Abstract
The prothoracicotropic hormone (PTTH) of Drosophila melanogaster is a modulator of ecdysteroid (molting hormone) synthesis and was isolated and characterized from extracts of whole larvae (≈4 × 105 larvae). The purification protocol included delipidation, salt-extraction, heat treatment, conventional column chromatography, and HPLC, and yielded about 50 μg of pure hormone. Biological activity was followed using a ring gland in vitro assay in which ecdysteroidogenesis by control ring glands as measured by radioimmunoassay was compared with ring gland incubations containing active fractions. The molecular weight of the purified PTTH was 45 kDa and N-terminal amino acid sequence analysis indicated that those analyzed sequences displayed no significant homology with known peptides or peptide hormones, including PTTH from the silkmoth, Bombyx mori. Western blot analysis indicated that the native form of Drosophila PTTH was a single 66-kDa polypeptide with N-linked carbohydrate chains and intrachain disulfide bonds. The purified 45-kDa peptide is the deglycosylated form, a result of glycosidase activity present during preparation of the PTTH extract. The deglycosylated form shows heterogeneity, presumably as a result of varying degrees of deglycosylation at the N terminus.
Keywords: neuropeptide, glycoprotein, ecdysone, metamorphosis
Prothoracicotropic hormone (PTTH), produced by specific neurosecretory cells (prothoracicotropes) in the insect brain, controls the cyclical progress of insect molting by modulating the synthesis of ecdysteroids (molting hormones) by the prothoracic glands via a second messenger transduction cascade (1, 2). Upon reaching its target tissue the ecdysone is hydroxylated to 20-hydroxyecdysone, the principal insect molting hormone (2).
Since Kopeć (3) suggested that the larval brain of the gypsy moth, Lymantria dispar, secretes a factor necessary for molting, attempts have been made to purify and characterize the “brain hormone” (subsequently called PTTH). Available knowledge of the identity and molecular structure of PTTH is based largely on experiments with the silkworm, Bombyx mori, and to a lesser extent, with the tobacco hornworm, Manduca sexta (1, 2, 4, 5). In the former case, a 30-kDa PTTH has been purified and cloned (6, 7), whereas in the case of Manduca, progress has been made (8) but the complete sequence remains elusive (9, 10). The prothoracicotropes in the Lepidoptera appear to be two large, lateral neurosecretory cells in each brain hemisphere (11–13).
PTTH is the primary signal carrier for initiating the molting cycle, and it would be most useful if the power of genetic analysis could be employed in studies of how environmental cues elicit PTTH synthesis and release, how PTTH brings about enhanced ecdysteroidogenesis, how PTTH synthesis and release are regulated, etc. However, the purification and characterization of Drosophila PTTH have not been attained, perhaps because of the insect’s small size and the fact that the larval ecdysteroid-producing gland is part of a complex, the ring gland (14), rather than existing as an individual structure (2). The ability of neural extracts to stimulate ecdysteroid synthesis by the larval ring glands in vitro provided a reliable physiological assay for the Drosophila PTTH (14), leading to this report on the purification and characterization of PTTH from Drosophila melanogaster.
MATERIALS AND METHODS
Reagents.
Column chromatographic and other chemicals were obtained from Sigma. Radioactive [23,24-3H]ecdysone (specific activity: 47.3 Ci/mmol; 1 Ci = 37 GBq) was purchased from New England Nuclear. The anti-ecdysone antibody (DUL-I) used in the radioimmunoassay (RIA) was from Trifolio-M (Lahnau, Germany), alkaline phosphatase-conjugated anti-mouse IgG was from Promega, and endoglycosidase was from Boehringer Mannheim.
Animals.
The Oregon-R (Or-R) wild-type strain of D. melanogaster was reared in uncrowded conditions in a plastic cage on standard medium containing corn meal, sugar, agar, yeast, and propionic acid as mold inhibitor. The animals were maintained at 70–80% humidity, 23 ± 1°C under a photoperiodic regimen (12-hr light/12-hr dark). Synchronization of developmental stage was achieved according to published methods (15). Third instar larvae were collected as starting material for PTTH purification and were stored at −70°C until use.
In Vitro Assay of PTTH Activity.
PTTH activity recovered from each purification step was assessed using the in vitro ring gland assay described (16). This assay uses five glands from wandering third instar larvae as a control (−PTTH) and five glands as the experimental (+PTTH) group with the degree of gland activation expressed as an activation ratio (Ar) defined as the amount of ecdysone synthesized by the experimental glands divided by that synthesized by control glands. Ring glands were dissected out and incubated for 2 hr in 20 μl of Grace’s medium (GIBCO) at 24°C under high humidity in the dark. Each incubation was terminated by removing the culture medium for assay of its ecdysone content by modification of previously described RIA procedures (17, 18). The labeled ligand was [23,24-3H]ecdysone and unlabeled ecdysone was used as the competing ligand. All RIA analyses were repeated at least six times.
Preparation of Larval Extracts and Heat Treatment.
