Abstract
Identification of clonal lymphocytic populations by polymerase chain reaction may be difficult in cases with scant cellular infiltrates or those with a heterogeneous population of cells. Here, we assessed the diagnostic utility of laser capture microdissection (LCM) and high-resolution microcapillary electrophoresis in the analysis of clonality of small biopsy specimens. Clonality was determined in 24 cases: five reactive tonsils, five reactive lymph nodes, six inflammatory skin lesions, and eight T-cell lymphomas. CD3-positive T lymphocytes were captured by LCM from paraffinized immunohistochemically stained sections. Genomic DNA was analyzed for T-cell receptor-γ gene rearrangement by polymerase chain reaction followed by high-resolution microcapillary electrophoresis with the DNA 500 LabChip and the Agilent Bioanalyzer. In the reactive specimens, T-cell receptor-γ polymerase chain reaction revealed monoclonal bands when 10 to 1000 cells were captured. This pattern changed to polyclonal when higher numbers of cells were microdissected (2000 to 10,000 cells). In contrast, lymphoma cells were consistently monoclonal whether low or high numbers were microdissected. Microcapillary electrophoresis coupled with LCM facilitated clonality analysis in equivocal cases. In two of eight lymphoma cases, LCM revealed diagnostic monoclonal bands, whereas routine T-cell receptor-γ assessment of whole tissue sections with 10% polyacrylamide gel electrophoresis demonstrated only minor clonal bands. We conclude that clonality determined by LCM is cell number-dependent. Biopsy specimens containing low numbers of reactive polyclonal T cells may produce pseudomonoclonal bands and therefore should be interpreted with great caution.
Recent advances in molecular biological techniques such as polymerase chain reaction (PCR) have assisted in the development of specific assays for the analysis of lymphoid infiltrates.1 It is generally accepted that the vast majority of lymphoid malignancies are clonal in origin, whereas reactive lymphoid proliferations contain no predominant single clone.2,3
The configuration of the T-cell receptor (TCR) gene rearrangement is unique to an individual T cell and its progeny and can be used as a marker of clonality. The T-cell receptor is a heterodimer with the majority of T cells expressing the αβ TCR, whereas less than 5% of all T cells express the γδ TCR proteins. In the germline configuration, these four TCR genes consist of many different variable (V), diversity (D), and joining (J) gene segments.4,5 Functional TCR genes are created in developing T lymphocytes by rearrangements of DNA that juxtapose V, D, and J segments. TCR-γ gene rearrangement is a preferential target for clonality analysis, since it is rearranged at an early stage of T-lymphocyte development in both TCR αβ and TCR γδ precursor cells. Moreover, the TCR-γ gene contains a limited number of Vγ and Jγ segments (14 Vγ, including 10 functional undergoing rearrangements and three pseudogenes, and five Jγ segments), thereby facilitating PCR amplification.6,7 Therefore, TCR-γ gene rearrangement is the most commonly used marker for DNA PCR analysis of T-cell clonality. In contrast to TCR-β, the TCR-γ locus does not contain a D segment, thereby limiting V-J junctional diversity to only one hypervariable N region. As a consequence, a relatively small number of nucleotide additions produce V-J junctional length, varying by only 20 to 30 bp, impeding analysis of clonal and polyclonal PCR products by routine polyacrylamide gel electrophoresis (PAGE). High-resolution electrophoresis techniques facilitate analysis of TCR-γ PCR products providing single-nucleotide resolution. In addition to normal physiological rearrangement processes during T-cell ontogenesis,8 TCR-γ rearrangements can be detected in greater than 90% of peripheral T-cell lymphoma and mycosis fungoides, T-cell acute lymphoblastic leukemia, T-cell prolymphocytic leukemia, T-cell large granular lymphocyte leukemia, but not in true natural killer cell proliferations.5,9,10,11
Identification of a clonal lymphocytic population may be difficult because of the paucity of the infiltrate as well as a heterogeneous background population of cells. Employment of laser capture microdissection (LCM) enables isolation of target T lymphocytes from other lymphoid cells in tissue samples.12,13,14 This methodology has been applied for molecular analysis of composite lymphomas, where two unrelated clonal lymphocytic populations occupy different but intimately interwoven microenvironments.15 In addition, it has been shown that LCM has improved clonality detection in cutaneous B- or T-cell lymphomas.16,17
The aim of this study was to assess the diagnostic utility of LCM and high-resolution microcapillary electrophoresis in the clonality analysis of various lymphoid infiltrates, including small biopsy specimens. We demonstrate that clonality determined by LCM is cell number-dependent. We conclude that biopsy specimens containing low numbers of reactive polyclonal T cells (less than 2000 cells) may produce pseudomonoclonal bands, and these cases should be interpreted with great caution.
Materials and Methods
Clinical Samples
All samples were archival formalin-fixed, paraffin-embedded tissue specimens that were collected for clinical purposes and retrieved from the files of the Department of Pathology at Rhode Island Hospital (Providence, RI). This project received institutional review board approval. Clonality was assessed in 24 cases, including five tonsils with reactive follicular hyperplasia removed for obstructive sleep apnea syndrome, five reactive lymph nodes including axillary lymph nodes obtained from prophylactic mastectomy specimens, and mesenteric lymph nodes from inflammatory bowel disease specimens, six inflammatory skin lesions, and eight cases of T-cell lymphoma (three nodal and five cutaneous). Pathological diagnoses were obtained from the hospital pathology database, and the histology was reviewed by two pathologists (E.Y. and M.B.R.). Lymphoma cases were classified according to the World Health Organization classification.18 Diagnostic workup of T-cell lymphoma cases included morphology, flow cytometry, and immunohistochemistry.
