Abstract
Background
Whether the cardioprotective characteristic of higher cardiorespiratory fitness (CRF) extends to adults with manifest hypertension (HTN) is poorly understood.
Methods
We examined the association between CRF and nonfatal cardiovascular disease (CVD) events in 8147 men and 1268 women, who, at baseline, were free of known CVD and had HTN based on a history of physician diagnosis or a measured resting blood pressure (BP) ≥140/90 mm Hg. The CVD events (myocardial infarction, stroke, coronary revascularization) were ascertained from mail-back surveys. The CRF was quantified as maximal treadmill exercise test duration and was grouped for analysis as low (lowest 20% of exercise duration), moderate (middle 40%), and high (upper 40%).
Results
A total of 71 CVD events occurred during 12,224 woman-years, and 837 CVD events occurred during 82,366 man-years of follow-up. Age and examination year adjusted CVD rates per 1000 person-years according to low, moderate, and high CRF groups were 10.8, 8.4, and 3.8 (trend P = .001) in women, and were 15.3, 10.9, and 7.2 (trend P < .001) in men. After further controlling for CVD risk factors, abnormal exercise electrocardiogram (ECG) responses, and family history of CVD, hazards ratios (95% CI) for CVD events across incremental CRF categories were 1.00 (referent), 0.88 (0.74 to 1.06), 0.70 (0.57 to 0.86), trend P < .001, in men, and were 1.00 (referent), 0.87 (0.48 to 1.58), 0.41 (0.20 to 0.84), trend P = .01, in women.
Conclusions
In adults with HTN, higher CRF is associated with lower risk of nonfatal CVD events, independent of other clinical risk predictors. Am J Hypertens 2007;20:608 - 615
Keywords: Physical fitness, hypertension, cardiovascular diseases, morbidity
Hypertension (HTN) is a prevalent major modifiable cardiovascular disease (CVD) risk factor among US adults.1,2 Nearly 1 in 3 or approximately 65 million US adults have HTN. From 1993 to 2003, the age-adjusted death rate from HTN increased about 30%, and the actual number of HTN-associated deaths rose 56%.1 The estimated total cost of HTN for 2006 exceeds $60 billion.1,2 Clearly, HTN is a major economic and public health burden.
Physical activity and cardiorespiratory fitness (CRF) levels are inversely associated with CVD morbidity and mortality.3,4 Higher physical activity and CRF also are associated with lower risk of new onset HTN.4-8 Moreover, accumulating evidence shows that exercise training can lower blood pressure (BP) in hypertensive adults.9,10 A few epidemiologic studies have reported protective associations between physical activity or CRF and CVD mortality in adults with HTN.11-14 To our knowledge, the relationship between CRF and nonfatal CVD events in adults with HTN has not been reported. Thus, we examined this issue in a cohort of adults with diagnosed HTN who are enrolled in the Aerobics Center Longitudinal Study (ACLS).
Methods
Study Population
Participants were 8147 men and 1268 women, aged 18 to 84 years, who completed a baseline clinical examination between 1971 and 2002 at the Cooper Clinic, Dallas, TX. The current analysis included participants who, at baseline, were free of known CVD, had normal resting electrocardiograms (ECG), and were able to complete an exercise stress test to at least 85% of their age-predicted maximal heart rate. All participants had HTN based on a history of physician diagnosis or a measured resting systolic or diastolic BP of ≥140 or ≥90 mm Hg, respectively. All participants responded to at least one follow-up mail-back health survey between 1982 and 2004. The majority of participants was white and from middle and upper socioeconomic strata. Written informed consent was obtained from participants before enrollment into the follow-up study, and the study was reviewed and approved annually by the Cooper Institute Institutional Review Board.
Clinical Examination
The baseline physician examination and clinical assessment were conducted after an overnight fast of at least 12 h.6,7 Body mass index (BMI, kg/m2) was computed from measured height and weight. After a brief period of sitting quietly, resting BP was measured in the seated position using auscultatory methods with a mercury sphygmomanometer. Systolic and diastolic pressure was recorded as the first and fifth Korotkoff sounds. Serum samples were analyzed for lipids and glucose using standardized bioassays. The presence of diabetes and dyslipidemia was based on a history of physician diagnosis or measured phenotypes that met clinical thresholds for each condition. Smoking habits and alcohol intake were obtained from a questionnaire. Drinks per week of alcohol intake were computed with one drink standardized to 12 ounces of beer, 5 ounces of wine, or 1.5 ounces of hard liquor.