Larvae (≈4 × 105, approximate wet weight 0.8 kg) were homogenized in 3 vol of cold acetone containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 100 μM l-1-tosylamido-2-phenylethyl chloromethyl ketone (TPCK) using a Waring blender at 4°C. The homogenate was centrifuged at 6000 × g for 10 min at 4°C, and the yellow supernatant was discarded. PTTH activity was recovered successfully from the acetone powder after it was solubilized with 5 vol of 2% NaCl containing 1 mM PMSF and 100 μM TPCK (pH 6.8). After each extraction, insoluble material was removed by centrifugation and subsequent heat treatment (95°C for 3 min with shaking). The supernatant after heat treatment was subjected to acetone precipitation and the precipitate assayed for PTTH activity after being dissolved in 0.05 M Tris·HCl (pH 7.8) and dialyzed against three changes of 10 vol of buffer.
Q-Sepharose Column Chromatography.
The concentrated protein solution was loaded onto a Q-Sepharose column (30 × 250 mm) equilibrated with 0.05 M Tris·HCl buffer (pH 7.8), and fractions were eluted with the same buffer and assayed for PTTH activity. All buffers used for chromatographic purification contained protease inhibitors (1 mM PMSF and 100 μM TPCK).
S-Sepharose Column Chromatography.
Following concentration and dialysis, the active fractions from the Q-Sepharose column were applied to an S-Sepharose column (25 mm × 150 mm), which was developed with a linear gradient of NaCl (0–0.4 M) in 0.05 M sodium acetate buffer (pH 5.6) at a flow rate of 90 ml/hr. Fractions eluted from the columns were monitored routinely by optical absorption at 280 nm and assayed for PTTH activity.
C18 Reverse-Phase HPLC (RPHPLC).
All fractions with PTTH activity from the previous step were pooled, concentrated, and lyophilized. The lyophilized sample (4 mg) was dissolved in 2 ml H2O containing 1 mM PMSF and 100 μM TPCK and applied to a 4.6 × 300 mm C18 column (Vydac, Hesperia, CA), equilibrated with 20% acetonitrile. Elution used a linear gradient of 20–40% acetonitrile in 0.05% trifluoroacetic acid (TFA) for 60 min at a flow rate of 1 ml/min. Fractions were collected and bioassayed.
Superdex G-75 Gel Filtration.
After lyophilization, the HPLC active fractions were dissolved in 0.05 M Tris·HCl buffer (pH 7.8) and applied to a Superdex G-75 gel-filtration column (Superfine, 15 × 610 mm) that has been equilibrated with the same Tris·HCl buffer at a flow rate of 60 ml/hr. Two-milliliter fractions were collected and bioassayed.
SDS/PAGE.
SDS/PAGE was carried out as described (19). Aliquots of various purified PTTH fractions were analyzed after treatment with or without reducing reagent (5% 2-mercaptoethanol in the sample buffer) in a 1-mm thick, 12% acrylamide gel. Gels were stained with Coomassie brilliant blue R-250.
Amino Acid Sequence Analysis.
Aliquots of PTTH purified as described above were subjected to SDS/PAGE after treatment with a reducing agent and transferred to a polyvinylidine difluoride (PVDF) membrane (Bio-Rad). The membrane was stained with Ponceau-S and the stained bands were cut out for sequence analysis. Amino acid sequence analysis was performed using HPLC (MilliGen) in tandem with a phenylthiohydantoin amino acid analyzer (MilliGen 6600B).
Preparation of Antiserum Against Purified 45-kDa PTTH.
Mouse polyclonal antiserum against the purified PTTH was obtained as described (20). A male C57Br/6J mouse was immunized by intramuscular injection of purified PTTH (10 μg) in complete Freund’s adjuvant and by three boosters of the purified PTTH (7 μg) in complete Freund’s adjuvant at 1-week intervals. Ten days after the last injection, serum was obtained from the mouse and stored at −20°C until use.
Western Blot Analysis.
Third instar larvae were homogenized in 300 μl of 2% NaCl (pH 6.8) containing 2 mM PMSF and 200 μM TPCK in an Eppendorff tube and sonicated briefly. The homogenate was heated in boiling water for 3 min, cooled rapidly and centrifuged at 24,000 × g for 30 min. Samples were analyzed by PAGE (12% polyacrylamide slab gel) and then electrophoretically transferred to a PVDF membrane (21, 22). The membrane was blocked with 2% bovine serum albumin in TBST buffer (10 mM Tris·HCl, pH 8.0/150 mM NaCl/0.1% Tween 20) for 1 hr and incubated with a 1:10,000 dilution of PTTH antiserum for 1 hr at room temperature. After washing with TBST, the membrane was incubated with a 1:5000 dilution of alkaline phosphatase-conjugated anti-mouse IgG serum (Promega) for 1 hr. The color reaction was carried out using 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium as substrates in alkaline phosphatase buffer (100 mM Tris·HCl, pH 9.5/100 mM NaCl/5 mM MgCl2).
Enzyme Digestion of Intact PTTH with Glycosidase.
Third instar larval extracts and brain extracts were dissolved in distilled water containing 1 mM PMSF and 100 μM TPCK and 10× reaction buffer [50 mM sodium phosphate buffer (pH 7.2)]. After the addition of N-glycosidase F or O-glycosidase, the reaction mixtures were incubated at 37°C for 1 hr and then subjected to immunoblotting using PTTH antiserum.
Immunohistochemistry.