Laser Capture Microdissection
LCM was performed on immunostained slides. Immunohistochemical staining for CD3 (T-cell lineage-specific marker) was performed with the CD3 monoclonal antibody (1:200 dilution; Dako Corp, Carpinteria, CA) using the DAKO Envision Plus system according to the company’s protocol. Heat-induced antigen retrieval was performed with a microwave pressure cooker for 10 minutes.
For LCM, immunohistochemistry-stained sections were air-dried, dehydrated through graded alcohols, and subjected to microdissection. Numbers of CD3+ cells varying from 10 to 10,000 were microdissected and captured on LCM HS Capsure Caps (Arcturus Engineering, Mountain View, CA) using an Autopix automated laser capture microdissection instrument (Arcturus). The individual cells were automatically counted during the dissection.
Genomic DNA was extracted from the microdissected cells as well as from the whole tissue sections with the QIAamp DNA Micro Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions with minor modifications. In brief, the thermoplastic films with captured cells were peeled from the caps, immersed in 50 μl of proteinase K (600 mAU/ml) and digested overnight at 55°C. After digestion, DNA was purified by spin column according to the kit instructions and finally diluted in 20 μl of elution buffer (10 mmol/L Tris-Cl, pH 8.5). Extraction from 2000 cells yielded approximately 20 ng of DNA (10 pg/one laser shot).
Polymerase Chain Reaction
DNA was analyzed for TCR-γ gene rearrangement by single multiplex PCR with four primers in one tube according to McCarthy et al19 with minor modifications. The combination of primers included two directed against V region: Vγ11 5′-TCTGG(G/A)GTCTATTACTGTGC-3′ complementary to a common sequence in Vγ 1-8, and Vγ101 5′-CTCACACTC(C/T)CACTTC-3′ complementary to a common sequence in Vγ10 and Vγ11. Two consensus primers were directed against J region: Jγ12 5′-CAAGTGTTGTTCCACTGCC-3′ complementary to a sequence in Jγ1/2 and Jp12 5′-GTTACTATGAGC(T/C)TAGTCC-3′ complementary to a common sequence in JγP1/P2. Briefly, each 25-μl PCR contained 5 μl of DNA extracted from the whole tissue section (0.1 μg/μl) or 5 μl of DNA extracted from the LCM specimens, 4 μmol/L of pooled V and J primers, 0.2 mmol/L of each deoxynucleoside-5′-triphosphate, 1.5 mmol/L MgCl2, and 0.125 U of AmpliTaq gold polymerase (Applied Biosystems, Foster City, CA). Amplification reactions were performed in a Bio-Rad iCycler (Bio-Rad Laboratories, Hercules, CA). After initial DNA denaturation at 94°C for 10 minutes, 45 amplification cycles were performed as follows: denaturation (94°C for 30 seconds), annealing (59°C for 30 seconds), and extension (72°C for 30 seconds). The final step consisted of a 10-minute extension at 72°C to complete all products. In each experiment, a monoclonal control (T-cell lymphoma), polyclonal control (tonsil), and PCR master mix without a DNA template were run in parallel with the test samples. The wild-type p53 gene was amplified by PCR for all samples examined, confirming the presence of amplifiable DNA. The primer pair of exon 7 of p53 gene amplified a 140-bp PCR product (forward primer: 5′-TCCTAGGTTGGCTCTGACT-3′; reverse primer: 5′-CAAGTGGGCTCCTGACCTG-3′).
To determine sensitivity, a clonal Jurkat T-cell lymphoma cell line DNA, known to carry rearrangements involving Vγ 1-8 and Vγ11, was mixed in decreasing proportions with lymphoid DNA from a reactive polyclonal tonsil or DNA from nonlymphoid placental DNA and subsequently subjected to PCR.
Microcapillary Electrophoresis
PCR products were detected by high-resolution microcapillary electrophoresis with the DNA 500 LabChip and the Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA). The Bioanalyzer is a novel instrument that uses Lab-on-a-Chip technology to perform gel electrophoresis in a microfabricated chip. Each chip contains an interconnected set of gel-filled channels that allow for molecular sieving of nucleic acids. Electrodes sit in the reservoirs that connect to the ends of the various channels. Electrical voltages are applied to fluid reservoirs by means of individual electrodes, each of which is connected to a separate high-voltage power supply. When an electric field is applied, the charged molecules move toward one of the electrodes. The molecules are separated electrophoretically in a sizing polymer sieve solution similar to capillary electrophoresis. Active control over voltage gradients directs the movement of materials using the phenomenon of electrophoretic flow. PCR products in 1 μl of reaction mixture were separated by fragment size and detected by highly sensitive laser-induced fluorescence emission using an intercalating dye that is added to the polymer. DNA fragments were normalized to two internal markers (Figure 1) and a ladder. All data, including electropherograms, PCR product size determination, and quantification were performed automatically with Agilent 2100 Bioanalyzer software. TCR-γ PCR products ranged in size from 75 to 110 bp. In parallel, samples of interest were analyzed by 10% PAGE at 10 W for 90 minutes, stained with 10 μg/ml ethidium bromide (Fisher Scientific, Pittsburgh, PA) and visualized under UV light. The gels were interpreted as polyclonal if electrophoresis resulted in a diffuse smear, as monoclonal if they demonstrated one (monoallelic) or two (biallelic) distinct dominant bands, and as oligoclonal if they had more than two dominant bands. Electropherograms were interpreted as clonal if a peak height was greater than two times the maximum height of the polyclonal background curve.20 The presence of an equivocal band of decreased intensity within a polyclonal background was defined as minor clone.
