Abstract
Ewing family tumors are molecularly characterized by expression of chimeric transcripts generated by specific chromosomal translocations, most commonly involving fusion of the EWS gene to a member of the ETS family of transcription factors (including FLI1, ERG, ETV1, E1AF, and FEV). Approximately 85% of reported cases of Ewing sarcoma bear an EWS-FLI1 fusion. In rare cases, FUS can substitute for EWS, with translocation t(16;21)(p11;q24) producing a FUS-ERG fusion with no EWS rearrangement. We report a case of Ewing sarcoma, presenting as a pathological fracture of the distal clavicle in a 33-year-old male, in which cytogenetic analysis revealed a single t(2;16)(q35;p11) balanced translocation. Fluorescence in situ hybridization using a commercially available diagnostic probe was negative for an EWS gene rearrangement; instead, break-apart fluorescence in situ hybridization probes for FUS and FEV were positive for a translocation involving these genes. Cloning and sequencing of the breakpoint region demonstrated an in-frame fusion of FUS to FEV. In conclusion, this represents the first reported case of Ewing family tumors demonstrating a variant translocation involving FUS and FEV and highlights the need to consider alternative permutations of fusion partners for molecular diagnosis of sarcomas.
Ewing sarcoma is a highly malignant small round cell tumor of bone. It is the second most common bone malignancy of childhood, with approximately 50% of cases occurring between 10 and 20 years of age, although it may occur in adults, including the elderly.1,2 Males are affected more frequently than females, with a ratio of approximately 1.5:1. Extraosseous soft tissue and visceral locations are also well described.3,4 Based on their shared immunophenotypes and molecular signatures, several diagnostic entities, previously considered distinct, are now amalgamated as the Ewing family of tumors (EFT). These include classic Ewing sarcoma of bone as well as extra-osseous Ewing sarcoma, peripheral primitive neuroectodermal tumor, and Askin tumor (peripheral primitive neuroectodermal tumor of chest wall).5
In light of the effectiveness of chemotherapeutics in its treatment, establishing the correct diagnosis of EFT as opposed to other small round cell sarcomas is of particular clinical relevance.1 The diagnostic pathological criteria for EFT include a spectrum of histological, immunophenotypic, and molecular features. The malignant cells display intense cytoplasmic membrane-associated immunoreactivity with antibodies to CD99. In approximately 85% of cases, the chromosomal translocation t(11;22)(q24;q12) can be detected by cytogenetic or molecular analysis of the tumor cells. This tumor-specific translocation results in an in-frame fusion of EWS, at chromosome band 22q12, with FLI1, a member of the ETS transcription factor family,6 at 11q24. The precise breakpoint sites within the EWS gene may have prognostic significance.7 Less commonly, EWS becomes fused with another ETS member, including ERG in approximately 5 to 10% of cases,8,9 and even less frequently with ETV1,10 E1AF (ETV4),11 or FEV.12 In a recent report, four cases of EFT showed a fusion of FUS to ERG through a t(16;21)(p11;q24) instead of the more typical EWS gene rearrangement.13 FUS, belonging to the TET family of RNA-binding proteins, shows considerable homology with EWS.14 Such a finding highlights the possibility of variant gene participation in both the 5′ and 3′ portions of EFT fusion transcripts. This has particular diagnostic relevance, since the current methods of reverse transcription-polymerase chain reaction (RT-PCR) and commonly used fluorescence in situ hybridization (FISH) probes may overlook the involvement of alternative translocation partners when such permutations are not considered. To illustrate further this important issue, we report here a case of EFT showing a novel t(2;16)(q35;p11) translocation that results in an in-frame fusion of FUS and FEV.
Materials and Methods
Clinical History
A 33-year-old Caucasian male fell while snowboarding, injuring his left, nondominant shoulder. X-rays revealed a distal clavicle fracture through a lytic lesion with permeative borders. Magnetic resonance imaging showed a 3.2-cm expansile lesion of the distal clavicle. The initial core needle biopsy showed reactive bone and a tiny 100-μm fragment of small round blue cells. After sections were taken for routine histology, immunohistochemistry was performed, with the tumor cells staining strongly positive for CD99 and negative for CD20; however, the specimen was cut through, and paraffin-based FISH analysis could not be performed. Because there was insufficient material for a definitive diagnosis, an open biopsy, placed directly over the distal clavicle, was performed and further tissue obtained. This allowed definitive diagnosis, and treatment was instituted for EFT.
