Summary
Establishment and maintenance of apical basal cell polarity are essential for epithelial morphogenesis and have been studied extensively using the Drosophila eye as a model system. Bazooka (Baz), a component of the Par-6 complex, plays important roles in cell polarity in diverse cell types including the photoreceptor cells. In ovarian follicle cells, localization of Baz at the apical region is regulated by Par-1 protein kinase. In contrast, Baz in photoreceptor cells is targeted to adherens junctions (AJs). To examine the regulatory pathways responsible for Baz localization in photoreceptor cells, we studied the effects of Par-1 on Baz localization in the pupal retina. Loss of Par-1 impairs the maintenance of AJ markers including Baz and apical polarity proteins of photoreceptor cells but not the establishment of cell polarity. In contrast, overexpression of Par-1 or Baz causes severe mislocalization of junctional and apical markers, resulting in abnormal cell polarity. However, flies with similar overexpression of kinase-inactive mutant Par-1 or unphosphorylatable mutant Baz protein show relatively normal photoreceptor development. These results suggest that dephosphorylation of Baz at the Par-1 phosphorylation sites is essential for proper Baz localization. We also show that the inhibition of protein phosphatase 2A (PP2A) mimics the polarity defects caused by Par-1 overexpression. Further, Par-1 gain-of-function phenotypes are strongly enhanced by reduced PP2A function. Thus, we propose that antagonism between PP2A and Par-1 plays a key role in Baz localization at AJ in photoreceptor morphogenesis.
Introduction
Genetic control of apical-basal cell polarity is essential for epithelial morphogenesis and asymmetric cell division during cell fate specification. It is also important for development of polarized subcellular structures with specialized functions such as the light sensing organelles of photoreceptor cells. A small number of evolutionarily conserved proteins play important roles in diverse types of apical-basal cell polarization. These polarity proteins form two major heterotrimeric cassettes consisting of Crumbs (Crb)-Stardust (Sdt)-Dpatj (Crb complex) and Par-6-aPKC-Baz (Par-6 complex) in the apical cell membrane. Recent studies have shown that these two protein complexes function in a coordinated fashion by direct protein-protein interactions (Hurd et al., 2003; Lemmers et al., 2004; Nam and Choi, 2003; Sotillos et al., 2004). However, the functional significance of these interactions and the mechanisms for precise subcellular targeting of these proteins remain to be elucidated. The Drosophila eye provides an excellent system to study in vivo functions of these interacting polarity proteins in control of cell polarity and organization of the rhabdomere, the light-sensitive apical structure of photoreceptor cells.
In Drosophila, about 800 ommatidial clusters comprising of 8 photoreceptor cells (R1-R8) are generated in the eye disc epithelium during the third instar larval stage, but morphogenesis of photoreceptor cells takes place mainly during the following pupal stage. By 37% pupal development (pd), the apical region of each photoreceptor cell is involuted by 90°, which reorients the apical side toward the center of the cluster (Longley and Ready, 1995). In photoreceptor cells, Crb complex proteins are localized immediately apical to AJs (Fig. 1A). At 55% pd, when the rhabdomeres begin to develop from the apical surface of photoreceptor cells, Crb complex proteins are positioned to the region called the rhabdomere stalk, which links the rhabdomere with the AJ. During this time, developing rhabdomeres undergo dramatic vertical extension from the distal region of photoreceptor cells to the proximal base of the retina.
Fig. 1. Requirement of Baz for localization of Par-6 and aPKC.
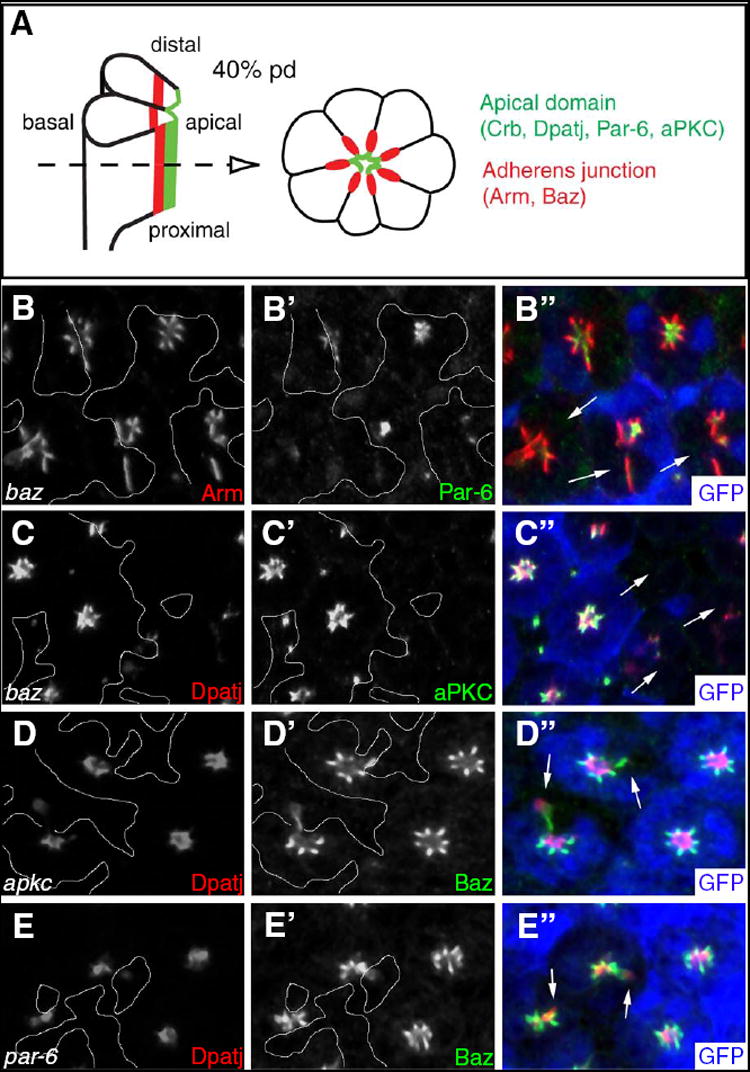
(A) Schematic view of longitudinal (left) and tangential (right) sections of a photoreceptor cluster in mid pupal stage (40h pd) prior to rhabdomere formation. At this stage, the apical side (green) of the cells and the region of AJ (red) is oriented toward the center of the ommaidial cluster, as photoreceptor cells have rotated 90° inward during earlier pupal stage. In a tangential section indicated by the dashed arrow, Crb, Sdt, Dpatj, Par-6 and aPKC colocalize to the apical domain whereas Arm and Baz are localized at AJs. Later, the green apical region becomes the rhabdomere stalk as the rhabdomeres develop from the apical surface of photoreceptors. (B, C) In baz mutant photoreceptor cells marked by the absence of GFP (blue in B” and C”), Par-6 (B’), aPKC (C’) and Dpatj (C) are absent or strongly reduced (arrows). In contrast, Arm (B) is mislocalized basolaterally. (D) apkc mutant clones show the presence of Baz but its mislocalization (arrows). Dpatj is mispositioned basal to Baz. (E) par-6 mutant clones also display misplaced Dpatj basal to Baz (arrows). Clone boundaries are marked by white lines.
