Abstract
Male CD-1 mice were administered interleukin-1β (IL-lβ) and bacterial endotoxin (lipopolysaccharide, LPS) and subsequently tested in the tail suspension test (TST), the Porsolt forced swim test (FST), and in the open field. IL-1β (100, 300 and 1000 ng/mouse) injected intraperitoneally (i.p.) 90 min before the test induced a dose-dependent increase in the time spent immobile in the TST and the time spent floating in the FST. These responses were statistically significant only at the higher doses of IL-1β (300 and 1000 ng). Nevertheless, all three doses of IL-1β significantly decreased line crossings and rears in the open field and depressed food intake and body weight. Very similar effects were induced by LPS. Doses of 1 and 5 μg i.p. increased immobility time in the TST and floating time in the FST, but the same doses strongly depressed locomotor activity and body weight.
These results indicate that both IL-1β and LPS can induce depression-like effects in the TST and the FST. However, the doses necessary to induce these changes reduced feeding and activity in an open field, so that the effects observed in the FST and TST could be attributed to a general reduction in locomotor activity. Thus the results obtained in these two animal tests commonly used to test antidepressant properties do not provide strong support for an IL-1 hypothesis of depression.
1. Introduction
A current hypothesis is that cytokines may be involved in depressive illness (Smith, 1991; Dantzer et al., 1999; Charlton, 2000). The focus of the hypothesis has been on one cytokine, interleukin-1 (IL-1), largely because administration of IL-1 induces sickness behavior (Kent et al., 1992), which has some features in common with depression (Dantzer et al., 1999; Dunn et al., 2005). Administration of bacterial lipopolysaccharide (LPS) which induces the production of IL-1 (Dinarello, 1988) also induces sickness behavior. The cytokine hypothesis of depression would be supported if cytokine and LPS administration induced depression-like activity in classical animal tests of depression.
The two most commonly used tests for depressants and antidepressants are the Porsolt forced swim test (FST), and the tail suspension test (TST) (Cryan et al., 2002). However, there is a paucity of data in the literature on the effects of IL-1 and LPS in these two tests, and only one study in mice. In the published reports, del Cerro and Borrell (1990) found that IL-1 administered i.c.v. to rats reduced floating in the FST, which they interpreted as an antidepressant effect. However, Huang and Minor (2000) reported that i.c.v. injection of IL-1β significantly increased floating in rats in the FST, and that this response was prevented by i.c.v. administration of the IL-1-receptor antagonist, IL-1ra. In a very recent study, Deak et al. (2005) reported that the behavior of rats during the forced swim test was not affected by LPS. In the only study in mice of which we are aware, systemically administered LPS increased floating (Jain et al., 2001).
Therefore, we studied the effects of administering various doses of IL-1β and LPS to mice on the FST and TST. The same animals were also observed in an open field (OFT), and food intake and body weight were monitored to control for general effects of IL-1β and LPS on locomotor activity and physiology. The results suggest that although IL-1β and LPS alter behavior in the FST and TST in a direction consistent with depression-like activity, significant responses occurred only at doses that markedly decreased locomotor activity and feeding.
2. Materials and methods
2.1. Animals
Two separate batches of CD-1 albino, VAF+ male mice were obtained from Charles River (R16 colony Raleigh-Durham facility) and upon arrival housed in an animal colony room maintained at 22±2 °C and with 12-h light–dark cycle with lights on at 7 A.M. They were kept in plastic cages with wood shaving bedding either in groups of 4 (first experiment) or singly (second experiment). Mice were given free access to water and Purina chow pellets. The animals were 4–7 weeks old at the beginning of the experiments. All experiments were conducted in accordance with the NIH Guide on the care and use of animals for research, and an in-house protocol approved by the LSUHSC-Shreveport Animal Care and Use Committee.
2.2. Materials
Escherichia coli lipopolysaccharide (LPS; 026:B6, Sigma L3755), and recombinant mouse IL-1β (R&D Systems, Minneapolis) dissolved in sterile saline (0.9% NaCl) were injected intraperitoneally (i.p.) in a volume of 0.1 ml. Control animals were injected with saline.
