Abstract
The aim of this study was to determine the frequency of microsatellite instability (MSI+) in tumors from a population-based series of young colorectal cancer patients and its correlation with the loss of expression of mismatch repair (MMR) proteins. The BAT-26 mononucleotide repeat was used to screen for MSI+ in all colorectal cancers diagnosed in Western Australia throughout a 5-year period in patients <60 years of age. MSI+ was found in 75 of 1003 (7.5%) cases, of which six contained a concomitant mutation in BRAF and were therefore excluded from further investigations as possible hereditary nonpolyposis colorectal cancer. Immunohistochemistry was used to evaluate expression of the four major MMR proteins (MLH1, MSH2, MSH6, and PMS2) in the remaining 69 MSI+ tumors. Complete loss of MLH1 and PMS2 expression or of MSH2 and MSH6 expression was found in 35 (51%) and 17 (25%) cases, respectively, whereas other patterns of complete loss were observed in eight cases (12%). Eight tumors (12%) were initially recorded as showing normal expression, but on review seven were reclassified as having abnormal staining because of heterogeneous patterns of MMR loss. Three of these seven cases had previously been found to have germline mutations. Because of possible misinterpretation of heterogeneous immunohistochemistry staining for MMR protein loss, MSI testing is recommended as the initial screen for population-based detection of hereditary nonpolyposis colorectal cancer.
The microsatellite instability (MSI+) phenotype in tumor DNA, also referred to as MSI-high, is a consequence of defects in the DNA mismatch repair system.1 MSI+ occurs in ∼10% of sporadic colorectal carcinomas (CRCs) and in almost all tumors associated with hereditary nonpolyposis colorectal cancer (HNPCC; Lynch syndrome). The majority of sporadic MSI+ CRCs arise in the proximal colon of older patients and are associated with acquired, methylation-induced transcriptional silencing of MLH1 gene expression.2 Germline mutations in MLH1 and MSH2 and to a lesser extent MSH6 and PMS2 account for almost all MSI+ CRCs associated with the HNPCC syndrome.3
The identification of potential inherited mismatch repair (MMR) gene mutation carriers is currently the major clinical application for MSI screening. Accurate diagnosis of this syndrome is of great importance for family risk management because regular colonoscopy has been demonstrated to improve the survival of mutation carriers.4 There is widespread concern, however, that most mutation carriers in the population are not being identified.5,6 Several reasons are likely to account for this, including the failure of clinicians to carefully document family cancer histories and to refer patients for genetic evaluation. For patients who are referred to family cancer clinics, tumors are usually tested for MSI in the first instance using polymerase chain reaction (PCR)-based methods. MSI testing is not available, however, in the large majority of routine pathology service laboratories, and these rely on immunohistochemistry (IHC) technique to detect loss of MMR protein expression as a surrogate marker for the presence of MSI.
Whether or not all or clinicopathological subsets of CRCs should be routinely tested for MMR defects to assist with the detection of HNPCC is still a matter of conjecture. There is also considerable debate as to whether MSI or IHC is the technically superior screening approach.7,8,9,10,11,12,13 With the aim of improving the rate of HNPCC detection in the state of Western Australia, our laboratory is conducting a population-wide study that uses MSI as the prescreen test followed by IHC for positive cases. All CRC patients <60 years of age and diagnosed in this state throughout a 5-year period were tested for MSI. The coexistence of a BRAF oncogene mutation in tumors found to be MSI+ was used as a criterion to exclude cases that were sporadic in origin.14 The remaining MSI+/wild-type BRAF cases were further evaluated for loss of MMR protein expression and subsequent germline testing for consenting individuals.