Whole-mount immunohistochemistry was performed by modifying a previously published method (12) and using the antiserum at a dilution of 1:1000 with a 1:500 dilution of alkaline phosphatase-conjugated anti-mouse IgG goat serum.
RESULTS
Purification of 45-kDa PTTH.
The purification protocol consisted of seven steps including delipidation, salt-extraction, heat treatment, conventional column chromatography and HPLC. The ring gland bioassay was used at each step. An ≈80-fold purification from the heat treatment step was attained yielding ≈50 μg of the putative hormone with a recovery of 1.2% (Table 1). Substances such as endogenous ecdysteroids in the crude extracts that could interfere with the bioassay were removed in the acetone powder step. The specific activity of the crude extracts prior to heat treatment could not be determined due to an endogenous contaminant in the sample that interfered with the in vitro assay. Therefore, the activity of the supernatant after heat treatment was set at 100%.
Table 1.
Summary of purification of the 45-kDa PTTH
| Purification procedure | Total protein, mg | Critical dose, μg | Specific activity,* unit/mg | Total activity,* unit | Yield, % | Purification, -fold |
|---|---|---|---|---|---|---|
| Acetone powder | 1.72 × 105 | — | — | — | — | — |
| 2% NaCl extract | 1.60 × 103 | — | — | — | — | — |
| Heat treatment | 340.00 | 8.0 | 125 | 42,500 | 100.00 | 1.00 |
| Q-Sepharose | 31.00 | 4.1 | 244 | 7,600 | 17.80 | 1.95 |
| S-Sepharose | 4.00 | 0.8 | 1,250 | 5,000 | 11.80 | 10.00 |
| HPLC | 1.00 | 0.5 | 2,000 | 2,000 | 4.71 | 16.00 |
| Superdex G-75 | 0.05 | 0.1 | 10,000 | 500 | 1.20 | 80.00 |
Third instar larvae of D. melanogaster, 4 × 105 (0.8 kg) were used as starting material.
One unit (i.e., critical dose) corresponds to the amount of hormone that is required to increase the ecdysone Ar from 1 to 2 during a 2-hr incubation.
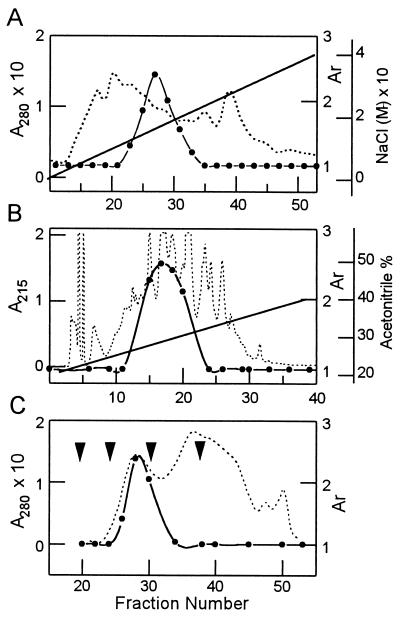
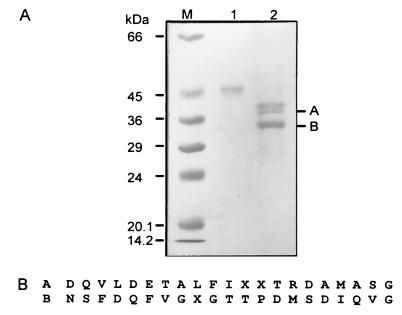
The supernatant after heat treatment was subjected to anion-exchange chromatography using Q-Sepharose. PTTH activity was detected in the “flowthrough” fraction from the column, but not in the bound fractions. After concentration and dialysis, the unadsorbed fraction from the Q-Sepharose was applied to a cation-exchange chromatography using S-Sepharose and eluted with a salt gradient. PTTH activity eluted in a peak between 0.12–0.16 M NaCl (Fig. 1A). The resulting purification in this step was about 10-fold, with essentially full recovery of PTTH activity. The active fractions from S-Sepharose chromatography were lyophilized and applied to a RPHPLC C18 column, and PTTH activity was eluted with 26–29% acetonitrile containing 0.05% TFA (Fig. 1B). Total PTTH activity was 2000 units and was active at a dose of 0.5 μg in the ring gland in vitro assay. After further purification by HPLC in which peaks 7–10 showed biological activity, the HPLC active fractions were lyophilized and then applied to a Superdex G-75 gel filtration column (Fig. 1C). The in vitro assay revealed that fractions 27–31 were active and yielded 50 μg of material with an overall recovery of 1.2%. To assess its purity, the fractions with PTTH activity were pooled and subjected to SDS/PAGE (Fig. 2A). The PTTH showed a broad band under nonreducing conditions corresponding to 45 kDa. Under reducing conditions, the 45-kDa band was no longer evident and three sharp bands (40, 38, and 33 kDa) and a few smaller ones (<10 kDa) were detected (data not shown).
Figure 1.
