Abstract
Inorganic mercury (Hg2+) is a prevalent environmental contaminant to which exposure to can damage rod photoreceptor cells and compromise scotopic vision. The retinal pigment epithelium (RPE) likely plays a role in the ocular toxicity associated with Hg2+ exposure in that it mediates transport of substances to the photoreceptor cells. In order for Hg2+ to access photoreceptor cells, it must be first be taken up by the RPE, possibly by mechanisms involving transporters of essential nutrients. In other epithelia, Hg2+, when conjugated to cysteine (Cys) or homocysteine (Hcy), gains access to the intracellular compartment of the target cells via amino acid and organic anion transporters. Accordingly, the purpose of the current study was to test the hypothesis that Cys and Hcy S-conjugates of Hg2+ utilize amino acid transporters to gain access into RPE cells. Time- and temperature-dependence, saturation kinetics, and substrate-specificity of the transport of Hg2+, was assessed in ARPE-19 cells exposed to the following S-conjugates of Hg2+: Cys (Cys-S-Hg-S-Cys), Hcy (Hcy-S-Hg-S-Hcy), N-acetylcysteine (NAC-S-Hg-S-NAC) or glutathione (GSH-S-Hg-S-GSH). We discovered that only Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy were taken up by these cells. This transport was Na+-dependent and was inhibited by neutral and cationic amino acids. RT-PCR analyses identified systems B0,+ and ASC in ARPE-19 cells. Overall, our data suggest that Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy are taken up into ARPE-19 cells by Na-dependent amino acid transporters, possibly systems B0,+ and ASC. These amino acid transporters may play a role in the retinal toxicity observed following exposure to mercury.
Keywords: retinal pigment epithelium, transport, inorganic mercury, amino acid transporters
INTRODUCTION
Mercury can exist in the environment in three different forms: elemental (Hg0), inorganic (Hg2+) and organic (primarily as methylmercury; CH3Hg +). Hg0 and Hg2+ are released into the environment from the emissions of coal-burning plants and mining processes. Some of the released Hg2+ is biotransformed by microbial methylation to form CH3Hg+ , which humans are exposed to via the consumption of contaminated water and/or food (ATSDR, 2003). Over 90′ of ingested CH3Hg+ is absorbed by the gastrointestinal tract (Kershaw et al., 1980). After being + is oxidized to Hg2+, either in plasma or within target absorbed, an appreciable fraction of CH3Hg cells, (Dunn and Clarkson, 1980; Gage, 1964; Norseth and Clarkson, 1970a,b; Omata et al., 1980). Similarly, Hg0 entering systemic circulation can also undergo oxidation to form Hg2+ (Engqvist et al., 1998; Magos et al., 1989; Ogata and Aikoh, 1983; Sichak et al., 1986). Since cellular exposure to Hg2+ can be detrimental, it is important to determine how mercuric ions are handled by target cells that are affected adversely by this metal.
Exposure of certain organs and cells to Hg2+ can result in serious toxicological consequences, including cell death. One such organ is the eye. The visual system has been shown to be susceptible to the toxicological effects of mercuric ions. In vivo studies in laboratory animals have shown that vision loss can result after exposure to Hg0, CH3Hg+ or Hg2+, (ATSDR, 2003; Evans and Garman, 1980; Finocchio et al., 1980; Fox and Sillman, 1979; Shaw et al., 1980; Tessier-Lavigne et al., 1985; Warfvinge and Bruun, 1996). Indeed, one of the earliest signs of mercury poisoning is an impairment of scotopic (night) vision (Evans and Garman, 1980). This aspect of vision is mediated primarily by rod photoreceptor cells, which are unable to respond appropriately to light following exposure to mercuric compounds (Evans and Garman, 1980; Tessier-Lavigne, 1985). In addition, chronic exposure to mercury may result in a perturbation of peripheral vision, followed by a more severe loss of central vision (Evans and Garman, 1980; Finocchio et al., 1980; Saldana et al., 2006). Surprisingly, the actual mechanisms by which mercuric ions gain access to the photoreceptor cells and cause subsequent vision loss are unclear.