Materials and Methods
Patients and Specimens
All CRC cases diagnosed in the state of Western Australia from 2000 to 2006 inclusive were identified by electronic searches of the public and private pathology service provider databases. Only patients <60 years of age at diagnosis were selected for the study. This age cutoff was chosen as a compromise between the feasibility of screening for MSI+ in large sample numbers while at the same time maximizing the capture of HNPCC cases and reducing sporadic cases. An additional 24 CRC patients <30 years of age and diagnosed in Western Australia during the period from 1993 to 1999 were also included in the study as potential high-risk cases for HNPCC. Archival tissue blocks obtained from surgical resection or biopsies were selected to contain maximal tumor content. All cases were screened for MSI and BRAF mutation. No information on family history of cancer was available at the time of MSI+ screening or IHC. Ethics approval for the project was obtained from the Human Research Ethics Committees of each hospital and from the Confidentiality of Health Information Committee. The ethical issues involved in the phenotypic screening (MSI and IHC) of archival tumor tissues without patient consent are described elsewhere.15
MSI and BRAF Mutation Screening
The MSI status for all tumors was determined using fluorescent-single strand conformation polymorphism (F-SSCP) to detect deletions in the BAT-26 mononucleotide repeat as described previously.16 F-SSCP was also used to screen for the common V600E point mutation in the BRAF oncogene.17 Several 10-μm sections cut from formalin-fixed, paraffin-embedded tumor blocks were digested at 50°C with proteinase K for at least 48 hours before heat inactivation (95°C, 10 minutes) of the enzyme. Primer sequences and PCR conditions for the amplification of BAT-26 and BRAF were the same as described previously.16,17
Immunohistochemical Staining for MLH1, PMS2, MSH2, and MSH6
IHC was performed on tumors from 2000 to 2004 found to be MSI+ and BRAF wild type and on all tumors diagnosed in 2005 or 2006. Tissue sections of 4-μm thickness were cut from the same tumor blocks used for MSI+ screening and placed onto silane-coated slides. After dewaxing and rehydration, they were stained for MLH1, PMS2, MSH2, and MSH6 using commercially available antibodies (clones G168-15, A16-4, G219-1129 and 44, respectively; BD PharMingen) at the recommended dilutions and using standard IHC methods. Antigen retrieval was performed using Target retrieval solution (DAKO, Botany, NSW, Australia) and a Decloaker pressure cooker (BioCare Medical, Stafford, QLD, Australia) at 121°C for 20 minutes. The detection system used was the Mach3 kit (BioCare Medical) as recommended by the supplier. Lymphocytes and normal colonic epithelium located adjacent to tumor cells served as internal controls for positive MMR protein expression. Cases were initially scored by a pathologist (J.H.) as positive for expression (MMR normal) if nuclear staining was present in any of the malignant cells. Cases were initially scored as negative for expression (loss of MMR expression) if all tumor cells showed complete loss of staining while the adjacent normal cells showed nuclear staining.
Results
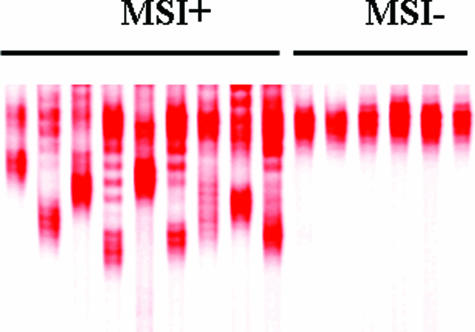
A total of 1059 tumors from CRC patients <60 years of age and diagnosed from 2000 to 2004 were screened for MSI+ using the BAT-26 marker alone, of which 1003 gave a result. Representative F-SSCP results are shown in Figure 1. Deletion of the BAT-26 allele was found in 75 of 1003 (7.5%) cases. The frequency of MSI+ in different age groups was 22.2% (≤29 years), 14.7% (30 to 39 years), 10.6% (40 to 49 years), and 4.4% (50 to 59 years). Mutations in BRAF (V600E) were found in 6 of 75 (8%) of the MSI+ tumors, and all were in patients 54 to 59 years of age. Based on previous observations,14 these were excluded from additional follow-up as possible HNPCC. The remaining 69 MSI+/BRAF wild-type tumors were further investigated by IHC for expression of the major MMR proteins. All cases showed appropriate positive staining of normal colonic epithelial cells and lymphocytes with all four antisera tested. In MSI+ tumors, loss of MLH1 expression usually occurs in conjunction with PMS2 loss, whereas MSH2 loss is usually accompanied by MSH6 loss. These common patterns of loss were observed in 35 (51%) and 17 (25%) cases, respectively (Table 1). Tumors in this category showed complete loss of staining for the two relevant proteins in all malignant cells. Another eight tumors showed complete loss of expression for MSH6 alone (three cases); PMS2 alone (two cases); MSH2 alone (one case); PMS2 and MSH6 (one case); or MLH1, PMS2, and MSH6 (one case). There were insufficient tumor cells in one case to allow accurate evaluation of staining. The remaining eight tumors were initially assessed as showing normal expression for all four MMR proteins, although a degree of staining heterogeneity was noted in some cases.
Figure 1.
Representative F-SSCP gel showing deletions in the BAT-26 mononucleotide repeat indicative of the MSI+ phenotype. Samples on the left are cases in which earlier detection of MSI+ status was independently confirmed by separate PCR and SSCP gel run.
Table 1.