Crb, together with Sdt and Dpatj (Hong et al., 2001; Nam and Choi, 2003; Nam and Choi, 2006; Richard et al., 2006), is required for extension of rhabdomeres and formation of AJ along the distal-proximal axis of the photoreceptor cell, although it is not essential for establishing apical basal cell polarity (Izaddoost et al., 2002; Pellikka et al., 2002). The mammalian homolog of Crb, CRB1, is also localized to the inner segment of photoreceptors, the structure analogous to the rhabdomere stalk, between the outer segment and the AJ (Pellikka et al., 2002). Furthermore, mutations in CRB1 causes retinal diseases including retinitis pigmentosa 12 and Leber Congenital Amaurosis (LCA) in humans (den Hollander et al., 2001; den Hollander et al., 1999).
In addition to Crb, Par-6 complex is also required for proper organization and maintenance of apical photoreceptor membranes in the eye (Hong et al., 2003; Nam and Choi, 2003). Genetic evidence suggests that Par-6 complex is required for localization of the Crb complex to the apical membrane whereas the Crb complex may be necessary for maintenance of Par-6 complex proteins in the apical region (Hong et al., 2003; Nam and Choi, 2003). However, it is unknown whether any one of the Par-6 complex proteins plays a primary role in the Par-6 complex function during the organization of photoreceptor cells. Interestingly, although Par-6 and aPKC, like the Crb complex, are localized to the apical membrane of photoreceptors, Baz is targeted to the AJ domain where Armadillo (Arm, Drosophila β-catenin) and DE-cadherin are localized (Nam and Choi, 2003). Recent studies have also shown the localization of Baz to AJ in the embryonic epithelia (Harris and Peifer, 2005). The differential localization of Baz and Par-6/aPKC raises the question of how Baz localization is regulated and whether Baz functions independently from Par-6 and aPKC.
In C. elegans, the Baz homolog, Par-3, is asymmetrically localized to the anterior cortex of the embryo whereas the serine/threonine protein kinase Par-1 is positioned to the posterior side (Kemphues et al., 1988). In Drosophila follicle cells, Baz localization to the apical membrane is also regulated by basolateral Par-1 (Benton and St Johnston, 2003). Thus, in both C. elegans embryo and Drosophila follicle cells, Par-3 and Par-1 localize in a complementary cellular pattern. Interestingly, Baz is a phosphorylation substrate for Par-1 kinase, and the apical localization of Baz requires Par-1 phosphorylation (Benton and St Johnston, 2003). In follicle cells, Par-1 is required to exclude Baz from the basolateral membrane. Loss of Par-1 results in the basolateral expansion of Baz-Par-6 complex, indicating that Par-1 is essential for the regulation of apical basal cell polarity (Benton and St Johnston, 2003). As opposed to the essential role for Par-1 in follicle cell polarity, no significant function of Par-1 in regulation of cell polarity in the developing eye disc has been identified (Bayraktar et al., 2006). However, it has not been studied whether Par-1 is required at a later time point in photoreceptor morphogenesis for the control of Baz localization during the pupal stage when the photoreceptor cells undergo massive reorganization of their apical basal cell structure.
Since Par-1 is a protein kinase, an interesting question is whether Baz localization might be regulated by an opposing phosphatase activity. Evidence from mammalian studies has suggested that the formation of tight junctions is regulated by the interaction of protein phosphatase 2A (PP2A) and aPKC (Nunbhakdi-Craig et al., 2002). However, understanding the in vivo role of PP2A in cell polarity requires additional study, and the use of an animal model such as Drosophila eye can be a powerful tool to address these questions. PP2A is a heterotrimeric serine/threonine phosphatase composed of invariant catalytic (‘C’) and structural (‘A’) subunits and a variable regulatory subunit (‘B’) that directs the AC core complex to different substrates (Janssens and Goris, 2001). In Drosophila, the catalytic subunit is encoded by the microtubule star (mts) gene. mts mutants die in embryogenesis with defects in mitosis (Snaith et al., 1996), but the function of Mts in apical basal cell polarity has not been examined.
To understand the role of Baz and the regulation of Baz localization during photoreceptor morphogenesis, we investigated the functional relationship among Baz, Par-1 and PP2A in early/mid pupal eye development. First, we show that Baz plays an essential role in the localization of Par-6-aPKC complex to the apical domain. Second, Par-1 is required for the localization and/or maintenance of Baz and proper morphogenesis of photoreceptors. Thirdly, Baz unphosphorylated at the Par-1 sites is preferentially targeted to AJ whereas phosphorylated Baz is ectopically localized. Lastly, we identify an important function of PP2A in photoreceptor cell organization and its role as an antagonizing factor for Par-1. This study establishes the role for Baz as a central player in the localization of cell polarity proteins during photoreceptor morphogenesis and provides new insight into how Baz is regulated by antagonistic roles of Par-1 and PP2A.
Materials and Methods
Genetics
Mitotic recombinations were induced by using FLP/FRT method for clonal analysis (Xu and Rubin, 1993). apkcK06304 and bazXi106 mutant clones were produced in the eye by eyeless (ey)-FLP in y w par-6Δ226 FRT9-2/ y w Ubi-GFP FRT9-2 ; ey-Flp/+, y w ey-Flp/+ ; FRT42D aPKCK06304/FRT42D Ubi-GFP, and y w bazXi106 FRT9-2/ y w Ubi-GFP FRT9-2 ; ey-Flp/+, respectively (Petronczki and Knoblich, 2001; Wodarz et al., 2000; Wodarz et al., 1999). Overexpression of Mts, dnMts, Par-1 or Baz was induced by crossing UAS-Mts, UAS-dnMts (Hannus et al., 2002), UAS-Par-1 (full length of Par-1 α form, Sun et al., 2001), UAS-GFP:Baz, UAS-GFP:BazS151A,S1085A (Benton and St Johnston, 2003). with GMR-Gal4 (Freeman, 1996). mtsXE2258, a null allele of mts (Shiomi et al., 1994; Wassarman et al., 1996) and par-1Δ16 or par-1W3, null alleles of par-1 (Cox et al., 2001; Huynh et al., 2001) were used. par-1W3 is a deletion disrupting both par-1 and mei-W68 (Shulman et al., 2000), whereas the par-1Δ16 is a par-1 specific deletion of complete kinase domain and most of the linker region of par-1 gene (Cox et al., 2001).