The CRF was quantified as the duration of a maximal treadmill exercise test using a modified Balke-Ware protocol.6,15 Exercise duration on this protocol is highly correlated with measured maximal oxygen uptake (r > 0.90).6 The percentage of age-predicted maximal heart rate (eg, 220 - age) that was achieved during exercise testing was 101% ± 8% in men and was 100% ± 9% in women. To standardize exercise performance, we estimated maximal metabolic equivalents (METs, 1 MET = 3.5 mL of O2 uptake/kg/min) from the final treadmill speed and grade.16 In previous ACLS reports, which have shown low CRF is an independent predictor of mortality and incident nonfatal disease,7,17 we have defined low, moderate, and high CRF exposures according to the lowest 20%, the middle, and the upper 40%, respectively, of the age- and sex-specific distribution of maximal exercise duration in the overall ACLS population (Table 1). To maintain consistency in our study methods and because a widely accepted clinical categorization of CRF does not exist, we used this approach. Abnormal exercise ECG responses were defined broadly and included rhythm and conduction disturbances and ischemic ST-T wave abnormalities, as described elsewhere.18 We have found 90% agreement between the ECG interpretation recorded in our database and that of a group of three physicians who read a random sample of 357 patient records.18
Table 1.
Age- and sex-specific maximal treadmill* exercise duration and estimated MET levels of cardiorespiratory fitness
| Men |
Women |
||||
|---|---|---|---|---|---|
| Age (y) | CRF group | Duration (min) | METs | Duration (min) | METs |
| 20-39 | Low | <15.0 | <10.4 | <10.3 | <8.2 |
| Moderate | 15.0-20.3 | 10.4-13.1 | 10.3-15.0 | 8.2-10.4 | |
| High | >20.3 | >13.1 | >15.0 | >10.4 | |
| 40-49 | Low | <13.5 | <9.9 | <8.9 | <7.6 |
| Moderate | 13.5-19.0 | 9.9-12.2 | 8.9-13.0 | 7.6-9.4 | |
| High | >19.0 | >12.2 | >13.0 | >9.4 | |
| 50-59 | Low | <11.0 | <8.5 | <7.0 | <6.7 |
| Moderate | 11.0-16.0 | 8.5-10.8 | 7.0-10.7 | 6.7-8.5 | |
| High | >16.0 | >10.8 | >10.7 | >8.5 | |
| ≥60 | Low | <7.8 | <7.2 | <5.5 | <5.8 |
| Moderate | 7.8-13.1 | 7.2-9.5 | 5.5-9.0 | 5.8-7.6 | |
| High | >13.1 | >9.5 | >9.0 | >7.6 | |
CRF = cardiorespiratory fitness; METs = metabolic equivalents.
Treadmill exercise testing was performed using a modified Balke-Ware protocol as described in the Methods section.
Assessment of Outcomes
The CVD events were ascertained from responses to mail-back health surveys. In the overall ACLS population, the aggregate survey response rate is 65% to 75% across seven survey periods. Nonresponse bias is a concern in epidemiologic surveillance and this issue has been investigated in the ACLS.19 Baseline health histories and clinical measures were similar between responders and nonresponders and between early and late survey responders.19 Total mortality rates also have been similar between survey responders and nonresponders (unpublished data). Overall, our surveillance experience appears comparable to that described in other long-term cohort studies.20 Nonfatal CVD end points were obtained using case-finding questions for a physician diagnosis of myocardial infarction (MI) or stroke, and for having a coronary revascularization procedure (coronary artery bypass graft or percutaneous coronary intervention). Participants were asked whether a physician had ever told them that they had a heart attack or stroke, or if they had undergone any coronary interventions. If yes, participants were asked to report the year of the diagnosis or procedure. Our method of ascertaining study end points and the case-finding questions are similar to those used in other established epidemiologic studies on CVD.21,22 In participants reporting multiple events, the first event was used for analysis. The primary outcome was total CVD events (MI, coronary revascularization, and stroke). We also examined coronary heart disease (CHD) events (MI, coronary revascularization) as a separate end point. In a random sample of these end points (n = 50 each), we applied standard definitions for documenting MI, revascularization, and stroke.23,24 The percentage of agreement between reported events and medical record review was 88%, 100%, and 89% for MI, revascularization procedures, and stroke, respectively.