Neoadjuvant chemotherapy and external beam radiation therapy were administered and well tolerated. Surgical resection of the distal clavicle was performed, dividing the clavicle on the medial side of the coracoclavicular ligaments and dividing the acromion sagittally. Examination of the specimen revealed that the lesion had been removed with a wide margin, and the tumor exhibited 100% pathological response. The patient made an excellent early recovery and was free of disease at last follow-up, 10 months following initial diagnosis.
Tissue Handling for Morphological and Genetic Studies
The needle core biopsy was formalin-fixed and paraffin-embedded in toto. The subsequent open biopsy additionally had a representative sample of fresh tumor tissue submitted for cytogenetic evaluation and a portion snap-frozen in liquid nitrogen and stored at −70°C for molecular studies. The remainder of the tissue was fixed in formalin for routine histology.
Cytogenetic Studies and Fluorescence in Situ Hybridization
Chromosomal analysis was performed on tissue from the open biopsy using standard tissue culture and harvesting procedures. Metaphases were stained by the GTG method. The karyotype alterations were described according to International System for Human Cytogenetic Nomenclature 1995.15 FISH was performed using commercially available dual-color break-apart probes for EWS and FUS (Vysis, Des Plaines, IL). An in-house probe was prepared that consisted of the bacterial artificial chromosomes (BACs) RP11-96D18 and FP11-42612 that flank the FEV gene. These BACs were labeled with SpectrumRed and SpectrumGreen (Vysis), respectively, to create a dual-color break-apart probe to detect possible rearrangements within the FEV gene. A second BAC probe combination was generated with BAC RP11-270E5 from band 2p12 (labeled with SpectrumGreen) and RP11-207M4 that spanned the FEV locus (labeled with SpectrumRed).
Sequencing Analysis of Fusion Transcript
Total RNA was extracted from a frozen portion of tumor tissue using a standard protocol with TRIzol reagent (Invitrogen, Carlsbad, CA). The RNA was reverse transcribed into cDNA with Superscript II (Invitrogen) and then used as template for PCR amplification of the fusion breakpoint. Primers were designed to flank the probable breakpoints within the FUS and FEV genes. The primer sequences used were as follows: FUS-IF, 5′-gtgcgcggacatggcctcaaacg-3′, derived from exon 1 of FUS; and FEV-IR, 5′-tgttgggcttgctcttgcgctc-3′, derived from exon 3 of FEV. The reaction was subjected to 35 cycles of PCR using the following conditions: denaturation at 94°C for 1 minute, annealing at 62°C for 30 seconds, and extension at 72°C for 45 seconds. The amplification product was resolved on 1% agarose gel electrophoresis, and a band of the expected size of ∼1.4 kb was excised and gel-purified. This was cloned into a TOPO TA vector, cultured overnight following transformation into TOP10 Escherichia coli cells. A minipreparation of the plasmid DNA was performed, and the insert was recovered using EcoRI digestion. Clones containing the appropriate-sized insert were submitted for sequencing.
Tissue Microarray
A tissue microarray (TMA) consisting of 168 cases of pediatric tumors was constructed consisting of 1.0-mm tissue cores, using standard methods.16 It included 22 cases of EFT, two of which were negative for both EWS-FLI1 and EWS-ERG fusions by RT-PCR on molecular diagnostic testing and three of which with unavailable molecular diagnostic results. Other tumor types included neuroblastoma (30 cases), ganglioneuroma (14 cases), medulloblastoma (14 cases), embryonal rhabdomyosarcoma (25 cases), alveolar rhabdomyosarcoma (21 cases), Wilms’ tumor (24 cases), fibromatosis (10 cases), congenital fibrosarcoma (five cases), and one case each of alveolar soft part sarcoma, clear cell sarcoma of kidney, and neurofibroma. All cases were diagnosed at the British Columbia Children’s Hospital pathology service and reviewed by a pediatric pathologist during the construction of the TMA. Sections from this TMA were analyzed by FISH for disruption of FUS using the commercially available break-apart probe described above (Vysis) and FEV using dual-color break-apart BACs RP11-96D18 (labeled with SpectrumRed) and RP11-426L12 (labeled with SpectrumGreen) that flank FEV on chromosome 2q35.
Results
Histology and Immunohistochemistry
H&E sections of the open biopsy material showed features of a small round blue cell tumor with destruction of bone (Figure 1A). The diagnostic suspicion of EFT was further supported by immunohistochemistry, which showed strong, crisp membranous staining for CD99 (Figure 1B).
Figure 1.
