SUMMARY
Cell fusion is fundamental for reproduction and organ formation. Fusion between most C. elegans epithelial cells is mediated by the EFF-1 fusogen. However, fusion between the anchor cell and the utse syncytium that establishes a continuous uterine-vulval tube proceeds normally in eff-1 mutants. By isolating mutants where the anchor-cell fails to fuse, we identified aff-1. AFF-1 ectopic expression results in fusion of cells that normally do not fuse in C. elegans. The fusogen activity of AFF-1 was further confirmed by its ability to fuse heterologous cells. AFF-1 and EFF-1 differ in their fusogenic activity and expression patterns but share eight conserved predicted disulfide bonds in their ectodomains, including a putative TGF-β-type-I-Receptor domain. We found that FOS-1, the Fos transcription factor ortholog that controls anchor-cell invasion during nematode development, is a specific activator of aff-1-mediated anchor-cell fusion. Thus, FOS-1 links cell invasion and fusion in a developmental cascade.
INTRODUCTION
Most multicellular organisms are comprised of three different germ layers that are organized during development from the outside inward and are separated by basement membranes. The external ectoderm covers and internalizes the mesoderm and endoderm layers. Organs derived from internal germ layers, however, often perform biological functions that rely on connections with the external world, necessitating formation of mixed-layer organs. A prominent example is the birthing of embryos, which first develop within the mesodermal gonad and are then expelled from the body via an ectodermal organ. Despite such a fundamental requirement for establishment of communication and canalization between internal organs and the ectoderm, the molecular mechanisms that govern this process remain poorly understood (Wolpert, 2007).
In C. elegans, uterine-vulval connection is established by the activity of a single cell, the anchor cell (AC), that lies at the interface between the ectodermal vulva and the mesodermal uterus. Upon specification, the AC induces vulva precursor cell (VPC) differentiation by a LIN-3/EGF signal that activates a LET-23/EGF receptor in the VPCs (Sternberg and Horvitz, 1986). This is followed by a second signaling phase where a LAG-2/Delta signal from the AC activates LIN-12/Notch at six surrounding uterine cells, resulting in their differentiation into π cells whose daughters connect to the vulva (Newman et al., 1994). Via these two signaling cascades, the AC synchronizes uterine-vulval development and fixes their relative positions (Figure 1A).
Figure 1. aff-1 Mutants Exhibit AC Fusion Failure Phenotype.
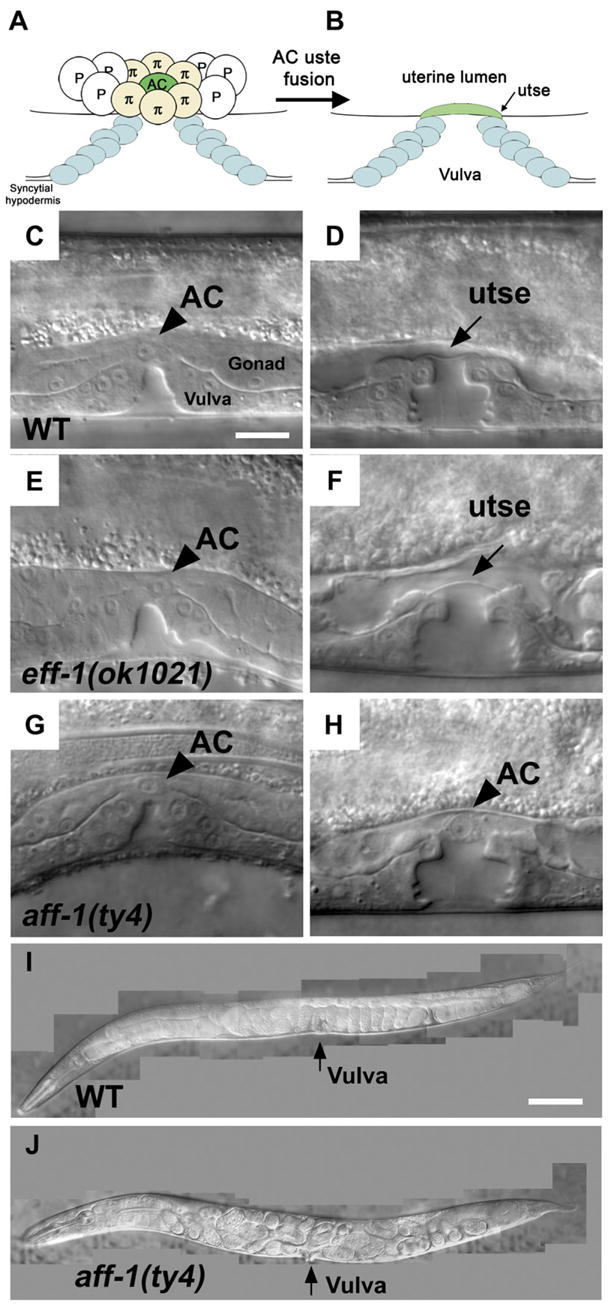
Animals in (C)–(H) are in early- to mid-L4 larval stage, and (I) and (J) are gravid adults. In all the panels hereafter, anterior is to the left; ventral down.
(A) Schematic view of AC (green) and utse cell precursors (π) before cell fusion. The vulval primordial epithelial cells (blue) invaginate connecting the epidermis (hypodermis) with the uterus.
(B) Formation of the utse and cell-cell fusion of AC with π cell daughters connect the uterus through the vulva.
(C, E, and G) In wild-type as in eff-1 and aff-1 null alleles the AC is correctly localized on the vulva apex (arrowhead).
(D) In wild-type animal, fusion of the AC to the utse syncytium resulted in the formation of a hymen/utse layer (arrow) between the vulval and uterine lumens.
(F) In eff-1 mutant, normal AC fusion resulted in hymen formation, indicating that eff-1 is not required for AC fusion.
(H) In aff-1 mutant, the AC failed to fuse and was retained at the uterus-vulva junction (arrowhead).
(I) Adult animal exhibiting normal development of embryos that are laid from the uterus at the 50–100 cell stage.
(J) Strong Egl phenotype of aff-1 mutant hermaphrodite. Embryos complete embryonic development in the uterus, and the larvae hatch inside the mother.
The scale bar represents 5 μm in (A)–(H) and 20 μm in (I) and (J).
Uterine-vulval connection, continuity, and attachment to the C. elegans body are established by two additional activities of the AC. First, the AC induces the localized breakdown of the basement membranes, separating the uterus from the VPCs. The AC then prompts the onset of basolateral processes that invade in between the two central VPCs’ descendants (Sherwood and Sternberg, 2003). This invasion is mediated by the activity of the FOS-1 transcription factor in the AC (Sherwood et al., 2005) in a process reminiscent of FOS regulation of cell invasion during normal mammalian development and in tumor metastasis. After completing its signaling and invasion phases, the AC lying between the vulva and uterus lumens must be removed to establish a continuous opening. Its removal is initiated by the fusion of eight π cell daughters, generating the utse syncytium (Figure 1A). The AC subsequently fuses to this syncytium, leaving only a thin layer of cytoplasm in the junction between the lumens (Sharma-Kishore et al., 1999) (Figure 1B). This thin laminar process is broken when the first embryo is laid to establish a direct channel between the two organs.
Cell fusion occurs during the development of multicellular organisms in crucial processes like fertilization and formation of multinucleate cells in muscles, placentas, and bones (Podbilewicz, 2006; Podbilewicz and White, 1994). Despite the detailed characterization of cell-cell fusion events in several organisms, the molecules that directly mediate this process remain uncharacterized (Podbilewicz, 2006; Podbilewicz and White, 1994). The only protein that has been characterized as a fusogen, directly involved in cell-cell fusion, is C. elegans EFF-1 (Epithelial Fusion Failure-1) (Mohler et al., 2002). In eff-1 mutant worms, most of the numerous fusion events characteristic of C. elegans development do not occur, while overexpression of eff-1 results in ectopic fusion of cells that do not normally fuse (del Campo et al., 2005; Shemer et al., 2004). Dynamic expression of eff-1 in fusing cells links execution of fusion with tight regulation of the process (Podbilewicz, 2006; Shemer and Podbilewicz, 2002). EFF-1 is a type I membrane protein with homologs in other nematodes. EFF-1 fuses heterologous Sf9 insect culture cells, demonstrating that it is a bona fide fusogen (Podbilewicz et al., 2006).
Here we show that in eff-1 null mutants, several fusion events occur normally. Anchor-cell fusion, the fusion of two vulval rings, and the fusion between the lateral seam cells occur in an EFF-1-independent manner. These processes must therefore be facilitated by the activity of an unidentified fusogen. We have identified a related protein, AFF-1, that is required for AC fusion and other specific fusion events in different organs. Differential and specific expression of these two related fusogens allows a dynamic regulation of most but not all of the 300 stereotyped somatic cell fusion events in C. elegans. In an aff-1 mutant background, AC fusion is blocked while aff-1 ectopic expression in normally nonfusing cells results in their fusion. This demonstrates both the necessity and sufficiency of aff-1 for cell fusion in vivo. Moreover, fusion of cultured Sf9 cells upon AFF-1 expression indicates that AFF-1 is a fusogen. The dynamic expression of aff-1 in the AC and utse is positively regulated by the FOS-1 transcription factor. Thus, FOS-1 governs AC fusion via AFF-1 fusogen, connecting tissue merging, cell invasion, and cell fusion during the formation of a tube.