Statistical Analysis
Baseline characteristics of the population were estimated by fitness category and by CVD status for men and women. Trends in covariates by fitness were estimated using F tests. Follow-up time among noncases was calculated as the difference between the date of the baseline examination and the date of the last returned survey where the participant was reported to be free of CVD. Follow-up time among cases was computed as the difference between the baseline examination date and the reported date of the CVD event. If a diagnosis date was not provided, we used the midpoint between the date of the case-finding survey and either the baseline examination date or the date of the last returned survey where the participant was reported to be free of CVD. The mean ± SD follow-up interval in years was 10.1 ± 7.7 for men and 9.6 ± 7.1 for women. Cox regression analysis was used to estimate hazards ratios and 95% confidence intervals of CVD events according to exposure categories. The proportional hazards assumption was examined by comparing the cumulative hazards plots grouped on exposure in women and in men; no appreciable violations were noted. We constructed an indicator variable to account for differences in survey response frequency. All multivariable analyses included as covariables: age (years), examination year, survey response frequency, smoking status (current smoker or not), alcohol intake (≥5 drinks/week or not), abnormal exercise ECG responses (present or not), and family history of CVD (present or not). We conducted an additional analysis that further adjusted for differences in the following five factors that may be intermediate in the causal pathway between CRF and CVD: resting systolic and diastolic BP (per mm Hg), diabetes and dyslipidemia (present or not for each), and BMI (per kg/m2). Tests of linear trends were computed using ordinal scoring. There were no a priori hypotheses on sex differences in CVD event rates or in the association between CRF and CVD risk. All P values are two-sided and P < .05 was regarded as statistically significant.
Results
There were 837 CVD events (288 MI, 183 strokes, 366 revascularizations) during 82,366 man-years of exposure and 71 CVD events (28 MI, 30 strokes, 13 revascularizations) during 12,224 woman-years of exposure. At baseline, low fit individuals (Table 2) were slightly younger than those in the moderate and high fitness categories and CVD risk factors were often less favorable. Compared with noncases, individuals who developed CVD were older, had lower CRF, and had higher prevalence of major CVD risk factors (Table 3).
Table 2.
Baseline characteristics of study participants by CRF category among men and women
| Men |
|||||
|---|---|---|---|---|---|
| Total (n = 8147) | Low CRF (n = 1855) | Moderate CRF (n = 3203) | High CRF (n = 3089) | P for trend | |
| Age (mean ± SD, y) | 47.4 ± 9.8 | 45.0 ± 9.5 | 47.4 ± 9.7 | 49.0 ± 9.7 | <.001 |
| Body mass index (mean ± SD, kg/m2) | 27.1 ± 4.0 | 29.9 ± 5.1 | 27.2 ± 3.3 | 25.4 ± 2.7 | <.001 |
| Blood pressure (mean ± SD, mm Hg) | |||||
| Systolic | 133 ± 14 | 134 ± 14 | 133 ± 14 | 133 ± 14 | <.001 |
| Diastolic | 90 ± 9 | 91 ± 9 | 90 ± 9 | 89 ± 8 | <.001 |
| Current smoker, no. (%) | 1357 (16.7) | 536 (28.9) | 558 (17.4) | 263 (8.5) | <.001 |
| Alcohol intake (≥5 drinks per week), no. (%) | 3238 (39.7) | 807 (43.5) | 1314 (41.0) | 1117 (36.2) | <.001 |
| Abnormal ECG during exercise, no. (%) | 596 (7.3) | 184 (9.9) | 241 (7.5) | 171 (5.5) | <.001 |
| Diabetes mellitus,* no. (%) | 677 (8.3) | 253 (13.6) | 277 (8.7) | 147 (4.8) | <.001 |
| Dyslipidemia,† no. (%) | 5569 (68.4) | 1619 (87.3) | 2337 (73.0) | 1613 (52.2) | .88 |
| Family history of premature CVD, no. (%) | 1346 (16.5) | 316 (17.0) | 517 (16.1) | 513 (16.6) | .70 |
|
Women |
|||||
| Total (n = 1268) | Low CRF (n = 235) | Moderate CRF (n = 437) | High CRF (n = 596) | P for trend | |
| Age (mean ± SD, y) | 50.3 ± 10.1 | 47.6 ± 10.4 | 49.4 ± 10.1 | 52.0 ± 9.7 | <.001 |
| Body mass index (mean ± SD, kg/m2) | 24.2 ± 4.5 | 27.3 ± 6.2 | 24.5 ± 4.2 | 22.9 ± 3.1 | <.001 |
| Blood pressure (mean ± SD, mm Hg) | |||||
| Systolic | 131 ± 17 | 130 ± 16 | 131 ± 17 | 132 ± 16 | .48 |
| Diastolic | 87 ± 10 | 87 ± 11 | 87 ± 10 | 88 ± 9 | .38 |
| Current smoker, no. (%) | 89 (7.0) | 31 (13.2) | 34 (7.8) | 24 (4.0) | <.001 |
| Alcohol intake (≥5 drinks per week), no. (%) | 217 (17.1) | 46 (19.6) | 105 (24.0) | 66 (11.1) | <.001 |
| Abnormal ECG during exercise, no. (%) | 121 (9.5) | 28 (1.9) | 44 (10.1) | 49 (8.2) | .24 |
| Diabetes mellitus,* no. (%) | 71 (5.6) | 19 (8.1) | 29 (6.6) | 23 (3.9) | .03 |
| Dyslipidemia,† no. (%) | 605 (47.7) | 158 (67.2) | 251 (57.4) | 196 (32.9) | .67 |
| Family history of premature CVD, no. (%) | 260 (20.5) | 35 (14.9) | 95 (21.7) | 130 (21.8) | .06 |
CRF = cardiorespiratory fitness; CVD = cardiovascular disease; ECG = electrocardiogram; SD = standard deviation.