A: Representative histology of the tumor specimen showing an aggressive small round blue cell tumor infiltrating through bone (H&E; ×40 magnification). B: Immunohistochemical staining of the tumor cells for CD99 showing diffusely strong membranous positivity (×400 magnification).
Cytogenetic Analysis
The cultured open biopsy tumor tissue yielded a small number of analyzable metaphases, all of which revealed a clonal karyotype representing a stemline with an apparently balanced t(2;16)(q35;p11) (Figure 2) and an evolved sideline with additional copies of chromosomes 4, 16, and 21. Pursuant to the histological features of EFT, FISH analysis was undertaken with a commercial probe for the EWS gene and did not show evidence of rearrangement at this locus (data not shown). In light of the karyotypic breakpoint at 16p11, a commercial dual-color break-apart probe for FUS was used that revealed a break at this site in 95% of interphase nuclei in the cultured specimen as well as in tumor metaphases (Figure 3A). In view of the breakpoint at 2q35 and the known locus of the ETS family gene FEV, an “in house” dual-color break-apart probe for this gene was generated and applied to the tumor tissue, confirming a rearrangement at this site (data not shown). The 2p12 (SpectrumGreen) and FEV (SpectrumRed) probe combination also showed disruption of the FEV probe signal (Figure 3B).
Figure 2.
Representative metaphase from the tumor sideline showing the t(2;16)(q35;p11) (arrows) and extra copies of chromosomes 4, 16, and 21.
Figure 3.
Representative FISH images. A: A sideline metaphase with +16 hybridized with the break-apart probe for FUS showing two red-green fused signals on the two intact copies of FUS on two #16 chromosomes, a green signal on the der(2) and a red signal on the der(16). B: A stemline metaphase hybridized with a 2p12 control probe (RP11-270E5-green) and the FEV BAC probe (RP11-207M4-red) showing splitting of the FEV probe with signals on the der(16) and the der(2).
In-Frame Fusion of FUS and FEV
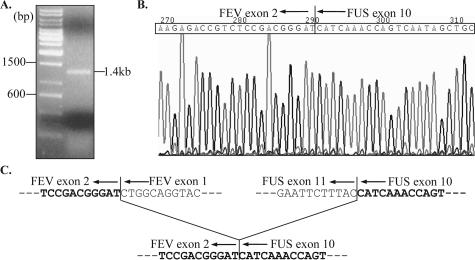
Figure 4A shows the 1.4-kb band of the RT-PCR amplification of the fusion transcript, corresponding to the predicted product size based on the primer design based on published sequences for the FUS and FEV cDNAs. Sequencing of this product showed a fusion of FUS exon 10 with FEV exon 2 (Figure 4B). The fusion is in-frame with disruption of both partner genes at exon-exon junctions (Figure 4C).
Figure 4.
Sequence analysis of the FUS-FEV fusion transcript. A: RT-PCR amplification product of the predicted 1.4-kb size. B: Sequence chromatogram of the fusion transcript at the breakpoint region (shown as the reverse complement sequence), illustrating the fusion of FUS (ending at the 3′-end of exon 10) to FEV (beginning at the 5′-end of exon 2). The fusion sequence is in-frame, as shown in (C).
Tissue Microarray FISH Analysis
Of the 168 pediatric solid tumor cases in the tissue microarray, 120 gave interpretable FISH results for FUS rearrangement, including 14 of 22 cases of EFT, and 142 cases were interpretable for FEV, including 16 of 22 cases of EFT. All were negative for both FUS and FEV rearrangements, including both cases of EFT that were negative on routine molecular diagnostic testing and all three cases of EFT where molecular diagnostic results were unknown.
Discussion
Although EFTs are most commonly characterized by the disease-specific t(11;22) EWS-FLI1 fusion, a growing list of variant ETS family genes may substitute for FLI1 in this translocation without altering the tumor phenotype. Variant translocations involving the TET family (EWS homologs) have also recently been described. These “promiscuous” molecular partnerships may have implications for the generation of false-negative results during diagnostic evaluation or when monitoring for minimal residual disease if the appropriate probes are not used. This situation is exemplified by the current case representing a novel translocation resulting in an in-frame fusion of FUS and FEV. Although both partner genes are separately involved in previously described EFT translocations, this new fusion combination confirms the notion that both partner genes in EFT translocations are interchangeable,17 highlighting the need to consider such permutations in the context of clinical molecular diagnostics.