Antibody staining and histology
The following primary antibodies were used: rabbit anti-PKCζ (Santa Cruz), 1:500; mouse anti-Arm, 1:100; anti-αSpectrin, 1:10; anti-Na+/K+ ATPase (Developmental Studies Hybridoma Bank),1:50; rabbit anti-Baz (Wodarz et al., 1999), 1:500; rat anti-Crb (Bhat et al., 1999), 1:400; sheep anti-GFP (Biogenesis), 1:100; rabbit anti-Mts (Shiomi et al., 1994), 1:100; rabbit anti-Par-6 (Pinheiro and Montell, 2004), 1:500; rabbit anti-Par-1 (Vaccari and Ephrussi, 2002), 1:1000; rabbit anti-Dlg (Lee et al., 2003), 1:200; mouse and rabbit anti-Dpatj (Bhat et al., 1999), 1:500. Secondary antibodies conjugated with Cy3, Cy5, or FITC were from Jackson Laboratories. Fluorescent immunostaining of pupal eyes and confocal analysis were performed as reported (Izaddoost et al., 2002; Nam and Choi, 2003).
Results
Baz is essential for apical targeting of Par-6 and aPKC in photoreceptors
During early-mid pupal stage of eye development prior to the formation of the rhabdomere, Baz is localized at AJ of photoreceptor cells. Baz is known to form a protein complex with Par-6 and aPKC, but it is not colocalized with them in photoreceptor cells, as Par-6/aPKC are targeted apical to the AJ (Fig. 1A) (Nam and Choi, 2003). Because the rhabdomere is not clearly formed at this stage, apical markers like Dpatj and Par-6 are localized in the most central region of each photoreceptor cluster surrounded by AJ markers, Arm and Baz, resulting in a characteristic star shape pattern in the tangential section (Fig. 1A).
Previous studies have shown that Baz is essential for localization of Crb complex and AJ formation during photoreceptor morphogenesis in the pupal eye (Hong et al., 2003; Nam and Choi, 2003). However, it is unknown whether Baz is required for the apical localization of its interacting partners, Par-6 and aPKC. To test this possibility, we analyzed the localization pattern of Par-6 and aPKC in loss-of-function (LOF) clones of baz null mutant cells. As the rhabdomeres develop, Baz is relocated from AJ to the rhabdomere at 65-70% pupal development (pd). Therefore, we examined mutant clones in earlier pupal eyes at approximately 40% pd when Baz is restricted to AJ. As shown in Fig. 1, loss of Baz caused a strong reduction of Par-6 (Fig. 1B) as well as aPKC (Fig. 1C) to lower or nearly undetectable levels, whereas basolateral membranes of mutant photoreceptors were affected less severely or relatively intact (Supplementary Fig. 1). In contrast to the strong reduction of Par-6 and aPKC protein levels in baz mutant clones, significant levels of Baz protein expression were present in par-6 or apkc null clones, although Baz protein was abnormally distributed to the basolateral region, and Dpatj was displaced basally (Fig. 1D and 1E, see also Supplementary Fig. 2). These data suggest that Baz is a nodal component for apical targeting of Par-6 and aPKC whereas Par-6 and aPKC might be required for the maintenance of Baz at AJ.
Par-1 is required for maintenance of apical and AJ proteins in pupal photoreceptor cells
Based on the critical role of Baz for the localization of apical polarity proteins, it is important to determine how localization of Baz protein is regulated. In ovarian follicle cells, Par-1 is required to exclude Baz from the basolateral membrane, thereby restricting Baz localization to the apical membrane (Benton and St Johnston, 2003). Because Baz localizes to AJ rather than the apical membrane domain in photoreceptors, we examined whether the localization and function of Par-1 in photoreceptors might be different from that in ovarian follicle cells. At 40% pd, Par-1 is broadly distributed in the cytoplasm of photoreceptor cells. Double-labeling for the AJ marker Arm or a basolateral marker α-Spec showed weak and diffused Par-1 staining near basolateral membrane, but its level was considerably elevated in the apical region near the center of each ommatidial cluster (Supplementary Fig. 3).
The enhanced distribution of Par-1 in the apical region of photoreceptor cells suggests that Par-1 might play a role in photoreceptor morphogenesis. Thus, we examined whether Par-1 is required for correct localization of apical/AJ markers and photoreceptor development. Using par-1Δ16 null mutation, we generated par-1 LOF clones in pupal eyes and examined mutant phenotypes at about 40% pd. Par-1 protein expression was not detected in par-1Δ16 null mutant clones (Fig. 2A), confirming that this allele is protein-null (Cox et al., 2001). par-1 mutant clones showed a range of abnormalities, depending on the position along the proximal-distal axis of the photoreceptor cells. In the distal region of photoreceptor cells, toward the periphery of the retina, Arm was mildly affected in approximately 80% of mutant ommatidia (N>100, Fig. 2B). About 20% (N>100) of ommatidia showed relatively normal pattern of Arm and Dpatj staining (Fig. 2B, arrowheads). However, serial confocal sections of such par-1 mutant ommatidia showed mislocalization of Arm staining to basolateral positions and occasional loss of AJ marker staining in the deeper proximal region of the retina (Fig. 2B, Supplementary Fig. 4). As shown in Fig. 2C, Baz is prominent in the distal section but greatly reduced at more proximal sections. An apical polarity protein, Crb, was also reduced or lost at proximal region of par-1 mutant ommatidia (Fig. 2C). These phenotypes suggest that Par-1 is necessary for the proper proximal-distal extension or maintenance of AJ and apical domains during the distal-proximal elongation of photoreceptors in pupal stages. However, despite such distal-proximal defects, Dpatj and Crb remained apical to Arm, indicating that apical basal cell polarity is not noticeably affected in par-1 mutant photoreceptor cells. Basolateral marker Dlg appears to be intact despite the ectopic presence of Arm, suggesting that a low level of ectoptic Arm is not sufficient to disrupt the basolateral membrane property (Fig. 2D and 2E). Clonal analysis of par-1W3, a deletion mutant of par-1 and adjacent mei-W68 genes (Cox et al., 2001), showed the same phenotype of par-1Δ16 (not shown), further supporting that the par-1Δ16 phenotypes are due to loss of Par-1 function.