Diabetes mellitus was defined as a fasting plasma glucose concentration ≥7.0 mmol/L (126 mg/dL), a history of physician diagnosis, or insulin use
Dyslipidemia was defined as one or more of the following: total cholesterol ≥6.20 mmol/L (240 mg/dL) or a history of physician diagnosis, triglycerides ≥2.26 mmol/L (200 mg/dL), or HDL <1.03 mmol/L (40 mg/dL).
Table 3.
Baseline characteristics of study participants by sex and cardiovascular disease event status
| Men |
Women |
|||
|---|---|---|---|---|
| Characteristic | Noncase (n = 7310) | Case (n = 837) | Noncase (n = 1197) | Case (n = 71) |
| Age (mean ± SD, y) | 46.9 ± 9.8 | 52.2 ± 8.5 | 50.1 ± 10.1 | 54.0 ± 9.2 |
| Body mass index (mean ± SD, kg/m2) | 27.2 ± 4.0 | 26.9 ± 3.6 | 24.3 ± 4.3 | 23.8 ± 3.8 |
| Maximal METs (mean ± SD) | 11.0 ± 2.4 | 10.2 ± 2.3 | 8.8 ± 2.1 | 7.7 ± 1.7 |
| Blood pressure (mean ± SD, mm Hg) | ||||
| Systolic | 133 ± 14 | 135 ± 15 | 131 ± 17 | 133 ± 13 |
| Diastolic | 90 ± 9 | 89 ± 9 | 87 ± 9 | 87 ± 9 |
| Current smoker, no. (%) | 1219 (16.7) | 138 (16.5) | 82 (6.9) | 7 (9.9) |
| Alcohol intake (≥5 drinks per week), no. (%) | 2926 (40.0) | 312 (37.3) | 199 (16.6) | 18 (25.4) |
| Abnormal ECG during exercise, no. (%) | 451 (6.2) | 145 (17.3) | 106 (8.9) | 15 (21.1) |
| Diabetes mellitus,* no. (%) | 571 (7.8) | 106 (12.7) | 67 (5.6) | 4 (5.6) |
| Dyslipidemia,† no. (%) | 4912 (67.2) | 657 (78.5) | 561 (46.9) | 44 (62.0) |
| Family history of premature CVD, no. (%) | 1193 (16.3) | 153 (18.3) | 248 (20.7) | 12 (16.9) |
CRF = cardiorespiratory fitness; CVD = cardiovascular disease; ECG = electrocardiogram; SD = standard deviation.
Diabetes mellitus was defined as a fasting plasma glucose concentration ≥7.0 mmol/L (126 mg/dL), a history of physician diagnosis, or insulin use
Dyslipidemia was defined as one or more of the following: total cholesterol ≥6.20 mmol/L (240 mg/dL) or a history of physician diagnosis, triglycerides ≥2.26 mmol/L (200 mg/dL), or HDL <1.03 mmol/L (40 mg/dL).
Rates and hazards ratios of CVD events according to CRF groups among men are shown in Table 4. An inverse gradient of total CVD event rates was observed across incremental CRF groups (trend P < .001). After adjusting for covariables, men with moderate and high CRF had a 14% and 34% lower risk of CVD events than men with low CRF (trend P < .001). The inverse association between CRF and CVD events remained significant after additional adjustment for BMI, resting BP, diabetes, and dyslipidemia (trend P < .001). Similar inverse patterns of association were observed between CRF and CHD events (P < .001).
Table 4.