In order for mercury to gain access to photoreceptor cells, it must first cross the retinal pigment epithelium (RPE). The RPE is a single layer of epithelial cells positioned strategically between the choriocapillaris and the neural retina. Specifically, the basolateral plasma membrane is apposed to the choroidal capillaries while the apical microvilli interdigitate with the outer segments of photoreceptor cells. Because of this location, the RPE is responsible for the transport of nutrients and wastes to and from the photoreceptor cells. Owing to tight junctions between cells, the movement of substances across the RPE is mediated mainly by specific protein transporters present on the basolateral and apical plasma membranes. Interestingly, the membrane localization of certain proteins in the RPE is opposite that of most epithelia (Bok, 1993; Chancy et al., 2000; Marmorstein, 2001; Smith et al., 1999). This unique distribution is important in maintaining the basolateral to apical flow of nutrients across the RPE. In addition to essential nutrients and ions, the RPE may also deliver toxic compounds and metals such as lead, cadmium, and mercury to the photoreceptors. Indeed, following exposure to Hg0, mercuric ions have been detected in the RPE and photoreceptor cells (Warfvinge and Bruun, 1996). This accumulation can have significant detrimental effects on the RPE and the adjacent photoreceptors (Erie et al., 2005; Fox and Sillman, 1979; Tessier-Lavigne et al., 1985; Warfvinge and Bruun, 1996, 2000).
The mechanisms by which mercuric ions are taken up into and exported out of RPE cells remain unclear. In other epithelia, Hg2+ and CH3Hg+, as S-conjugates of thiol-containing molecules, have been shown to gain access to intracellular compartments of cells through the actions of amino acid and/or organic anion transporters (Clarkson, 1993; Bridges and Zalups, 2005). Mercuric ions bind readily to the reduced sulfur atom of thiol-containing molecules, such as cysteine (Cys), homocysteine (Hcy), and glutathione (GSH) to form thermodynamically stable linear II coordinate covalent complexes (Fuhr and Rabenstein, 1973). The Cys and Hcy conjugates of Hg2+ (Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy, respectively) are similar structurally to cystine and homocystine. Therefore, we hypothesized that these complexes are taken up at the site of one or more transporters of cystine and/or homocystine. Indeed, studies in Madin-Darby canine kidney (MDCK) cells indicate that the luminal uptake of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy in proximal tubular cells is mediated by the amino acid transporter, system b0,+ (Bridges et al., 2004; Bridges and Zalups, 2004). System b0,+ is the principal sodium-independent transporter of cystine on the luminal plasma membrane of proximal tubular cells, which are the primary cellular targets that take up and are affected adversely by Hg2+.
Inasmuch as humans continue to be exposed to CH3Hg+ , Hg0 and Hg2+ and, since this exposure can result in visual impairment, the purpose of the current study was to identify mechanisms in RPE that may play a role in the delivery of mercuric ions to photoreceptor cells. Considering that CH3Hg+ and Hg0 oxidize to form Hg2+ within the body, the primary focus of this study was to examine the handling of Hg2+. Initially, we tested the hypothesis that amino acid carriers play a role in the transport of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy across the RPE. Indeed, analyses of time- and temperature-dependence, saturation kinetics and substrate-specificity of the transport of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy provide indirect evidence supporting the theory that these two conjugates are transported by one or more Na+-dependent amino acid transporters present in the plasma membrane of RPE cells.
MATERIALS AND METHODS
Tissue Culture
ARPE-19 cells (American Type Culture Collection, Manassas, VA) are an immortalized line of human RPE cells that accurately reflect RPE cells in vivo (Dunn et al., 1996, 1998). Cells were cultured in Dulbecco’s modified Eagle’s medium/Ham’s F-12 50/50 mixture (Mediatech, Herndon, VA) supplemented with 10′ fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 100 U/ml penicillin, and 100 μg/ml streptomyocin (Invitrogen, Carlsbad, CA). Cells were sub-cultured by incubation in 0.25′ trypsin (Invitrogen) in phosphate buffered saline (PBS) containing 0.5 mM ethylenediaminetetraacetic acid (EDTA). Prior to experimentation, cells were seeded at 0.3 x 106 cells per well in 24-well plates and cultured for 24 h to obtain confluent monolayers. Cultures were maintained at 37° C in a humidified atmosphere of 5′ CO2 and 95′ O2.
Reverse-transcriptase Polymerase Chain Reaction Analyses of Transporter Expression
RT-PCR analyses were carried out in order to confirm the presence of RNA encoding ATB0,+ and ASC2 in ARPE-19 cells. It is assumed that the presence of RNA encoding these two transporters is indicative of the expression of the ATB0,+ and ASC2 proteins.
Total RNA was isolated from the cells using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. RT-PCR analyses were performed using the Gene Amp kit from Applied Biosystems (Foster City, CA). The presence of ATB0,+ and ASC2 was assayed using primers specific to each transporter to yield 425- and 405-base-pair fragments, respectively. The primers for each transporter are as follows: ATB0,+, 5’-CGT GCC CGG CCC ATT CTA AG-3’ and 5’AAA ACC ACC ATT GAG CAC CCT ACA-3’; ASC2, 5’-GGG GCA GGA GGT GGA GGG GAT GA-3’ and 5’-AGC GTG GCG GAA CTG GAA GAG GTC-3’. For both sets of primers, the PCR conditions were as follows: 94°C, 30s; 57°C, 30s; 72°C, 1 min for 35 cycles. RT-PCR products were electrophoresed on a 0.8′ agarose gel stained with ethidium bromide.