2.3. Behavioral tests and experimental procedure
Mice were subjected to three behavioral tests: the tail suspension test (TST), the forced swim test (FST) and the open field test (OFT). Body weight was monitored, and food intake was determined in animals housed individually.
2.3.1. Tail suspension test (TST)
We used the test essentially as described by Steru et al. (1985). A short piece of paper adhesive tape (about 6 cm) was attached along half the length of the tail (about 3 cm). The free end of the adhesive tape was attached to a 30 cm long rigid tape (made from the paper tape folded several times) which was attached to a seesaw lever linked to a spring strain gauge that activated the hand of a spring balance. The animal was surrounded by a white-painted wooden enclosure (H: 45 cm, W: 40 cm, D: 40 cm), such that the mouse’s head was about 20 cm above the floor. Mice were observed for 6 min. Wriggling of the animal to avoid the aversive situation produced movements of the hand on the balance that were recorded by an observer who could not see the animal directly. As recently pointed out by Mayorga and Lucki (2001), one of the confounding factors in the tail suspension test is tail-climbing behavior. The tail climbing periods tend to be scored as immobility by a mechanical device although they clearly constitute avoidance behavior. Very few of the CD-1 mice displayed tail climbing, but when this was observed, the data were omitted.
2.3.2. Open field test (OFT)
Spontaneous locomotor activity was quantified for 6 min in an open field, a Plexiglas box 59 × 59 cm with its floor divided into 16 squares illuminated by white light. Four squares were defined as the center and the 12 squares along the walls as the periphery. Each mouse was gently place in the very center of the box and activity was scored as a line crossing when a mouse removed all four paws from one square and entered another.
2.3.3. Forced swim test (FST)
The forced swim test was performed according to the method of Porsolt et al. (1977). A vertical glass cylinder (25 cm high, 14 cm in diameter) was filled with 30 °C water to a depth of 20 cm. The water depth was chosen so that the animals must swim or float without their hind limbs or tail touching the bottom. For testing, each mouse was placed in the cylinder for 6 min, and the latency to float and the duration of floating (i.e. the time during which mice made only the small movements necessary to keep their heads above water) was scored. As indicated by Porsolt, only the data scored during the last 4 min were analyzed and presented.
2.3.4. Experimental procedure
In the animal room, each mouse was removed from its cage, gently weighed, given an i.p. injection, and returned to its home cage (singly housed mice) or placed in a holding cage (grouped animals). IL-1β was injected 90 min, and LPS 120 min before the TST. These time points were chosen based on previous behavioral, endocrine and neurochemical studies. When mice were presented with sweetened milk, the response to IL-1 appeared around 15–20 min, and persisted for approximately 2 h, with the maximum response between 30 and 90 min (Swiergiel and Dunn, 2002). The increase in plasma corticosterone occurred over a similar time period with a peak around 2 h, closely paralleling the brain noradrenergic response, while the increases in tryptophan and in serotonin metabolism occurred between 90 min and 4 h (Dunn, 1988, 1992). For LPS, we chose 120 min because this was typically the time of the peak response in sweetened milk drinking, elevation of plasma corticosterone, and brain noradrenergic activity (Dunn, 1992). In group-housed animals, the tail suspension test was performed first, followed by a 6-min resting period in a holding cage and then the forced swim test. Open field activity was observed several days later 90 min after the IL-1β and LPS injections. One experiment was performed in singly housed animals (n =6). In this experiment, all three tests were performed in one testing session: TST, 6-min rest in the home cages; OFT, 6-min rest; and then the FST. The FST was always performed last because we considered it the most stressful, and thus more likely to affect subsequent tests. It also takes some considerable time for the fur to dry completely. In contrast, the TST seemed benign and appeared to have little effect on the subsequent behavior of the mouse.
All observations were conducted in a darkened room, adjacent to, but separate from, the animal colony room, between 10 AM and 3 PM. The apparati were illuminated with white light (20 lx intensity at the floor level). The open field and forced swim tests were videotaped, and the tail suspension test was scored live. Observers who scored behaviors were unaware of the experimental treatments. Upon completion of the tests, mice were returned to their home cages and in the case of individually housed mice, a weighed amount of food pellets provided. The animals and the remaining food pellets were weighed 24 h after the injections.