RESULTS
AC Fusion Failure in aff-1 Mutants
Previous studies demonstrated that AC fusion occurs normally in eff-1 hypomorph mutants np29 and hy21 (Choi et al., 2006; Shemer et al., 2004). To investigate this further, we examined AC fusion in worms possessing the eff-1 allele ok1021, which is functionally null (A.S. and B.P., unpublished data). We found that in the eff-1(ok1021) mutant background, the AC fuses and forms a thin membrane, similar to the wild-type structure (Figures 1C–1F), indicating that AC fusion is mediated by a fusogen distinct from EFF-1.
Failure of AC fusion results in fertilized embryos that cannot exit from the uterus and complete their development inside the body of the mother (Cinar et al., 2003). This was the basis for forward genetic screens in which genes required either directly or indirectly for AC fusion were isolated. One group of genes includes the cog-2/egl-13 (Cinar et al., 2003; Hanna-Rose and Han, 1999), lin-11 (Newman et al., 1999) and lin-29 (Newman et al., 2000) transcription factors, and smo-1, encoding a regulator of protein localization (Broday et al., 2004). These mutations disrupt π cell differentiation and localization, including the fusion of their daughters with the AC. nsf-1, encoding N-ethylmaleimide-sensitive factor, was isolated as a gene required autonomously for AC fusion (Choi et al., 2006). NSF acts as a regulator of vesicle fusion through binding and disassembly of the SNARE complexes (Sollner et al., 1993). It is unclear whether NSF-1 mediates AC fusion directly or by regulating the traffic and surface expression of a yet-unidentified AC fusogen.
To identify factors that directly mediate AC fusion, we performed a forward genetic screen for an EGg Laying defective (Egl) mutant phenotype. The collection of Egl mutations was refined further for mutations that affect the AC specifically, by following AC fusion directly. We isolated one mutation, ty4, in which the AC failed to fuse in 97% (n = 135) of the mutant worms (Figures 1G, 1H, and 2B). The ty4 mutation was mapped to a genetic interval of 0.24 map unit (about 300 kb) on chromosome II (see Supplemental Experimental Procedures in the Supplemental Data available with this article online). We performed complementation analyses with mutations in this region that correspond to genes that may function in cell fusion. The only mutation failing to complement ty4 is tm2214, a 1 kb deletion in the C44B7.3 predicted gene (Figure 2A). Like ty4, tm2214 mutant worms exhibit a completely penetrant Egl phenotype (Figure 1J; n = 48 and n = 96, respectively), and in 98% of the worms examined, the AC failed to fuse (n = 135; Figure 2B). Sequencing the C44B7.3 gene in the ty4 mutant revealed a single base substitution of G to A in the putative ATG start codon, resulting in replacement of the initiator methionine with an isolucine residue. ty4 therefore presumably represents a null allele. Similarity in the severity of the phenotypes of ty4, tm2214, and ty4/tm2214 transheterozygote alleles suggests that the two alleles are functionally null. The AC fusion failure phenotype of ty4 mutant worms was rescued by introducing an 8 kb genomic fragment harboring the promoter and coding region of the C44B7.3 gene (Figures 2A and 2B). Finally, the AC fusion failure phenotype was detected in worms fed with dsRNA directed against the C44B7.3 transcript (Figure 2B; Table S1). These results demonstrate that the C44B7.3 gene is specifically required for AC fusion, prompting us to rename it aff-1 (Anchor-cell Fusion Failure-1).
Figure 2. aff-1 Activity Is Required for AC Fusion.

(A) Scheme of aff-1 gene structure with mutations and construct annotations. The first methionine is substituted to isoleucine in ty4 mutation while the tm2214 deletion (red line) introduced a stop codon after alanine 47. The sequence that was used as the template for dsRNA experiments is marked in green and the 8 kb PCR-based rescue fragment in blue.
(B) Phenotypic analysis of aff-1 alleles. AC fusion failure and low fertility in ty4 and tm2214 mutants. aff-1 dsRNA phenocopy aff-1 mutant phenotype. ty4 phenotype is rescued by an 8 kb fragment from aff-1. Error bars indicate standard deviation. Asterisks indicate difference from wild-type with statistical significance of p < 0.001 according to unpaired two-tailed t test.
(C) Sequence alignment of C. elegans fusogens with their putative homologs from P. pacificus and selected members of the TGF-β type I receptors. The alignment is limited to the structurally defined part of the Hs BRIA sequence. Secondary structures presented under the alignment refer to the solved crystal structure of BRIA. Nine cysteines (pink) are conserved between all the aligned proteins, suggesting that these proteins share a similar structural fold. An additional cysteine followed by asparagine (green) that are part of TGF-β binding domain are not conserved in EFF-1 and AFF-1 proteins. Abbreviations: Ce, C. elegans; Pp, P. pacificus; Dm, D. melanogaster; Xe, X. laevis; Hs, H. sapiens; BRIA, BMP receptor IA extracellular domain; TGF, TGF-βRI. Alignment color code was according to the ClustalX color scheme Jalview software. Accession numbers: Ce AFF-1: EF205023; Pp AFF-1: contig1480; Ce EFF-1: GI:19071563. Pp EFF-1: Contig2476 (http://www.pristionchus.org/cgi-bin/seq_retrieval.pl); Dm Baboon: gi|33589356; Xe ALK4: gi|49903662; TGF Hs: gi|4759226; BRIA Hs: gi|48425316. See Experimental Procedures.
Sequence analysis revealed that aff-1 encodes a predicted type-I transmembrane protein, with a domain organization similar to that of the EFF-1 fusogen (Figure S3). While the two proteins exhibit only moderate overall primary sequence similarity (26% identity; 46% similarity), we note a striking conservation of cysteine and proline residue positions throughout the two sequences (Figure S3). These observations suggest that AFF-1 and EFF-1 share significant structural similarity and may therefore constitute members of a fusogen family, with distinct functions during development (Figure S4). Figure 2C shows that the ectodomain of AFF-1 contains a domain of ~100 residues with a characteristic pattern of cysteines conserved between EFF-1 and AFF-1 homologs in different nematode species. Moreover, this putative structural region of AFF-1 and EFF-1 ectodomains is a Transforming Growth Factor-β type I Receptor-like (TGF-β RI) structural domain conserved in invertebrates and vertebrates.
AFF-1 Is Required Directly for AC Fusion
Abnormal development or localization of either the AC, the vulval, or the uterine cell types can result indirectly in AC fusion failure. Alternatively, such failure can occur by specifically disrupting the AC fusion process. To explore which of these possibilities is affected by impairment of aff-1 function, we examined different aspects of vulval, AC, and π/utse cell development and localization prior to the process of AC fusion, in aff-1 mutant worms. In the two aff-1 mutant alleles examined, the presence of a bloated shaped AC indicated fusion failure, and the AC did not fuse even during later stages of development (Figure 3H). In 7%–9% (n = 135) of the worms, the unfused AC degenerated (Figure S1) even though AC fate determination, VPC induction, AC invasion, vulva invagination, and eversion occur normally (Figures 3 and 4A–4D). Thus, aff-1 mutants show specific AC fusion failure.
Figure 3. aff-1 Is Required for AC Fusion prior to Cytoplasmic Mixing.
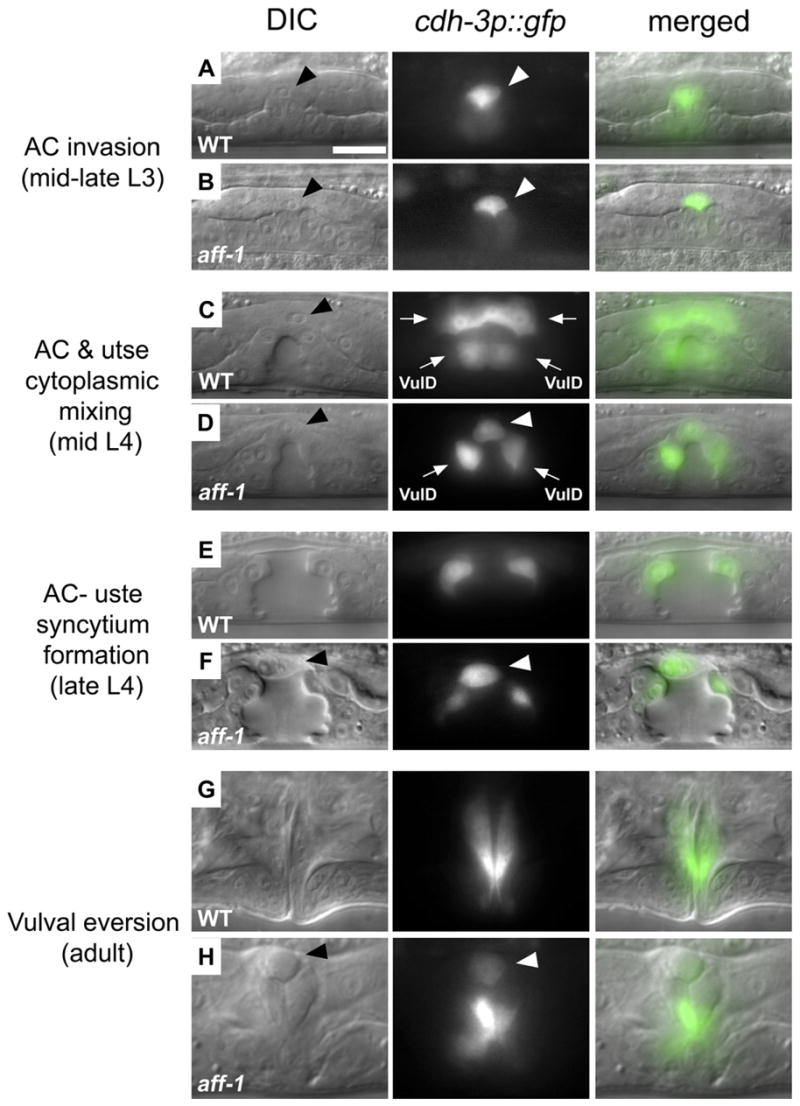
AC, arrowhead; utse syncytium, arrows.