Patterns of MMR Protein Expression in 69 MSI+ Tumors from Young CRC Patients
| Pattern of loss (n)* | MLH1† | PMS2 | MSH2 | MSH6 |
|---|---|---|---|---|
| Common (52) | ||||
| 35 | − | − | + | + |
| 17 | + | + | − | − |
| Other (8) | ||||
| 3 | + | + | + | − |
| 2 | + | − | + | + |
| 1 | + | − | + | − |
| 1 | + | + | − | H |
| 1 | − | − | + | − |
| Heterogeneous (7) | ||||
| 2 | + | + | H | H |
| 2 | H | H | + | + |
| 1 | + | H | + | + |
| 1 | U | H | + | + |
| 1 | + | + | + | H |
| No loss (1) | ||||
| 1 | + | + | + | + |
One case contained insufficient tumor tissue for proper evaluation by IHC.
−, loss of expression in all tumor cells; +, expression in all tumor cells; H, heterogeneous loss of expression in tumor cells; U, unsatisfactory IHC result.
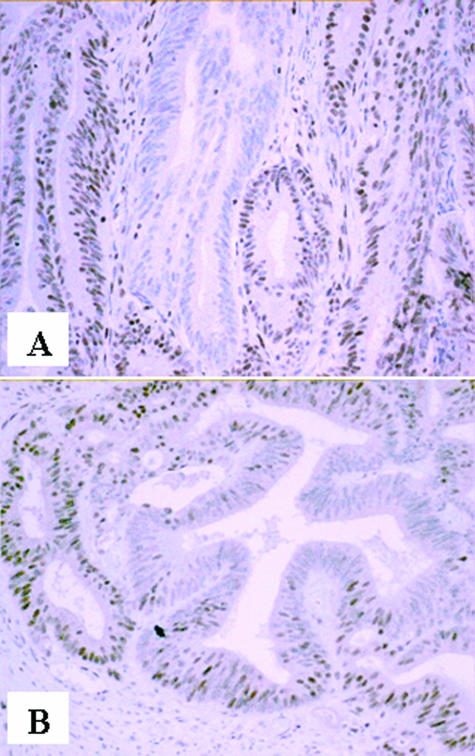
The single Familial Cancer Program serving the entire state of Western Australia (population, 2 million) was notified of the 69 young CRC patients (<60 years) with MSI+/BRAF wild-type tumors identified in this study. Eighteen (26%) were already known to this service as germline MMR gene mutation carriers (12 MSH2, five MLH1, one MSH6) in either the proband (n = 13) or members of their immediate family (n = 5). Three mutation carriers were among the eight tumors initially reported as showing normal MMR protein expression: one case each for MLH, MSH2, and MSH6. This prompted additional review of all eight MSI+ cases showing apparently normal expression. Heterogeneous staining patterns were observed for seven cases and included examples from each of the four MMR proteins (Table 2 and Figure 2). Two patterns of staining heterogeneity were observed. In two of the tumors there were confluent areas of staining loss involving multiple adjacent gland profiles (zonal loss; Figure 2A), whereas five tumors showed intraglandular variation in staining with strongly immunoreactive cells admixed with unstained cells (focal loss; Figure 2B). In some cases, the neoplastic cells toward the periphery of tumor aggregates or the advancing tumor margin appeared more consistently immunoreactive, whereas central glands were often negative. However, there was no clear correlation between the distribution of staining and morphological features associated with the MSI phenotype such as mucinous differentiation or presence of tumor-infiltrating lymphocytes. The remaining case was a biopsy sample of an in situ tumor that showed apparently normal expression of all four MMR proteins. This case had previously been diagnosed at 18 years of age with a small bowel tumor, and his family has a known MSH2 germline mutation.
Table 2.
Heterogeneous Staining Patterns for MMR Proteins in MSI+ CRC
| Case | MLH1 | PMS2 | MSH2 | MSH6 |
|---|---|---|---|---|
| 1079* | Moderate intensity in 25% cells, heterogeneity within glands | Moderate intensity in 25% cells, heterogeneity within glands | Strong uniform staining | Variable staining intensity |
| 909† | Strong uniform staining | Strong uniform staining | Variable staining intensity with areas of focal loss | Focal areas with loss of staining |
| 308* | Strong uniform staining | Strong uniform staining | Mostly strongly positive, <10% of glands showed heterogeneity | Mostly strongly positive, <10% of glands showed heterogeneity |
| 500‡ | Mostly strongly positive | Strong uniform staining | Heterogeneous staining both zonal and within individual glands | Heterogeneous staining both zonal and within individual glands |
| 857§ | Weak nuclear signal in ∼25% of cells, heterogeneity within glands | Weak-moderate signal in ∼25% of cells, heterogeneity within glands | Strong uniform staining | Strong uniform staining |
| 727¶ | Focal areas of reduced intensity in <1% of glands | Complete loss of staining in ∼10% of glands; heterogeneity in <10% | Strong uniform staining | Focal areas of complete loss in <1% of glands |
| 184∥ | Unsatisfactory stain | Areas of focal loss | Strong uniform staining | Strong uniform staining |
Patient lost to follow-up.