2.4. Data analysis
One-way analysis of variance (ANOVA) was performed using SuperAnova (Abacus Concepts, Inc.), followed by Fisher’s Least Significant Difference t-test. All data are reported as the mean±standard error of the mean.
3. Results
3.1. Behavioral effects of IL-1β
3.1.1. Tail suspension test (TST)
In group-housed animals, intraperitoneal administration of all three doses of mIL-1β 90 min before observation increased the duration of immobility in the TST (F3,61=4.85, p <0.01; Fig. 1). Post hoc analysis revealed statistically significant effects of only the 300 and 1000 ng doses (p <0.05). In a separate experiment in singly housed mice, IL-1β increased the immobility time, but only the 1000 ng dose significantly increased the immobility (p <0.01, data not shown).
Fig. 1.
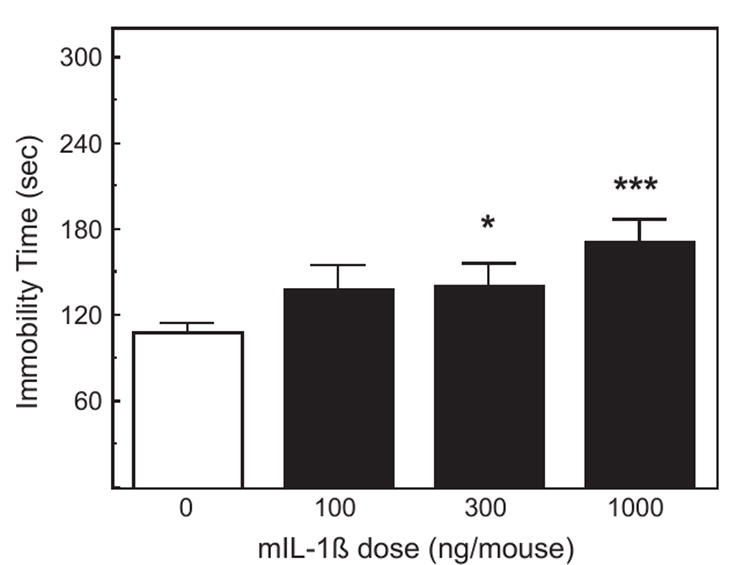
The effect of IL-1β on activity in the tail suspension test. Pooled data from two experiments. Mice (n =8– 10 in the first experiment, n =5–6 in the second experiment) were injected i.p. with saline, 100, 300 or 1000 ng of mIL-1β 90 min prior to the TST. The duration of immobility was scored during a 6-min test. *Significantly different from zero dose: *p <0.05; ***p <0.001.
3.1.2. Forced swim test (FST)
In group-housed animals, mIL-1β given 102 min before testing increased the duration of floating in the FST (F3,40=6.51, p <0.01; Fig. 2). Only the 300 and 1000 ng doses of IL-1β significantly increased floating (p <0.05 and p <0.001, respectively). In a repeat experiment, IL-1β also increased floating, but only the 1000 ng dose induced a significant effect (p <0.01). Similar results were obtained in singly housed mice, but only 1000 ng of IL-1β significantly increased the duration of floating (p <0.05).
Fig. 2.
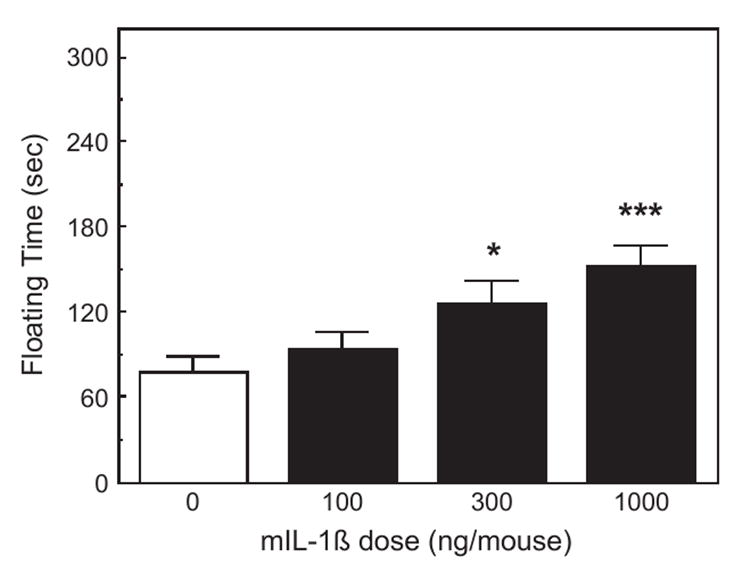
The effect of IL-1β on activity in the forced swim test. Mice (n =8) were injected i.p. with saline, 100, 300 or 1000 ng of mIL-1β 102 min prior to the FST. The same animals as in Fig. 1 tested 6 min after the TST. The duration of floating was scored during the last 4 min of a 6-min test. *Significantly different from zero dose: *p <0.05; ***p <0.001.