Nomarski (left) fluorescence (center) and overlaid (right) images of vulval-uterine area in critical intermediates of AC development during L3 to adult.
(A) AC invasion in the L3 stage was detected by a cadherin promoter driving GFP expression (cdh-3p::GFP; [Sherwood and Sternberg, 2003]).
(B) In aff-1 mutant, AC invasion is normal.
(C) In wild-type, cytoplasmic mixing between AC and utse cells is detected by diffusion of the AC marker cdh-3p::GFP to the utse (arrows); VulD represents vulval ring “D.”
(D) In aff-1 mutant, cdh-3p::GFP retention in the AC demonstrates that cytoplasmic mixing does not occur (arrowhead).
(E) At the vulval “Christmas tree” stage, the AC and utse syncytium form a thin layer between vulva and uterus lumens in wild-type.
(F) In aff-1 mutant, this layer is not formed and the unfused AC lies at the vulva-uterus junction (arrowhead).
(G) Normal adult vulva after eversion.
(H) Unfused AC remains at the apex of the everted aff-1 vulva (arrowhead). All panels are at the same magnification; the scale bar corresponds to 5 μm.
Figure 4. aff-1 Is Required for Fusion Events in Other Tissues.
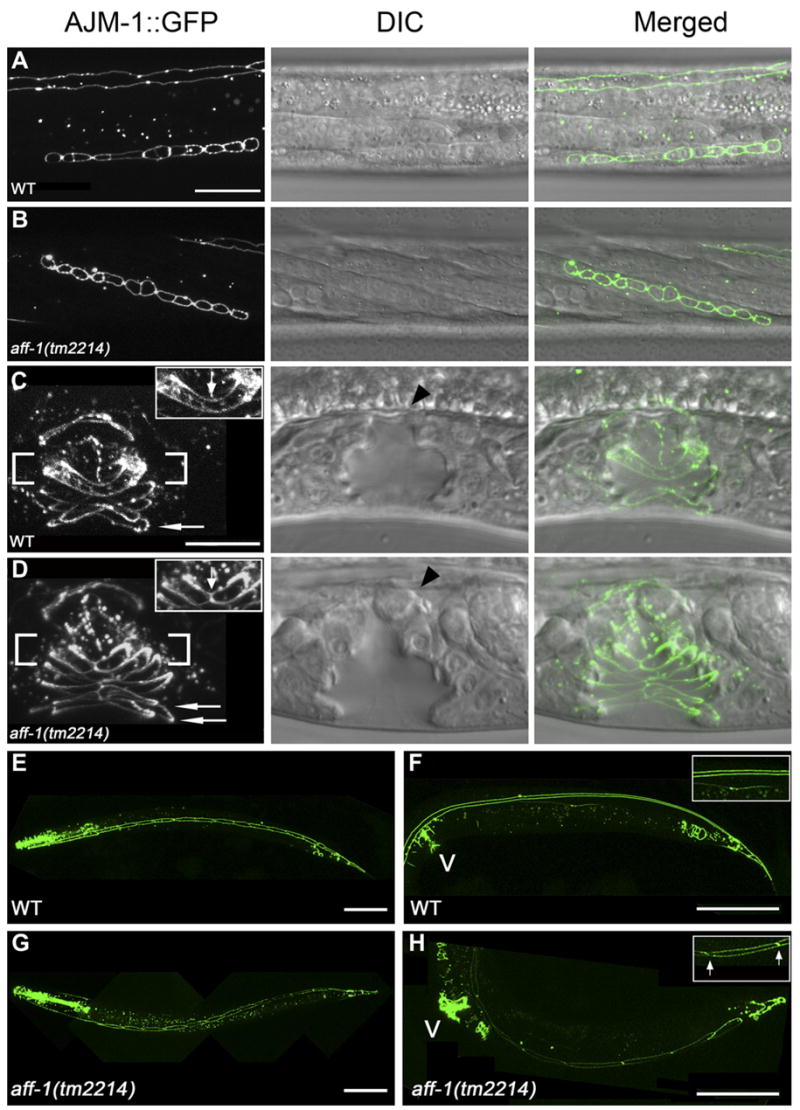
(A–D) Nomarski (center) and the corresponding fluorescence image in selected stages of vulval development of the apical junction marker AJM-1::GFP that marks epithelia cell borders.
(A) In wild-type worms, 12 primordial vulval cells are located at the ventral side at late L3 stage.
(B) A similar pattern in aff-1 mutants shows that aff-1 does not affect VPCs proliferation. In addition, the fusion of 3° fate VPCs to the epidermis is normal.
(C) Fusion of vulval cells results in the formation of vulval rings in wild-type (for example vulD ring, fusion marked with an arrow in inset). vulA represents a single ventral ring (arrow).
(D) In aff-1 deletion, the two D cells did not fuse; hence the D ring is unfused (arrow in insert). The A cells fail to fuse before ring formation and two vulA rings form instead of one (arrows).
(E–H) Selected stages of seam cell development in wild-type (E and F) and aff-1 mutant worms (G and H) examined by the AJM-1::GFP marker.
(E) In wild-type L3 stage, 16 seam cells are on each side of the body, separated by apical junctions (left view).
(F) During late L4/early adult, seams undergo cell fusion that results in a long syncytium marked by two parallel lines of AJM-1::GFP; see insert and top of (A).
(G) In aff-1 mutant, early seam development is similar to wild-type.
(H) During late L4, the seam syncytia did not form, so individual cells are detected and remained unfused in adults. Insert shows detail with unfused apical junctions, arrows.
(A, B) and (C, D) are panels with same magnification. The scale bars in (A) and (C) represent 10 μm. (E–H) Scale bar corresponds to 50 μm. V, vulva.
To determine whether π/utse differentiation was affected in aff-1 mutants, we followed the expression pattern of egl-13p::gfp, which marks the nuclei of utse cells. We found that π/utse differentiation and localization is normal in aff-1 mutants (data not shown). Additionally, the formation of the utse syncytium, nuclei distal migration, and utse transition into a thin-layer syncytium occurs normally in an aff-1 background (Figure S1), suggesting that utse syncytiogenesis is facilitated by an aff-1-independent mechanism.
Studies of the mechanism of cell fusion in C. elegans and during other membrane fusion events revealed that this process is executed via several intermediate steps, including pore formation and pore extension (Mohler et al., 1998; Shemer et al., 2004). In C. elegans, cytoplasmic mixing of the fusing cells occurs early on, before the cell shape changes characteristic of syncytia formation (Shemer et al., 2004). To investigate whether cytoplasmic mixing between the AC and utse occurs in an aff-1 mutant background, we used the AC-specific cytoplasmic marker cdh-3p::GFP. In cdh-3p::GFP wild-type worms, GFP diffusion from the AC to the utse syncytium is detected (Figure 3C, top arrows), demonstrating the cytoplasmic mixing event. In contrast, in an aff-1 mutant background, GFP is retained in the AC, indicating that there is no cytoplasmic mixing between the two cell types (Figure 3D, arrowhead). This suggests that AFF-1 is required for early stages of AC fusion, prior to the formation of fusion pores that are large enough to allow diffusion of GFP from the cytoplasm of the AC to the utse cells.
Taken together, these results demonstrate that aff-1 is required specifically during the process of AC fusion and does not participate in developmental processes occurring before or in parallel to AC fusion. Thus, in the sequence of events leading to fusion, AFF-1 is required prior to pore formation and cytoplasmic content mixing.
AFF-1 Is Required for Specific Vulval and Late Epidermal Seam Cell Fusion
In addition to AC fusion failure, other blocks in specific fusion events were observed in aff-1 mutant worms. For example, we found specific blocks in fusion of the vulval A and D rings (vulA and vulD) and in the terminal fusion between the entire lateral seam cells, which normally results in the formation of a single epithelial seam syncytium on each side of the animal (Figure 4). As in the case of the AC, these late fusion events occur normally during the late L4-adult transition in an eff-1 null allele but not in aff-1 mutant worms (Figures 4D and 4H). The development of these cells before fusion is normal compared with the wild-type (Figure 4), suggesting that aff-1 acts directly in the fusion process at the late L4 to adult molt. In summary, aff-1 is required not only for AC fusion but also for other fusion events that are eff-1 independent. In light of the dual activity of AFF-1 and EFF-1 in different fusion events, a mutation in aff-1 or eff-1 alone does not represent complete inhibition of all fusion events. eff-1 aff-1 double-mutant worms have extremely low viability and can only be maintained due to escapers in contrast to eff-1 and aff-1 mutant worms that are viable and semiviable, respectively (Figure 2B and Table S1). utse syncytiogenesis and sperm-egg fusion occur normally in eff-1 and aff-1 mutant backgrounds, possibly due to the activity of other fusion factors (Figure S1 and Table S1). This suggests that additional fusogen(s) exist in C. elegans.