Known MSH6 germline mutation.
Known MSH2 germline mutation.
Known MLH1 germline mutation.
Germline mutation test result pending.
Patient deceased before blood sample could be obtained for testing.
Figure 2.
Heterogeneous IHC staining patterns for MSI+ CRC. A: Zonal loss for PMS2. B: Intraglandular heterogeneity for MSH6.
A second CRC cohort from <60-year-old patients who were diagnosed in 2005 or 2006 (n = 208) was evaluated simultaneously for MSI and IHC to evaluate the degree of concordance between the two techniques. Both analyses were performed blinded to the results of the other test. Using BAT-26 alone, 20 of 208 (9.6%) tumors were found to be MSI+ (Table 3). The same number of tumors showed loss of MMR expression using the criteria of complete loss of expression in tumor cells in the presence of staining of adjacent nonmalignant cells. Discordant results were observed for only 2 of 208 (1%) cases. These comprised one MSI− tumor that showed complete loss of MSH6 expression and one MSI+ tumor with clonal loss of both MSH2 and MSH6. The latter case can therefore be considered equivalent to the seven MSI+ tumors in the previous cohort (2000 to 2004 diagnosis period) that showed heterogeneous staining patterns. Importantly, among the remaining 187 MSI− tumors that did not show complete loss of expression, 140 (75%) displayed heterogeneous or clonal patterns of loss for at least one of the four MMR proteins.
Table 3.
Concordance between MSI and IHC in CRC from Young Patients (<60 Years)
Defined as any expression for all four MMR proteins. Includes cases showing weak, clonal, or heterogeneous staining, or variable staining intensity.
Clonal losses of both MSH2 and MSH6.
Defined as complete loss of at least one MMR protein.
Complete loss of MSH6 expression only.
Discussion
Most estimates of the proportion of CRCs that are attributable to HNPCC range from 1 to 3%18,19,20,21,22,23 with frequencies in unselected patients <50 years of age and <45 years of age estimated at 14 and 17%, respectively.12,21 Identification of pathogenic mutations in MMR genes provides a definitive genetic test that can assist with the management of affected families. Because mutation screening is expensive, clinical guidelines based mainly on age and family history of cancer have been proposed to help with the prioritization of individuals for genetic testing (Amsterdam and Bethesda criteria). Although this approach to the detection of HNPCC has relatively good specificity, it suffers from poor sensitivity with the result that most MMR gene mutations in the community remain undiagnosed. The reasons for this have been outlined elsewhere.5,6
With the aim of increasing the detection rate for HNPCC in the Western Australian population, our group is trying the use of MSI as the initial test to select young CRC patients that are to be offered MMR genetic testing. During the course of this work, it became apparent that a relatively high incidence of errors can occur in the interpretation of IHC results for MMR protein expression because of heterogeneity of staining patterns. This presents as a major difficulty for population-based HNPCC detection programs that use IHC as the initial screening test in the absence of supporting data from MSI testing. Because routine pathology laboratories are at the front line of population-based screening for HNPCC and are almost totally reliant on the IHC technique, we propose that serious consideration be given to alternative strategies that are based on MSI analysis.
In a process that was totally blinded to both family history and germline mutation status, more than 1000 consecutive CRCs from relatively young patients (<60 years) were evaluated for MSI+. Approximately 7.5% tested positive and, because of their young age, were therefore considered strong candidates for HNPCC. BRAF mutation testing was useful for excluding a small proportion of these MSI+ cases (6 of 75, 8%) as being acquired rather than hereditary in origin.14 The presence of BRAF mutation serves as a surrogate marker for methylation of the MLH1 promoter. All six MSI+ patients with BRAF mutation were 54 to 59 years of age, suggesting this marker is unlikely to be of value for the exclusion of sporadic MSI+ cases in patients below this age group. Patients <60 years of age comprise ∼25% of nonselected CRC cohorts, implying that ∼1.8% of all CRCs fall into the high-risk category for HNPCC as defined by the criteria of young age, MSI+, and wild-type BRAF. Although the germline mutation status is not yet known for all of the high-risk cases, this value is within the range of 1 to 3% cited above for the frequency of HNPCC observed in other population-based studies.18,19,20,21,22,23 Only 18 of 69 (26%) of the high-risk cases identified in the present study were previously known as germline mutation carriers to the single referral center for HNPCC in Western Australia. Screening for germline mutations in 24 patients who have so far been contacted and given consent is still ongoing. To date (March, 2007), six new germline mutations have been identified in previously unknown HNPCC families (three MLH1, one MSH2, one MSH6, and one PMS2).