Chromatographic separations of PTTH. Solid line denotes gradient, solid circles PTTH activity recovered, and broken line absorbance. (A) Ion-exchange chromatography using an S-Sepharose column. The column was eluted with a linear gradient of 0–0.4 M NaCl in a 0.05 M sodium acetate buffer (pH 5.6). PTTH activity was recovered from fractions eluted with a gradient of 0.12–0.16 M NaCl. (B) RPHPLC using a C18 column. PTTH was eluted with a linear gradient of 20–40% acetonitrile in 0.05% TFA for 60 min at 25°C at a flow rate of 1 ml/min. (C) Gel filtration chromatography using a Superdex G-75 column. The 45-kDa PTTH was eluted in fractions 27–31, each fraction being 2 ml. First arrow denotes void volume, second arrow the fraction where bovine serum albumin (66.8 kDa) eluted during column calibration, third arrow the fraction where carbonic anhydrase (29 kDa) eluted during column calibration, and fourth arrow where cytochrome c (12.4 kDa) eluted during column calibration. Activation ratio (Ar) = The quantity of ecdysone synthesized by the ring glands in Grace’s medium with PTTH/The quantity of ecdysone synthesized by the ring glands in Grace’s medium alone (control)
Figure 2.
(A) SDS/PAGE analysis of the purified 45-kDa PTTH under both nonreducing and reducing (2-mercaptoethanol) conditions. Lanes: M, molecular mass markers; 1, 1.0 μg of pure PTTH under nonreducing conditions; 2, 3.0 μg of pure PTTH under reducing conditions. To conserve space the figure was cropped and the smaller fragments—e.g., 10 kDa are not shown. (B) N-terminal amino acid sequence of two reduced PTTH fragments, A and B.
Amino Acid Sequence Analysis.
To determine the N-terminal amino acid sequence needed to design a primer for gene cloning, the reduced peptide fragments were prepared and analyzed as described in Materials and Methods. Amino acid sequence analysis revealed that 21 amino acid residues could be detected in each of the two A and B fragments (Fig. 2B). The size of each sequenced fragment is about 2310 Da. If both the 38- and 33-kDa fragments were cut at the N-terminal end, there would be no overlapping of the sequences. If the cut occurred within 2500 Da of the N terminus there also would be no overlapping of the sequence. Although two smaller fragments (<10 kDa) were subjected to amino acid sequence analysis, no amino acid residue signals were detected—i.e., the N termini were blocked.
In Vitro Activation of Ring Gland by Purified PTTH.
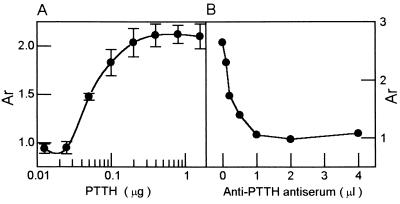
The purified 45-kDa PTTH elicited a significant, dose-dependent increase in ecdysteroid secretion by ring glands in vitro. Serial dilutions of purified 45-kDa PTTH evoked a range of responses that approximates a saturation curve when assayed on ring glands (Fig. 3A). The purified 45-kDa moiety stimulated ecdysone synthesis and secretion at a dose of 0.05 μg, whereas 0.2 μg elicited maximal activity. When the in vitro assay was performed with PTTH fractions obtained during the purification processes, the relatively concentrated protein inhibited ecdysone synthesis by the ring gland in some cases, somewhat similar to what has been reported for Manduca sexta PTTH (23). However, when purified 45-kDa PTTH was assayed at a high concentration, no such inhibition effect was noted (data not shown).
Figure 3.
Effect of antiserum on PTTH activity in the in vitro assay. (A) Dose-dependent effect of the purified 45-kDa PTTH on ecdysone secretion by the ring glands in vitro. Each datum point represents the mean (±SEM) of four separate determinations. (B) Inhibition of the activity of purified PTTH by the anti-PTTH antiserum. Various doses of anti-PTTH anti-serum and purified PTTH (0.5 μg) were incubated together and the mixture was then assayed using the ring gland in vitro assay as in A.
Immunoblot Analysis.
Using the mouse antiserum raised against the purified 45-kDa PTTH, Western blot analysis revealed the high specificity of the antibody for the 45-kDa PTTH, as well as the reduced fragments (data not shown). When whole body larval extracts were reduced with 2-mercaptoethanol and subjected to electrophoresis under normal or reducing conditions, a major band was noted in crude extracts at 66 kDa, as well as several minor bands. When the crude larval extract without protease inhibitors was incubated at 37°C for 1 hr, the 66-kDa component was no longer evident (data not shown).
To examine the specificity of the anti-PTTH serum against the 45-kDa PTTH, serial dilutions of anti-PTTH antiserum were incubated with the latter, and after incubation the sample was subjected to the ecdysone RIA. The data showed that the anti-PTTH antiserum inhibited the activity of the purified PTTH in a dose-dependent manner (Fig. 3B). PTTH activity was inhibited at a dose of 0.2 μl of antiserum, and maximal inhibition was observed at a dose of 2.0 μl. These results indicate that the antibody recognizes an epitope of the purified 45-kDa PTTH essential for biological activity.
Characterization of the Native PTTH–Glycoside.