AFF-1 Is Sufficient to Fuse Cells that Normally Do Not Fuse In Vivo
We have shown that AFF-1 is required for specific fusion events in C. elegans. To test whether AFF-1 is also sufficient to fuse cells in C. elegans, we cloned the full-length aff-1 ORF under the regulation of a heat-shock promoter. This construct was expressed in embryos by standard heat-shock protocols, along with the apical junction protein AJM-1::GFP, which marks epithelial cell boundaries (Podbilewicz, 2006). For controls, we examined AJM-1::GFP distribution in non-heat-shocked embryos with the same genotype and in heat-shocked AJM-1::GFP embryos. Ectopic expression of aff-1 resulted in the disappearance of AJM-1::GFP from the boundaries of hypodermal cells and the redistribution of the marker in large entities that presumably represent the formation of ectopic syncytia (Figure 5B; 26 out of 77 embryos). Normal fusion pattern in the controls demonstrated that the effect is specific to aff-1 ectopic expression (Figure 5A; n = 100). To exclude the possibility that aff-1 acts via the activation of eff-1, we overexpressed aff-1 in eff-1 null mutant embryos and detected the same level of ectopic fusion (Figure 5D; in 40% of the embryos, n = 25); hence AFF-1 fuses cells via an eff-1-independent mechanism. In order to examine the dynamic fusogenic activity of aff-1, we monitored fusion directly by detecting cytoplasmic mixing between fusing cells. We used a strain in which the eff-1 promoter drives expression of cytoplasmic GFP in individual cells of the embryonic hypodermis. Dynamic GFP diffusion from the hypodermal cells to the embryonic seam cells, which normally do not express aff-1, concomitant with AJM-1::GFP disappearance from the cell junctions shows that AFF-1 induces bona fide cell fusion (Figure 5F, Movie S2). These results demonstrate that AFF-1 is sufficient for the induction of cell fusion in C. elegans in an eff-1-independent manner.
Figure 5. AFF-1 Fuses C. elegans Hypodermal Cells and Heterologous Insect Cells.
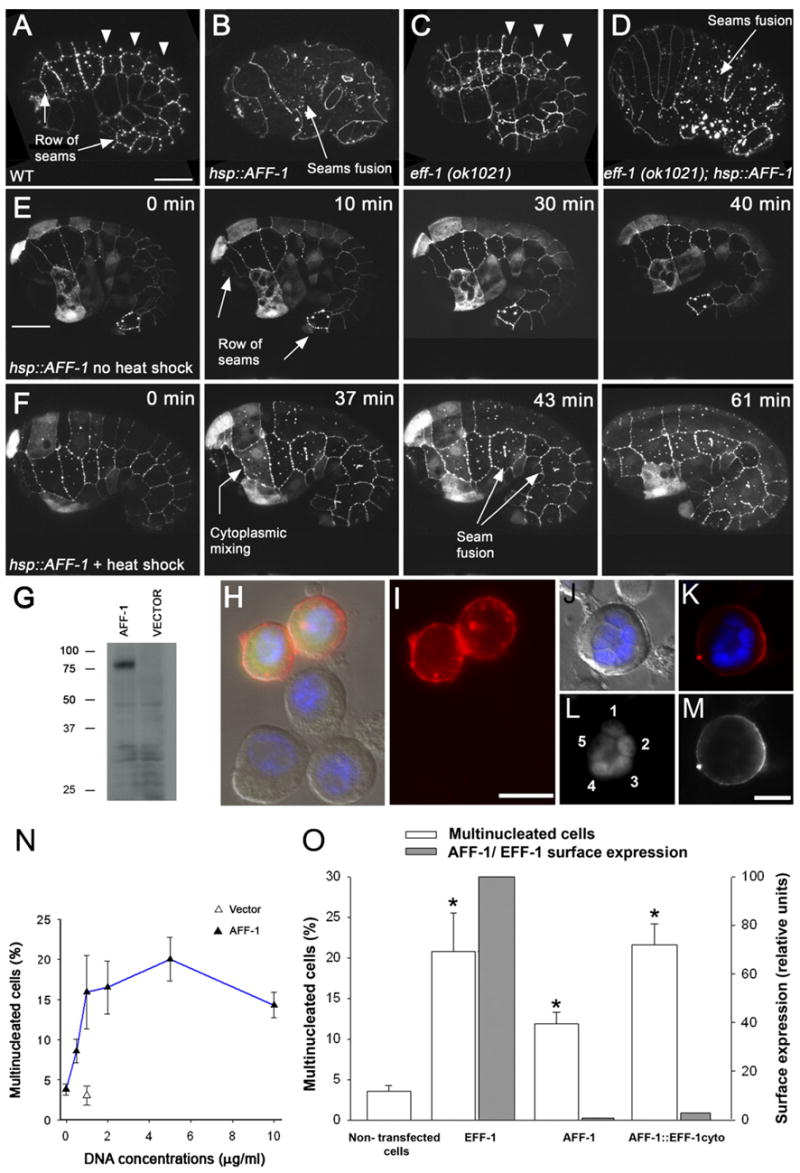
(A–F) Comma to 1.5-fold stage embryos, anterior to the left and ventral down.
(A–D) Confocal projections of embryos from different genetic backgrounds all marked with the apical junction marker AJM-1::GFP. Punctuated staining is due to the background of the GFP.
(A) In wild-type embryos, dorsal hypodermal cells undergo fusion (arrowheads). The seam cells do not fuse during embryogenesis (arrows).
(B) hsp::aff-1 embryos after heat shock. The disappearance of apical junction between individual cells suggests that AFF-1 causes fusion of the hypodermal cells.
(C) eff-1 mutant embryos where most embryonic fusions do not occur. Arrowheads mark some unfused dorsal cells.
(D) The lack of eff-1 does not attenuate AFF-1-induced fusion, indicating that AFF-1 acts in an eff-1-independent mechanism.
(E and F) Individual frames from time-lapse movies (see Supplemental Data) of control (E) and hsp::aff-1 worms (F) marked by AJM-1::GFP and by eff-1p::GFP that is distributed in the cytoplasm of individual hypodermal cells. The time after heat shock appears on top right.
(E) In non-heat-shocked embryos, dorsal fusion is normal while individual seam cells did not fuse (arrows; see Movie S1).
(F) In the intermediate step of the heat shock effect (37 min), diffusion of GFP from ventral hypodermal cell to a single seam cell (arrow) concomitant with apical junction removal between these cells indicates that aff-1 is sufficient to induce cell fusion ectopically. In addition, fusion between seams is observed (arrows). The cytoplasmic GFP diffuses through hyp6, hyp7, and seam cells (Movie S2).
(G) AFF-1 protein tagged with V5-6XHis epitopes was expressed in Sf9 cells and detected from the cell lysate as a single specific band of apparent MW of 75 kDa by western blot with anti-V5 antibodies.
(H) Immunofluorescence with anti-V5 antibodies (red), DAPI staining (blue) on aff-1-expressing cells (green). The lower three cells do not express the construct.
(I) AFF-1 protein (red) is distributed at the cell surface and in intracellular puncta.
(J–M) AFF-1 expression in a pentanucleate cell.
(J) Cell nuclei are marked by DAPI staining (blue) merged with DIC.
(K) AFF-1 protein immunostaining (red) and DAPI (blue).
(L) Five distinct nuclei (1–5) are detected in the syncytium.
(M) AFF-1 protein is localized to the plasma membrane.
(N) Ectopic expression of AFF-1 results in multinucleated Sf9 cells 24 hr after transfection. Percentages of multinucleation with respect to aff-1 DNA concentration are shown (filled triangles and blue line). The multinucleation of control cultures transfected with empty vector is marked by an empty triangle.
(O) Cell surface AFF-1 induces multinucleation more potently than EFF-1.
Percentages of multinucleated cells (empty columns) and surface expression in relative units (gray columns) of empty vector, EFF-1, AFF-1 and a chimera between AFF-1 extracellular domain and EFF-1 transmembrane and cytoplasmic domain (AFF-1::EFF-1cyto; see Supplemental Data).
(A–F) Scale bars represent 10 μm; (H and I) 20 μm; (J–M) 10 μm.
Error bars represent standard error and stars represent statistical significance of p < 0.05.
C. elegans AFF-1 Fuses Heterologous Insect Cells
The necessity of AFF-1 for specific fusion events, combined with its ability to promote ectopic cell fusions, suggests that this protein acts directly in the cell fusion process. To explore this further, we expressed the AFF-1 protein in heterologous cultures of Sf9 insect cells that do not usually undergo cell fusion (Podbilewicz et al., 2006). Western blot analysis detected a single band corresponding to AFF-1 protein at apparent MW of 75 kDa (Figure 5G). Surface biotinylation and immunofluorescence revealed that the protein is distributed both in intra-cellular compartments and at the surface of Sf9 transfected cells (Figures 5H and 5I). Fusion in culture was estimated by evaluating the relative quantity of multinucleated cells after transfection. Using confocal and fluorescence microscopy, we demonstrated that transfection with aff-1 generated multinucleate cells containing two to six nuclei (Figures 5J–5M and Figure S6). We detected 20% multinucleation in cells transfected with aff-1 (Sf9-AFF-1 cells) in comparison to only 3% in cells that were transfected with an empty vector (Figure 5N). To investigate whether AFF-1-induced multinucleation is a result of cell-cell fusion or a failure of cell division, we incubated Sf9-AFF-1 cells in the presence of 5′-fluoro-2′-deoxyuridine (FdUrd) that blocks cell division at the transition between G1 and S phases (Podbilewicz et al., 2006). We found that Sf9-AFF-1 cells treated with FdUrd had a similar proportion of multinucleation as untreated cells (15.8% ± 2.8% without FdUrd and 15% ± 2.4% with FdUrd). Thus, the transfected cells incubated in FdUrd did not show an apparent decrease in the number of multinucleated cells, supporting a mechanism of multinucleation independent from failure in cytokinesis in the presence of karyokinesis.