Approximately 76% of the high-risk (MSI+) cases showed complete loss of expression for the common MLH1/PMS2 or MSH2/MSH6 combinations and a further 12% showed complete loss of a single protein or rarer double or triple protein loss combinations (Table 1). These patterns of loss have been described by other groups and are typical of MSI+ tumors from HNPCC cases.12,23,24,25 The loss of MSH6 alone has previously been associated with the MSI-low phenotype.12 Four cases of MSH6 loss alone were found in the present study including one that showed heterogeneous staining. These occurred in MSI+ tumors detected using BAT-26 alone, suggesting that the use of this marker will allow the detection of at least some MSH6 germline mutations. The two cases with rare combinations of MMR protein loss (PMS2/MSH6 and MLH1/PMS2/MSH6) cannot be readily explained. They may represent a combination of germline and somatic genetic changes, or alternately, they could reflect technical difficulties with IHC in these cases.
The major finding of the present study is that ∼10% of MSI+ tumors in young CRC patients displayed atypical staining patterns characterized by heterogeneous expression of MMR proteins (Figure 2 and Table 2). These cases were initially considered to show normal staining because a proportion of the tumor cells were immunoreactive. The fact that such tumors were MSI+ and that at least three of seven (42%) cases were also later shown to contain a germline mutation demonstrated an incorrect interpretation of the IHC findings. These observations confirm several previous reports of normal IHC expression in tumors with germline MMR mutations, although two of these studies investigated MLH1 and MSH2 expression only,10,26 whereas the third investigated MLH1, MSH2, and MSH6 expression only.11 Evaluation of all four major MMR proteins may have resulted in stronger concordance between the IHC result and germline mutation status in these studies. However, our finding of examples of heterogeneous staining for each of the four MMR proteins (Tables 1and 2) suggests that false negatives would occur even with the use of a wider panel of markers. Germline mutation data were not available for four of the seven MSI+ cases with heterogeneous staining in the present study. Further work is required to determine the frequency and causes of heterogeneous staining in MSI+ tumors from patients with germline mutations.
Most screeners have adopted the position that any nuclear staining of MMR proteins in tumor cells represents normal expression, whereas loss of expression is recorded only when nuclear staining is absent in all malignant cells.8,27,28,29,30,31 In light of the present results and previous reports,10,11,26 this is clearly a flawed definition and in the absence of information on MSI status could easily lead to an incorrect diagnosis for a significant minority of individuals at high risk for HNPCC. Furthermore, the risk of misinterpretation is likely to be greater if IHC assessment is based on a small biopsy specimen where the heterogeneous staining pattern may be less apparent. These issues are of particular concern for population-based screening in which IHC is performed by routine pathology laboratories without the benefit of parallel MSI testing. Similar concerns about the accuracy of IHC when used as a routine first screen for HNPCC detection in the absence of MSI data have been reported by others.7,11,26,32 Other well-known limitations of IHC include the lack of standardization in regard to fixation and staining protocols.
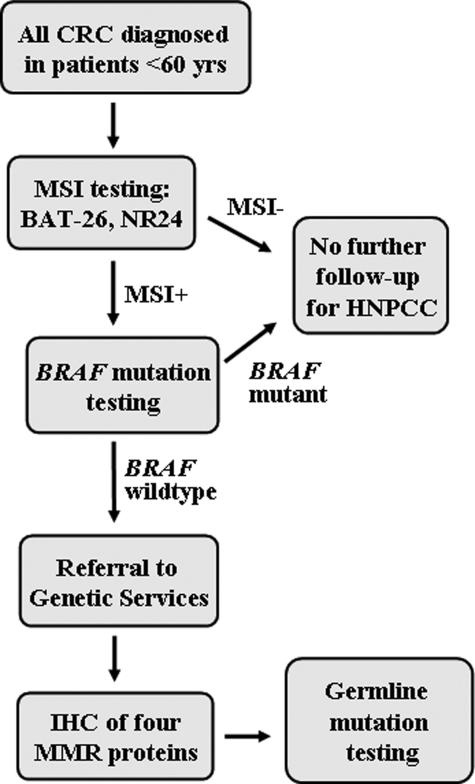
We propose an alternate strategy for population-based HNPCC screening that uses MSI as the initial test for all CRC patients <60 years of age, regardless of family history (Figure 3). Pathology laboratories would be required to send sections of routinely processed tumor tissue to a specialist molecular pathology laboratory for MSI testing rather than perform in-house IHC testing. IHC would only be performed on the relatively small proportion (7%) of cases subsequently found to be MSI+ and to have wild-type BRAF. The first advantage of this approach is that it bypasses the requirement to accurately validate the family history. Even using the guidelines set out by the Amsterdam criteria, several population-based studies have shown that only 50% or less of MMR mutations are detected.12,19,33 The second advantage is that MSI results are far more clear-cut than IHC with no subjective assessment involved (compare Figures 1and 2). A very low incidence (3 of 1059, 0.3%) of BAT-26 polymorphism was encountered in the present study of a mainly Caucasian population and manifested as a slight shift in the wild-type banding pattern on F-SSCP gels. These were readily discounted as polymorphisms rather than somatic deletions by running the patients’ germline DNA side-by-side with the tumor DNA. A third advantage is that BRAF mutation testing could be performed by the same specialist molecular pathology laboratory on the small percentage of cases found to be MSI+, although the present results indicate this is probably only worthwhile for patients 50 to 60 years of age. Finally, MSI testing is likely to be more cost-effective for population-based screening than IHC performed on the four major MMR proteins. The method is amenable to high-throughput analysis, and because there is no clinical requirement for a rapid test result, samples can be batched for even greater cost-effectiveness.