Although the clinical, morphological, and immunophenotypic features of EFTs are often characteristic, the identification of a fusion translocation using cytogenetic and molecular techniques is frequently needed as confirmatory evidence, particularly for unusual morphological variants.3 Currently, the methods used in clinical practice include RT-PCR of specific fusion transcripts and FISH to detect disruption of the EWS gene. Although such methods are useful for the great majority of EFT cases because of the predominance of a small subset of fusion transcript variants, these methods would require modification to detect rare variants such as the one described in this report. Therefore, a negative result generated by such tests should not preclude the diagnosis of EFT in the context of typical morphological and immunophenotypic features. Rather, such cases highlight the continuing value of using classic karyotype analysis in sarcoma diagnosis because of the ability of cytogenetic analysis to interrogate the cancer genome for balanced chromosomal translocations. Combining knowledge of the variant participant gene families associated with EFT together with the accurate map location of genes in relation to chromosomal breakpoint sites provides the opportunity to identify novel EFT-specific rearrangements, as has been demonstrated with this case, which may be critical to the provision of an accurate diagnosis and appropriate clinical management.
Furthermore, this case highlights the importance of considering FUS as a fusion partner in the molecular diagnosis of EFT. Although this represents the only reported case of EFT showing involvement of FUS aside from the initial report of Shing et al,13 it is possible that the frequency of FUS rearrangement is under-reported because its possible involvement in EFT is not routinely questioned in most centers. This case reiterates, therefore, the need to investigate FUS involvement using FISH in suspected EFT cases where EWS rearrangement is not found.
The predicted structure of this FUS-FEV transcript is consistent with other EFT fusion transcripts, because the structure includes the required N-terminal serine-tyrosine-glutamine-glycine (SYQG) transactivation domain of FUS and the DNA-binding domain of FEV (Figure 5).12,18 However, it incorporates a greater number of FUS exons than previously reported for FUS-ETS gene fusions, with the breakpoint located within the RNA-recognition motif of FUS.17 As a result, there is inclusion of the majority of this domain in the fusion product. The presence of this domain has yet to be described in fusion transcripts of EFT involving FUS and is extremely rare in other malignancies with FUS fusion transcripts, with one reported case of myxoid liposarcoma in which the fusion transcript contained up to exon 13 of FUS.19 This alternative transcript structure again highlights the genotypic flexibility of EFT gene fusions. The inclusion of additional FUS sequences may confer alternative functional or clinical-pathological characteristics, but this has yet to be determined.
Figure 5.
Schematic representation of the structure of the TET family proteins EWS and FUS and the ETS family protein FEV (adapted from Janknechkt17), as well as the novel FUS-FEV fusion transcript described in this article. Exons are demarcated by dotted lines, with numeric annotations above denoting the exon number. Domains important in the function of the fusion transcript include the SYQG-rich transcriptional activating domain of the TET proteins and the DNA-binding domain of the ETS protein. Other domains include the RNA-recognition motifs, arginine-glycine-glycine-rich (RGG-rich) regions involved in nuclear import signaling, the zinc finger (Zn) nucleic acid-binding domain, and an alanine-rich (Ala-rich) region involved in transcriptional repression. Small arrowheads represent the most common breakpoint sites in EFT. Large arrowheads denote the breakpoints seen in the novel FUS-FEV transcript.
In conclusion, we have identified a novel translocation in a case of Ewing sarcoma resulting in an in-frame fusion of FUS to FEV, providing further evidence that both partner genes in EFT-associated fusion transcripts are interchangeable, and emphasizing this consideration for the purposes of diagnosis, genotype-phenotype correlation, and future investigations into the biology of this entity.
Acknowledgments
We thank Ms. K. Garbut and Ms. S. Clemens for the cytogenetic and EWS FISH analyses, Ms. C. Salski for technical assistance in the FUS and FEV FISH analyses, and Ms. H. Wildgrove for technical assistance with molecular analysis.
Footnotes
See related Commentary on page 437
T.L.N. is supported by the Royal College of Physicians and Surgeons of Canada Clinician Investigator Program.