To compare the fusogenic activity and potency of AFF-1 with that of EFF-1, we measured the percent of multinucleation in plates transfected with EFF-1 or AFF-1 (Podbilewicz et al., 2006). We found that 48 hr posttransfection with 0.5 μg/ml DNA, AFF-1 expression on the surface was ~100 times lower than EFF-1 expression, as estimated by western blotting after surface biotinylation (gray bars, Figure 5O). In the same cells, the efficiency of multinucleation was only two times higher for cells transfected with EFF-1 than for cells expressing AFF-1 (white bars, Figure 5O). Low expression of AFF-1 might reflect its cytotoxic effects, observed as loss of cells for higher concentrations of DNA and/or longer posttransfection times (data not shown). To increase the surface expression of AFF-1, we expressed a chimera containing AFF-1 ectodomain with EFF-1 transmembrane and cytoplasmic tail (AFF-1::EFF-1cyto, see Supplemental Data). The AFF-1::EFF-1cyto chimera showed an increase in surface expression together with a significant increase in the level of multinucleated cells. While the level of surface expression was 30 times lower for AFF-1::EFF-1cyto than for EFF-1, we observed a similar level of multinucleation (Figure 5O). Thus, comparable cell fusion efficiency at a much lower surface density suggests that AFF-1 is a more potent fusogen than EFF-1. In conclusion, AFF-1 surface expression in insect cells results in cell fusion and formation of syncytia in vitro, demonstrating that AFF-1 is a bona fide fusogen.
aff-1 Is Expressed in the AC and utse Cells as They Fuse
To follow aff-1 expression, we fused a 4.5 kb fragment of the aff-1 promoter to GFP and monitored its distribution in transgenic worms. The extent of the aff-1 promoter was deduced from sequence similarities between C. elegans aff-1 and its putative ortholog from C. briggsae. Specific and continuous expression was detected in the AC from the invasion of the vulval primordium at mid-L3 until its fusion with the utse cells (Figures 6A–6C). As the vulva completes its invagination in the L4, the utse syncytium starts to express aff-1, resulting in coexpression of aff-1 in both cells prior to their fusion (Figure 6D). Since utse aff-1 expression and AC-utse fusion occur almost simultaneously, it is possible that aff-1 expression detected in the utse is actually a contribution from the AC cytoplasm after the fusion event. To test this, we examined utse aff-1 expression in lin-29(n482) mutant worms where AC-utse fusion does not occur (Newman et al., 2000). aff-1 expression in the utse in these mutants indicated that aff-1 is specifically expressed in the utse cells and is not a consequence of AC to utse cytoplasmic GFP diffusion after fusion (Figure 6F). Moreover, the lin-29 transcription factor is not required for aff-1 expression in π/utse cells, as demonstrated by aff-1 utse expression in lin-29 mutant worms (Figure 6F). Taken together, the phenotypes in aff-1 and lin-29 mutant backgrounds, and aff-1 expression in the AC followed by expression in the utse, are consistent with a direct function of AFF-1 in the AC-utse fusion process.
Figure 6. Correlation between aff-1 Expression and Its Fusogenic Effect.
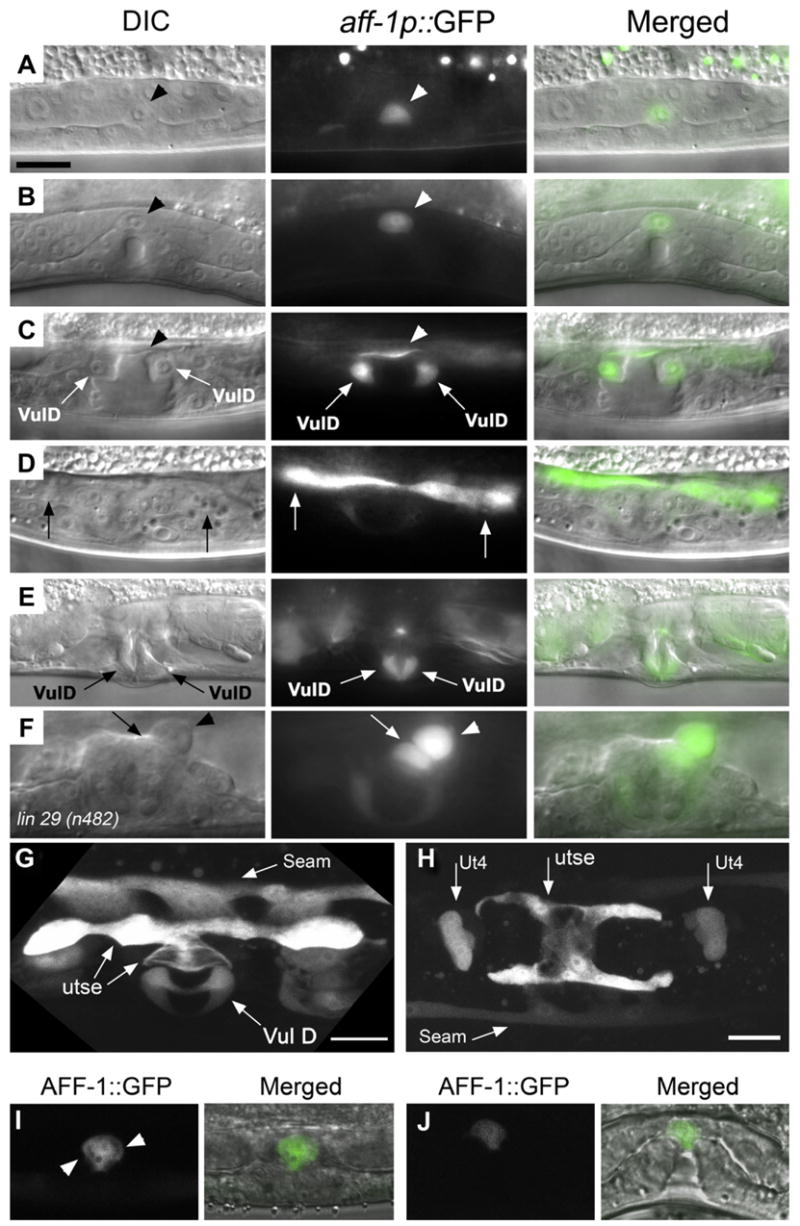
(A–F) Nomarski (left) aff-1 promoter GFP fusion (aff-1p::GFP) fluorescence signal (center) and overlaid (right) images.
(A) At mid-L3, aff-1 is expressed specifically in the AC (arrowhead).
(B) AC expression is retained while the vulva invaginates during early L4.
(C) Expression of the aff-1 transcriptional reporter is induced in the vulval D ring cells (arrows) and in the AC-utse syncytium as it is formed in late L4 stage (arrowhead).
(D) A different focal plane from the same worm shown in (C) where the AC-utse expression is highlighted (arrows).
(E) The vulD expression of aff-1 is retained in adult worms (arrows).
(F) In lin29(n482) mutants, AC and utse fusion do not occur (Newman et al., 2000). A single AC (arrowhead) is localized adjacent to a single utse cell (arrow). aff-1 signal in utse cells that did not fuse with the AC cell demonstrates that aff-1 is autonomously expressed in the utse cells.
(G) Confocal projection of aff-1 expression pattern in L4 larva (dorsolateral view). aff-1 is expressed in VulD ring, the utse, and in the seams. Seam cell expression starts at mid-L4 just before aff-1-dependent fusion.
(H) Confocal projection of the H-shaped utse syncytium where aff-1 is expressed (ventral view). In addition, aff-1 expression is detected in two rows of lateral seam cells and in the two uterine ut4 toroids (Ut4).
(I) Subcellular localization of AFF-1::GFP protein in the AC during invasion (lateral view of confocal image). The fusion protein was specifically expressed in the AC by the anchor-cell-specific promoter pAC (Kirouac and Sternberg, 2003). AFF-1::GFP protein is localized in intracellular compartments and at the plasma membrane (arrowheads).
(J) Similar subcellular localization of AFF-1::GFP was detected at the time of AC fusion in a confocal image (lateral view).
(A–F) Scale bar 5 μm; (G) 10 μm; (H) 20 μm.