Fig. 2. Localization of Par-1 and loss of function phenotype of par-1 in photoreceptors.
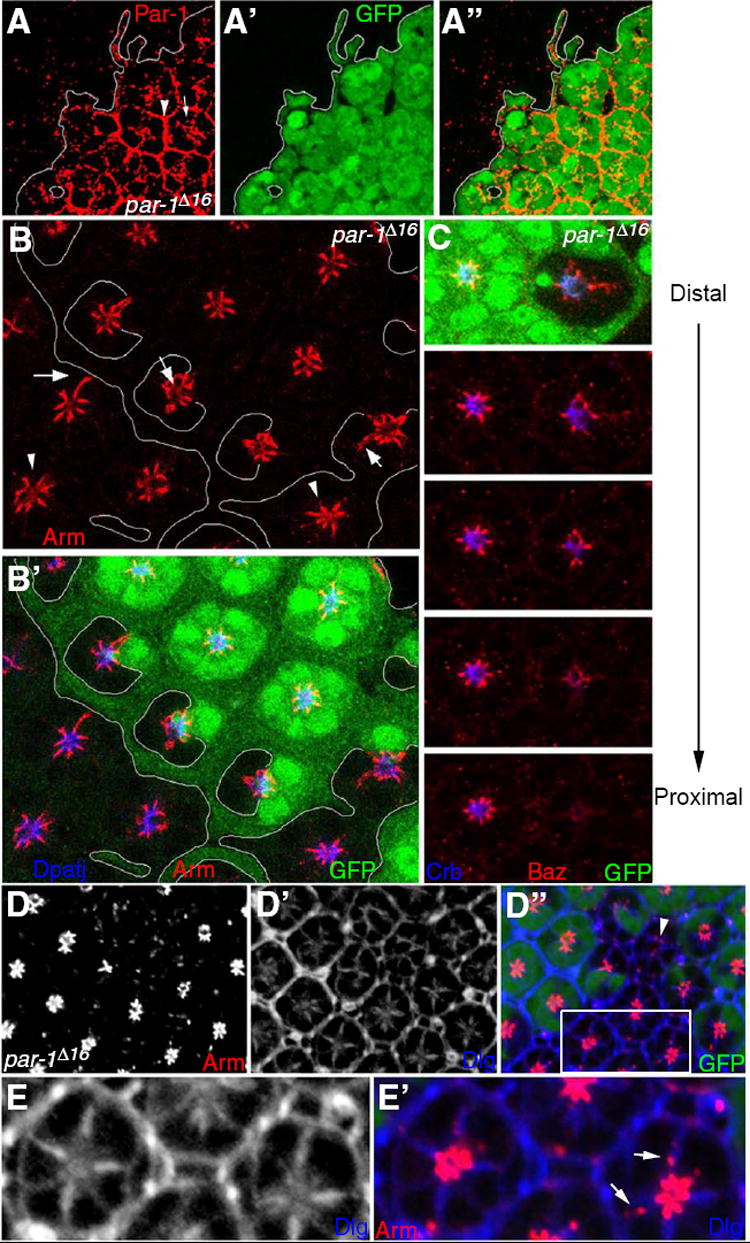
(A) Par-1 expression was examined in par-1 mosaic eye at 40% pd. The white line indicates the clone boundary. In par-1+ wild-type ommatidia marked by the expression of GFP clone marker (A’), Par-1 is strongly expressed in the interommatidial cells (arrowhead). In photoreceptor cells, Par-1 is weakly localized along the basolateral cell membranes but is enriched in the central (apical) region of photoreceptor clusters (arrow). par-1- mutant cells without GFP expression show no Par-1 expression (A). (B) Pattern of Arm (red) and Dpatj (blue) in par-1 mutant clones marked by GFP (green, B’) and lines. Note that although Arm is often expanded basolaterally (arrows) some clusters look relatively normal (arrowheads). (C) A series of cross-sections of a mosaic eye from the distal to the proximal shows that Baz (red) and Crb (blue) are gradually lost in the proximal sections of a par-1 mutant clone as marked by the absence of GFP (green). (D) 40h pd retina with par-1 mutant clones stained for Arm and Dlg. This image is a confocal section at the middle region along the proximal-distal axis of the retina. Some mutant photoreceptors show significant loss of Arm (arrowheads) or ectopic Arm at basolateral positions (arrows) (D and D”). (D’) Basolateral membrane marked by Dlg staining. Dlg is also detected in the apical region. (E and E’) High magnification of the box region in (D). Dlg staining is not affected by the presence of ectopic Arm localization in the basolateral membrane (arrows).
Par-1 overexpression causes mislocalization of Baz and disruption of cell polarity
Our data suggest that Par-1 is required for maintenance of AJ and apical markers during distal-proximal extension of the photoreceptor apical domain but not for regulation of apical basal polarity per se. The possibility remains that Par-1 may be involved in controlling cell polarity but its function in cell polarity may be compensated by unknown redundant factors. To test whether Par-1 can induce changes in cell polarity, we overexpressed Par-1 in differentiating retinal cells by GMR-Gal4 and examined whether it alters the localization pattern of Baz and other polarity proteins. Overexpression of wild-type Par-1 at 25°C resulted in dramatic disruption of the apical basal pattern of photoreceptor cells (Fig. 3A, B). The AJ region marked by Arm was displaced irregularly and was diffused more basally or apically and into the cytoplasm from the normal AJ position. The Dpatj apical marker was also mislocalized and diffused, often more basally to AJ (Fig. 3B”, arrows). Baz, which normally colocalizes at the AJ with Arm, was mislocalized in a domain overlapping the Arm mislocalization (Fig. 3C). Misplaced positioning of the AJ and apical markers suggests that overexpression of Par-1 leads to a disruption of apical basal photoreceptor cell polarity. This phenotype was highly consistent with 100% (n>100) of ommatidia showing similar defects in cell polarity. In contrast to the severe defects caused by overexpressing wild-type Par-1, no significant effects were observed when a kinasenull Par-1 mutant, Par-1(KN) (Sun et al., 2001), was expressed with the same GMR-GAL4 control under similar conditions (Fig. 3D). As in the wild-type eye, Arm was tightly localized to AJ, and Dpatj was restricted to the apical region. Therefore, the mislocalization of AJ and apical markers induced by Par-1 appears to be due to the Ser/Thr kinase activity of Par-1.