3.1.3. Open field test (OFT)
In group-housed animals, this test was performed 5 days later in the same mice, but with the treatments randomized. IL-1β significantly depressed all measures of activity (Fig. 3): central (F3,21=5.40, p <0.01), peripheral (F3,21=6.98, p <0.01), and total line crossings (F3,21=8.69, p <0.001), as well as the number of rears (F3,21=4.71, p <0.05). Post hoc analyses indicated that all three doses of IL-1β induced these effects. Very similar results were obtained in singly housed mice. IL-1β reduced the numbers of line crossings and rears, but while rears were significantly reduced by all three doses (p <0.01), line crossings were reduced only by the 300 and 1000 ng doses (p <0.01).
Fig. 3.
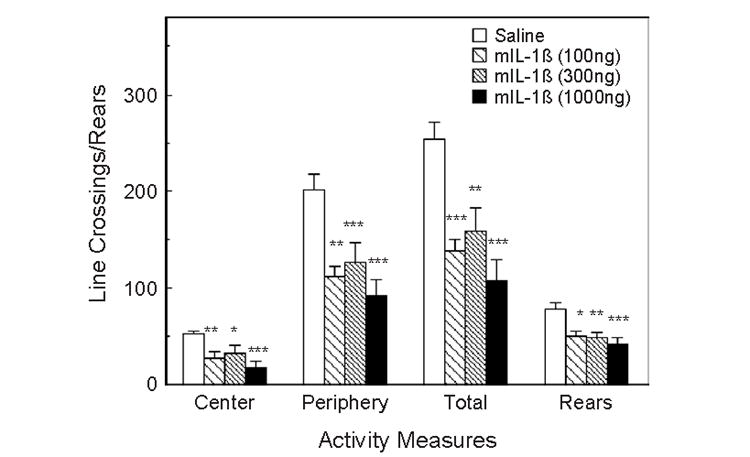
The effect of IL-1β on activity in the open field test. Mice (n =8) were injected i.p. with saline, 100, 300 or 1000 ng of mIL-1β 90 min prior to the test. The same mice as in Figs. 1 and 2, but injected and tested 5 days later. Activity was scored in a 6-min test. *Significantly different from zero dose: *p <0.05; **p <0.01; ***p <0.001.
3.1.4. Food intake and body weight
Food intake and body weight change during a 24-h period following the injections were assessed in singly housed mice. IL-1β depressed both parameters (F3,12=7.80, p <0.01 and F3,12=4.24, p <0.05, Fig. 4). During this experiment, 5 of 26 mice that had been injected with the 1000 ng dose displayed symptoms of shock and could not be tested, so the use of this dose of IL-1 was discontinued.
Fig. 4.