Evaluation of Transport
Uptake experiments using ARPE-19 cells were carried out as described previously (Bridges et al., 2001a,b; Naggar et al., 2003). At the beginning of each experiment, culture medium was removed and cells were washed with warm (37°C) uptake buffer (25 mM Hepes, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5 mM glucose, pH 7.5/Tris). In some experiments, NaCl was replaced with 140 mM N-methyl-D-glucamine (NMDG) in order to determine the role of Na+ in the uptake of specific mercuric species. Cells were incubated with 250 μL of an individual mercuric conjugate for 30 min at 37°C, unless otherwise noted. At the end of the incubation, the radiolabeled substances were aspirated from the culture dishes and the remaining cells were washed twice with cold (4°C) uptake buffer containing 1 mM sodium 2,3-dimercaptopropane-1-sulfonate (DMPS; Sigma, Saint Louis, MO), a well-known chelator of mercuric ions (Aposhian, 1983; Ruprecht, 1997). After the final washes had been completed, cells were solubilized with 1′ SDS in 0.2 N NaOH and the suspension was added to a scintillation vial containing 5 mL of Optifluor scintillation fluid (Perkin Elmer, Boston, MA). The radioactivity of each sample was determined by liquid scintillation spectrometry using a Beckman LS6500 scintillation counter (Beckman, Fullerton, CA)
Mercuric conjugates of Cys, Hcy, glutathione (GSH) and N-acetylcysteine (NAC) were generated by mixing 5 μM HgCl2, containing [ 203Hg2+], with 12.5 μM of the respective thiol. This mixture was allowed to incubate at room temperature for at least 5 min. The 203Hg2+ (2.36 mCi/mg) was generated by neutron activation of 202HgO at the Missouri University Research Reactor (Aslamkhan et al., 2003; Belanger et al., 2001).
In order to determine the species of Hg2+ taken up by RPE cells, uptake of Hg2+ was measured as a conjugate of one of several common thiol-containing biomolecules. Cells were incubated with 203Hg2+, as a conjugate of Cys (Cys-S-Hg-S-Cys), Hcy (Hcy-S-Hg-S-Hcy), GSH (GSH-S-Hg-S-GHS), or NAC (NAC-S-Hg-S-NAC) in the presence NaCl or NMDG for 30 min at 37°C or 4°C.
The effects of time and temperature on the transport of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy were evaluated by measuring the Na+-dependent and independent uptake of each conjugate at 37°C and 4°C for times ranging from 5 to 60 min. Experiments designed to measure the saturation kinetics for the transport of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy were performed by incubating cells with Cys-S-[203Hg]-S-Cys or Hcy-S-[203Hg]-S-Hcy for 30 min at 37°C or 4°C in the presence of unlabeled Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy (0.5, 1, 5, 10, 25, 50, 75, or 100 μM). These experiments were carried out in the presence and absence of Na+. Substrate-specificity analyses were carried out by incubating cells with Cys-S-[203Hg]-S-Cys or Hcy-S-[203Hg]-S-Hcy in the presence of one of the following unlabeled amino acids: methionine, cysteine, leucine, phenylalanine, isoleucine, alanine, arginine, lysine, serine, glycine, homocysteine, glutamate, aspartate, N-methylaminoisobutyric acid (MeAIB); or the organic anion, para-aminohippurate (PAH). These compounds were chosen based on their charge and utilization by specific amino acid transporters. MeAIB and PAH are classic inhibitors of system A and the organic anion transporter (OAT1 and OAT3), respectively. System A has not been identified as a mechanism for the transport of mercuric compounds (Cannon et al., 2001) while OAT has been shown to transport several conjugates of Hg2+ and CH3Hg+, (Aslamkhan et al., 2003; Zalups and Ahmad, 2004, 2005a, 2005b). In addition, some cells were exposed to Cys-S-[203Hg]-S-Cys or Hcy-S-[203Hg]-S-Hcy in the presence of a combination of two unlabeled amino acids (methionine and lysine, methionine and cysteine, methionine and arginine, alanine and cysteine, arginine and cysteine, or lysine and cysteine). In these experiments, all amino acids were used at a concentration of 3 mM and all experiments were carried out in the presence and absence of NaCl.