Fig. 3. Par-1 overexpression causes severe disruption of Arm and Dpatj localization.
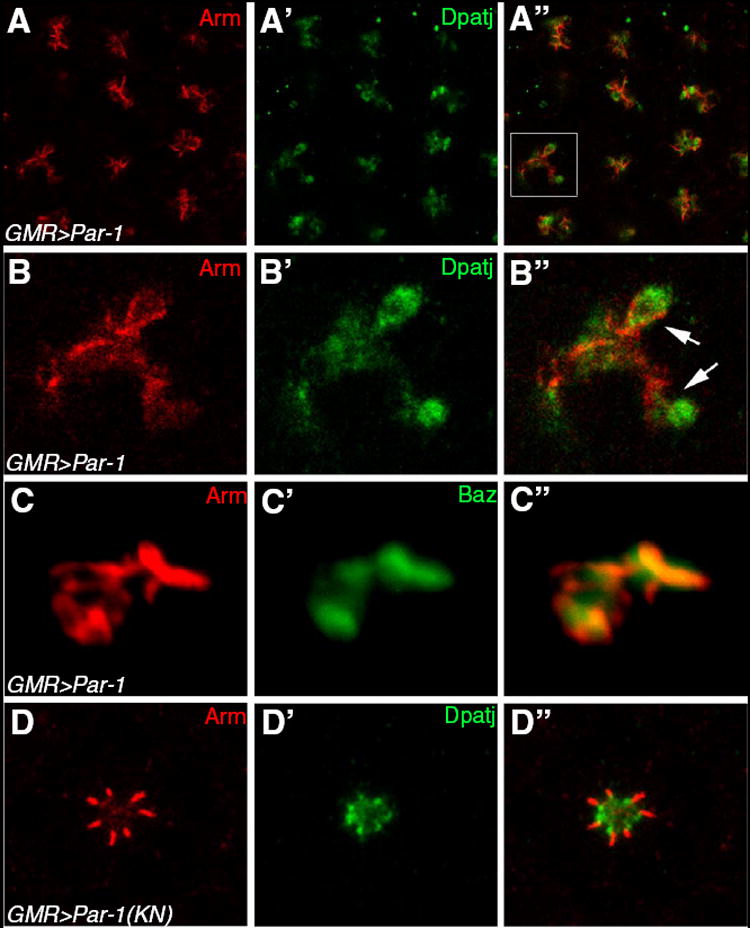
(A) Par-1 overexpression by GMR-Gal4 causes diffusion and expansion of Arm (A, red) and Dpatj (A’, green). (B) A magnified view of the square region of (A). Often, Dpatj is displaced basal to Arm, suggesting the loss of cell polarity (A”, arrows). (C) GMR>Par-1 stained for Arm and Baz shows significant overlap of these markers. (D) Par-1(KN), a kinase-inactive mutant of Par-1, shows a near normal pattern of Arm and Dpatj.
Unphosphorylatable Baz protein is localized to the normal position at AJ
As Par-1 kinase activity is important for inducing Baz mislocalization, and Baz is a biochemical substrate for Par-1 protein kinase (Benton and St Johnston, 2003), it is possible that Par-1 phosphorylation of Baz may be responsible for the mislocalization of AJ and apical markers (Fig. 4). To test this possibility, we expressed GFP-tagged wild-type Baz (BazWT) in differentiating retinal cells and examined whether the GFP-Baz proteins are normally localized to AJ or are recruited to ectopic positions in the photoreceptor cells. As shown in Fig. 4A, most GFP-BazWT was severely displaced to apical or basolateral regions. The apical marker Dpatj was also diffused and mislocalized basolaterally from the apical domain, implying the disruption of apical basal cell polarity (Fig. 4A-A”’). This phenotype of BazWT overexpression was similar to the defects caused by overexpression of Par-1 (Fig. 3A, B), suggesting that the mislocalization effects of Baz overexpression might be correlated with the Par-1 kinase activity to Baz.
Fig. 4. Localization of GFP-Baz and GFP-BazSA.

(A) Overexpression of wild-type GFP-Baz by GMR-Gal4 causes an expansion of Arm (A, red) and Dpatj (A’, green), as well as cell polarity defects (A”). (B) The unphosphorylated form of GFP-Baz (GFP-BazSA) shows relatively normal localization of GFP-Baz (B”, green), although weak GFP-BazSA staining is occasionally found at ectopic positions (arrows). Most Dpatj (B’, blue) staining is also normally localized to the apical region, (C) Localization of Arm (C, red) and Dpatj (C’, blue) in the heterozygote of GMR-Gal4/+ as control. The table in the panel (D) shows the frequency of severely affected ommatidia in each genotype.
To test this idea further, we examined the distribution of a unphosphorylatable BazS151A,S1085A (BazSA) that has serine-to-alanine substitutions at the Par-1 phosphorylation sites, S151 and S1085 (Benton and St Johnston, 2003). As hypothesized, most BazSA protein was predominantly localized to the normal AJ positions basal to the apical Dpatj domain (100% ommatidia, N>100) (Fig. 4B). Consistent with the relatively normal localization of BazSA, adult flies with BazSA overexpression showed near normal eyes (not shown), although the expression levels of BazWT and BazSA were similar based on the level of GFP tag. (Fig. 4A” and 4B”).
Inhibition of PP2A phenocopies Par-1 overexpression
Our data shown above suggest that the absence of phosphorylation at the Par-1 phosphorylation sites of Baz is required to restrict Baz localization to AJ and to maintain photoreceptor cell polarity. A potential mechanism for inhibiting the Par-1 kinase function may involve the activity of a protein phosphatase(s). One candidate phosphatase, PP2A, has been implicated in a variety of functions, including establishment or maintenance of apical basal cell polarity in mammalian epithelial cells (Nunbhakdi-Craig et al., 2002). Therefore, we examined the possibility of whether Mts, the catalytic subunit of Drosophila PP2A, might be required for proper localization of Baz and the organization of cell polarity by antagonizing the Par-1 function. Because mts null mutant cells in the eye fail to survive even to a mid-pupal stage (Hannus et al., 2002; Sathyanarayanan et al., 2004; Wassarman et al., 1996), we took an alternative approach to inactivate wild-type Mts function by expressing a dominant-negative mutant form of Mts (dnMts). dnMts was generated by a truncation in the N-terminal region of Mts and shown to interfere with the PP2A activity specifically (Hannus et al., 2002; Sathyanarayanan et al., 2004).