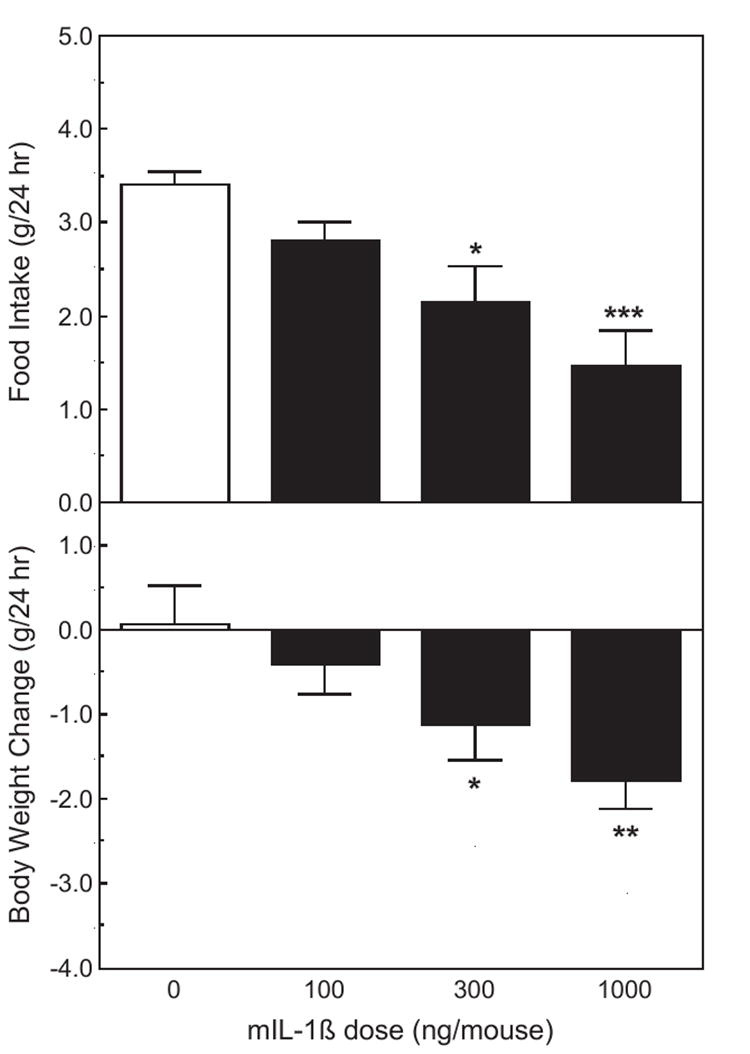
The effect of IL-1β on 24 h food intake and body weight change. The same treatments as in Fig. 3. Upper panel: food intake. Lower panel: body weight change. *Significantly different from zero dose: *p <0.05; **p <0.01; ***p <0.001.
3.2. Behavioral effects of LPS
Tests were performed only in group-housed animals, and each mouse received only one injection of LPS.
3.2.1. Tail suspension test (TST)
Administration of LPS i.p. 120 min before observation increased the duration of immobility in the TST (F2,21=4.12, p <0.05). Post hoc analysis revealed that both the 1 and 5 μg doses of LPS increased immobility (p <0.05, Fig. 5).
Fig. 5.
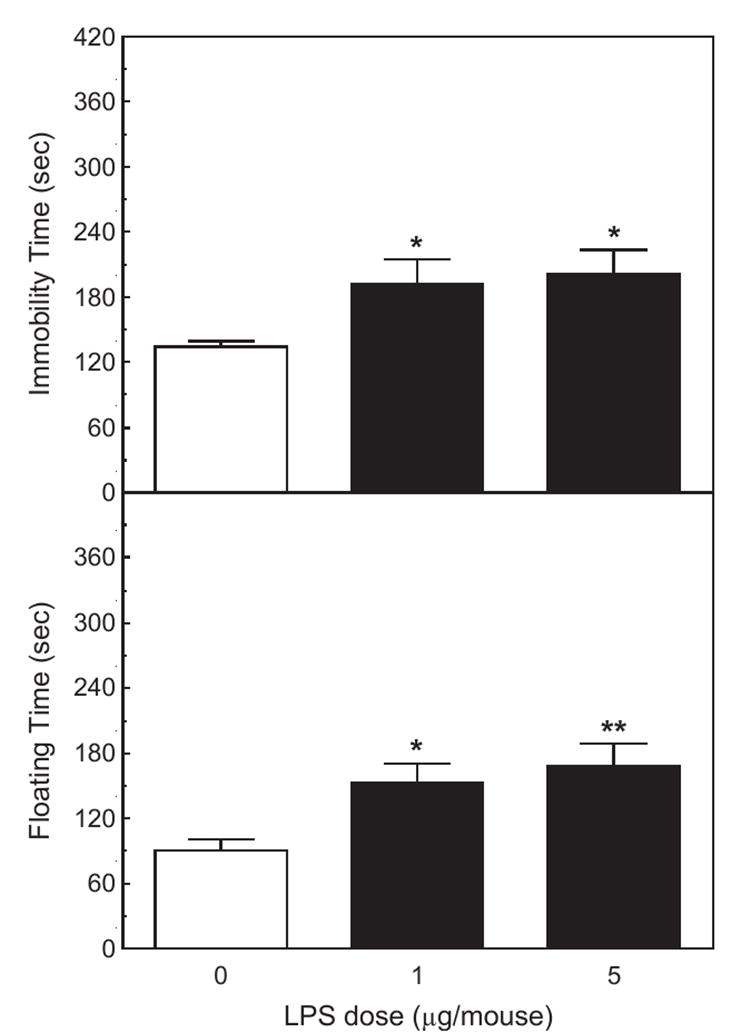
The effect of LPS on activity in the tail suspension and the forced swim tests. Mice (n =8) were injected i.p. with saline, 1 or 5 μg of LPS 120 min before the TST and 144 before the FST. Upper panel: the duration of immobility was scored during a 6-min test. Lower panel: the duration of floating was scored during the last 4 min of a 6-min forced swim test. *Significantly different from zero dose: *p <0.05; **p <0.01.