Moreover, the transport of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy was also measured in the presence of increasing concentrations of cystine or homocystine, respectively. Cells were exposed to 5 μM Cys-S-[203Hg]-S-Cys or Hcy-S-[203Hg]-S-Hcy in the presence of unlabeled cystine or homocystine (0, 25, 50, 75, 100, 250, 500, or 750 μM). Alternatively, cells were incubated with 5 μM [35S]-cystine (0.6 mCi/mg; Amersham, Piscataway, NJ) in the presence of unlabeled Cys-S-Hg-S-Cys (0, 5, 10, 25, 50, 100, 250, or 500 μM). These experiments were all carried out in the presence of NaCl. The uptake of radiolabeled homocystine in the presence of unlabeled Hcy-S-Hg-S-Hcy was not examined due to the unavailability of a radiolabeled form of homocystine.
Assessment of Cellular Viability
The toxicological effects of HgCl2, Cys-S-Hg-S-Cys, Hcy-S-Hg-S-Hcy, and GSH-S-Hg-S- GSH on ARPE-19 cells were measured using a methylthiazoletetrazolium (MTT) assay, as described previously (Bridges et al., 2004; Bridges and Zalups, 2004). ARPE-19 cells were seeded at a density of 0.3 x 106 cells/mL in 96-well culture dishes (200 μL/well). After growing to confluence (24 h), cells were washed twice with warm, uptake buffer containing NaCl and were then treated with HgCl2, Cys-S-Hg-S-Cys, Hcy-S-Hg-S-Hcy, or GSH-S-Hg-S-GSH (0, 5, 25, 50, 100 or 250 μM). The cells were incubated for 30 min at 37°C in a humidified atmosphere of 5′ CO2. Cells were then washed twice with cold (4°C) uptake buffer and incubated with 0.5 mg/ml MTT for 2 h at 37°C, in a humidified atmosphere of 5′ CO2. After this incubation, 100 μL of solubilization buffer (10′ Triton X-100 and 0.1 N HCl in isopropyl alcohol) were added to each well and the mixture was allowed to incubate for 16 h at room temperature. Each plate was read at 490 nm in a BioTek μQuant spectrophotometric plate reader (BioTek, Winooski, VT).
Data Analyses
All experiments were performed at least twice with triplicate measurements for each condition. Data for each parameter assessed were analyzed first with the Kolmogorov-Smirnov test for normality and then with Levene’s test for homogeneity of variance. The corresponding means for each set of data were subsequently compared using a one- or two-way analysis of variance (ANOVA). When significant F-values were obtained, differences among means were assessed using Tukey’s multiple-comparison procedure. A p value of <0.05 was considered statistically significant.
RESULTS
Uptake of Mercuric Conjugates of Thiol-Containing Biological Molecules
Uptake of Hg2+, as Cys-S-Hg-S-Cys, Hcy-S-Hg-S-Hcy, GSH-S-Hg-S-GSH, or NAC-S-Hg-S-NAC was measured in the cells in the presence of NaCl or NMDG at either 37°C or 4°C (Figure 1). Uptake of Cys-S-Hg-S-Cys at 37°C was approximately threefold greater in the presence of NaCl than in the presence of NMDG. Similarly, the inward transport of Hcy-S-Hg-S-Hcy at 37°C was more than twofold greater under Na+-dependent conditions compared with that under Na+-independent conditions. Interestingly, significant differences were also noted between the Na+-dependent and Na+-independent transport of GSH-S-Hg-S-GSH, as well as that of NAC-S-Hg-S-NAC.
Figure 1.