Figure 3.
Proposed flowchart for the population-based detection of HNPCC based on routine screening for MSI as the first test.
We observed a very strong concordance (99%) between results from MSI and IHC testing (Table 3). To achieve this, analysis of four MMR proteins was required by IHC, and the criterion of complete loss of staining for at least one MMR protein was used to score a tumor as being a possible HNPCC case. A high proportion (75%) of MSI− tumors showed heterogeneity of staining for one or more MMR proteins. In light of the findings from this study, all such cases would have required additional investigation by MSI to exclude the possibility of false-negative reporting. Primarily for this reason, we believe that MSI testing is superior to IHC as the prescreen for population-based detection of HNPCC.
The major caveat to the above MSI-based approach is that the sensitivity for population-based detection of HNPCC using MSI remains to be determined. In the present study using BAT-26 alone, the frequency of MSI+ observed for patients <45 years of age was 15.5% (31 of 200). Although these 31 cases may not all be found to carry germline MMR gene mutations, it is interesting to note that a recent study of 105 unselected CRC patients <45 years of age reported a germline mutation frequency of 17.1% (18 of 105).12 Furthermore, a recent study of 262 CRCs reported that 27 of 28 (96.4%) cases with loss of MMR protein expression also showed deletions of BAT-26.34 The one case not detected by this marker showed loss of PMS2 alone. A large Spanish study recently found that simultaneous assessment of BAT-26 and another mononucleotide repeat marker, NR24, resulted in 96% sensitivity for the detection of loss of MMR protein expression.35 Finally, in the present study we found a very high concordance (206 of 208, 99%) between MSI+ status determined by BAT-26 alone and complete loss of MMR expression (Table 3). Only 1 of 208 (0.5%) tumors showed complete loss of expression of an MMR protein (MSH6) in the absence of MSI+ (Table 3). Such rare cases may be detected by additional screening with the NR24 marker.
In summary, heterogeneous IHC staining for MMR proteins can lead to incorrect interpretation of the results and thereby reduce the sensitivity for detection of HNPCC in population studies. This is likely to be a major limitation for routine pathology laboratories that perform IHC in the absence of corroborating data from MSI testing. If IHC alone is used for screening, it cannot be assumed that focal, heterogeneous, or weak staining patterns reliably exclude HNPCC. Unless these concerns can be adequately addressed, our recommendation is that population-based screening programs for the detection of HNPCC strongly consider using MSI as the initial test.
Acknowledgments
We thank Mike Walsh for advice with the immunohistochemical protocols and the West Australian pathology laboratories for their assistance with this project.
Footnotes
Supported by the National Health and Medical Research Council of Australia (grant 353552) and the Royal Australasian College of Surgeons (surgeon-scientist fellowship to M.M.).
