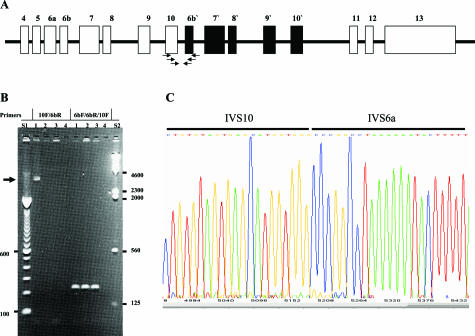
Theoretically, such a duplication would lead to the generation of a CFTR DNA containing exon 10 followed by exon 6b. This is demonstrated in Figure 2A. To confirm this putative arrangement, we performed PCR using an exon 10 forward primer and an exon 6B reverse primer19 and successfully amplified a fragment of ∼3.8 kb from the patient sample DNA but not from samples not harboring the duplication (Figure 2B). The amplification of this fragment provides a second confirmation for the presence of this large duplication in the patient’s DNA.
Figure 2.
Detection of duplication junction fragment by PCR amplification and DNA sequencing. A: Schematic representation of the duplicated region, where duplicated exons are referred to with 6b′, 7′, etc. The figure also shows the primer walking strategy (arrows) used to amplify the junction fragment in B. B: Under PCR conditions using primers 10F and 6bR,3 a fragment of ∼3.8 kb was detected from the proband (lane 1) but not from two random DNA samples (lanes 2 and 3). When primers from 10F, 6bF, and 6bR were mixed together in a separate PCR, conditions of the PCR allowed the amplification only of the fragment representing exon 6b. C: A DNA sequencing trace showing sequences of IVS10 and IVS6a at the breakpoint of the duplication.
DNA Sequencing of Junction Fragment
DNA sequencing analysis verified the presence of the duplications as sequences from IVS10 and IVS6a were detected. The exact breakpoint of the duplication, however, was not identified. Using the information we obtained from DNA sequencing, we designed a different PCR to amplify the junction fragment using primers closer to the junction point based on information obtained from sequencing the first fragment. Using this second PCR and DNA sequencing, we were able to identify the breakpoint of the duplication (Figure 2C). The fragments duplicated started 415 bp downstream of exon 6a, in IVS6a, and spanned exons 6b, 7, 8, 9, and 10, breaking at 2987 bp downstream of exon 10 in IVS10. The duplicated region is 26,817 bp.
The duplication is predicted to cause an out-of-frame addition of eight amino acids after codon E528 of exon 10, followed by a TGA stop codon. This would lead to the production of a truncated CFTR protein containing only the first transmembrane domain (TM1) and a portion of nucleotide binding domain 1 (NBD1) and lacking the remainder of the protein.
We attempted to identify the downstream junction fragment of the duplication. Using primers that we predicted would flank the duplication breakpoint, we were able to identify the expected size fragment. DNA sequencing showed that the fragment contains normal DNA sequence with no intervening unidentified DNA sequence.
Analysis of Samples from Ambry Genetics
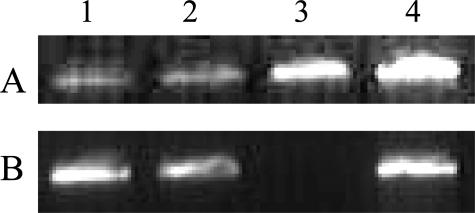
We received two anonymized DNA samples from two probands from Ambry Genetics that were genotyped by multiplex ligation probe amplification assay as harboring a duplication of exon 6b to 10. We examined whether the duplications identified in the two independent probands from Ambry Genetics harbored the same breakpoint as the one identified in our laboratory. Using the primers designed to amplify the junction fragment, we were able to show that their breakpoint is identical in both our sample and the two samples from Ambry Genetics (Figure 3). This strongly suggests that the duplication of exons 6b to 10 is a founder duplication. We further attempted to identify downstream junction fragment (Figure 3). We amplified the expected product using primers that flank the theoretical downstream duplication breakpoint based on sequence analysis (Figure 2). All samples showed the same amplified product, and DNA sequencing did not identify any non-IVS10 DNA sequence.
Figure 3.
Detection of downstream (A) and upstream (B) junction fragments in three probands harboring duplication of exons 6b to 10. Sample 1 is from the proband from Quest Diagnostics. Samples 2 to 4 are from Ambry Genetics. Samples 1, 2, and 4 harbor the duplications, whereas sample 3 is a normal sample. All samples showed amplification of sequences in IVS10 (A), whereas only the probands showed the amplification of upstream junction fragment (B) between IVS10 and IVS6a. DNA sequencing on junction fragment products (data not shown) showed all probands harboring the same breakpoint, suggesting a founder duplication.