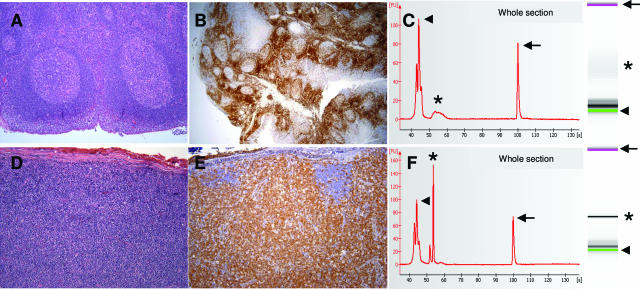
Figure 1.
Clonality analysis in whole tissue sections from a reactive tonsil (A–C) and from nodal T-cell lymphoma (D–F). A and D show typical histology on hematoxylin and eosin sections. B and E demonstrate immunohistochemical staining for CD3. C and F show the electropherogram (on the left) and corresponding microcapillary electrophoresis image (on the right). The horizontal axis of the electropherogram indicates migration time (in seconds); the vertical axis displays relative fluorescence intensity. The first peak on the electropherogram and green band on the gel image (arrowheads) are the marker peak of 15 bp. The last peak on the electropherogram and the pink band on the gel image (arrows) are the marker peak of 600 bp. The peaks for interpretation on electropherograms and correlative bands on the gel image are labeled by asterisks. C shows a polyclonal pattern with a characteristic bell-shaped curve on the electropherogram and smear on microcapillary electrophoresis image. F shows a monoclonal rearrangement with a characteristic spike on the electropherogram and single band on microcapillary electrophoresis.
Results
Clonality in Whole Tissue Sections from Reactive Lymph Nodes and Tonsils and from Lymphoma Specimens
Clonality was assessed initially in whole tissue sections from histologically benign lymph nodes and tonsils (five each). In all cases, an initial quantity of 500 ng of target DNA yielded a polyclonal pattern with a smear on microcapillary electrophoresis gel image and bell-shaped curve on the corresponding electropherogram (Figure 1, A–C). In all three cases of the nodal T-cell lymphoma, TCR-γ analysis of DNA extracted from the whole tissue sections showed monoclonal rearrangement with a characteristic single band on microcapillary electrophoresis gel image and spike on electropherogram (Figure 1, D–F). Clonal PCR products ranged from 75 to 110 bp in size.
Sensitivity Studies
The sensitivity of the technique was evaluated by two DNA mixing assays. First, clonal Jurkat T-cell line DNA was mixed with lymphoid DNA from a reactive polyclonal tonsil in proportions varying from 20 to 0.2% (Figure 2A). Monoclonal rearrangements were visible down to the point where clonal Jurkat DNA comprised only 2% of the total lymphoid DNA (which is equivalent to 2 in 100 cells). Monoclonal bands clearly dominated over polyclonal background down to the 3% dilution, whereas a polyclonal background was distinguishable in lower concentrations of Jurkat cells (less or equal to 2%). Second, clonal Jurkat T-cell line DNA was mixed with nonlymphoid placental DNA in proportions varying from 10 to 0.02% (Figure 2B). Here, the sensitivity was higher as opposed to the lymphoid DNA, with monoclonal peaks detectable down to a concentration of 0.1% (equivalent to 1 in 1000 cells).
Figure 2.
Sensitivity assays using the clonal Jurkat T cell line DNA admixed with lymphoid DNA from reactive tonsils (A) or with nonlymphoid placental DNA (B). Results of microcapillary electrophoresis are represented as a gel image and electropherogram. Serial DNA dilutions are provided at the top of the gel image. Location of the bands for interpretation on the gel image is labeled by asterisks. The first and last peaks on electropherograms are marker peaks of 15 and 600 bp, respectively. In A, monoclonal peaks are visible at 2% dilution, in B, they are visible at 0.1% dilution.
Clonality of LCM T Cells from Reactive Lymph Nodes and Tonsils and from Lymphoma Specimens
For LCM, numbers of cells varying from 10 to 10,000 were captured. A total of 72 samples were microdissected from two reactive lymph nodes, three tonsils, and three nodal lymphoma cases. One laser shot of approximately 8 to 10 μm in diameter (size of normal lymphocyte) yielded one to three cells. To confirm the minimal cell number necessary to obtain a sufficient amount of DNA for PCR analysis, we captured different numbers of cells varying from 1 to 20. We determined that 10 captured cells were sufficient to obtain a PCR product in the TCR-γ gene rearrangement assay using non-nested PCR. In addition, tissue sections of various thicknesses, including 5, 7, and 10 μm, were assessed for LCM. Although 10-μm sections yielded a larger amount of DNA, the cells were captured more efficiently from the thinner sections. Therefore, sections of 7-μm thickness were used for LCM in further studies.