Rates and hazards ratios for cardiovascular disease events by CRF groups in hypertensive men and women
| Outcomes CRF status | Person-years of follow-up | Events | Rate* | HR† | 95% CI | HR‡ | 95% CI |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| Total CVD | |||||||
| Low CRF | 16,268 | 205 | 15.3 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 31,902 | 350 | 10.9 | 0.86 | 0.72-1.03 | 0.88 | 0.74-1.06 |
| High CRF | 33,763 | 282 | 7.2 | 0.66 | 0.54-0.80 | 0.70 | 0.57-0.86 |
| P linear trend | <.001 | <.001 | <.001 | ||||
| CHD | |||||||
| Low CRF | 16,472 | 180 | 12.9 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 32,222 | 295 | 9.1 | 0.87 | 0.71-1.05 | 0.89 | 0.73-1.08 |
| High CRF | 34,103 | 232 | 5.9 | 0.67 | 0.54-0.82 | 0.71 | 0.57-0.89 |
| P linear trend | <.001 | <.001 | .002 | ||||
| Women | |||||||
| Total CVD | |||||||
| Low CRF | 2160 | 29 | 10.8 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 4239 | 32 | 8.4 | 0.94 | 0.52-1.70 | 0.87 | 0.48-1.58 |
| High CRF | 5757 | 20 | 3.8 | 0.49 | 0.24-0.97 | 0.41 | 0.20-0.84 |
| P linear trend | .001 | .03 | .01 | ||||
| CHD | |||||||
| Low CRF | 2193 | 13 | 6.5 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 4318 | 21 | 4.7 | 0.96 | 0.46-1.97 | 0.96 | 0.46-2.01 |
| High CRF | 5781 | 12 | 2.0 | 0.47 | 0.20-1.13 | 0.47 | 0.19-1.17 |
| P linear trend | .004 | .08 | .09 |
CHD = coronary heart disease; CI = confidence interval; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; HR = hazards ratio.
Rate is expressed as per 1000 person-years and adjusted for age and examination year
Adjusted for the above plus survey indicator, current smoking (yes or not), alcohol intake (≥5 drinks/wk, or not), family history of CVD (present or not), and abnormal exercise electrocardiogram responses (present or not)
Adjusted for the above plus body mass index (kg/m2), resting systolic and diastolic blood pressure (mm Hg), diabetes, or dyslipidemia (present or not for each).
In women (Table 4), rates of total CVD events were inversely associated with CRF (trend P = .001). After adjustment for covariables, women with moderate and high CRF had a 6% and 51% lower risk of CVD events than women with low CRF (trend P = .03). The CRF remained inversely associated with CVD risk after additional adjustment for intermediate risk factors (trend P = .01). The CRF also was inversely associated with rates of CHD events (trend P = .004); however, the significant inverse trend was attenuated by adjustment for covariables (P = .08) and for intermediate risk factors (P = .09).
We next examined whether the severity of baseline HTN modified the association between CRF and CVD incidence (Figs. 1 and 2). For this analysis, measured BPs and reported HTN status at baseline were used to group participants into the following categories:2 controlled HTN (BP < 140/90 mm Hg with history of physiciandiagnosed HTN), stage 1 HTN (BP 140/90 to 160/100 mm Hg, with or without physician diagnosis), or stage 2 HTN (BP ≥160/100 mm Hg, with or without physician diagnosis). There was weak statistical evidence of interaction between CRF and HTN severity in predicting CVD events in men (χ2df = 1 = 3.4, P = .06) and in women (χ2df = 1 = 2.4, P = .12), although statistical power to examine interaction effects likely was inadequate. In men, a significant inverse gradient of age and examination year-adjusted CVD rates were seen across incremental CRF levels within each HTN group. Across HTN strata, men in the lowest CRF group had a 1.6- to 2.6-fold higher rate of total CVD events than men in the highest CRF group. In women, CRF was inversely associated with CVD rates within HTN groups; however, the rate differences were not statistically significant.
FIG. 1.
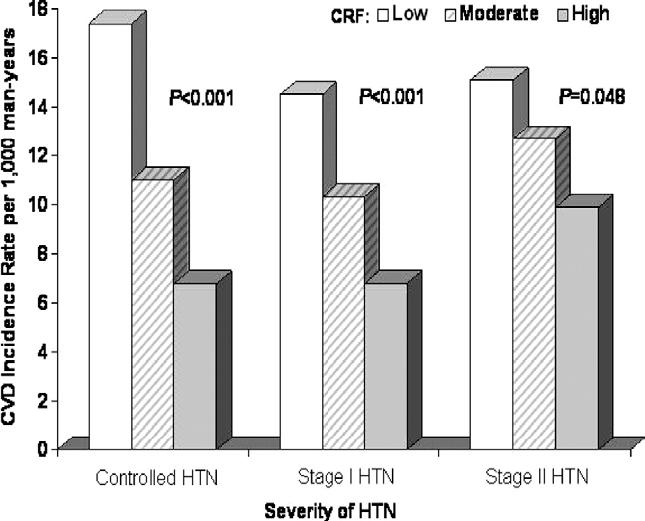
Age and examination year adjusted rates of total CVD events (per 1000 man-years) by levels of cardiorespiratory fitness and severity of HTN in 8147 hypertensive men. The number of men (and cases) in the low, moderate, and high CRF groups were 418 (47), 697 (86), and 546 (51) in controlled HTN; were 1027 (111), 1973 (196), and 2172 (184) in stage 1 HTN; and were 410 (47), 533 (68), and 371 (47) in stage 2 HTN. CRF = cardiorespiratory fitness; CVD = cardiovascular disease; HTN = hypertension.