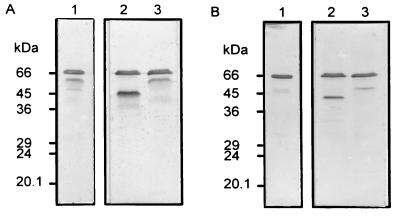
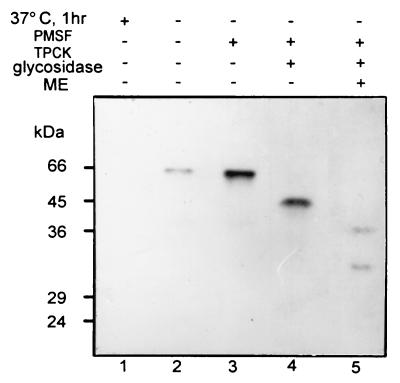
To determine why both the purified 45-kDa PTTH and the prominent 66-kDa component present in larval extracts reacted to the antibody during Western blot analysis, the possibility of a carbohydrate moiety was examined. The mobility of the 66-kDa component of larval extracts was not affected by reducing conditions (Fig. 4, lanes 1) and when the sample was incubated with N-glycosidase under nonreducing conditions, a new 45-kDa component appeared along with the 66-kDa band (Fig. 4A, lane 2). On the other hand, when the N-glycosidase digested sample was reduced, the 45-kDa band was no longer evident but a 40-kDa band appeared (Fig. 4B, lane 2). The above events were not observed when the 66-kDa peptide was incubated with O-glycosidase (Fig. 4, lanes 3). The band below the 66-kDa band (Fig. 4A, lanes 1 and 3) appeared only intermittently and is probably a partially degraded product of the 66-kDa band.
Figure 4.
Characterization of the native PTTH glycopeptide. Third instar larval extract was incubated with endoglycosidase for 1 hr at 37°C, and the samples were then subjected to SDS/PAGE under nonreducing (A) or reducing (B) conditions followed by immunoblotting. Lanes: 1, incubation medium contained protease inhibitors (2 mM PMSF and 200 μM TPCK); 2, incubation medium contained N-glycosidase (3 units); 3, incubation medium contained O-glycosidase (0.75 unit).
These results suggest that the Drosophila native PTTH is a 66-kDa peptide containing an N-linked carbohydrate moiety, and that the purified 45-kDa molecule is the deglycosylated form of PTTH. Furthermore, the native 66-kDa form appears to be a single polypeptide with intrachain disulfide bonds. Because the carbohydrate portion of the 66-kDa PTTH was not completely digested by an excess amount of N-glycosidase, the larval extracts probably contain high levels of glycoprotein as well as the previously mentioned unknown substances that interfere with the RIA.
To ensure that the identified PTTH is indeed from the brain of Drosophila, extracts of third instar larval brains were analyzed by immunoblotting under the same conditions as in Fig. 4. In both the presence or absence of protease inhibitors, a major 66-kDa band was immunostained (Fig. 5, lanes 2 and 3). However, when brain extracts were incubated at 37°C for 1 hr without protease inhibitor, the 66-kDa peptide disappeared (Fig. 5, lane 1). When the sample was digested with N-glycosidase, the 66-kDa band was replaced by the 45-kDa band (Fig. 5, lane 4). When the digested sample was reduced, the 45-kDa band gave rise to 40- and 33-kDa peptides (Fig. 5, lane 5). These 40- and 33-kDa components appear to be identical to the reduced fragments of the purified 45-kDa PTTH. These data show that PTTH does exist in the Drosophila brain and indicate that the native PTTH is a 66-kDa glycoprotein containing N-linked carbohydrate chains.
Figure 5.
Western blot analysis of PTTH in brain extracts using anti-PTTH antiserum. Protein (10 μg) extracted from brains of third instar larvae was separated by SDS/PAGE and subjected to immunoblotting. Lanes: 1, extract was incubated at 37°C for 1 hr without protease inhibitors before Western blot analysis; 2, extract analyzed in absence of protease inhibitors; 3, brain extract analyzed in the presence of protease inhibitors (2 mM PMSF and 200 μM TPCK); 4, extract was treated with N-glycosidase at 37°C for 1 hr under nonreducing conditions before analysis; 5, extract was treated with N-glycosidase at 37°C for 1 hr under reducing conditions.
Effects of Protease and Deglycosidase on PTTH Stability.
To investigate further the protease effect on the PTTH of larval extracts, these larval extracts were incubated with or without protease inhibitor at 37°C for various periods (0–24 hr) and then subjected to SDS/PAGE and immunoblot analysis (data not shown). An immunoreactive 66-kDa band decreased in intensity as the incubation time increased (0–40 min) in the absence of protease inhibitors, and was no longer observed after 60 min. These results indicate that the PTTH is degraded during sample preparation by an endogenous proteinase(s) if the latter are not inhibited. When larval extracts were incubated in the presence of protease inhibitors, the 66-kDa peptide (and a 60-kDa band) stained intensely (data not shown). After a 3 hr incubation, the 60-kDa moiety stained more intensely than the 66-kDa PTTH and a broad band of about 45-kDa appeared. By 12 hr, both the 66- and 60-kDa bands were absent but the broad band(s) of about 45-kDa persisted. Under reducing conditions, a 33-kDa band appeared. These results confirm the conclusion that the 66-kDa PTTH is digested by endogenous deglycosidase during the preparation of larval extracts and that the heterogeneous forms of the 45-kDa PTTH noted may result from the partial digestion of the carbohydrate chain. These results also suggest strongly that purified 45-kDa PTTH is the deglycosylated form of the native 66-kDa PTTH.