TCR-γ gene rearrangement analysis showed a tendency toward a monoclonal pattern when the number of microdissected cells varied between 10 and 1000 cells. This pattern changed to polyclonal when higher numbers of cells were microdissected (2000 to 10,000 cells). Figure 3A demonstrates a representative tonsil in which pseudomonoclonal bands were detected when 100, 200, 500, and 1000 cells were captured for analysis. These bands were of various sizes and were not reproducible on repeated PCR.
Figure 3.
TCR-γ PCR analysis of LCM cells from a reactive tonsil (A) and from a nodal lymphoma (B). Results of microcapillary electrophoresis are represented as a gel image and electropherogram. The number of captured cells is provided at the top of the gel image. Location of the bands for interpretation on the gel image is labeled by asterisks. The first and last peaks on electropherograms are marker peaks of 15 and 600 bp, respectively. CD3+ captured cells are shown in the panels with the corresponding electropherograms. A tendency toward a monoclonal pattern is seen when low numbers of cells (10 to 1000) were captured from the reactive tonsil (A). This pattern changed to polyclonal when higher numbers of cells were microdissected. LCM-captured lymphoma cells were consistently monoclonal whether low or high numbers of cells were microdissected (B).
In contrast, LCM lymphoma cells demonstrated a consistent monoclonal pattern, whether lower or higher numbers of cells were microdissected. The monoclonal peaks were identical in size between the different number of LCM cells and those from whole tissue extracts. In a representative case demonstrated in Figure 3B, there was a tendency to show TCR-γ rearrangement in both alleles, giving rise to two clonal products, when the number of microdissected cells was more or equal to 2000. The monoclonal bands were reproducible on repeated PCR analysis.
Clonality of LCM Cells in Small Skin Biopsies
All six cases of inflammatory skin lesions showed a polyclonal pattern when whole tissue sections were analyzed. In two of six cases, various numbers of microdissected cells (10 to 10,000) were analyzed for TCR-γ gene rearrangement. The lymphoid infiltrate in these cases appeared to be pseudomonoclonal when less than 2000 cells were used for analysis (Figure 4A). These pseudomonoclonal bands were not reproducible on repeated PCR. In contrast, the LCM of cutaneous T-cell lymphoma cells was constantly monoclonal whether low or high numbers of cells were microdissected (Figure 4B).
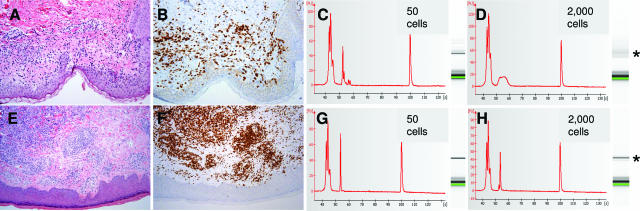
Figure 4.
Clonality analysis of LCM cells in small skin biopsies in a representative case of dermatitis (A–D) and a cutaneous T-cell lymphoma (E–H). A and E show typical histology on hematoxylin and eosin sections. B and F demonstrate immunohistochemical staining for CD3. C shows a pseudomonoclonal peak on the electropherogram and a single band on microcapillary electrophoresis gel image (asterisk) when 50 cells were used for analysis. The first and last peaks on electropherograms are marker peaks of 15 and 600 bp, respectively. This pattern changed to polyclonal when 2000 or more cells were captured (D). In contrast, cutaneous T-cell lymphoma cells were consistently monoclonal whether low or high numbers of cells were microdissected (G and H).
Five of eight cases of T-cell lymphoma showed a single monoclonal peak (or a very prominent monoclonal peak in a polyclonal background) on routine TCR-γ assessment of whole tissue sections with 10% PAGE (Figure 5A, lanes 1 to 3, 5, and 6); biallelic clonal products were seen in one case (Figure 5A, lane 4). In these cases, microcapillary electrophoresis demonstrated clonality in total agreement with PAGE; however, microcapillary electrophoresis provided superior resolution with more distinct bands on the gel image and exact amplicon sizes on the electropherograms (Figure 5B, lanes 1 to 6). In two cases with clinical, histological and immunohistochemical evidence of cutaneous T-cell lymphoma, the PCR products detected by routine 10% PAGE were interpreted initially as a minor clonal population (Figure 5A, lanes 7 and 8). In these cases, microcapillary electrophoresis of the whole tissue section PCR products revealed a clearer image (Figure 5, B, lanes 7 and 8; and C, electropherograms 7 and 8). The signal was even stronger when DNA was amplified from the laser capture microdissected CD3-positive T lymphocytes (2000 cells were captured for analysis). As shown in Figure 5A, lanes 9 and 10, amplicons from LCM cells demonstrated better resolution with distinct bands on 10% PAGE. Microcapillary electrophoresis of LCM cells showed further improvement over traditional PAGE as well as over the whole tissue section amplicons, providing superior resolution with sharp distinct bands and higher peaks on electropherograms (Figure 5, B, lanes 9 and 10; and C, corresponding electropherograms).
Figure 5.