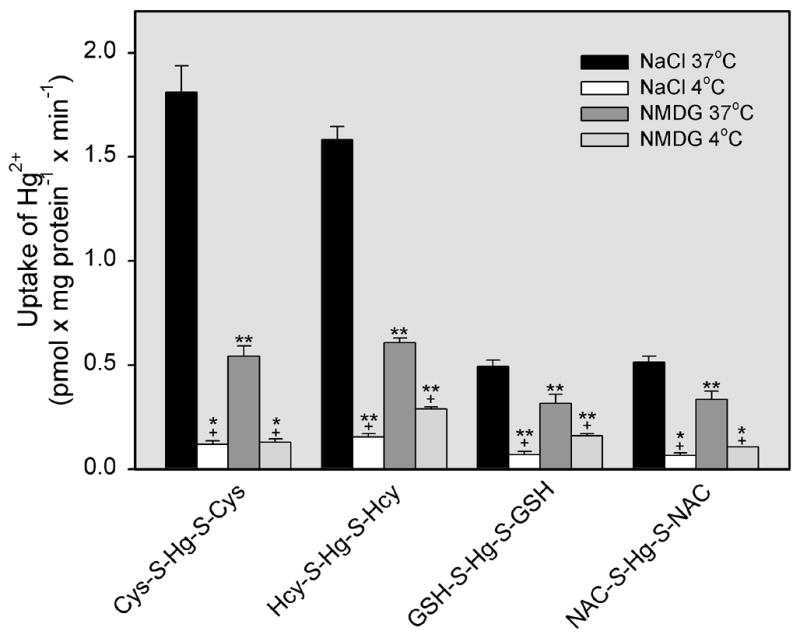
Uptake of inorganic mercury (Hg2+) as a conjugate of cysteine (Cys-S-Hg-S-Cys), homocysteine (Hcy-S-Hg-S-Hcy), glutathione (GSH-S-Hg-S-GSH), or N-acetylcysteine (NAC-S-Hg-S-NAC) in ARPE-19 cells. Cells were exposed to one of these conjugates (5 μM) for 30 min at 37°C in the presence of NaCl or N-methyl-D-glucamine (NMDG). Results are presented as means ± standard error. Data represent two experiments performed in quadruplicate. *, significantly different (p < 0.05) from the mean for the corresponding group of cells (exposed to the same conjugate) in the presence of NaCl or NMDG at 37°C. **, significantly different (p < 0.05) from the means of all other corresponding groups of cells. +, significantly (p < 0.05) from the mean of the corresponding groups of cells exposed to the same buffer.
Under Na+-dependent conditions, uptake of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy at 37°C was approximately threefold greater than that in cells exposed to GSH-S-Hg-S-GSH or NAC-S-Hg-S-NAC at 37°C. When cells were exposed to Cys-S-Hg-S-Cys, Hcy-S-Hg-S-Hcy, GSH-S-Hg-S-GSH, or NAC-S-Hg-S-NAC at 4°C in the presence of NMDG or NaCl, the uptake of Hg2+ was relatively similar among all groups.
Uptake of Hg2+ as Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy
Time-course analyses for the Na+-dependent and independent uptake of Cys-S-Hg-S-Cys (Figure 2A) or Hcy-S-Hg-S-Hcy (Figure 2B) in ARPE-19 cells were carried out at 4°C and 37°C. These data show that, at 37°C, the rates of uptake of either conjugate increased significantly over 60 min of exposure. At nearly every time studied, the uptake of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy in the presence of NaCl was significantly greater at 37°C than at 4°C. Furthermore, the uptake of these conjugates at 37°C in Na+-dependent conditions was also significantly greater than uptake of these conjugates in the presence of NMDG at either 4°C or 37°C.
Figure 2.
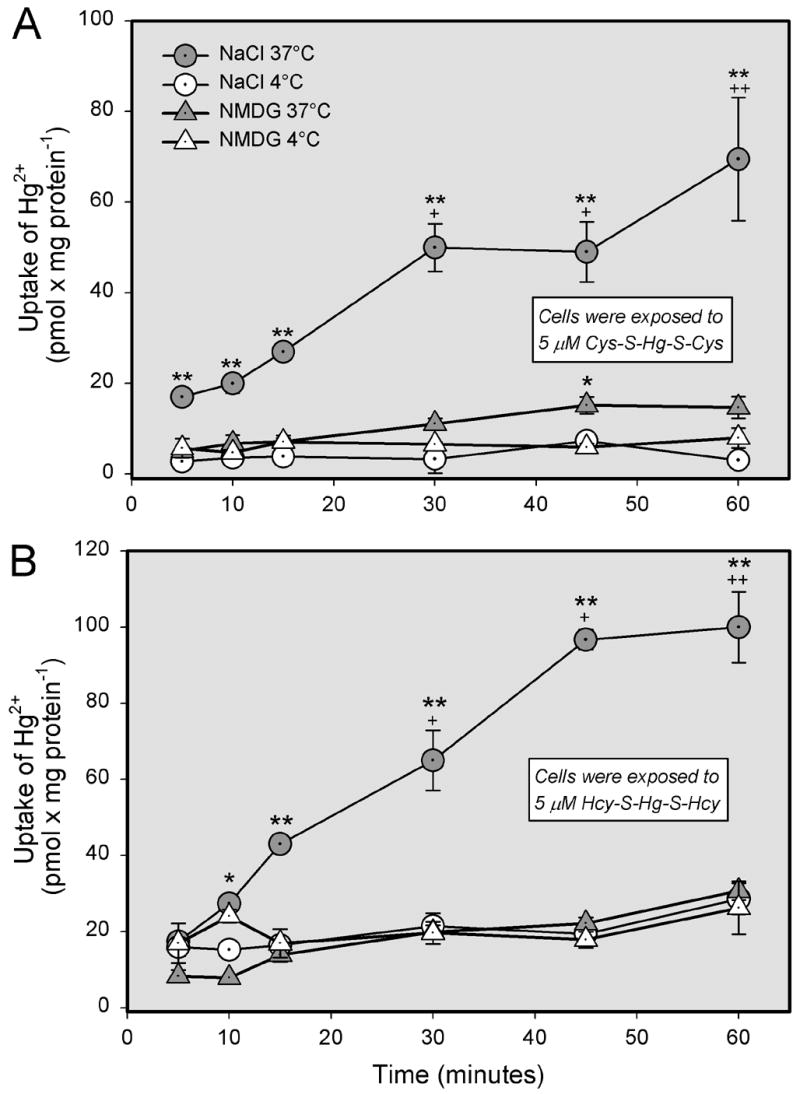