Activity Assay of the Degraded PTTH Fragment.
To determine if degraded PTTH retains activity, larval extracts were incubated with or without protease inhibitor (PMSF or TPCK) at 37°C for 24 hr and then divided into two samples. One was subjected to immunoblotting and the other was bioassayed via the in vitro ring gland RIA paradigm. It should be noted that PMSF is more effective than TPCK in reducing endogenous proteolytic activity and each inhibitor yields its own unique degradation pattern. In the absence of 2-mercaptoethanol and with PMSF, degradation does not proceed past the 45-kDa band, but in the presence of 2-mercaptoethanol and PMSF the 33-kDa band appears. With TPCK the 45-kDa band was not seen and degradation proceeded to 22-kDa fragments in the presence or absence of 2-mercaptoethanol. The 22-kDa fragments showed no PTTH activity upon assay, but the 45-kDa peptide did stimulate ecdysteroid synthesis and secretion as did the native 66-kDa PTTH. These data reveal that PMSF is a more effective protease inhibitor than TPCK in this system. A combination of both inhibitors was used in subsequent experiments. The results of this study suggest that the deglycosylated PTTH is functionally indistinguishable from the native PTTH and that the glycosyl group of the native form has another role, perhaps as a deterrent to proteolytic degradation.
Immunoblot Analysis of the Developmental Expression of PTTH.
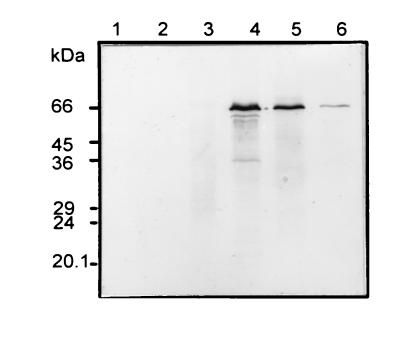
To examine possible developmental changes in PTTH content, extracts from several developmental stages were analyzed by SDS/PAGE under nonreducing conditions followed by immunoblotting. The 66-kDa PTTH was first detected in third instar larvae and reached its maximal level at this stage (Fig. 6). The PTTH level then decreased gradually but was detectable even in the adult. PTTH was not detected in embryos or in extracts of first and second instar larvae. These data are in accord with the pattern of ecdysteroid synthesis during development (24).
Figure 6.
Developmental expression of the 66-kDa PTTH. Protein extracts (28 μg) from each stage were subjected to SDS/12% PAGE, followed by immunoblotting using the anti-PTTH antiserum. Lanes: 1, eggs; 2, first instar larvae; 3, second instar larvae; 4, third instar larvae; 5, pupae; 6, adults.
Cross-Reactivity of Anti-Drosophila PTTH Antiserum with Brain Extracts of Bombyx mori.
To examine interspecific PTTH relationships between two insect orders, Lepidoptera and Diptera, brain extracts from fifth instar larvae of Bombyx mori and third instar larvae of Drosophila were analyzed by immunoblotting using the anti-PTTH serum. Western blot analysis revealed a positive signal in the brain of Drosophila, but not in the brain of Bombyx mori (data not shown).
Immunohistochemical Localization of PTTH.
A pair of small dorso–medial neurosecretory cells in the brains of third instar larvae were highly immunoreactive to the anti-PTTH antiserum (data not shown). Serial sections suggested that a pair of immunoreactive tracks extended from the center of the medial region of the brain hemisphere to the surface that is connected to the ring gland. Whole mount immunohistochemistry against Drosophila third instar larval brain, ventral ganglion, and ring gland complexes was also performed, and only the central nervous system showed immunoaffinity to the antibody preparation. In general, the data were similar to that generated using Bombyx anti-PTTH antibody (25). No immunoreactivity was detected in the control preparations that were treated with preimmune mouse serum.
DISCUSSION
Approximately 50 μg of a Drosophila 45-kDa peptide with PTTH activity were obtained from 4 × 105 (0.8 kg) whole body extracts of third instar larvae using a seven-step procedure. In the case of the only other PTTH purified and characterized, that of the silkworm Bombyx mori, 15 μg of pure PTTH was obtained from 5 × 105 heads (3.7 kg wet weight) (5). The weight of the starting material was about 4.5 times that of the Drosophila material, but about three times as much PTTH was finally obtained from the fly larval extracts as that from Bombyx adult heads. Conventional liquid chromatography was not sufficient to isolate the pure hormone since the elution patterns of PTTH activity with conventional chromatography showed that the 45-kDa moiety was highly heterogeneous. This is not surprising, however, because such heterogeneity has been found for other insect peptide hormones (5, 12, 23, 26). However, the use of RPHPLC and the final procedure of gel filtration served to remove small contaminating molecules. The purified 45-kDa peptide stimulated ecdysone synthesis and secretion by ring glands in vitro at a dose of 50 ng. Maximal activity was obtained with a dose of 200 ng, this amount being comparable to the lowest effective dose of Bombyx PTTH on the Bombyx prothoracic glands (5, 7), and this ≈10 nM concentration is in agreement with the physiological doses of other insect neurohormones as well.