Comparison of 10% PAGE (A) and microcapillary electrophoresis (B, gel image; C, electropherograms). MW, DNA molecular weight marker; Pos, positive control (T-cell lymphoma); Neg, negative control (reactive tonsil). Lanes 1–3: Nodal T-cell lymphoma. Lanes 4–8: Cutaneous T-cell lymphoma. Location of the bands for interpretation on the gel image is labeled by asterisks. Ten percent PAGE of the whole tissue sections demonstrates bands that have been interpreted as minor clonal (A, lanes 7 and 8). LCM provides better resolution in these cases (A, lanes 9 and 10). Microcapillary electrophoresis of the whole tissue section PCR products exhibits diagnostic monoclonal bands (B, lanes 7 and 8; and C, lanes 7 and 8). The signal is stronger when the DNA is amplified from the LCM cells (B, lanes 9 and 10; and C, lanes 9 and 10). The first and last peaks on electropherograms are marker peaks of 15 and 600 bp, respectively.
Discussion
A PCR-based clonality analysis of T-cell receptor gene rearrangements is an essential ancillary technique in distinguishing benign from malignant lymphoid infiltrates. This is of particular importance when a diagnosis cannot be rendered based on morphology and immunophenotype alone. In small biopsy specimens, mechanical distortion and cauterization artifacts may preclude morphological interpretation, whereas the small amount of tissue present may not be sufficient for a broad immunohistochemical panel. Although PCR techniques provide high sensitivity, because as few as 1 to 10 cells are required for amplification of rearranged DNA by PCR, it has been recognized that small amounts of DNA may increase the risk of pseudoclonality, which may be a critical factor in the interpretation of PCR results.21,22,23,24,25 Beside pitfalls in clonality interpretation introduced by low amounts of DNA, there can be a bias introduced by technical aspects, especially using primers to the different V region families in separate parallel assays as in the commonly used BIOMED procedure.5 In this study using LCM, we determined that at least 2000 T lymphocytes should be present in the biopsy specimen to eliminate the possibility of false-positive results. Moreover, we demonstrated that LCM coupled with microcapillary electrophoresis facilitates TCR-γ gene rearrangement analysis.
Techniques for isolation of pure cell populations include gradient control cell separation (Ficoll), expression-based cell separation (flow cytometry), or microdissection of target cells from frozen tissue or paraffin-embedded material.26 Several microdissection technologies have been used to isolate pure cell populations, including manual hydraulic micromanipulation,27 microdissection with glass pipette,28 or using a craft glue.29 Since its introduction a decade ago, LCM has enabled isolation of single target cells.12 The combination of LCM with immunohistochemistry allows for the targeting of cells with a particular immunophenotype,30,31 which proves to be advantageous in cases where the target cells cannot be distinguished from other cells based on morphology alone. In this study, T lymphocytes were first immunolabeled by immunohistochemistry with a T-cell lineage-specific marker (CD3) and subsequently captured by LCM. In previous studies, microdissection techniques have been used in the analysis of single lymphoid cells such as Hodgkin and Reed-Sternberg cells, small lymphoid aggregates, germinal centers, and composite lymphomas containing two different neoplastic processes with divergent phenotype.15,24,32,33 Microdissection of single Hodgkin and Reed-Sternberg cells has proven to be of great value for the detection of monoclonal IgH gene rearrangements in Hodgkin’s lymphoma.28 In other studies, LCM of germinal centers has facilitated clonality detection in morphologically and phenotypically aberrant germinal centers.34,35 Yamauchi et al36 provided molecular evidence that a common and single clone is present in microdissected neoplastic follicles of follicular lymphoma. Another application of LCM has resulted in the separation between two morphologically and/or phenotypically distinct neoplastic populations such as composite (biphenotypic) non-Hodgkin’s lymphoma or a combination of Hodgkin’s lymphoma and non-Hodgkin’s lymphoma.15,37 Finally, it has been shown that LCM improved clonality detection in cutaneous T- or B-cell lymphomas.16,17 In the study by Yazdi et al,16 an entire lymphocytic infiltrate from the hematoxylin and eosin section was microdissected for clonality analysis, as opposed to our study where only CD3+ lymphocytes were targeted.