Time- and temperature-dependence of the transport of inorganic mercury (Hg2+) as a conjugate of cysteine (Cys-S-Hg-S-Cys) or homocysteine (Hcy-S-Hg-S-Hcy) in ARPE-19 cells. Cells were exposed to 5 μM Cys-S-Hg-S-Cys (A) or Hcy-S-Hg-S-Hcy (B) in the presence of NaCl for various times at either 4°C or 37°C. Results are presented as means ± standard error. Data represent two experiments performed in triplicate. *, significantly different (p < 0.05) from the mean for the corresponding group of cells exposed at 4°C. **, significantly different (p < 0.05) from the means of all other corresponding groups of cells. + or ++, significantly (p < 0.05) from the mean for the same treatment group at previous time points.
The saturation kinetics of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy transport were studied and compared in the presence of NaCl or NMDG at 37°C and 4°C. At 37°C, the overall rate of uptake of Cys-S-Hg-S-Cys (Figure 3A) was significantly greater in the presence of NaCl than in the presence of NMDG. At 4°C, there was no difference in the amount of Hg2+ associated with cells exposed to either NaCl or NMDG. Using the Michaelis-Menten equation, the maximal velocity (Vmax) for the Na+-dependent uptake of Cys-S-Hg-S-Cys at 37°C was calculated to be 111 ± 29.5 pmol x mg protein−1 x min−1 while the Michaelis-Menten constant (Km) was 160 ± 62.4 μM. These values were calculated by subtracting the uptake of Cys-S-Hg-S-Cys in the presence of NaCl at 37°C from the uptake of Cys-S-Hg-S-Cys in the presence of NMDG at 37°C. In order to distinguish among the activities of individual transporters, kinetic data obtained under Na+-dependent and independent conditions at 37°C were plotted by the Eadie-Hofstee method (Figure 4A). The data points representing Na+-dependent transport were obtained by subtracting the NMDG data from the NaCl data. Based on these plots, it appears that there are at least two distinct, Na+-dependent, transport processes responsible for the uptake of Cys-S-Hg-S-Cys. There also appear to be two processes involved in the Na+-independent uptake of Cys-S-Hg-S-Cys, although this uptake represents a minor component of Cys-S-Hg-S-Cys entry.
Figure 3.
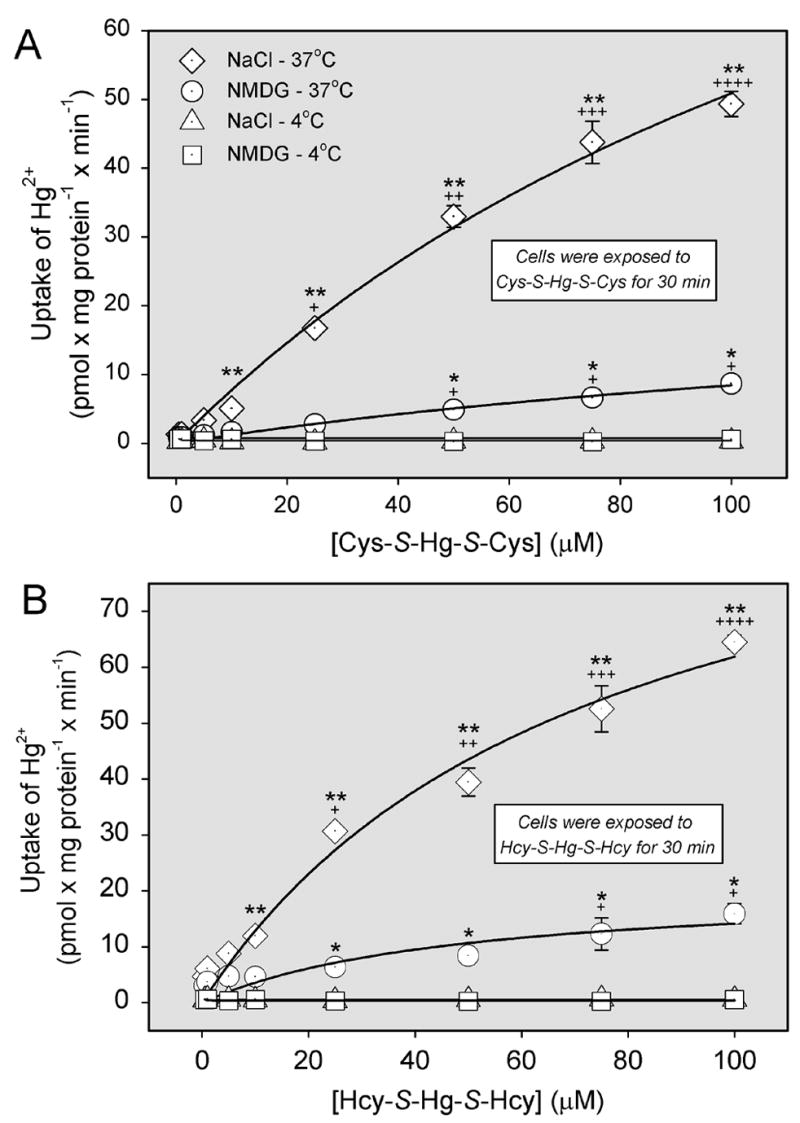