FIG. 2.
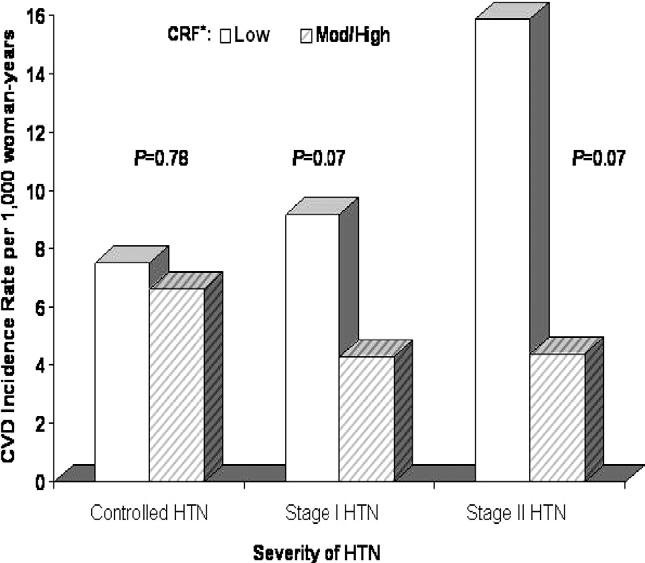
Age and examination year adjusted rates of total CVD events (per 1000 woman-years) by levels of cardiorespiratory fitness and severity of HTN in 1268 hypertensive women. *Moderate and high fitness were collapsed because of the small number of CVD events in these cells. The number of women (and cases) in the low and moderate/high CRF groups were 91 (6) and 317 (22) in controlled HTN; were 109 (9) and 590 (25) in stage 1 HTN; and were 35 (4) and 126 (5) in stage 2 HTN. CRF = cardiorespiratory fitness; CVD = cardiovascular disease; HTN = hypertension.
Discussion
The CRF is inversely associated with the risk of all-cause and CVD mortality in initially asymptomatic women and men.3,4 Exposure to cardiovascular risk factors has been variable in these cohorts. Therefore, statistical power often is insufficient to examine the association between CRF and CVD within population groups that have higher CVD rates because of the presence of clinically manifest disease, such as HTN. In a previous ACLS report, CVD death rates of 26.7, 14.2, and 11.9 per 10,000 man-years were seen across low, moderate, and high CRF groups (trend P = .02), respectively, in hypertensive men.12 Inverse patterns of association between physical activity or CRF exposures and CVD mortality generally have been reported in the few other published studies in hypertensive adults.11,13,14 This suggests that physical activity and CRF may favorably influence the etiologic pathway between HTN and fatal clinical CVD events. The risk of CVD mortality may be different from that associated with having a nonfatal event.25 To more thoroughly evaluate the role of physical activity or CRF in primary CVD prevention, it is important to examine relationships with nonfatal CVD events and not merely with mortality.
The present study demonstrated that higher CRF was associated with significantly lower rates of nonfatal CVD events in adults with clinically documented HTN. The inverse association between CRF and CVD events was present in women and in men and persisted after considering the potential confounding effects of other established CVD risk predictors. The severity of baseline HTN did not modify the inverse pattern of association between CRF and CVD. Protective associations also were seen between CRF and nonfatal CHD. Some of the inverse trends in women were attenuated in multivariable analyses due to low statistical power. This is one of the largest prospective studies, and to our knowledge the first in women, to relate an objectively measured CRF exposure with the occurrence of nonfatal CVD in a population sample of adults with HTN.
The Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure guidelines include both pharmacologic and lifestyle interventions for primary HTN prevention and for managing the risk of HTN-related morbidity and mortality.2 Although promoting higher physical activity levels is mentioned with diet and stress management as components of antihypertensive lifestyle modification, historically the major hygienic focus has been on diet modification. The CRF is an objective physiologic measure that reflects the functional effects of physical activity habits, genetics, and diseases status.26 Previous ACLS investigations have shown favorable associations between CRF and BP-related health issues. The CRF is inversely associated with the prevalence of elevated clinical BP,27 the incidence of HTN,6,7 and with the rates of total and CVD mortality in men with diagnosed HTN.12,28 The present results extend these previous findings to nonfatal CVD end points in women and men with documented HTN.