Although LCM significantly increases PCR sensitivity by enrichment of the target cell population, it also may increase the risk of pseudoclonality due to low amounts of DNA. The most probable explanation of pseudomonoclonal bands when the amount of target cell DNA is very low is preferential amplification of a few TCR-γ genes, so that restriction of the product occurs and mono- or oligoclonal bands are detected. Quantitation of template DNA has been addressed in several previous studies of B-cell clonality.21,22,23,24 To our knowledge, the effect of low DNA quantity on T-cell clonality was reported in only one abstract,37 possibly reflecting the fact that the majority of lymphoid malignancies encountered in the West are of B cell lineage.18 In those studies, it was shown that dilution of target DNA from reactive polyclonal tonsils and lymph nodes results in the appearance of oligoclonal and monoclonal bands. In previous reports the DNA was amplified from normal peripheral blood lymphocytes21 as well as from fresh-frozen tissues22 or formalin-fixed paraffinized tissues.23,24 Our results are consistent with those reported by Wan et al21 and Elenitoba-Johnson et al.24 In these studies, the number of PCR cell targets was roughly estimated from the DNA concentration based on 6 pg of DNA/diploid cell38 and the proportion of target lymphocytes within the lymphoid infiltrate.21,24 Wan and colleagues21 demonstrated that polyclonal results were reliably obtained when using DNA from normal peripheral blood lymphocytes when the starting quantity of template DNA was 40 ng (equivalent of 800 target B cells). Using DNA obtained from formalin-fixed paraffinized reactive tonsils and lymph nodes, Elenitoba-Johnson et al24 found that polyclonal results were reproducible at starting amounts of template DNA of 5 ng or higher (equivalent of 830 target B cells in case that all cells in the specimen are B cells). Based on the amount of 6 pg of DNA/diploid cell, DNA yield from 2000 cells in our study can be estimated as 12 ng. Increased amount of DNA obtained from target T lymphocytes in our study (20 ng) would be explained by the occasional harvesting of more than one lymphocyte per laser shot. In this study, we used formalin-fixed paraffinized tissues, which are associated with increased DNA degradation and thereby decreased proportion of amplifiable DNA. Therefore, the amount of amplifiable DNA may be less than 20 ng/2000 cells. In contrast to our study, Taylor et al22 suggested that in conditions when the proportion of B cells in the sample is unknown, the amount of DNA should be 100 ng or greater. Compared with our data, this observation is rational when the target cells represent a minority of the lymphoid infiltrate, such as a small number of polyclonal B lymphocytes comprising less than 5% of the peripheral blood cells, in the case of T-cell lymphoma (Sezary syndrome), or when lymphocytes represent a very small proportion of the cell population such as the case of nonlymphoid tumors, as was described previously in colorectal cancer.39 In another report, it has been proposed that 500 lymphocytes microdissected from formalin-fixed paraffinized tissues should be sufficient for clonality analysis.14 This number of cells is ample in the case of lymphomas but, as we demonstrated in this study, may produce pseudomonoclonal bands in cases containing reactive infiltrates. Therefore, in these cases clonality should be confirmed by repeated PCRs.
Lab-on-a-Chip analysis of PCR products by microcapillary electrophoresis offers superior resolution and improved sensitivity, thus representing a significant improvement over traditional PAGE techniques. Our results are consistent with those reported previously by Beaubier et al.40 We demonstrated that microcapillary electrophoresis can further enhance sensitivity of clonality analysis by PCR. Thus, we were able to detect as few as 2% of clonal T cells in a mixed lymphocytic infiltrate and 0.1% in a mixture of nonlymphoid DNA. Increased PCR sensitivity may also be a serious pitfall in the interpretation of clonality in scant lymphoid infiltrates from small tissue biopsies, fine needle aspirates, and in small groups of cells microdissected from tissue sections. Low numbers of reactive B cells in small biopsy specimens may generate pseudomonoclonal bands in histologically and phenotypically benign specimens such as stomach, skin, salivary gland, orbit, bone marrow, or microdissected germinal centers.23,24,25,32,41,42,43,44,45 High rates of T-cell monoclonality in skin biopsies were found by Dippel et al46 in apparently benign inflammatory skin conditions. Our results confirm that clonality determined by PCR is cell number-dependent, and small formalin-fixed paraffinized biopsy specimens containing less than 2000 target cells may produce pseudomonoclonal bands. Therefore, in small tissue samples we recommend first estimating the number of target cells and, if necessary, using serial sections to obtain reliable numbers of targets.
Footnotes
Supported by the Center of Biomedical Research Excellence, Center for Cancer Research Development, Molecular Pathology, National Center for Research Resources (grant P20 RR017695-02).
References
- Medeiros LJ, Carr J. Overview of the role of molecular methods in the diagnosis of malignant lymphomas. Arch Pathol Lab Med. 1999;123:1189–1207. doi: 10.5858/1999-123-1189-OOTROM. [DOI] [PubMed] [Google Scholar]