T.L.N. and M.J.O. contributed equally to this work
References
- Grier HE. The Ewing family of tumors. Ewing’s sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am. 1997;44:991–1004. doi: 10.1016/s0031-3955(05)70541-1. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Kandel RA, Bell RS, Mathews RE, Ghazarian DM. Extraskeletal Ewing sarcoma in a 77-year-old woman. Arch Pathol Lab Med. 2001;125:1358–1360. doi: 10.5858/2001-125-1358-EESIAY. [DOI] [PubMed] [Google Scholar]
- Folpe AL, Goldblum JR, Rubin BP, Shehata BM, Liu W, Dei Tos AP, Weiss SW. Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol. 2005;29:1025–1033. [PubMed] [Google Scholar]
- O’Sullivan MJ, Perlman EJ, Furman J, Humphrey PA, Dehner LP, Pfeifer JD. Visceral primitive peripheral neuroectodermal tumors: a clinicopathologic and molecular study. Hum Pathol. 2001;32:1109–1115. doi: 10.1053/hupa.2001.28247. [DOI] [PubMed] [Google Scholar]
- Khoury JD. Ewing sarcoma family of tumors. Adv Anat Pathol. 2005;12:212–220. doi: 10.1097/01.pap.0000175114.55541.52. [DOI] [PubMed] [Google Scholar]
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, Aurias A, Thomas G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- de Alava E, Kawai A, Healey JH, Fligman I, Meyers PA, Huvos AG, Gerald WL, Jhanwar SC, Argani P, Antonescu CR, Pardo-Mindan FJ, Ginsberg J, Womer R, Lawlor ER, Wunder J, Andrulis I, Sorensen PH, Barr FG, Ladanyi M. EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing’s sarcoma. J Clin Oncol. 1998;16:1248–1255. doi: 10.1200/JCO.1998.16.4.1248. [DOI] [PubMed] [Google Scholar]
- Sorensen PH, Lessnick SL, Lopez-Terrada D, Liu XF, Triche TJ, Denny CT. A second Ewing’s sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet. 1994;6:146–151. doi: 10.1038/ng0294-146. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, de Alava E, Ladanyi M, Wexler LH, Kovar H, Paulussen M, Zoubek A, Dockhorn-Dworniczak B, Juergens H, Wunder JS, Andrulis IL, Malik R, Sorensen PH, Womer RB, Barr FG. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing’s sarcoma. J Clin Oncol. 1999;17:1809–1814. doi: 10.1200/JCO.1999.17.6.1809. [DOI] [PubMed] [Google Scholar]
- Jeon IS, Davis JN, Braun BS, Sublett JE, Roussel MF, Denny CT, Shapiro DN. A variant Ewing’s sarcoma translocation (7;22) fuses the EWS gene to the ETS gene ETV1. Oncogene. 1995;10:1229–1234. [PubMed] [Google Scholar]
- Urano F, Umezawa A, Yabe H, Hong W, Yoshida K, Fujinaga K, Hata J. Molecular analysis of Ewing’s sarcoma: another fusion gene, EWS-E1AF, available for diagnosis. Jpn J Cancer Res. 1998;89:703–711. doi: 10.1111/j.1349-7006.1998.tb03274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Couturier J, Pacquement H, Michon J, Thomas G, Magdelenat H, Delattre O. A new member of the ETS family fused to EWS in Ewing tumors. Oncogene. 1997;14:1159–1164. doi: 10.1038/sj.onc.1200933. [DOI] [PubMed] [Google Scholar]
- Shing DC, McMullan DJ, Roberts P, Smith K, Chin SF, Nicholson J, Tillman RM, Ramani P, Cullinane C, Coleman N. FUS/ERG gene fusions in Ewing’s tumors. Cancer Res. 2003;63:4568–4576. [PubMed] [Google Scholar]
- Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993;363:640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- ISCN An International System for Human Cytogenetic Nomenclature. Miteliman FE, editor. Basel: S. Karger; 1995 [Google Scholar]
- Hsu FD, Nielsen TO, Alkushi A, Dupuis B, Huntsman D, Liu CL, van de Rijn M, Gilks CB. Tissue microarrays are an effective quality assurance tool for diagnostic immunohistochemistry. Mod Pathol. 2002;15:1374–1380. doi: 10.1097/01.MP.0000039571.02827.CE. [DOI] [PubMed] [Google Scholar]
- Janknecht R. EWS-ETS oncoproteins: the linchpins of Ewing tumors. Gene. 2005;363:1–14. doi: 10.1016/j.gene.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Rabbitts TH, Forster A, Larson R, Nathan P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat Genet. 1993;4:175–180. doi: 10.1038/ng0693-175. [DOI] [PubMed] [Google Scholar]
- Panagopoulos I, Mertens F, Isaksson M, Mandahl N. A novel FUS/CHOP chimera in myxoid liposarcoma. Biochem Biophys Res Commun. 2000;279:838–845. doi: 10.1006/bbrc.2000.4026. [DOI] [PubMed] [Google Scholar]