References
- Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RJ, Farrington SM, Dunlop MG, Campbell H. Mismatch repair genes hMLH1 and hMSH2 and colorectal cancer: a HuGE review. Am J Epidemiol. 2002;156:885–902. doi: 10.1093/aje/kwf139. [DOI] [PubMed] [Google Scholar]
- Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomaki P, De La Chapelle A, Mecklin JP. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Riley BD, Weissman SM, Coronel SM, Kinarsky Y, Lynch JF, Shaw TG, Rubinstein WS. Hereditary nonpolyposis colorectal carcinoma (HNPCC) and HNPCC-like families: problems in diagnosis, surveillance, and management. Cancer. 2004;100:53–64. doi: 10.1002/cncr.11912. [DOI] [PubMed] [Google Scholar]
- Terdiman J. It is time to get serious about diagnosing Lynch syndrome (hereditary nonpolyposis colorectal cancer) with defective DNA mismatch repair in the general population. Gastroenterology. 2005;129:741–744. doi: 10.1016/j.gastro.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Chapusot C, Martin L, Puig PL, Ponnelle T, Cheynel N, Bouvier AM, Rageot D, Roignot P, Rat P, Faivre J, Piard F. What is the best way to assess microsatellite instability status in colorectal cancer? Study on a population base of 462 colorectal cancers. Am J Surg Pathol. 2004;28:1553–1559. doi: 10.1097/00000478-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Ward RL, Turner J, Williams R, Pekarsky B, Packham D, Velickovic M, Meagher A, O’Connor T, Hawkins NJ. Routine testing for mismatch repair deficiency in sporadic colorectal cancer is justified. J Pathol. 2005;207:377–384. doi: 10.1002/path.1851. [DOI] [PubMed] [Google Scholar]
- Jass JR. Re: Ward et al. Routine testing for mismatch repair deficiency in sporadic colorectal cancer is justified (letter to the editor). J Pathol 2005, 207:377–384. J Pathol. 2006;208:590–591. doi: 10.1002/path.1933. [DOI] [PubMed] [Google Scholar]
- Mangold E, Pagenstecher C, Friedl W, Fischer HP, Merkelbach-Bruse S, Ohlendorf M, Friedrichs N, Aretz S, Buettner R, Propping P, Mathiak M. Tumors from MSH2 mutation carriers show loss of MSH2 expression but many tumors from MLH1 mutation carriers exhibit weak positive MLH1 staining. J Pathol. 2005;207:385–395. doi: 10.1002/path.1858. [DOI] [PubMed] [Google Scholar]
- Shia J, Klimstra DS, Nafa K, Offit K, Guillem JG, Markowitz AJ, Gerald WL, Ellis NA. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germ-line mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005;29:96–104. doi: 10.1097/01.pas.0000146009.85309.3b. [DOI] [PubMed] [Google Scholar]
- Southey MC, Jenkins MA, Mead L, Whitty J, Trivett M, Tesoriero AA, Smith LD, Jennings K, Grubb G, Royce SG, Walsh MD, Barker MA, Young JP, Jass JR, St. John DJ, Macrae FA, Giles GG, Hopper JL. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol. 2005;23:6524–6532. doi: 10.1200/JCO.2005.04.671. [DOI] [PubMed] [Google Scholar]
- Evans GD, Lalloo F, Mak T, Speake D, Hill J. Is it time to abandon microsatellite instability as a pre-screen for selecting families for mutation testing for mismatch repair genes? J Clin Oncol. 2006;24:1960–1962. doi: 10.1200/JCO.2005.05.3207. [DOI] [PubMed] [Google Scholar]
- Deng G, Bell I, Crawley S, Gum J, Terdiman JP, Allen BA, Truta B, Sleisenger MH, Kim YS. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004;10:191–195. doi: 10.1158/1078-0432.ccr-1118-3. [DOI] [PubMed] [Google Scholar]
- Zeps N, Iacopetta B, Schofield L, George J, Goldblatt J. Waiver of individual patient consent in research: when do potential benefits to the community outweigh private rights? Med J Aust. 2007;186:88–90. doi: 10.5694/j.1326-5377.2007.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Iacopetta B, Grieu F. Routine detection of the replication error phenotype in clinical tumor specimens using fluorescence-SSCP. Biotechniques. 2000;28:566–570. doi: 10.2144/00283cr02. [DOI] [PubMed] [Google Scholar]
- Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer. 2006;5:2. doi: 10.1186/1476-4598-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MP, Pedroni M, Benatti P, Percesepe A, Di Gregorio C, Foroni M, Rossi G, Genuardi M, Neri G, Leonardi F, Viel A, Capozzi E, Boiocchi M, Roncucci L. Hereditary colorectal cancer in the general population: from cancer registration to molecular diagnosis. Gut. 1999;45:32–38. doi: 10.1136/gut.45.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salovaara R, Loukola A, Kristo P, Kaariainen H, Ahtola H, Eskelinen M, Harkonen N, Julkunen R, Kangas E, Ojala S, Tulikoura J, Valkamo E, Jarvinen H, Mecklin JP, Aaltonen LA, de la Chapelle A. Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2000;18:2193–2200. doi: 10.1200/JCO.2000.18.11.2193. [DOI] [PubMed] [Google Scholar]