Overexpression of dnMts by GMR-Gal4 in developing eyes resulted in severe defects in the apical basal pattern of most photoreceptor clusters (N>100), as indicated by abnormal positioning of Dpatj and Arm markers (Fig. 5A, B). In most cases, the apical and AJ domains were mislocalized. Arm staining was mislocalized to basolateral or more apical positions as compared to Dpatj (Fig. 5B; arrows), suggesting that the apical basal polarity is altered. Baz was also mislocalized together with Arm when dnMts was overexpressed (Fig. 5C). In contrast to severe mislocalization of apical and AJ markers in the photoreceptor cells of pupal eyes, overexpression of dnMts in larval eye discs caused no significant change in relative localization of polarity markers Dpatj and Arm. Eye discs from GMR>dnMts larvae cultured at 18°C are normal, but transient overexpression of dnMts during pupal stage by a temperature shift to 29°C caused similar defects in mid-pupal eye as shown in Fig. 5A-5C. These observations indicate that the DN-Mts phenotype is likely to be due to an inhibition of PP2A function specifically during a later time window of pupal development.
Fig. 5. Effects of PP2A inhibition by dominant-negative Mts.

(A) dnMts overexpression by GMR-Gal4 causes the expansion of Arm (A, red) and Dpatj (A’, green), as well as cell polarity defects (A”, arrows). (B) High magnification of the rectangle area in (A”). Note that Arm and Dpatj are diffused and abnormally positioned. (C) GMR>dnMts stained for Arm and Baz showing their overlap. (D) Localization of Arm (A, red) and Dpatj (A’, green) in GMR-Gal4/+ control.
Remarkably, the pattern of apical and AJ marker mislocalization induced by Par-1 overexpression was almost indistinguishable from that of dnMts (Fig. 3B, 5B). This result is consistent with the idea that Par-1 and PP2A have opposing functions in the localization of polarity proteins.
PP2A function is antagonistic to Par-1 and Baz
To further probe the suggestion that Par-1 and Mts have opposing functions, we examined whether these two genes show an antagonistic genetic interaction. Since the eye phenotype of Par-1 overexpression is similar to that of PP2A inhibition, we expected that coexpression of Par-1 and dnMts would enhance the phenotype of either Par-1 overexpression or dnMts. Indeed, concomitant expression of dnMts and Par-1 resulted in 100% lethality, an outcome not seen by overexpressing either protein alone. To confirm such genetic interaction in the eye, we overexpressed Par-1 in the mts/+ heterozygous background to reduce the Mts level. Par-1 overexpression led to a reduction of the eye size and roughening of external morphology (Fig. 6B). This phenotype was strongly enhanced by the reduced mts gene dosage (+/mts), resulting, with 100% penetrance, in flies with smaller and rougher eyes (Fig. 6C).
Fig. 6. Genetic interaction of Par-1 and Mts.

(A-C) Scanning electron microscope images of GMR>Par-1(KN) (A), GMR>Par-1 (B) and GMR>Par-1; mts/+ (C). (D) Localization of Arm (A, red) and Dpatj (A’, green) in a kinase-inactive mutant form of Par-1 (Par-1(KN)) overexpressed by GMR-Gal4. (E) Par-1 overexpression by GMR-Gal4 causes severe mislocalization of Arm (E, red) and Dpatj (E’, green), resulting in cell polarity defects (E”, arrows). The same images shown in Fig. 3B are provided here for phenotype comparison purposes. (F) Par-1 overexpression by GMR-Gal4 in the mts/+ heterozygous background (GMR>Par-1; mts/+) shows further reduction or loss of Arm (F, red) and Dpatj (F’, green), compared to GMR>Par-1 (E).
We also examined whether the enhanced pupal eye phenotype by Par-1 overexpression in +/mts background correlated with the phenotypes in the pupal photoreceptor cells. As shown earlier, Par-1 overexpression causes mispositioning of the AJ and apical markers in pupal eyes (Fig. 6E). The reduced mts gene dosage under this condition (GMR>Par-1; mts/+) resulted in a strong reduction or loss of AJ and apical markers in photoreceptor cells at 40% pd (Fig. 6F), suggesting that these markers are not targeted to the membrane or may not be stably maintained. This result further supports the idea of an antagonistic relationship between Par-1 and PP2A.
Discussion
Par-6, aPKC and Baz play important roles in asymmetric polarization of cells. These conserved proteins act in a complex in Drosophila and other animal species, but it is largely unknown how this complex is formed and targeted to specific membrane domains. In this study, we have analyzed the function of Baz, Par-1 and PP2A in apical targeting of polarity proteins during photoreceptor morphogenesis in Drosophila and found antagonistic function of Par-1 and PP2A in controlling the localization of Baz.
Requirement of Baz for Par-6 and aPKC localization in the photoreceptor cells
Our clonal analysis suggests that Baz is crucial for targeting or maintenance of Par-6 and aPKC. In contrast, Baz protein expression was not significantly reduced in par-6 or apkc null clones, although Baz protein distribution was mislocalized to the basolateral region. This implies that Baz plays a nodal role among the Par-6 complex proteins (Fig. 1C and 1D). . A caveat in this analysis is that par-6 and apkc mutant clones are very small (1-2 ommatidia) compared to the relatively large baz mutant clones, raising the possibility that par-6 and apkc mutant clones analyzed may represent rare escaper cells that survive with weaker phenotypes. However, this may not be the case because nearly identical phenotypes were seen in more than 50 par-6 or apkc mutant clones (Supplementary Fig. 2). Our data suggesting the central role for Baz in Par-6 and PKC localization are also consistent with studies on embryonic epithelia, in which Baz localization to the membrane precedes localization of Par-6, implying Par-6-independent membrane localization of Baz (Harris and Peifer, 2005). The requirement of Baz for the localization of Par-6/aPKC but not vice versa suggests that initial Baz localization may be independent of Par-6 and aPKC, as reported in embryonic epithelia (Harris and Peifer, 2005).