3.2.2. Open field test (OFT)
LPS injected 132 min before testing significantly depressed all measures of activity (Fig. 6): central (F2,14=15.2, p <0.001), peripheral (F2,14=11.1, p <0.001), and total line crossings (F2,14=13.1, p <0.001), as well as the number of rears (F2,14=4.046, p <0.05).
Fig. 6.
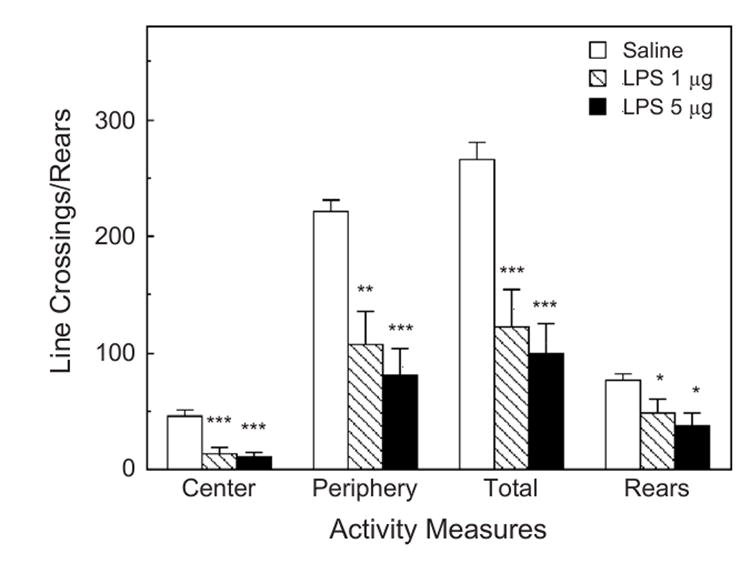
The effect of LPS on activity in the open field test. Mice were injected 132 min prior to the OFT. The same animals tested in Fig. 5. Activity was scored during a 6-min test. *Significantly different from zero dose: *p <0.05; **p <0.01; ***p <0.001.
3.2.3. Forced swim test (FST)
LPS injected 144 min before testing increased the duration of floating in the FST (F2,21=5.83, p <0.01). Both the 1 and 5 μg doses significantly increased the duration of floating (p <0.05 and p <0.001, respectively; Fig. 5).
3.2.4. Body weight
Both doses of LPS strongly depressed body weight (F2,21=14.73, p <0.001; Fig. 7). Food intake could not be assessed in group-housed animals.
Fig. 7.