- Samowitz WS, Curtin K, Lin HH, Robertson MA, Schaffer D, Nichols M, Gruenthal K, Leppert MF, Slattery ML. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology. 2001;121:830–838. doi: 10.1053/gast.2001.27996. [DOI] [PubMed] [Google Scholar]
- Katballe N, Christensen M, Wikman FP, Orntoft TF, Laurberg S. Frequency of hereditary non-polyposis colorectal cancer in Danish colorectal cancer patients. Gut. 2002;50:43–51. doi: 10.1136/gut.50.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JM, Kim CY, Christensen ER, Tester DJ, Parc Y, Burgart LJ, Halling KC, McDonnell SK, Schaid DJ, Walsh Vockley C, Kubly V, Nelson H, Michels VV, Thibodeau SN. The frequency of hereditary defective mismatch repair in a prospective series of unselected colorectal carcinomas. Am J Hum Genet. 2001;69:780–790. doi: 10.1086/323658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- Ruszkiewicz A, Bennett G, Moore J, Manavis J, Rudzki B, Shen L, Suthers G. Correlation of mismatch repair genes immunohistochemistry and microsatellite instability status in HNPCC-associated tumors. Pathology. 2002;34:541–547. doi: 10.1080/0031302021000035965-2. [DOI] [PubMed] [Google Scholar]
- Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, Jass JR, Hopper J, Gallinger S, Bapat B, Redston M, Thibodeau SN. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- Wahlberg SS, Schmeits J, Thomas G, Loda M, Garber J, Syngal S, Kolodner RD, Fox E. Evaluation of microsatellite instability and immunohistochemistry for the prediction of germ-line MSH2 and MLH1 mutations in hereditary nonpolyposis colon cancer families. Cancer Res. 2002;62:3485–3492. [PubMed] [Google Scholar]
- Marcus VA, Madlensky L, Gryfe R, Kim H, So K, Millar A, Temple LK, Hsieh E, Hiruki T, Narod S, Bapat BV, Gallinger S, Redston M. Immunohistochemistry for hMLH1 and hMSH2: a practical test for DNA mismatch repair-deficient tumors. Am J Surg Pathol. 1999;23:1248–1255. doi: 10.1097/00000478-199910000-00010. [DOI] [PubMed] [Google Scholar]
- Stahl J. Mismatch repair proteins and microsatellites hit clinical practice. Adv Anat Pathol. 2000;7:85–93. doi: 10.1097/00125480-200007020-00003. [DOI] [PubMed] [Google Scholar]
- Rigau V, Sebbagh N, Olschwang S, Paraf F, Mourra N, Parc Y, Flejou JF. Microsatellite instability in colorectal carcinoma. The comparison of immunohistochemistry and molecular biology suggests a role for hMSH6 [correction of hMLH6] immunostaining. Arch Pathol Lab Med. 2003;127:694–700. doi: 10.5858/2003-127-694-MIICC. [DOI] [PubMed] [Google Scholar]
- Wright CL, Stewart ID. Histopathology and mismatch repair status of 458 consecutive colorectal carcinomas. Am J Surg Pathol. 2003;27:1393–1406. doi: 10.1097/00000478-200311000-00001. [DOI] [PubMed] [Google Scholar]
- Jover R, Paya A, Alenda C, Poveda MJ, Peiro G, Aranda FI, Perez-Mateo M. Defective mismatch-repair colorectal cancer: clinicopathologic characteristics and usefulness of immunohistochemical analysis for diagnosis. Am J Clin Pathol. 2004;122:389–394. doi: 10.1309/V9PG-K2Y2-60VF-VULR. [DOI] [PubMed] [Google Scholar]
- Bacher JW, Flanagan LA, Smalley RL, Nassif NA, Burgart LJ, Halberg RB, Megid WM, Thibodeau SN. Development of a fluorescent multiplex assay for detection of MSI-high tumors. Dis Markers. 2004;20:237–250. doi: 10.1155/2004/136734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomaki P, Chadwick RB, Kaariainen H, Eskelinen M, Jarvinen H, Mecklin JP, de la Chapelle A. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- Hatch SB, Lightfoot HM, Jr, Garwacki CP, Moore DT, Calvo BF, Woosley JT, Sciarrotta J, Funkhouser WK, Farber RA. Microsatellite instability testing in colorectal carcinoma: choice of markers affects sensitivity of detection of mismatch repair-deficient tumors. Clin Cancer Res. 2005;11:2180–2187. doi: 10.1158/1078-0432.CCR-04-0234. [DOI] [PubMed] [Google Scholar]
- Xicola RM, Llor X, Pons E, Castells A, Alenda C, Pinol V, Andreu M, Castellvi-Bel S, Paya A, Jover R, Bessa X, Giros A, Duque JM, Nicolas-Perez D, Garcia AM, Rigau J, Gassull MA, Gastrointestinal Oncology Group of the Spanish Gastroenterological Association Performance of different microsatellite marker panels for detection of mismatch repair-deficient colorectal tumors. J Natl Cancer Inst. 2007;99:244–252. doi: 10.1093/jnci/djk033. [DOI] [PubMed] [Google Scholar]