When subjected to SDS/PAGE under reducing conditions, the sum of the apparent molecular weights of the reduced fragments exceeded the molecular weight of the purified 45-kDa peptide, as did that of the Bombyx PTTH (5). Amino acid sequence analysis revealed several overlapping signals for the 45-kDa peptide, but each signal was for a corresponding fragment under reducing conditions. These data suggest that the purified deglycosylated PTTH exists as a single 45-kDa polypeptide that was cleaved at a minimum of three different sites by an endoprotease during the purification procedure. These fragments are probably held together by intradisulfide bonds.
It has been reported that both Bombyx PTTH and Manduca PTTH are homodimers (5, 10, 26) and that Bombyx PTTH probably contains a carbohydrate chain bound to an asparagine at position 41 of the peptide, as deduced from the cDNA sequence (5, 27). However, the apparent native PTTH of Drosophila is a monomeric 66-kDa glycoprotein. The mobility of this protein band during PAGE was not altered under reducing conditions. In addition, the putative glycosylation of PTTH was tested by removing any N-linked oligosaccharide with a specific endoglycosidase and determining the effect on size and heterogeneity. The data revealed that the major 66-kDa band as well as several minor, smaller bands were shifted to the 45-kDa band under nonreducing conditions, a result consistent with deglycosylation of the 66-kDa moiety. These data indicate that the Drosophila PTTH is a glycoprotein containing N-linked carbohydrate chains, and this possibility is supported by the observation that the incubation of larval extracts with protease inhibitors for 6 hr resulted in the presence of only 45-kDa components. These heterogeneous 45-kDa “isoforms” remained intact until the incubation time was extended to 24 hr. The 45-kDa peptides were subsequently converted to a 33-kDa component under reducing conditions. The data support the hypothesis that the PTTH of Drosophila is a 66-kDa glycoprotein containing N-linked carbohydrate chains and intradisulfide bonds. It is likely that the larval extracts contain endoglycosidases, and the 45-kDa PTTH variants arose due to different degrees of carbohydrate chain cleavage resulting in different shapes and thus apparent multiple molecular weights.
It is also evident that the PTTH can undergo proteolysis during the purification process due to the presence of endogenous proteases, since the 66-kDa PTTH disappeared within 1 hr after incubation at 37°C in the absence of protease inhibitors. The effect of protease inhibitors on the degradation of PTTH as an indicator of overall proteolytic suppression was analyzed, and PMSF appears to be the most effective.
In many glycoproteins, the carbohydrate moiety endows the polypeptide with important physical characteristics such as conformational stability, protease resistance, charge, and water binding capacity (28). However, in the case of the Drosophila PTTH, the glycosyl group is not requisite for biological activity since the 45-kDa peptide was functionally indistinguishable from the native form, insofar as ring gland stimulation was concerned. This is also true of the Bombyx PTTH (5). Therefore, the glycosyl group may serve only as a guardian against protease activity and perhaps aid in the stabilization of the structure.
Previously, two smaller peptides were identified as candidates for the Drosophila PTTH, and weak PTTH activity was associated with a larger peptide (29). This reduced activity may result from the absence of protease inhibitors in the larval extract. In this study, lower molecular weight moieties with PTTH activity were not noted, perhaps because of their removal during the initial stages of purification or because they are degradative products of the 66-kDa PTTH.
Insect postembryonic development is initiated and coordinated by the precise temporal release of PTTH that is synthesized by specific neurosecretory cells in the insect brain and released from a neurohemal organ (2, 4, 11–13). In the case of Drosophila, PTTH expression was maximal in third instar larvae and decreased gradually throughout pupal–adult development. At the adult stage, it was detected, albeit at a low level, consistent with observations on the Bombyx PTTH. The prothoracic gland portion of the ring gland undergoes degeneration and cell death during adult development and is absent in adults (14), so that the adult “PTTH” may play a role separate from the stimulation of ecdysteroidogenesis in the adult fly, as it may in the adult moth.
Although the immunocytochemical analysis using the PTTH antibody is not yet complete, positive signals were observed in the posterior–lateral area of the protocerebrum. However, signals were also observed in other regions of the brain, perhaps due to the transit of axonal tracts emanating from the lateral cell bodies. In general, the picture resembled that observed in the Drosophila brain when Bombyx antibody was used as the probe (25). The reason for this similarity is presently unknown.
Acknowledgments
We thank the Institute of Molecular Biology and Genetics (IMBG) for technical assistance, Won Seok Son for photographic assistance, Pat Cabarga for clerical help, and Dr. David Schooley for constructive comments. This work was supported in part by grants from the Korea Science Engineering Foundation (KOSEF) through the Research Center for Cell Differentiation and from the Basic Science Research Program (Korea Ministry of Education). L.I.G. was supported by National Institutes of Health Grants DK-30118 and RR06627 and National Science Foundation Grant IBN-9300164.
Footnotes
Abbreviations: Ar, activation ratio; PTTH, prothoracicotropic hormone; RIA, radioimmunoassay; RPHPLC, reverse-phase HPLC; PMSF, phenylmethylsulfonyl fluoride; TPCK, l-1-tosylamido-2-phenylethyl chloromethyl ketone.