- Collins RD. Is clonality equivalent to malignancy: specifically, is immunoglobulin gene rearrangement diagnostic of malignant lymphoma? Hum Pathol. 1997;28:757–759. doi: 10.1016/s0046-8177(97)90145-3. [DOI] [PubMed] [Google Scholar]
- Knowles DM. Immunophenotypic and immunogenotypic approaches useful in distinguishing benign and malignant lymphoid proliferations. Semin Oncol. 1993;20:583–610. [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik M. New York: Garland Publishing,; Immunobiology. (ed 6) 2004:137–141. [Google Scholar]
- van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, van Krieken JH, Droese J, Gonzalez D, Bastard C, White HE, Spaargaren M, Gonzalez M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- Chen Z, Font MP, Loiseau P, Bories JC, Degos L, Lefranc MP, Sigaux F. The human T-cell V gamma gene locus: cloning of new segments and study of V gamma rearrangements in neoplastic T and B cells. Blood. 1988;72:776–783. [PubMed] [Google Scholar]
- Lawnicki LC, Rubocki RJ, Chan WC, Lytle DM, Greiner TC. The distribution of gene segments in T-cell receptor gamma gene rearrangements demonstrates the need for multiple primer sets. J Mol Diagn. 2003;5:82–87. doi: 10.1016/s1525-1578(10)60456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom B, Verschuren MC, Heemskerk MH, Bakker AQ, van Gastel-Mol EJ, Wolvers-Tettero IL, van Dongen JJ, Spits H. TCR gene rearrangements and expression of the pre-T cell receptor complex during human T-cell differentiation. Blood. 1999;93:3033–3043. [PubMed] [Google Scholar]
- Vega F, Medeiros LJ, Jones D, Abruzzo LV, Lai R, Manning J, Dunmire V, Luthra R. A novel four-color PCR assay to assess T-cell receptor γ gene rearrangements in lymphoproliferative lesions. Am J Clin Pathol. 2001;116:17–24. doi: 10.1309/5WFQ-N12E-DT05-UX1T. [DOI] [PubMed] [Google Scholar]
- Luo V, Lessin SR, Wilson RB, Rennert H, Tozer C, Benoit B, Leonard DG. Detection of clonal T-cell receptor gamma gene rearrangements using fluorescent-based PCR and automated high-resolution capillary electrophoresis. Mol Diagn. 2001;6:169–179. doi: 10.1054/modi.2001.27056. [DOI] [PubMed] [Google Scholar]
- Lamberson C, Hutchison RE, Shrimpton AE. A PCR assay for detecting clonal rearrangement of the TCR-gamma gene. Mol Diagn. 2001;6:117–124. doi: 10.1054/modi.2001.25321. [DOI] [PubMed] [Google Scholar]
- Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA. Laser capture microdissection. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- Fend F, Kremer M, Specht K, Quintanilla-Martinez L. Laser microdissection in hematopathology. Pathol Res Pract. 2003;199:425–430. doi: 10.1078/0344-0338-00441. [DOI] [PubMed] [Google Scholar]
- Elenitoba-Johnson KS. Assessment of clonal relationships in malignant lymphomas. Methods Enzymol. 2002;356:224–240. doi: 10.1016/s0076-6879(02)56936-6. [DOI] [PubMed] [Google Scholar]
- Fend F, Quintanilla-Martinez L, Kumar S, Beaty MW, Blum L, Sorbara L, Jaffe ES, Raffeld M. Composite low grade B-cell lymphomas with two immunophenotypically distinct cell populations are true biclonal lymphomas: a molecular analysis using laser capture microdissection. Am J Pathol. 1999;154:1857–1866. doi: 10.1016/S0002-9440(10)65443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi AS, Medeiros LJ, Puchta U, Thaller E, Flaig MJ, Sander CA. Improved detection of clonality in cutaneous T-cell lymphomas using laser capture microdissection. J Cutan Pathol. 2003;30:486–491. doi: 10.1034/j.1600-0560.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- Gallardo F, Pujol RM, Bellosillo B, Ferrer D, Garcia M, Barranco C, Planaguma M, Serrano S. Primary cutaneous B-cell lymphoma (marginal zone) with prominent T-cell component and aberrant dual (T and B) genotype: diagnostic usefulness of laser-capture microdissection. Br J Dermatol. 2006;154:162–166. doi: 10.1111/j.1365-2133.2005.06947.x. [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press,; 2001 [Google Scholar]
- McCarthy KP, Sloane JP, Kabarowski JH, Matutes E, Wiedemann LM. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992;1:173–179. [PubMed] [Google Scholar]
- Greiner TC, Rubocki RJ. Effectiveness of capillary electrophoresis using fluorescent-labeled primers in detecting T-cell receptor gamma gene rearrangements. J Mol Diagn. 2002;4:137–143. doi: 10.1016/s1525-1578(10)60694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan JH, Sykes PJ, Orell SR, Morley AA. Rapid method for detecting monoclonality in B cell lymphoma in lymph node aspirates using the polymerase chain reaction. J Clin Pathol. 1992;45:420–423. doi: 10.1136/jcp.45.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Spagnolo DV, Kay PH. B-cell target DNA quantity is a critical factor in the interpretation of B-cell clonality by PCR. Pathology. 1997;29:309–312. doi: 10.1080/00313029700169165. [DOI] [PubMed] [Google Scholar]
- Zhou XG, Sandvej K, Gregersen N, Hamilton-Dutoit SJ. Detection of clonal B cells in microdissected reactive lymphoproliferations: possible diagnostic pitfalls in PCR analysis of immunoglobulin heavy chain gene rearrangement. Mol Pathol. 1999;52:104–110. doi: 10.1136/mp.52.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenitoba-Johnson KSJ, Mitchell RS, Bohling SD, Brown MS, Robetorye RS. PCR analysis of the immunoglobulin heavy chain gene in polyclonal processes can yield pseudoclonal bands as an artifact of low B cell number. J Mol Diagn. 2000;2:92–96. doi: 10.1016/S1525-1578(10)60622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihal M, Mikkola D, Wood GS. Detection of clonally restricted immunoglobulin heavy chain gene rearrangements in normal and lesional analysis of the B cell component of the skin-associated lymphoid tissue and implication for the molecular diagnostic of cutaneous B cell lymphomas. J Mol Diagn. 2000;2:5–10. doi: 10.1016/S1525-1578(10)60609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JL, Finkelstein SD. Microdissection techniques for molecular testing in surgical pathology. Arch Pathol Lab Med. 2004;128:1372–1378. doi: 10.5858/2004-128-1372-MTFMTI. [DOI] [PubMed] [Google Scholar]