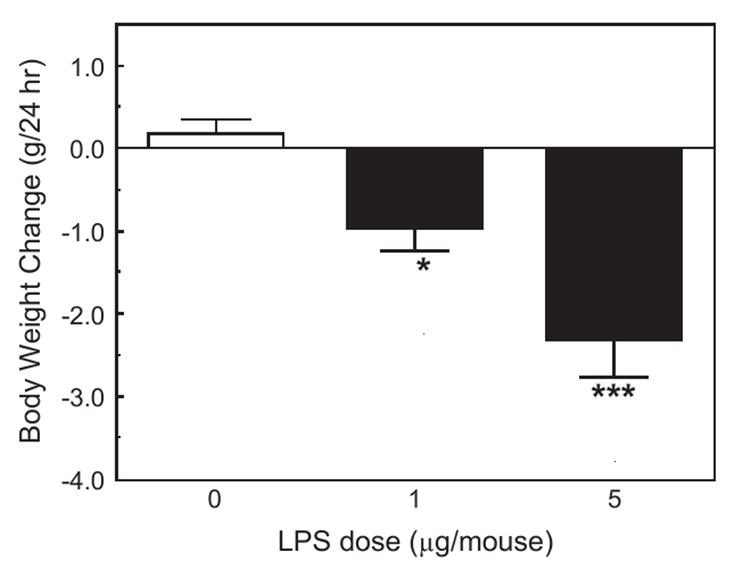
The effect of LPS on 24 h body weight change in the same mice from which results are presented in Figs. 5 and 6. *Significantly different from zero dose: *p <0.05; ***p <0.001).
4. Discussion
The results of the present study show that intraperitoneal injections of IL-1β and LPS increased the time spent immobile in the tail suspension test, and in the time spent floating in the forced swim test (Figs. 1, 2 and 5). Both effects are characteristic of treatments that induce depression. However, the doses of IL-1β and LPS necessary to induce statistically significant effects (300 ng/mouse of IL-1β and 1 μg/mouse of LPS) were relatively high, and induced marked decreases in locomotor activities in the open field (Figs. 3 and 6), as well as reducing feeding and body weight (Figs. 4 and 7). A salient observation was that even the lowest dose of mIL-1β (100 ng) strongly decreased locomotor activity in the open field, yet it failed to induce significant increases in immobility in the TST and the time spent floating in the FST. In a number of previous experiments, 100 ng doses of IL-1β have been shown to produce robust neuroendocrine and neurochemical responses (Dunn, 1988, 1992), and to reduce sweetened milk consumption (Swiergiel et al., 1997). In independent experiments in another laboratory (the Institute of Genetics and Animal Breeding in Warsaw), we also observed that 1 or 5 μg of LPS injected into Swiss-Webster mice increased immobility time in the TST and floating time in the FST, but the same doses dramatically depressed locomotor activity (by around 80%).
It has been postulated that inflammation, and specifically, cytokines, may play a role in depression (Dantzer, 2001). However, there is little published evidence that even the most frequently implicated cytokine (IL-1) affects behavior in standard paradigms used to assess depressive symptoms and test antidepressants.
del Cerro and Borrell (1990) reported that IL-1 (5 U) administered i.c.v. reduced floating in rats in the FST by more than twofold. Such an effect would normally be interpreted as an antidepressant effect. In direct contradiction to these results, Huang and Minor (2000) reported (in abstract form only) that i.c.v. injection of 2 ng of IL-1β to rats significantly increased floating in the FST, and that this response was prevented by i.c.v. administration of 6 μg of IL-1ra or by i.p. caffeine, the latter implicating adenosine receptors. Jain et al. (2001) reported that systemically administered LPS increased floating in the FST in mice. However, they used a very high dose of LPS (50 μg/mouse), and no assessment of locomotor activity was performed. In their study, the increases in floating were prevented by the antidepressants, desmethylimipramine and fluoxetine, as well as the cyclooxygenase inhibitors, nimesulide, naproxen, and rofecoxib. Very recently, Deak et al. (2005) reported that the behavior of rats during the forced swim test was not affected by LPS (10 or 100 μg/kg i.p.), whereas social investigation of a juvenile was profoundly decreased. Although the failure to observe effects of LPS in the FST is not consistent with our results in mice, the dissociation between the effects of LPS in the FST and on social behavior resembles the partial dissociation we observed between locomotor activity and behavior in the TST and FST.
Several reports relate indirectly to a role of cytokines in behavior in the FST. Tannenbaum et al. (2002) demonstrated that chronic stress increased sickness behavior and the duration of floating in the FST. Although the authors reported that chronic stress augmented the sickness behavior induced by i.p. IL-1β, they did not report the effects of IL-1β in the FST. Deak et al. (2003) showed that exposure to the FST did not alter the concentrations of IL-1β measured in the rat brain or in peripheral tissues, and concluded that the production of IL-1 in the brain was unlikely to play a role in the behavioral consequences in the forced swim test.