The cardioprotection of higher CRF was observed across a broad range of elevated BPs. In men with stage 2 HTN, CVD incidence rates in moderately and highly fit men were 17% and 35% lower, respectively, than those in low fit men; similarly, lower event rates of 29% to 41% and 57% to 65% were seen in moderately and highly fit men, respectively, with controlled HTN and with stage 1 HTN. Women with moderate and high CRF experienced CVD rates that were 14% to 75% lower than those in women with low CRF who had controlled, stage 1, or stage 2 HTN. Statistical power of these group analyses in women, however, was limited by a small number of events. Comparatively, clinical trial data indicate that antihypertensive drug therapy is associated with an average CVD risk reduction of 20-40%.2 Thus, based on observational findings from the present study and other studies,11-14 higher levels of physical activity and CRF may confer similar CVD risk reduction in hypertensive adults, as is seen with antihypertensive drug therapy. The cardiovascular benefits in hypertensive adults may be even greater in response to a combined pharmacologic and physical activity intervention. Data from randomized controlled trials on the independent and joint effect of drug therapy and exercise training on CVD events in hypertensive patients would strengthen these suggestive observational findings. The findings reported here and elsewhere11-14 reinforce national recommendations29 and underscore the importance of promoting higher CRF levels, presumably through greater physical activity, for primary CVD prevention in high-risk population groups, such as individuals with HTN.
The current study has strengths and limitations that should be considered. One of the strengths of the study is the extensive baseline examination. Undetected subclinical disease at baseline is a concern in prospective studies. It is less likely in our cohort because of the comprehensive physician examination and objective measurements completed by participants. Other strengths include the large sample of women and men with a clinically documented HTN, the use of maximal exercise testing to quantify CRF, the large number of person-years of follow-up, and a generally adequate number of events for sex-specific analyses. The CRF was associated with CVD events independent of other risk factors, which strengthens causal inferences. Biological plausibility for the observed associations may be through enhanced endothelial cell function and coronary flow reserve, reduced myocardial oxygen demand under a variety of circumstances, higher myocardial arrhythmia threshold, improved endogenous thrombolytic activity, and lower levels of circulating atherothrombotic cytokines, which promote a more stable coronary plaque milieu.4
The homogeneity of the ACLS population on sociodemographic factors deserves comment. On one hand this enhances the internal validity of our findings by reducing the degree of confounding by these factors. On the other hand, our findings may not apply to individuals who are not white or who are from lower socioeconomic strata. We are not aware of biological reasons that the association between CRF and CVD would be greatly different across race-ethnicity or sociodemographics. Care should be taken if attempting to generalize these results to other populations.
We did not have sufficient information on medication usage, menopausal status, or dietary habits to include in our analysis. It is possible that residual confounding by these factors may exist, although it seems unlikely that it would account for all of the observed association between fitness and CVD risk. Due to the widespread geographic distribution of patients evaluated at the Cooper Clinic, we were unable to verify all reported CVD events. However, based on a random sample of verified events, it appears that an acceptable level of agreement exists between the participants’ self-reported histories and their medical records. The present study related a single baseline CRF exposure with the 10-year occurrence of primary CVD events. Changes during the follow-up period in CRF or other risk-reducing behaviors, in the awareness and treatment of HTN, and in the clinical definition of HTN may, in part, explain the pattern of CVD event rates seen across exposure categories. Changes in CRF status and other health-related behaviors likely would result in nondifferential misclassification in a prospective study, which would weaken rather than strengthen the exposure-outcome association. Changes in the clinical definition of HTN, increased awareness, and improved treatment of HTN did occur during the period 1971 to 2004. To account in part for these potential temporal effects, we included in our analyses examination year and a term for survey response pattern. It was not possible to conduct a more extensive examination on the effect that these issues may have had on CVD events during the follow-up interval.
We conclude that CRF is inversely associated with the incidence of nonfatal CVD events in women and men with HTN. The association is graded, biologically plausible, and observed within strata of greater HTN severity. These findings suggest that functional capacity is an important prognostic indicator in hypertensive adults beyond traditional risk factors. The CRF can be enhanced in most individuals, including those with HTN,30 through participation in physical activities such as brisk walking, cycling, and jogging, for 30 min or more on most days of the week.29 Given the high population prevalence of HTN and because hypertension control rates still are far below the Healthy People 2010 Goal of 50%, the public health burden attributed to HTN continues to be large and may well increase in coming years. We, therefore, believe that clinicians should consider the potential independent cardioprotective benefits of greater physical activity and CRF, and vigilantly counsel their sedentary hypertensive patients to become more physically active and improve their CRF as a cornerstone of HTN management and primary CVD prevention.
Acknowledgments
We thank the Cooper Clinic physicians and technicians for collecting the baseline data.
This work was supported by NIH Grants AG06945 and HL62508, and by the Communities Foundation of Texas on recommendation of Nancy Ann and Ray L. Hunt.