Discussion
Recently, several comprehensive studies were published describing the detection of various large rearrangements in the CFTR gene.3,4,5 However, very few duplications have been identified, and none with junction breakpoints characterized (Cystic Fibrosis Mutation Database, http://www.genet.sickkids.on.ca/cftr/Home.html). We describe the detection and characterization of a novel duplication in the CFTR gene in a female CF patient whose sister died of CF. This large duplication was identified by SQF PCR after extensive DNA sequencing of the promoter, and the 27 CFTR exons and flanking intronic regions failed to identify a second mutation. The identification of the large duplication, after DNA sequencing failed to identify a second mutated allele, confirms the importance of analyzing CF patients’ DNA for such mutations. Furthermore, it might be useful in cases where the penetrance of missense mutations is not fully established to screen for large deletions or duplications as was reported previously.3 In addition, in cases of homozygosity for rare or novel mutations, screening for large DNA rearrangements could prove useful as the apparent homozygosity could be due the presence of a large deletion involving the exon where the rare/novel mutation would have occurred.
With two extra patients’ DNA samples sequenced and verified to harbor the same duplication breakpoint, a founder origin of this duplication is likely. We attempted to identify the mechanism behind the duplication. We scanned the regions flanking the breakpoints of the duplication in IVS6a and in IVS10 for repetitive sequences and ALU repeats in an attempt to identify a possible mechanism for occurrence of this duplication. We used the Institute for Systems Biology RepeatMasker software (http://www.repeatmasker.org) to scan for repetitive elements. The software identified two elements of the SINE group and one simple repeat in IVS6a, whereas 13 SINE, 14 LINE, one LTR, and four DNA elements were detected, as well as five simple repeats and four low complexity repeats in IVS10. The first few bases of the IVS6a junction shown in Figure 2C are the last seven bases of an Alu element. Alignment of this Alu element and IVS10 did not show sufficient homology to allow for a homologous recombination event to occur. Flanking the duplication break point is a sequence of GGG from IVS10 and CCC from IVS6a. Whether these and the presence of repeats in IVS6a and IVS10 facilitated the generation of the large duplication is a possibility. It is likely that the duplication occurred because of a nonhomologous recombination event.
In summary, we identified a novel large duplication in the CFTR gene in a CF patient carrying ΔF508 on the other allele. The duplication was detected in two independent probands from a different laboratory. All samples harbored the same junction breakpoint, suggesting a founder mutation. We also describe a simple molecular test for the detection of this duplication in patient samples. The identification of this and other rearrangement breakpoints in the CFTR gene4,20 will facilitate testing for such mutations in clinical laboratories.
Acknowledgments
We would like to thank Kelsey Starn for technical assistance in performing DNA sequencing.
Footnotes
Some of the authors are employees of Quest Diagnostics Inc., where they hold stock options.
References
- Cutting GR. Cystic fibrosis. Rimoin DL, Connor JM, Pyeritz RE, Korf B, editors. London: Churchill Livingstone,; Principles and Practice of Medical Genetics. 2002:2685–2717. [Google Scholar]
- McGinniss MJ, Chen C, Redman JB, Buller A, Quan F, Peng M, Giusti R, Hantash FM, Huang D, Sun W, Strom CM. Extensive sequencing of the CFTR gene: lessons learned from the first 157 patient samples. Hum Genet. 2005;118:331–338. doi: 10.1007/s00439-005-0065-1. [DOI] [PubMed] [Google Scholar]
- Hantash FM, Redman JB, Starn K, Anderson B, Buller A, McGinniss MJ, Quan F, Peng M, Sun W, Strom CM. Novel and recurrent rearrangements in the CFTR gene: clinical and laboratory implications for cystic fibrosis screening. Hum Genet. 2006;119:126–136. doi: 10.1007/s00439-005-0082-0. [DOI] [PubMed] [Google Scholar]
- Audrézet MP, Chen JM, Raguénès O, Chuzhanova N, Giteau K, Le Marechal C, Quéré I, Cooper DN, Férec C. Genomic rearrangements in the CFTR gene: extensive allelic heterogeneity and diverse mutational mechanisms. Hum Mutat. 2004;23:343–357. doi: 10.1002/humu.20009. [DOI] [PubMed] [Google Scholar]