Dynamic Expression of aff-1 in a Selected Group of Cells
To determine the expression of aff-1 transcripts in C. elegans, we expressed a transcriptional aff-1promoter::GFP reporter (see Supplemental Data). aff-1 expression is first detected in the embryonic hyp5 cell and later during larval development in various cell types, including pharyngeal muscles (Pm3 and Pm5), uterine rings (Ut2 and Ut4), head and tail neurons, sheath cells of chemo-sensory neurons, and male tail neurons (Figure S2). aff-1 is also expressed in vulval vulD (Figures 6C and 6E; arrows) and the seam cells (Figures 6G and 6H) shortly before these cells fuse. In general, the myoepithelial cells of the pharynx and the epithelial cells in the uterus, vulva, and hypodermis that express aff-1 undergo fusion. In contrast, there is no evidence for cell fusion in neurons that express aff-1. The dynamic expression of aff-1 suggests that aff-1-mediated cell fusion is regulated by transcriptional cues. The complementary expression of aff-1 in cells that fuse independently of eff-1 suggests that eff-1 and aff-1 activities are independent and that their combined autonomous activities account for most somatic cell fusion events in C. elegans.
To determine the cellular and subcellular localization of AFF-1 in C. elegans, we expressed a translational AFF-1::GFP using a genomic fragment containing the predicted aff-1 promoter and the ORF, including introns and regulatory sequences (see Supplemental Data). We found that AFF-1::GFP was weakly expressed on the plasma membrane and in intracellular organelles in hyp5, seam cells, vulD vulval precursors, and AC before, during, and after cell-cell fusion (Figure S5 and data not shown). Since we found weak expression in different independent transgenic lines, we expressed AFF-1::GFP under a strong AC-specific promoter (Kirouac and Sternberg, 2003). We found the reporter to be strongly expressed in intracellular organelles, and on the surface of the AC from the time of invasion of the vulval primordium to the fusion to the utse (Figures 6I and 6J). Both aff-1 reporters were expressed in hyp5, seam cells, vulD cells, and AC. Taken together, the transcriptional and translational aff-1 reporter constructs support the model in which AFF-1 is dynamically expressed in a specific group of cells that undergo cell fusion during normal development.
FOS-1 Regulates AFF-1-Mediated AC-utse Fusion
To identify the mechanism controlling aff-1 expression in the AC, we looked for candidate transcription factors. The early expression of aff-1 in the AC starting from the preinvasion stage suggests that aff-1 is regulated by transcription factors that act early during AC development. One such candidate is the FOS-1A transcription factor that is expressed early in the AC to promote basement membrane removal during invasion (Sherwood et al., 2005). To test whether FOS-1 may control aff-1 expression, we looked for FOS-1-binding sites in aff-1 promoter using Transfac algorithm (Supplemental Experimental Procedures). We identified a consensus TGA(T)TCA Fos-binding site in position −1866 of aff-1 promoter that is present in an identical position within the aff-1 promoter from C. briggsae, supporting the hypothesis that FOS-1 controls aff-1 expression. To further examine this hypothesis, we looked for aff-1p::GFP expression in the fos-1(ar105) mutant that specifically disrupts fos-1a (Sherwood et al., 2005). Strikingly, aff-1 expression is strongly reduced or undetectable in the AC and other uterine cells in this fos-1 mutant (Figures 7D and 7E; n = 33). In contrast, aff-1 expression is retained in VulD and the seam cells (Figure 7E; n = 25), indicating that fos-1a positively controls aff-1 expression in the AC and utse cells. Taken together, these results suggest that FOS-1A controls AC fusion via aff-1 expression. To test whether fos-1 is required for AC fusion, we analyzed fos-1 mutants and found that the AC failed to fuse (Figures 7A–7C, arrowheads; n = 20). Thus, the activity of fos-1 positively regulates two different aspects of AC development: invasion and fusion.
Figure 7. FOS-1 Controls AC Fusion via aff-1 Expression in utse and AC.
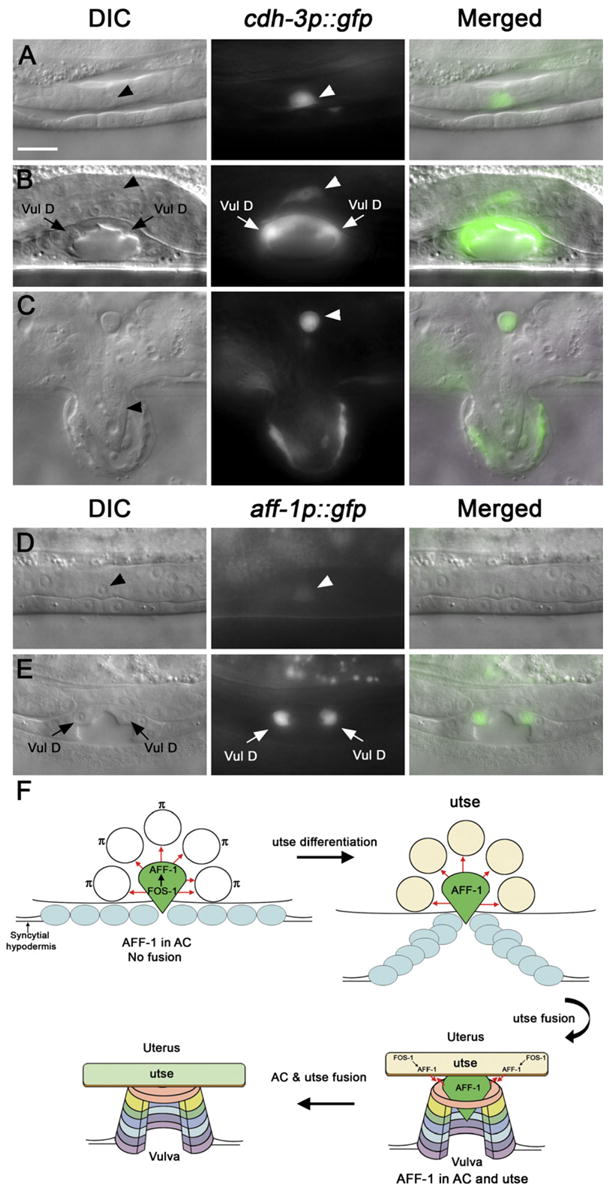
(A–C) Nomarski (left), AC marker cdh-3p::GFP (center), and overlaid (right) images of fos-1(ar105) mutant L3 to adult.
(A) AC invasion did not occur in fos-1 mutants, indicated by the retention of basement membrane between the gonad and the ectoderm (Sherwood et al., 2005) (arrowhead).
(B) In fos-1 mutant L4 larva, the cdh-3p::GFP-labeled AC did not fuse (arrowhead).
(C) This fusion failure persists until adulthood (arrowhead).
(D and E) Images of fos-1(ar105) mutants. No-marski (left), aff-1 promoter GFP (center), and merged (right).
(D) At the stage of AC invasion (L3), AC does not invade in fos-1 mutant (arrowhead; 100%, n = 36) and expresses aff-1 very weakly or is undetectable (97%, n = 33) in comparison to wild-type (see Figure 6A).
(E) aff-1 was not detected in the AC or other uterine cells while still retaining expression in VulD ring and in the seam cells (not shown) compared with wild-type (L4; Figures 6B–6D).
(F) Schematic representation of FOS-1-mediated regulation of aff-1 RNA (green) and AFF-1 protein (red arrows). FOS-1 controls aff-1 expression during AC invasion. During π/utse differentiation, AFF-1 is expressed in the AC only. After utse AFF-1-independent fusion, the utse syncytium starts expression of aff-1 RNA. Once AC and utse syncytium both express AFF-1, AC-utse fusion occurs.
(A)–(E) are with the same magnification. Scale bar represents 5 μm.
DISCUSSION
The unique development of the gonadal AC in C. elegans serves as a model for numerous fundamental cellular and developmental processes (Kimble, 1981; Seydoux and Greenwald, 1989; Sharma-Kishore et al., 1999; Sherwood et al., 2005; Sternberg and Horvitz, 1986). AC development is terminated by fusion to the utse syncytium (Figure 7F). The discrete morphology of the AC makes it an attractive model for cell fusion studies at single-cell resolution. We propose that AFF-1, together with the related EFF-1 fusogen, represent founding members of a family of developmental fusogens that induce fusion using similar mechanisms. AFF-1 and EFF-1 proteins presumably share a common ectodomain structure that includes a fold similar to TGF-β-type I Receptors. This putative domain represents nearly one-fifth of the EFF-1 and AFF-1 ectodomains. This conserved structure may be utilized by the as-yet-unidentified fusogens in other phyla. In addition, the dual activity of fos-1 as a regulator of both AC invasion and fusion uncovers a novel molecular connection between these cellular processes. This surprising link may have implications in the understanding of diverse developmental processes such as tube morphogenesis, placentation, angiogenesis, and metastasis (Wolpert, 2007).
Foundation of a Family of Developmental Fusogens
The general structure of EFF-1 and AFF-1 proteins is similar: a signal sequence that is followed by a long extracellular portion, a predicted transmembrane domain, and an intracellular tail (Figure S3). The viral fusogen-like domains in EFF-1 that were previously hypothesized as possible fusion-related domains (Mohler et al., 2002) are only moderately conserved in AFF-1 proteins (Figure S3). In contrast, a striking conservation in the position and number of all 16 cysteines in the extracellular domain of AFF-1 and EFF-1 proteins, along with partial conservation of 11 out of 20 extracellular prolines, may stabilize a similar three-dimensional structure required for fusion facilitation. While there are clear homologs in other nematodes, AFF-1 and EFF-1 exhibit only minor similarity to proteins from other vertebrates and invertebrates, especially to proteins from the TGF-β/activin/BMP type I receptor superfamily (Figure 2C). A TGF-β-RI-like binding domain (Keah and Hearn, 2005) may define an interacting extracellular region conserved between C. elegans fusogens EFF-1 and AFF-1 that may be shared with unidentified invertebrate and vertebrate fusogens. Interestingly, TGF-β has been recently found to negatively regulate cell fusion in human endometrial carcinomas (Strick et al., 2007). However, the fact that some critical residues in the TGF-β-RI-like binding domain of FF family members are not conserved (e.g., the Cys-Asn residues; Figure 2C) undercuts the idea that the FF family members will have similar protein-protein interactions as TGF-β-RIs. Perhaps common activities or protein-protein interactions were shared by a common ancestor, but were lost as each family evolved more efficient ways to execute their respective functions.