References
- 1.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr., Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Kohl HW., III Physical activity and cardiovascular disease: evidence for a dose response. Med Sci Sports Exerc. 2001;33:S472–S483. doi: 10.1097/00005768-200106001-00017. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services . Physical Activity and Health: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- 5.Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 6.Blair SN, Goodyear NN, Gibbons LW, Cooper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA. 1984;252:487–490. [PubMed] [Google Scholar]
- 7.Barlow CE, Lamonte MJ, Fitzgerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006;163:142–150. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 8.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–3100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 9.Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33:S484–S492. doi: 10.1097/00005768-200106001-00018. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa-Takata K, Ohta T, Tanaka H. How much exercise is required to reduce blood pressure in essential hypertensives: a dose-response study. Am J Hypertens. 2003;16:629–633. doi: 10.1016/s0895-7061(03)00895-1. [DOI] [PubMed] [Google Scholar]
- 11.Engstrom G, Hedblad B, Janzon L. Hypertensive men who exercise regularly have lower rate of cardiovascular mortality. J Hypertens. 1999;17:737–742. doi: 10.1097/00004872-199917060-00003. [DOI] [PubMed] [Google Scholar]
- 12.Church TS, Kampert JB, Gibbons LW, Barlow CE, Blair SN. Usefulness of cardiorespiratory fitness as a predictor of all-cause and cardiovascular disease mortality in men with systemic hypertension. Am J Cardiol. 2001;88:651–656. doi: 10.1016/s0002-9149(01)01808-2. [DOI] [PubMed] [Google Scholar]
- 13.Evenson KR, Stevens J, Thomas R, Cai J. Effect of cardiorespiratory fitness on mortality among hypertensive and normotensive women and men. Epidemiology. 2004;15:565–572. doi: 10.1097/01.ede.0000129527.53181.c8. [DOI] [PubMed] [Google Scholar]
- 14.Fang J, Wylie-Rosett J, Alderman MH. Exercise and cardiovascular outcomes by hypertensive status: NHANES I epidemiological follow-up study, 1971-1992. Am J Hypertens. 2005;18:751–758. doi: 10.1016/j.amjhyper.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 16.American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- 17.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons LW, Mitchell TL, Wei M, Blair SN, Cooper KH. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86:53–58. doi: 10.1016/s0002-9149(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 19.Macera CA, Jackson KL, Davis DR, Kronenfeld JJ, Blair SN. Patterns of non-response to a mail survey. J Clin Epidemiol. 1990;43:1427–1430. doi: 10.1016/0895-4356(90)90112-3. [DOI] [PubMed] [Google Scholar]
- 20.Hartge P. Participation in population studies. Epidemiology. 2006;17:252–254. doi: 10.1097/01.ede.0000209441.24307.92. [DOI] [PubMed] [Google Scholar]
- 21.Paffenbarger RS, Jr, Hyde RT, Wing AL, Steinmetz CH. A natural history of athleticism and cardiovascular health. JAMA. 1984;252:491–495. [PubMed] [Google Scholar]
- 22.Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 23.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 24.Kelly-Hayes M, Robertson JT, Broderick JP, Duncan PW, Hershey LA, Roth EJ, Thies WH, Trombly CA. The American Heart Association Stroke Outcome Classification. Stroke. 1998;29:1274–1280. doi: 10.1161/01.str.29.6.1274. [DOI] [PubMed] [Google Scholar]
- 25.Pasternak RC, Abrams J, Greenland P, Smaha LA, Wilson PW, Houston-Miller N. 34th Bethesda Conference: Task force #1—Identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol. 2003;41:1863–1874. doi: 10.1016/s0735-1097(03)00358-9. [DOI] [PubMed] [Google Scholar]
- 26.LaMonte MJ, Ainsworth BE, Reis JP. Measuring physical activity. In: Zhu W, Woods T, editors. Measurement Theory and Practice in Kinesiology. Human Kinetics; Champaign, IL: 2006. pp. 237–272. [Google Scholar]
- 27.Jurca R, LaMonte MJ, Church TS, Earnest CP, FitzGerald SJ, Barlow CE, Jordan AN, Kampert JB, Blair SN. Associations of muscle strength and aerobic fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36:1301–1307. doi: 10.1249/01.mss.0000135780.88930.a9. [DOI] [PubMed] [Google Scholar]
- 28.Blair SN, Kohl HW, Barlow CE. Physical fitness and all-cause mortality in hypertensive men. Ann Med. 1991;23:307–312. doi: 10.3109/07853899109148065. [DOI] [PubMed] [Google Scholar]
- 29.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity) Arterioscler Thromb Vasc Biol. 2003;23:E42–E49. [Google Scholar]
- 30.Duncan JJ, Farr JE, Upton J, Hagan RD, Oglesby ME, Blair SN. The effects of aerobic exercise on plasma catecholamines and blood pressure in patients with mild essential hypertension. JAMA. 1985;254:2609–2613. [PubMed] [Google Scholar]