Similar relationships among Par-6 complex proteins have also been observed during asymmetric cell division in C. elegans. PAR-3, the homolog of Baz, is properly localized in the absence of either PKC-3 (aPKC) or PAR-6, whereas it is indispensable for localization of both PKC-3 and PAR-6 (Hung and Kemphues, 1999). These studies suggest that Baz/Par-3 is a major component in the control of cell polarity in diverse systems, including the photoreceptors in Drosophila. However, the Par-6 complex may exist in different compositions with unique functions, depending on various developmental contexts. For instance, Baz/Par-3 and Par-6 colocalize to the apical cortex of dividing neuroblasts in the Drosophila CNS (Bilder, 2001) and in dividing cells in C. elegans embryos (Watts et al., 1996), whereas Baz is distinctly localized to the AJ basal to the Par-6 domain in the apical membrane of photoreceptors (Nam and Choi, 2003). It is also striking that whereas Baz is not critically required for photoreceptor differentiation in the larval eye imaginal disc, it becomes crucial during pupal eye development. These data suggest that Baz is required for specific developmental events such as junctional reorganization and rhabdomere formation, although it is expressed in photoreceptors from the time of neuronal fate specification as well as in undifferentiated cells prior to retinal development.
Role of Par-1 in the localization of Baz at AJ
Par-1 is a key regulator of Baz localization in ovarian follicle cells. In this system, Par-1 is localized to the basolateral membrane and is essential for exclusion of Baz expression from the basolateral membrane (Benton and St Johnston, 2003). During early embryogenesis, Par-1 is transiently restricted to the lateral membrane, but at mid-gastrulation it is localized near the apical domain immediately below the region of spot adherens junction (SAJ). Par-1 is required for the restriction of SAJ, preventing the expansion of the E-Cad SAJ marker into the lateral membrane, but not affecting Crb-Dpatj apical markers (Bayraktar et al., 2006).
In contrast to follicle and embryonic epithelia, in eye imaginal discs, par-1 LOF clones show no significant apical basal polarity defects, suggesting that Par-1 is not required for cell polarity in eye imaginal disc epithelia (Bayraktar et al., 2006). This raises a question of whether Par-1 function is dispensable for photoreceptor morphogenesis. In this study, we focused our analysis on pupal eye development, since some cell polarity genes such as crb are not required in larval imaginal discs (Izaddoost et al., 2002), although they become essential later during the pupal stage when the retina undergoes dramatic reorganization of cell junctions and the apical basal pattern in the photoreceptor cells. Our analysis of pupal eyes suggests that Par-1 is required for the distal-proximal growth or maintenance of apical and AJ domains of photoreceptor cells, as Baz and apical markers often fail to form continuous AJ and rhabdomeres along the distal-proximal axis of the retina in par-1 null mutant clones. These phenotypes are similar to the defects shown previously in the eyes of Crb complex mutants (Hong et al., 2003; Izaddoost et al., 2002; Nam and Choi, 2003; Nam and Choi, 2006; Pellikka et al., 2002). Like par-1 mutations, loss of these gene functions also affects the extension/maintenance of AJ and rhabdomeres but not apical basal cell polarity. Since Baz is essential for proper targeting of Par-6 and Crb complex proteins, loss of Par-1 function may result in mislocalization of Crb complex through affecting Baz localization, although it is possible that Par-1 may also be directly involved in localization of Crb complex proteins independent of Baz. In the eye imaginal disc, it has been reported that Par-1 is localized to the apical-marginal zone and AJ (Bayraktar et al., 2006). In the pupal eye, we also found that Par-1 is enriched in the apical region of photoreceptor clusters, although a low level of Par-1 is also detected broadly along the basolateral membrane (Fig. 2A). Thus, Par-1 localization is not restricted to the basolateral membrane but appears to be regulated in a complex pattern in different cell types.
Antagonistic interaction of Par-1 and PP2A
In ovarian follicle cells, phosphorylation of Baz by Par-1 is required for proper localization of Baz to the apical region of the cells, and BazSA mutated proteins are abnormally localized to the basolateral membrane (Benton and St Johnston, 2003). In contrast, our data show that under conditions of overexpression, Baz protein mutated at Par-1 phosphorylation sites is targeted to the AJ whereas wild-type Baz is ectopically localized. Overexpressed wild-type Baz may be abnormally targeted to non-AJ sites, but it is also possible that ectopic Baz may recruit AJ proteins to form ectotpic AJs. Nonetheless, our data suggest that, in photoreceptor cells, Par-1-dependent phosphorylation is not essential for initial localization of Baz to AJ. Instead, dephosphorylation of Baz may be a key for the localization of Baz to AJ. This explanation is consistent with the data that the pattern of AJ and apical markers is severely disrupted by overexpression of Par-1 but not by loss of Par-1, suggesting that Baz phosphorylation by Par-1 must be suppressed to maintain photoreceptor cell polarity. Our data provide genetic evidence to support that Mts is a major enzyme responsible for antagonizing the effects of Par-1-dependent Baz phosphorylation.
PP2A has been implicated in the regulation of tight junction formation in MDCK epithelial cells by interacting with aPKC (Nunbhakdi-Craig et al., 2002). However, it is unlikely that the Mts role in Baz localization is mediated through aPKC. First, wild-type and mutant BazSA are localized to completely different sites even though both have an intact phosphorylation site for aPKC. Second, the phenotype of Par-1 overexpression is mimicked by inhibition of Mts (Figs 3 and 5) but not by loss of aPKC (Fig. 1D). The idea of specific antagonism between Par-1 and Mts is also supported by the enhancement of the Par-1 overexpression phenotype by reduction of mts gene dosage (Fig. 6) but not apkc (data not shown). Thus, we propose a model in which the localization of Baz to AJ in photoreceptors cells during early pupal eye development depends on the removal of Par-1-mediated phosphorylation by Mts PP2A activity. In this model, Mts plays a pivotal role in regulation of Baz localization and consequent maintenance of photoreceptor cell polarity. On the contrary, Par-1 does not play an essential role for the initial targeting of Baz to AJ, but it is required for growth or stability of AJs and rhabdomeres during photoreceptor morphogenesis. It will be interesting to see whether the antagonistic interaction of Par-1 and PP2A plays an important role in regulation of Baz localization and function in various developmental contexts in Drosophila and other animal species.
Supplementary Material
Fig. 7. A model for the function of Par-1 and PP2A in Baz localization.