Thus there have been relatively few reports of the occurrence of depression-like effects of IL-1 and LPS on behavior in the TST and FST, and those reports have not been consistent. The differences among these reports could be ascribed to differences in the behavioral procedures used, the different scoring criteria used, and, perhaps, the different species or strains of mice (see Wahlsten et al., 2003).
A deficit in locomotor activity would very likely affect performance in the tail suspension and the forced swim tests. However, the question arises whether the mechanism of action of IL-1β and LPS is similar in all three tests. Clearly at some level the mechanisms must differ, but some common pathways are also likely to be involved. The most obvious way to approach this question experimentally is by testing the effects of a variety of antagonists on each behavior.
It is concluded that IL-1β and LPS elicit behavioral changes in the FST and TST that resemble those that occur following treatments thought to induce depression, such as chronic stress. However, such responses were only observed at doses of the compounds that induced reductions in locomotor activity in the open field, in feeding, and in body weight. Thus one cannot state with confidence that the apparent behavioral depression is independent of a general reduction in activity, and is a specific depressogenic effect.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (NS 35370). We thank Igor Leskov, M.D. for skilled technical assistance.
References
- Charlton BG. The malaise theory of depression: major depressive disorder is sickness behavior and antidepressants are analgesic. Med Hypotheses. 2000;54:126–30. doi: 10.1054/mehy.1999.0986. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Wollman EE, Vitkovic L, Yirmiya R. Cytokines, stress, and depression. In: Dantzer R, Wollman E, Yirmiya R, Dantzer R, Wollman E, Yirmiyas R, editors. Cytokines, Stress, and Depression. Vol. 461. New York: Kluwer Academic/Plenum Publishers; 1999. pp. 317–29. [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG. Exposure to forced swim stress does not alter central production of IL-1. Brain Res. 2003;972:53–63. doi: 10.1016/s0006-8993(03)02485-5. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–34. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- del Cerro S, Borrell J. Interleukin-1 affects the behavioral despair response in rats by an indirect mechanism which requires endogenous CRF. Brain Res. 1990;528:162–4. doi: 10.1016/0006-8993(90)90212-t. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biology of interleukin 1. FASEB J. 1988;2:108–15. [PubMed] [Google Scholar]
- Dunn AJ. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism paralleling the increased plasma corticosterone. Life Sci. 1988;43:429–35. doi: 10.1016/0024-3205(88)90522-x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exp Ther. 1992;261:964–9. [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Huang Q, Minor TR. Adenosine mediates interleukin-1beta induced behavioral depression in rats. Brain Behav Immun. 2000;14:101. [Google Scholar]
- Jain NK, Kulkarni SK, Singh A. Lipopolysaccharide-mediated immobility in mice: reversal by cyclooxygenase enzyme inhibitors. Methods Find Exp Clin Pharmacol. 2001;23:441–4. doi: 10.1358/mf.2001.23.8.662131. [DOI] [PubMed] [Google Scholar]
- Kent S, Blutheé R-M, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–8. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology. 2001;155:110–2. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn. 1977;229:327–36. [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Distinct roles for cyclooxygenases1 and 2 in interleukin-1-induced behavioral changes. J Pharmacol Exp Ther. 2002;302:1031–6. doi: 10.1124/jpet.102.036640. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Smagin GN, Dunn AJ. Influenza virus infection of mice induces anorexia: comparison with endotoxin and interleukin-1 and the effects of indomethacin. Pharmacol Biochem Behav. 1997;57:389–96. doi: 10.1016/s0091-3057(96)00335-8. [DOI] [PubMed] [Google Scholar]
- Tannenbaum B, Tannenbaum GS, Sudom K, Anisman H. Neurochemical and behavioral alterations elicited by a chronic intermittent stressor regimen: implications for allostatic load. Brain Res. 2002;953:82–92. doi: 10.1016/s0006-8993(02)03273-0. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Rustay NR, Metten P, Crabbe JC. In search of a better mouse test. Trends Neurosci. 2003;26:132–6. doi: 10.1016/S0166-2236(03)00033-X. [DOI] [PubMed] [Google Scholar]