- Küppers R, Zhao M, Hansmann ML, Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LX, Diss TC, Peng HZ, Isaacson PG. Clonality analysis of defined B-cell populations in archival tissue sections using microdissection and the polymerase chain reaction. Histopathology. 1994;24:323–327. doi: 10.1111/j.1365-2559.1994.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Turbett GR, Barnett TC, Dillon EK, Sellner LN. Single-tube protocol for the extraction of DNA or RNA from paraffin-embedded tissues using a starch-based adhesive. Biotechniques. 1996;20:846–850. doi: 10.2144/96205st04. 852–853. [DOI] [PubMed] [Google Scholar]
- Fend F, Emmert-Buck MR, Chuaqui R, Cole K, Lee J, Liotta LA, Raffeld M. Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol. 1999;154:61–66. doi: 10.1016/S0002-9440(10)65251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fend F, Kremer M, Quintanilla-Martinez L. Laser capture microdissection: methodical aspects and applications with emphasis on immuno-laser capture microdissection. Pathobiology. 2000;68:209–214. doi: 10.1159/000055925. [DOI] [PubMed] [Google Scholar]
- Küppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, Hansmann ML. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci USA. 1994;91:10962–10966. doi: 10.1073/pnas.91.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer M, Cabras AD, Fend F, Schulz S, Schwarz K, Hoefler H, Werner M. PCR analysis of IgH-gene rearrangements in small lymphoid infiltrates microdissected from sections of paraffin-embedded bone marrow biopsy specimens. Hum Pathol. 2000;31:847–853. doi: 10.1053/hupa.2000.8445. [DOI] [PubMed] [Google Scholar]
- Pruneri G, Mazzarol G, Manzotti M, Viale G. Monoclonal proliferation of germinal center cells (incipient follicular lymphoma) in an axillary lymph node of a melanoma patient. Hum Pathol. 2001;32:1410–1413. doi: 10.1053/hupa.2001.28965. [DOI] [PubMed] [Google Scholar]
- Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002;99:3376–3382. doi: 10.1182/blood.v99.9.3376. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Nakatsuka S, Miyanaga I, Hoshida Y, Sakamoto H, Aozasa K, Osaka Lymphoma Study Group Clonality analysis of follicular lymphoma using laser capture microdissection method. Int J Mol Med. 2002;10:649–653. [PubMed] [Google Scholar]
- Hussey CE, Wittwer CT, Segal GH. Low cell number may cause misinterpretation of polymerase chain reaction assays designed to determine monoclonality (abstract). Am J Clin Pathol. 1996;106:150–151. [Google Scholar]
- Jeffreys AJ, Wilson V, Neumann R, Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988;16:10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling FC, Clarke CE, Lillicrap D. Positive immunoglobulin gene rearrangement study by the polymerase chain reaction in a colonic adenocarcinoma. Am J Clin Pathol. 1992;98:116–119. doi: 10.1093/ajcp/98.1.116. [DOI] [PubMed] [Google Scholar]
- Beaubier NT, Hart AP, Bartolo C, Willman CL, Viswanatha DS. Comparison of capillary electrophoresis and polyacrylamide gel electrophoresis for the evaluation of T and B cell clonality by polymerase chain reaction. Diagn Mol Pathol. 2000;9:121–131. doi: 10.1097/00019606-200009000-00001. [DOI] [PubMed] [Google Scholar]
- Torlakovic E, Cherwitz DL, Jessurun J, Scholes J, McGlennen R. B-cell gene rearrangement in benign and malignant lymphoid proliferations of mucosa-associated lymphoid tissue and lymph nodes. Hum Pathol. 1997;28:166–173. doi: 10.1016/s0046-8177(97)90101-5. [DOI] [PubMed] [Google Scholar]
- Ahrens K, Braylan R, Almasri N, Foss R, Rimsza L. IgH PCR of zinc formalin-fixed, paraffin-embedded non-lymphomatous gastric samples produces artifactual “clonal” bands not observed in paired tissues unexposed to zinc formalin. J Mol Diagn. 2002;4:159–163. doi: 10.1016/S1525-1578(10)60697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter JH, Wick MR, Adesokan PN, Fitzgibbon JF, Zhu X, Humphrey PA. Assessment of clonality in cutaneous lymphoid infiltrates by polymerase chain reaction analysis of immunoglobulin heavy chain gene rearrangement. Am J Clin Pathol. 1997;108:60–68. [PubMed] [Google Scholar]
- Hsi ED, Siddiqui J, Schnitzer B, Alkan S, Ross CW. Analysis of immunoglobulin heavy chain gene rearrangement in myoepithelial sialadenitis by polymerase chain reaction. Am J Clin Pathol. 1996;106:498–503. doi: 10.1093/ajcp/106.4.498. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Kikuchi M, Sumiyoshi Y, Kobari S, Yoneda S, Takeshita M, Kimura N. Clonality of benign lymphoid hyperplasia in orbit and conjunctiva. Pathol Res Pract. 1994;190:436–443. doi: 10.1016/S0344-0338(11)80205-0. [DOI] [PubMed] [Google Scholar]
- Dippel E, Assaf C, Hummel M, Schrag HJ, Stein H, Goerdt S, Orfanos CE. Clonal T-cell receptor gamma-chain gene rearrangement by PCR-based GeneScan analysis in advanced cutaneous T-cell lymphoma: a critical evaluation. J Pathol. 1999;188:146–154. doi: 10.1002/(SICI)1096-9896(199906)188:2<146::AID-PATH334>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]