References
- 1.Gilbert L I, Combest W L, Smith W A, Meller V H, Rountree D B. BioEssays. 1988;8:153–157. doi: 10.1002/bies.950080506. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert L I, Rybczynski R, Tobe S. In: Metamorphosis: Postembryonic Reprogramming of Amphibians and Insect Cells. Gilbert L I, Tata J R, Atkinson B G, editors. San Diego: Academic; 1996. pp. 59–107. [Google Scholar]
- 3.Kopeć S. Biol Bull. 1922;42:323–342. [Google Scholar]
- 4.Bollenbacher W E, Granger N A. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut G A, Gilbert L I, editors. Vol. 7. Oxford: Pergamon; 1985. pp. 109–151. [Google Scholar]
- 5.Ishizaki H, Suzuki A. Prog Brain Res. 1992;92:1–14. doi: 10.1016/s0079-6123(08)61160-7. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki A, Nagasawa H, Kataoka H, Hori Y, Isogai A, Tamura S, Guo F, Zhong X, Ishizaki H, Fujishita M, Mizoguchi A. Agric Biol Chem. 1982;46:1107–1109. [Google Scholar]
- 7.Kawakami A, Kataoka H, Oka T, Mizoguchi A, Kimura-Kawakami M, Adachi T, Iwamai M, Nagasawa H, Suzuki A, Ishizaki H. Science. 1990;247:1333–1335. doi: 10.1126/science.2315701. [DOI] [PubMed] [Google Scholar]
- 8.Bollenbacher W E, Katahira E J, O’Brien M, Gilbert L I, Thomas M K, Agui N, Baumhover A H. Science. 1984;224:1243–1245. doi: 10.1126/science.6732895. [DOI] [PubMed] [Google Scholar]
- 9.Muelheisen D P, Gray R S, Katahira E J, Thomas M K, Bollenbacher W E. Peptides. 1993;14:531–541. doi: 10.1016/0196-9781(93)90142-4. [DOI] [PubMed] [Google Scholar]
- 10.Muelheisen D P, Katahira E J, Gray R S, Bollenbacher W E. Experientia. 1994;50:159–163. doi: 10.1007/BF01984956. [DOI] [PubMed] [Google Scholar]
- 11.Mizoguchi A, Oka T, Kataoka H, Nagasawa H, Suzuki A, Ishizaki H. Dev Growth Differ. 1990;32:591–598. doi: 10.1111/j.1440-169X.1990.00591.x. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien M A, Katahira E J, Hanagan T R, Arnold L W, Haughton G, Bollenbacher W E. J Neurosci. 1988;8:5840–5843. doi: 10.1523/JNEUROSCI.08-09-03247.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai J-D, Mizoguchi A, Gilbert L I. Inverteb Reprod Dev. 1994;26:187–196. [Google Scholar]
- 14.Dai J-D, Gilbert L I. Dev Biol. 1991;144:309–326. doi: 10.1016/0012-1606(91)90424-2. [DOI] [PubMed] [Google Scholar]
- 15.Ashburner M, Tompson J N. The Genetics and Biology of Drosophila. London: Academic; 1978. [Google Scholar]
- 16.Henrich V C, Pak M D, Gilbert L I. J Comp Physiol B. 1987;157:543–549. doi: 10.1007/BF00700973. [DOI] [PubMed] [Google Scholar]
- 17.Warren J T, Gilbert L I. Insect Biochem. 1986;16:65–82. [Google Scholar]
- 18.Reum L, Koolman J. In: Ecdysone: From Chemistry to Mode of Action. Koolman J, editor. Stuttgart: Thieme; 1989. pp. 131–143. [Google Scholar]
- 19.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Harlow E, Lane D. In: Antibodies: A Laboratory Manual. Harlow E, Lane D, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 53–138. [Google Scholar]
- 21.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnette W N. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 23.Kingan T G. Life Sci. 1981;28:2585–2594. doi: 10.1016/0024-3205(81)90715-3. [DOI] [PubMed] [Google Scholar]
- 24.Hodgetts R B, Sage B, O’Connor J D. Dev Biol. 1977;60:310–317. doi: 10.1016/0012-1606(77)90128-2. [DOI] [PubMed] [Google Scholar]
- 25.Z̃itñan D, Sehnal F, Bryant P J. Dev Biol. 1992;156:117–135. doi: 10.1006/dbio.1993.1063. [DOI] [PubMed] [Google Scholar]
- 26.Nagasawa H, Kataoka H, Isogai A, Tamura S, Suzuki A, Ishizaki H, Mizoguchi A, Fujiwara Y, Suzuki A. Science. 1984;226:1344–1345. doi: 10.1126/science.226.4680.1344. [DOI] [PubMed] [Google Scholar]
- 27.Ishibashi J, Kataoka H, Isogai A, Kawakami A, Saegusa H, Yasi Y, Mizoguchi A, Ishizaki H, Suzuki A. Biochemistry. 1994;33:5912–5919. doi: 10.1021/bi00185a031. [DOI] [PubMed] [Google Scholar]
- 28.Cumming, D. A. (1991) Glycobiology (IRL, Oxford).
- 29.Pak J W, Chung K W, Lee C C, Kim K, Namkoong Y, Koolman J. J Insect Physiol. 1992;38:167–176. [Google Scholar]