During early-mid pupal stage, Par-6 and Crb complex proteins are targeted to the apical region of photoreceptor cells, except that Baz is localized to AJ between the apical and the basolateral domains. Baz protein phosphorylated by Par-1 is displaced from the AJ whereas dephosphorylation of Baz at the S151/S1085 Par-1 sites allows Baz localization to AJ. Mts, the catalytic subunit of PP2A, antagonizes Par-1 function by dephosphorylating Baz and/or by inactivating Par-1.
Acknowledgments
We gratefully acknowledge H. J. Bellen, M. A. Bhat, K.-O. Cho, Y.-N. Jan, S. Eaton, D. St Johnston, J. A. Knoblich, E. Knust, B. Lu, D. J. Montell, T. Uemura, A. Wodarz, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for providing flies and antibodies. We thank Z. Chen and Y. Chao for technical assistance, and K.-O. Cho, Y.-C. Hsu, J. Lim, B.-J. Park, A. Singh, D. Wycuff and L. Yang for critical reading and comments on the manuscript. Confocal microscopy was supported by a grant from the NIH to D. B. Jones. This work was supported by grants from the NIH R01EY012210 and the Retina Research Foundation to K.-W. Choi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachmann A, et al. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–43. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- Bayraktar J, et al. Par-1 kinase establishes cell polarity and functions in Notch signaling in the Drosophila embryo. J Cell Sci. 2006;119:711–21. doi: 10.1242/jcs.02789. [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Bhat MA, et al. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–45. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Bilder D. Cell polarity: squaring the circle. Curr Biol. 2001;11:R132–5. doi: 10.1016/s0960-9822(01)00060-4. [DOI] [PubMed] [Google Scholar]
- Cox DN, et al. Drosophila par-1 is required for oocyte differentiation and microtubule organization. Curr Biol. 2001;11:75–87. doi: 10.1016/s0960-9822(01)00027-6. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. 2001;69:198–203. doi: 10.1086/321263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12) Nat Genet. 1999;23:217–21. doi: 10.1038/13848. [DOI] [PubMed] [Google Scholar]
- Elbert M, et al. The yeast par-1 homologs kin1 and kin2 show genetic and physical interactions with components of the exocytic machinery. Mol Biol Cell. 2005;16:532–49. doi: 10.1091/mbc.E04-07-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–60. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Hannus M, et al. Planar cell polarization requires Widerborst, a B’ regulatory subunit of protein phosphatase 2A. Development. 2002;129:3493–503. doi: 10.1242/dev.129.14.3493. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol. 2005;170:813–23. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, et al. Distinct roles of Bazooka and Stardust in the specification of Drosophila photoreceptor membrane architecture. Proc Natl Acad Sci U S A. 2003;100:12712–7. doi: 10.1073/pnas.2135347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, et al. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–8. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- Hung TJ, Kemphues KJ. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development. 1999;126:127–35. doi: 10.1242/dev.126.1.127. [DOI] [PubMed] [Google Scholar]
- Hurd TW, et al. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5:137–42. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- Huynh JR, et al. PAR-1 is required for the maintenance of oocyte fate in Drosophila. Development. 2001;128:1201–9. doi: 10.1242/dev.128.7.1201. [DOI] [PubMed] [Google Scholar]
- Izaddoost S, et al. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–83. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–39. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues KJ, et al. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–20. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Lee OK, et al. Discs-Large and Strabismus are functionally linked to plasma membrane formation. Nat Cell Biol. 2003;5:987–93. doi: 10.1038/ncb1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C, et al. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15:1324–33. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam SC, Choi KW. Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development. 2003;130:4363–72. doi: 10.1242/dev.00648. [DOI] [PubMed] [Google Scholar]
- Nam SC, Choi KW. Domain-specific early and late function of Dpatj in Drosophila photoreceptor cells. Dev Dyn. 2006;235:1501–7. doi: 10.1002/dvdy.20726. [DOI] [PubMed] [Google Scholar]
- Nishimura I, et al. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell. 2004;116:671–82. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, et al. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–78. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellikka M, et al. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–9. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Knoblich JA. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol. 2001;3:43–9. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- Pinheiro EM, Montell DJ. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development. 2004;131:5243–51. doi: 10.1242/dev.01412. [DOI] [PubMed] [Google Scholar]
- Richard M, et al. DPATJ plays a role in retinal morphogenesis and protects against light-dependent degeneration of photoreceptor cells in the Drosophila eye. Dev Dyn. 2006;235:895–907. doi: 10.1002/dvdy.20595. [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan S, et al. Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell. 2004;116:603–15. doi: 10.1016/s0092-8674(04)00128-x. [DOI] [PubMed] [Google Scholar]
- Shiomi K, et al. Alternative cell fate choice induced by low-level expression of a regulator of protein phosphatase 2A in the Drosophila peripheral nervous system. Development. 1994;120:1591–9. doi: 10.1242/dev.120.6.1591. [DOI] [PubMed] [Google Scholar]
- Shulman JM, et al. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell. 2000;101:377–88. doi: 10.1016/s0092-8674(00)80848-x. [DOI] [PubMed] [Google Scholar]
- Snaith HA, et al. Deficiency of protein phosphatase 2A uncouples the nuclear and centrosome cycles and prevents attachment of microtubules to the kinetochore in Drosophila microtubule star (mts) embryos. J Cell Sci. 1996;109(Pt 13):3001–12. doi: 10.1242/jcs.109.13.3001. [DOI] [PubMed] [Google Scholar]
- Sotillos S, et al. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J Cell Biol. 2004;166:549–57. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Ephrussi A. The fusome and microtubules enrich Par-1 in the oocyte, where it effects polarization in conjunction with Par-3, BicD, Egl, and dynein. Curr Biol. 2002;12:1524–8. doi: 10.1016/s0960-9822(02)01079-5. [DOI] [PubMed] [Google Scholar]
- Wassarman DA, et al. Protein phosphatase 2A positively and negatively regulates Ras1-mediated photoreceptor development in Drosophila. Genes Dev. 1996;10:272–8. doi: 10.1101/gad.10.3.272. [DOI] [PubMed] [Google Scholar]
- Watts JL, et al. par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development. 1996;122:3133–40. doi: 10.1242/dev.122.10.3133. [DOI] [PubMed] [Google Scholar]
- Wodarz A, et al. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol. 2000;150:1361–74. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, et al. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–7. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