Saturation kinetics of the transport of inorganic mercury (Hg2+) as a conjugate of cysteine (Cys-S-Hg-S-Cys) or homocysteine (Hcy-S-Hg-S-Hcy) in ARPE-19 cells. Cells were exposed to 5 μM Cys-S-Hg-S-Cys (A) or Hcy-S-Hg-S-Hcy (B) in the presence of unlabeled Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy, respectively, for 30 min at 37°C and 4°C. Uptake was measured in the presence of NaCl or N-methyl-D-glucamine (NMDG). Curves were obtained using nonlinear regression analyses with the Michaelis-Menten equation: y = ax/(b+x). Panel A: NaCl: y = 111x/(160 + x), r2 = 0.995. NMDG: y = 24.8x/(195.0 + x), r2 = 0.989. Panel B: NaCl: y =85x/(78 + x) r2 = 0.995. NMDG: y = 21x/(48 + x) r2 = 0.937. Results are presented as means ± standard error. Data represent two experiments performed in triplicate. *, significantly different (p < 0.05) from the mean for the corresponding group of cells exposed at 4°C. **, significantly different (p < 0.05) from the means of all other corresponding groups of cells. +, ++, +++, or ++++ significantly (p < 0.05) from the mean for the same treatment group at previous concentrations.
Figure 4.
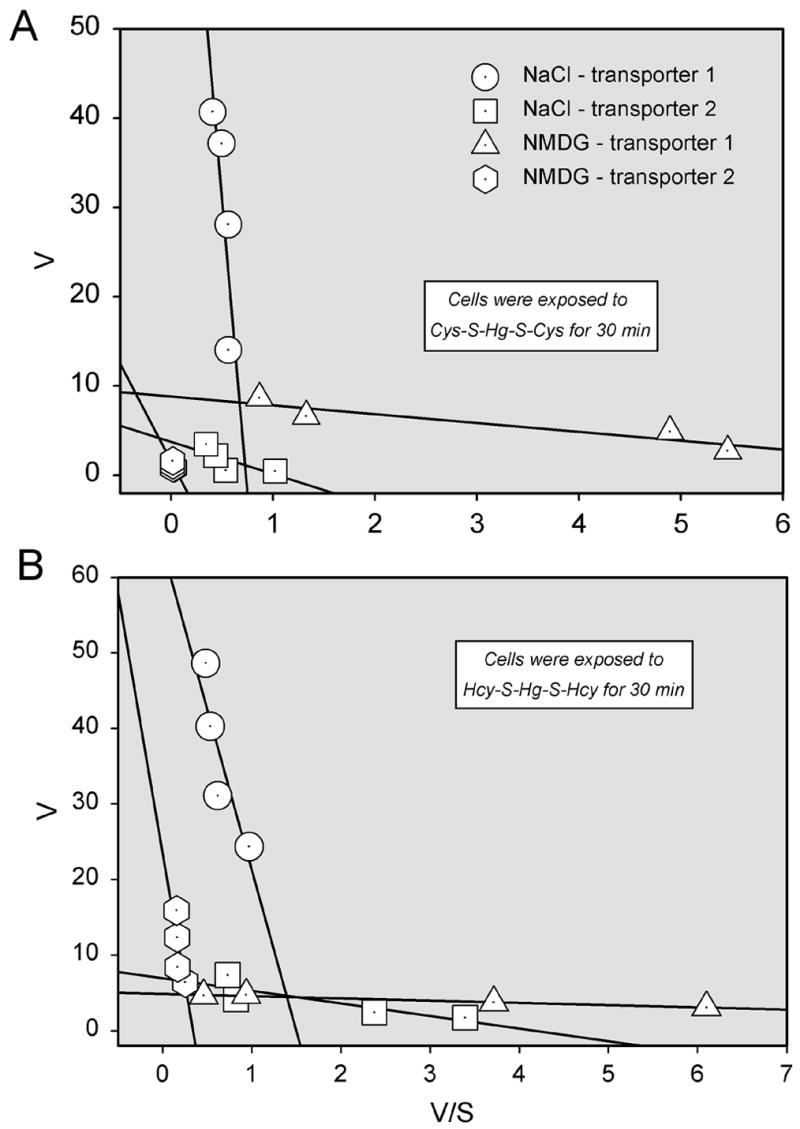
Eadie-Hofstee plot derived from the examination of saturation kinetics of the transport of inorganic mercury (Hg2+) as a conjugate of cysteine (Cys-S-Hg-S-Cys) (A) or homocysteine (Hcy-S-Hg-S-Hcy) (B) in ARPE-19 cells at 37°C. Linear regression analyses were used to generate lines with the following formula: y = ax + b. Panel A: NaCl (filled circles): y = 96.93 – 132.2x, r2 = 0.66; NaCl (filled triangles): y = 3.72 - 3.5x, r2 = 0.55. NMDG (open triangles): y = 8.82 – 0.99x, r2 = 0.86; NMDG (open circles) y = 1.56 – 21.83x, r2 = 0.28. Panel B: NaCl (filled circles): y = 63.99 – 42.67x, r2 = 0.781; NaCl (filled triangles): y = 6.92 – 1.66x, r2 = 0.726; NMDG (open circles): y = 23.6 – 68.8x, r2 = 0.554 NMDG (open triangles): y = 4.85 – 0.297x, r2 = 0.987 Results are presented as means ± standard error. Data represent two experiments performed in triplicate. *, significantly different (p < 0.05) from the mean for the corresponding group of cells exposed to NMDG. +, ++, +++, or ++++ significantly (p < 0.05) from the mean for the same cell type at previous concentrations.