- Niel F, Martin J, Dastot-Le Moal F, Costes B, Boissier B, Delattre V, Goossens M, Girodon E. Rapid detection of CFTR gene rearrangements impacts on genetic counselling in cystic fibrosis. J Med Genet. 2004;41:e118. doi: 10.1136/jmg.2004.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantash FM, Milunsky A, Wang Z, Anderson B, Sun W, Anguiano A, Strom CM. A large deletion in the CFTR gene in CBAVD. Genet Med. 2006;8:93–95. doi: 10.1097/01.gim.0000200945.54234.d7. [DOI] [PubMed] [Google Scholar]
- Dörk T, Macek M, Jr, Mekus F, Tümmler B, Tzountzouris J, Casals T, Krebsová A, Koudová M, Sakmaryová I, Macek M, Sr, Vávrová V, Zemková D, Ginter E, Petrova NV, Ivaschenko T, Baranov V, Witt M, Pogorzelski A, Bal J, Zékanowsky C, Wagner K, Stuhrmann M, Bauer I, Seydewitz HH, Neumann T, Jakubiczka S. Characterization of a novel 21-kb deletion, CFTRdele2,3(21 kb), in the CFTR gene: a cystic fibrosis mutation of Slavic origin common in Central and East Europe. Hum Genet. 2000;106:259–268. doi: 10.1007/s004390000246. [DOI] [PubMed] [Google Scholar]
- Chevalier-Porst F, Bonardot AM, Chazalette JP, Mathieu M, Bozon D. 40 kilobase deletion (CF 40 kb del 4-10) removes exons 4 to 10 of the Cystic Fibrosis Transmembrane Conductance Regulator gene. Hum Mutat. 1998;(Suppl 1):S291–S294. doi: 10.1002/humu.1380110191. [DOI] [PubMed] [Google Scholar]
- Lerer I, Laufer-Cahana A, Rivlin JR, Augarten A, Abeliovich D. A large deletion mutation in the CFTR gene (3120+1Kbdel8.6Kb): a founder mutation in the Palestinian Arabs. Mutation in brief no. 231. Online. Hum Mutat. 1999;13:337. doi: 10.1002/(SICI)1098-1004(1999)13:4<337::AID-HUMU13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mickle JE, Macek M, Jr, Fulmer-Smentek SB, Egan MM, Schwiebert E, Guggino W, Moss R, Cutting GR. A mutation in the cystic fibrosis transmembrane conductance regulator gene associated with elevated sweat chloride concentrations in the absence of cystic fibrosis. Hum Mol Genet. 1998;7:729–735. doi: 10.1093/hmg/7.4.729. [DOI] [PubMed] [Google Scholar]
- Morral N, Nunes V, Casals T, Cobos N, Asensio O, Dapena J, Estivill X. Uniparental inheritance of microsatellite alleles of the cystic fibrosis gene (CFTR): identification of a 50 kilobase deletion. Hum Mol Genet. 1993;2:677–681. doi: 10.1093/hmg/2.6.677. [DOI] [PubMed] [Google Scholar]
- Shrimpton AE, Borowitz D, Swender P. Cystic fibrosis mutation frequencies in upstate New York. Hum Mutat. 1997;10:436–442. doi: 10.1002/(SICI)1098-1004(1997)10:6<436::AID-HUMU4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Costes B, Girodon E, Vidaud D, Flori E, Ardalan A, Conteville P, Fanen P, Niel F, Vidaud M, Goossens M. Prenatal detection by real-time quantitative PCR and characterization of a new CFTR deletion, 3600+15kbdel5.3kb (or CFTRdele19). Clin Chem. 2000;46:1417–1420. [PubMed] [Google Scholar]
- Feuillet-Fieux MN, Ferrec M, Gigarel N, Thuillier L, Sermet I, Steffann J, Lenoir G, Bonnefont JP. Novel CFTR mutations in black cystic fibrosis patients. Clin Genet. 2004;65:284–287. doi: 10.1111/j.1399-0004.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- Hantash FM, Redman JB, Sun W, Strom CM: Characterization of a Novel Large Duplication in the CFTR Gene. Presented at the 2005 Annual North American Cystic Fibrosis Conference, 2005 October 21–23, Baltimore, MD [Google Scholar]
- Keiles S: Frequencies of CFTR, PRSS1, and SPINK1 Mutations in Patients with Pancreatitis CFTR Gross Deletions Update, and the Newest Genes Available at Ambry Genetics. Presented at the American College of Medical Genetics, 2006 March 23–26, San Diego, CA [Google Scholar]
- Cuppens H, Teng H, Raeymaekers P, De Boeck C, Cassiman JJ. CFTR haplotype backgrounds on normal and mutant CFTR genes. Hum Mol Genet. 1994;3:607–614. doi: 10.1093/hmg/3.4.607. [DOI] [PubMed] [Google Scholar]
- Dörk T, Fislage R, Neumann T, Wulf B, Tümmler B. Exon 9 of the CFTR gene: splice site haplotypes and cystic fibrosis mutations. Hum Genet. 1994;93:67–73. doi: 10.1007/BF00218916. [DOI] [PubMed] [Google Scholar]
- Strom CM, Huang D, Chen C, Buller A, Peng M, Quan F, Redman J, Sun W. Extensive sequencing of the cystic fibrosis transmembrane regulator gene: assay validation and unexpected benefits of developing a comprehensive test. Genet Med. 2003;5:9–14. doi: 10.1097/00125817-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Férec C, Casals T, Chuzhanova N, Macek M, Jr, Bienvenu T, Holubova A, King C, McDevitt T, Castellani C, Farrell PM, Sheridan M, Pantaleo SJ, Loumi O, Messaoud T, Cuppens H, Torricelli F, Cutting GR, Williamson R, Ramos MJ, Pignatti PF, Raguénès O, Cooper DN, Audrezet MP, Chen JM. Gross genomic rearrangements involving deletions in the CFTR gene: characterization of six new events from a large cohort of hitherto unidentified cystic fibrosis chromosomes and meta-analysis of the underlying mechanisms. Eur J Hum Genet. 2006;14:567–576. doi: 10.1038/sj.ejhg.5201590. [DOI] [PubMed] [Google Scholar]