In summary, EFF-1 and AFF-1 are the founding members of a family of fusogens in C. elegans and probably in other nematodes. All the members of the FF family share eight conserved predicted disulfide bonds in their ectodomains, including a putative TGF-β-type-I-Receptor-like domain (Figure 2C). The TGF-β-R1-like domain probably represents a conserved fold that might have implications for the mechanisms of developmental cell fusion.
Why Does C. elegans Need More Than One Fusogen?
Based on the conservation of extracellular cysteine and proline residues in the EFF-1 and AFF-1 proteins (Figures S3 and S4), which may stabilize a similar structure, one may hypothesize that fusion between aff-1- and eff-1-expressing cells may take place. However, several lines of evidence suggest that this heterotypic fusion does not occur and provide an explanation that emerges from biological context for the existence of these two similar fusogens. If heterotypic fusion between EFF-1 and AFF-1 occurred, the aff-1-expressing seam cells would fuse with the surrounding eff-1-expressing hyp7. However, this is not observed, so AFF-1 activity in the seam cells might allow seam cell fusion without the loss of seam cell identity (Figure 4H). In other cell types examined, such as VulD and embryonic hyp5 cell, there are EFF-1-expressing syncytia adjacent to AFF-1-expressing syncytia, but heterotypic fusions do not occur (Figure 6C and Figure S2). The regulated expression of two fusogens might establish the formation of developmental barriers between adjacent syncytia that may represent a general characteristic of developmental fusion.
The discovery of a new developmental fusogen in C. elegans implies that one general-purpose fusogen EFF-1 is not enough to account for all somatic cell fusions. AFF-1 is indeed a specialized fusogen required for particular fusion events. AFF-1-mediated fusions usually involve small membrane domains and limited timing of action, and are tightly controlled by transcriptional and posttranslational mechanisms (e.g., FOS-1 and NSF-1, respectively). Indeed, our experiments in heterologous insect cells suggest that AFF-1 is a more potent but also more toxic fusogen than its relative EFF-1.
FOS-1 Controls Cell Invasion and Cell Fusion
FOS-1A is expressed and required autonomously in the AC for its invasion activity in C. elegans. FOS-1A activates a network of target genes that facilitates cell invasion, a process reminiscent of Fos functions in mammalian development. We demonstrated an additional function of FOS-1 transcriptional network-regulating cell fusion after cell invasion (Figure 7F). Hence, the two sequential processes are regulated by the same transcription factor. Cell fusion may be a general safety mechanism to extinguish the invasive cell behavior of cells during normal development. For example, the formation of the syncytial trophoblast in the placenta may extinguish the invasive behavior of the cytotrophoblasts and may also restrict the invasive potential of embryonic cells (Cross et al., 1994). The dual activity of fos-1-regulating cell invasion followed by cell fusion uncovers a novel cascade of events that may also be utilized in the normal development of vertebrates and in tumor progression.
EXPERIMENTAL PROCEDURES
aff-1 Gene Characterization and Mutants Isolation
Gene prediction was verified by sequencing three C44B7.3 ESTs (yk474, yk1083, and yk1627). Two full-length ESTs revealed that the ORF starts at an ATG laying 45 bp 5′ from the wormbase annotated ATG (Wormbase Release WS167, http://www.wormbase.org/). This results in an addition of a signal peptide to the predicted protein.
The ty4 mutant was isolated in an EMS-based screen described previously (Choi et al., 2006; Cinar et al., 2003). ty4 was mapped to a genetic interval of about 0.24 map units (about 300 kb) in the center of chromosome II by Tc1 mapping followed by three-factor and deficiency mapping (Supplemental Data). To identify the ty4 mutation, the entire coding region of the aff-1 gene (3 kb, containing 12 exons and 11 introns), as well as 5 kb of aff-1 UTRs and promoter, was amplified using ty4 mutant as template by single worm PCR and sequenced using relevant primers. The tm2214 deletion was generated and generously provided by Shohei Mitani, Tokyo Women’s Medical University School of Medicine, Japan (http://www.nbrp.jp/index.jsp). PCR amplification and sequencing of tm2214 mutants revealed that a deletion of 999 bp occurs (from base 147 to 1146 in the C44B7.3 sequence where 1 is the A of the ATG) that introduced a stop codon after alanine 47 in the AFF-1 protein. ty4 and tm2214 alleles were outcrossed five and ten times, respectively.
For ty4 rescue experiments, an 8 kb genomic fragment of aff-1 that includes the promoter and coding region was amplified from wild-type worms. This PCR product was coinjected with pRabGFPrim3′ injection marker (Choi et al., 2006) into ty4 mutants.
Phenotypic Analysis
To measure the percentage of Egl worms, we picked hermaphrodites as L4 larvae onto separate plates. The worms were examined daily for the next 4 days, and all progeny were counted as in Brenner (1974). To analyze AC invasion and fusion, L3 to L4 larvae were examined using Nomarski optics and the AC marker cdh-3p::GFP (Pettitt et al., 1996). Cell fusion events in other tissues were examined using the apical junction marker AJM-1::GFP. Nuclear position in the utse was examined using egl-13p::GFP (Cinar et al., 2003; Hanna-Rose and Han, 1999).
RNAi experiments were performed as described in Timmons et al. (2001). A 1.5 kb fragment from aff-1 gene was cloned in L4440 (pPD129.36) cloning vector (kindly provided by A. Fire) and was transformed into the HT1115 bacteria strain. After IPTG induction, the bacterial culture was seeded on NGM agar plates. Worms from the RNAi-sensitive strain eri-1(mg366) IV were individually plated, and phenotypes were scored in the F1 progeny.
For heat shock experiments, 20–40 laying hermaphrodites were grown on agar plates at 20°C for 2 hr. After removal of the laying animals, the plates were sealed and subjected to 32°C for 30 min in a water bath and were visualized by transferring post-heat-shock embryos to an egg salt drop on a poly-L-lysine-coated slide or a 3% aga-rose-coated slide. Cell fusion was assayed immediately after heat shock treatments.
Lethality was scored for ty4 and tm2214. For ty4: 22% L1 rod-like lethal and 2% embryonic lethal (n = 400). For tm2214: 6% L1 rodlike lethal and 0% embryonic lethal (n = 1946).
Identification of the Homology to TGF-β-RI Extracellular Domain
The amino acid sequence of EFF-1A was used to find homologs in Pristionchus pacificus using BLAST (http://www.pristionchus.org/cgi-bin/blast_iframe.pl).
A significant alignment was obtained for contig2476 (E Value1e-33). We then proceeded to identify the amino acid sequence of the corresponding protein. The program “Augustus” (http://augustus.gobics.de/) was used for the gene prediction using the training set for C. elegans. We were able to predict a protein 570 aa in length which shows 40% identity to EFF-1A and 22% identity to AFF-1. We used PSI BLAST (Altschul et al., 1997) to verify that this homolog shows significant homology to all known EFF/AFF family (FF Family) members, and indeed, using this sequence, we were able to retrieve eight protein sequences all belonging to the FF family after one iteration. We then proceeded to review the various proteins aligned below the default threshold score of 0.005 (the standard cut-off score of BLAST is an E Value of 10). One protein RE55648p (Drosophila melanogaster), a member of the TGF-β-receptor family, with an E Value of 2 was of particular interest because of the high conservation specifically in Cysteine residues and an identity of 24% over a sequence length of 84 aa. To evaluate whether this homology is statistically significant and whether this Cysteine pattern is specific, we aligned this domain, using ClustalW, with other FF family members and created a pattern of the Cysteine and some similar residues:
CXC[EDH]X(0,1)CX(1,3)[TS][ENS]X(1,2)CX(2)[EDS]X[YFH]X(0,5)CX (9,17)CX(12)CX(8,10)CC
We then utilized this pattern or variations of it to run PHI BLAST (Altschul et al., 1997). We retrieved RE55648p and other members of the TGF-β receptor type I superfamily.
aff-1 eff-1 Double Mutants Have Very Low Viability
Out of 1381 F1 progeny from a balanced strain containing eff-1(ok1021) aff-1(tm2214)/mln1 [myo-2::gfp; pes-10::gfp] II, we obtained only ten non-GFP-homozygous double mutants. From the ten double mutants, we obtained only six F2 animals. The combined progeny (escapers) were all Dpy Unc with Eff tail and comprised two gravid Egl, two L2, and two larvae with posterior paralyzed body. Based on these results, we conclude that cell fusion in C. elegans is required for viability.
Cell Culture Assay
Sf9 cells were grown to 50% confluency on 35×10 mm tissue culture plates as recommended by manufacturers. Cells were transfected with cellfectin and with plasmid at 3 μg/ml (either pIZT-Empty vector, or pIZT-AFF-1), as recommended by Invitrogen, and were analyzed at different times from 18 to 96 hr posttransfection as in Podbilewicz et al. (2006).
To assay syncytium formation and to correlate it with the expression of GFP reporter present in the plasmids used for the transfection, we stained cell nuclei with Hoechst (1 ìg/ml, H3570, Molecular Probes) or DAPI for 10 min at 22°C. Multinucleated cells were assayed between 24 to 48 hr posttransfection, since after extended durations in culture we found high levels of toxicity with pIZT-aff-1 DNA. We obtained GFP(+) fluorescence (transfected cells), DIC, Hoechst, and phase-contrast images. Low expression of GFP in fusing AFF-1 cells hindered identification of the transfected cells as GFP-expressing cells. Thus, we scored the efficiency of multinucleation for all cells rather than only for cells transfected with AFF-1 protein and, as shown in Podbilewicz et al. (2006) significantly underestimated fusion rates.
The extents of multinucleation defined as the ratio between the number of nuclei in multinucleate cells and the total number of nuclei, are presented as means ± standard errors of at least seven experimental replicates from the same transfection (Podbilewicz et al., 2006).
Imaging and Software
Fluorescence and Nomarski images were captured using a Hamamatsu ORCA-ER on a Nikon E800 microscope or Zeiss Axiovert 200. Images were merged using Photoshop 8. Confocal microscopy used BioRad MRC1024 or Zeiss LSM 510 META. Sequence alignments were done using ClustalW (http://www.ebi.ac.uk/clustalw/) and were edited by the Jalview software (Clamp et al., 2004).
Supplementary Material
Supplemental Data include supplemental experimental procedures, one table, six figures, and two movies and can be found with this article online at http://www.developmentalcell.com/cgi/content/full/12/5/683/DC1/.
Acknowledgments
We thank Y. Kohara for EST clones, A. Fire for vectors, S. Mitani and the NBRP for the aff-1(tm2214) deletion allele, T. Stiernagle and the Caenorhabditis Genetics Center for nematode strains, F. Rey for advice on structural domain interpretation, M. Akerman for bioinformatics advice, and M. Suissa, S. Joshua, K. Brunschwig, D. Cassel, Y. Gruenbaum, E. Schejter, and B-Z Shilo for critically reading the manuscript. This research was supported by grants from the Israel Science Foundation (B.P.) and by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health (L.V.C.).
A.S. and J.C. contributed equally to this work. A.S. did the molecular biology work along with phenotypic characterization of all the worm strains. J.C and A.P.N. isolated and characterized the ty4 allele and did the ty4 rescue experiments. E.L. and L.C designed, performed, and analyzed cell fusion assays in culture. E.L. and C.V did biochemical experiments. O.A. did the heat shock experiments and bioinformatics. A.S. and B.P. designed and analyzed the experiments and wrote the paper. All authors discussed the results and commented on the manuscript.
Footnotes
Accession Numbers
The GenBank accession number for AFF-1 open reading frame is EF205023.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broday L, Kolotuev I, Didier C, Bhoumik A, Gupta BJ, Sternberg PW, Podbilewicz B, Ronai Z. The small ubiquitin like modifier (SUMO) is required for gonadal and uterine-vulval morphogenesis in C. elegans. Genes Dev. 2004;18:2380–2391. doi: 10.1101/gad.1227104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Richards KL, Cinar HN, Newman AP. N-ethylmaleimide sensitive factor is required for fusion of the C. elegans uterine anchor cell. Dev Biol. 2006;297:87–102. doi: 10.1016/j.ydbio.2006.04.471. [DOI] [PubMed] [Google Scholar]
- Cinar HN, Richards KL, Oommen KS, Newman AP. The EGL-13 SOX domain transcription factor affects the uterine {pi} cell lineages in Caenorhabditis elegans. Genetics. 2003;165:1623–1628. doi: 10.1093/genetics/165.3.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp M, Cuff J, Searle SM, Barton GJ. The Jalview Java alignment editor. Bioinformatics. 2004;20:426–427. doi: 10.1093/bioinformatics/btg430. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- del Campo JJ, Opoku-Serebuoh E, Isaacson AB, Scranton VL, Tucker M, Han M, Mohler WA. Fusogenic activity of EFF-1 is regulated via dynamic localization in fusing somatic cells of C. elegans. Curr Biol. 2005;15:413–423. doi: 10.1016/j.cub.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Hanna-Rose W, Han M. COG-2, a Sox domain protein necessary for establishing a functional vulval-uterine connection in Caenorhabditis elegans. Development. 1999;126:169–179. doi: 10.1242/dev.126.1.169. [DOI] [PubMed] [Google Scholar]
- Keah HH, Hearn MT. A molecular recognition paradigm: promiscuity associated with the ligand-receptor interactions of the activin members of the TGF-beta superfamily. J Mol Recognit. 2005;18:385–403. doi: 10.1002/jmr.715. [DOI] [PubMed] [Google Scholar]
- Kimble J. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev Biol. 1981;87:286–300. doi: 10.1016/0012-1606(81)90152-4. [DOI] [PubMed] [Google Scholar]
- Kirouac M, Sternberg PW. cis-Regulatory control of three cell fate-specific genes in vulval organogenesis of Caenorhabditis elegans and C. briggsae. Dev Biol. 2003;257:85–103. doi: 10.1016/s0012-1606(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Shemer G, del Campo J, Valansi C, Opoku-Serebuoh E, Scranton V, Assaf N, White JG, Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion in C. elegans. Dev Cell. 2002;2:355–362. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Simske JS, Williams-Masson EM, Hardin JD, White JG. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr Biol. 1998;8:1087–1090. doi: 10.1016/s0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- Newman AP, Acton GZ, Hartwieg E, Horvitz HR, Sternberg PW. The lin-11 LIM domain transcription factor is necessary for morphogenesis of C. elegans uterine cells. Development. 1999;126:5319–5326. doi: 10.1242/dev.126.23.5319. [DOI] [PubMed] [Google Scholar]
- Newman AP, Inoue T, Wang MQ, Sternberg PW. The Caenorhabditis elegans heterochronic gene lin-29 coordinates the vulval-uterine-epidermal connections. Curr Biol. 2000;10:1479–1488. doi: 10.1016/s0960-9822(00)00827-7. [DOI] [PubMed] [Google Scholar]
- Newman AP, White JG, Sternberg PW. The Caenorhadbitis elegans lin-12 gene mediates induction of ventral uterine specialization by the anchor cell. Development. 1994;121:263–271. doi: 10.1242/dev.121.2.263. [DOI] [PubMed] [Google Scholar]
- Pettitt J, Wood BW, Plasterk RHA. cdh-3, a gene encoding a member of the cadherin superfamily, functions in epithelial cell morphogenesis in Caenorhabditis elegans. Development. 1996;122:4149–4157. doi: 10.1242/dev.122.12.4149. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B. Cell fusion. WormBook, ed The C. elegans Research Community, WormBook. 2006 doi: 10.1895/wormbook.1.52.1. http://www.wormbook.org. [DOI]
- Podbilewicz B, Leikina E, Sapir A, Valansi C, Suissa M, Shemer G, Chernomordik LV. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev Cell. 2006;11:471–481. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B, White JG. Cell fusions in the developing epithelia of C. elegans. Dev Biol. 1994;161:408–424. doi: 10.1006/dbio.1994.1041. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Greenwald I. Cell autonomy of lin-12 function in a cell fate decision in C. elegans. Cell. 1989;57:1237–1245. doi: 10.1016/0092-8674(89)90060-3. [DOI] [PubMed] [Google Scholar]
- Sharma-Kishore R, White JG, Southgate E, Podbilewicz B. Formation of the vulva in C. elegans: a paradigm for organogenesis. Development. 1999;126:691–699. doi: 10.1242/dev.126.4.691. [DOI] [PubMed] [Google Scholar]
- Shemer G, Podbilewicz B. LIN-39/Hox triggers cell division and represses EFF-1/Fusogen-dependent vulval cell fusion. Genes Dev. 2002;16:3136–3141. doi: 10.1101/gad.251202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer G, Suissa M, Kolotuev I, Nguyen KCQ, Hall DH, Podbilewicz B. EFF-1 is sufficient to initiate and execute tissue-specific cell fusion in C. elegans. Curr Biol. 2004;14:1587–1591. doi: 10.1016/j.cub.2004.07.059. [DOI] [PubMed] [Google Scholar]
- Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell. 2003;5:21–31. doi: 10.1016/s1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Horvitz HR. Pattern formation during vulval development in C. elegans. Cell. 1986;14:761–772. doi: 10.1016/0092-8674(86)90842-1. [DOI] [PubMed] [Google Scholar]
- Strick R, Ackermann S, Langbein M, Swiatek J, Schubert SW, Hashemolhosseini S, Koscheck T, Fasching PA, Schild RL, Beckmann MW, Strissel PL. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-beta. J Mol Med. 2007;85:23–38. doi: 10.1007/s00109-006-0104-y. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Principles of development. 3. Oxford, New York: Oxford University Press; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data include supplemental experimental procedures, one table, six figures, and two movies and can be found with this article online at http://www.developmentalcell.com/cgi/content/full/12/5/683/DC1/.