Analysis of saturation kinetics for the transport of Hcy-S-Hg-S-Hcy (Figure 3B), revealed that the uptake of this conjugate at 37°C in the presence of NaCl (as compared to NMDG) was significantly greater at every concentration of Hcy-S-Hg-S-Hcy examined. The Vmax for this uptake was calculated to be 84.6 ± 12.8 pmol x mg protein−1 x min−1, while the Km was 78.3 ± 21.9 μM. These values were obtained by subtracting the Na+-dependent uptake of Hcy-S-Hg-S-Hcy from the corresponding Na+-dependent uptake. The amount of Hg2+ associated with cells exposed to NaCl at 4°C was not significantly different from that of cells exposed to NMDG. Data obtained under Na+-dependent and independent conditions at 37°C were then plotted using the Eadie-Hofstee method in order to differentiate among individual transport processes (Figure 4B). As before, in order to more accurately represent Na+-dependent transport, NMDG data was subtracted from the NaCl data. Like that of Cys- S-Hg-S-Cys, the Na+-dependent transport of Hcy-S-Hg-S-Hcy also appears to occur via two distinct processes.
Substrate-specificity for the transport of Cys-S-Hg-S-Cys (Figure 5A) or Hcy-S-Hg-S-Hcy (Figure 6A) in the presence and absence of Na+ was also assessed. Uptake of either of these conjugates in the presence of Na+ was significantly reduced by the addition of the following unlabeled neutral or cationic amino acids: methionine, cysteine, leucine, phenylalanine, isoleucine, alanine, arginine, lysine, serine, glycine, and homocysteine. Interestingly, exposure to the anionic amino acid, glutamate, also diminished uptake of each conjugate, although exposure to aspartate, which is also anionic, did not. Neither MeAIB (a substrate of system A) nor PAH (a substrate of OAT1 and OAT3) inhibited significantly the uptake of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy. In the absence of Na+, only cysteine significantly inhibited the uptake of Cys-S-Hg-S-Cys, while the uptake of Hcy-S-Hg-S-Hcy was inhibited by methionine, cysteine, arginine, serine, and homocysteine.
Figure 5.
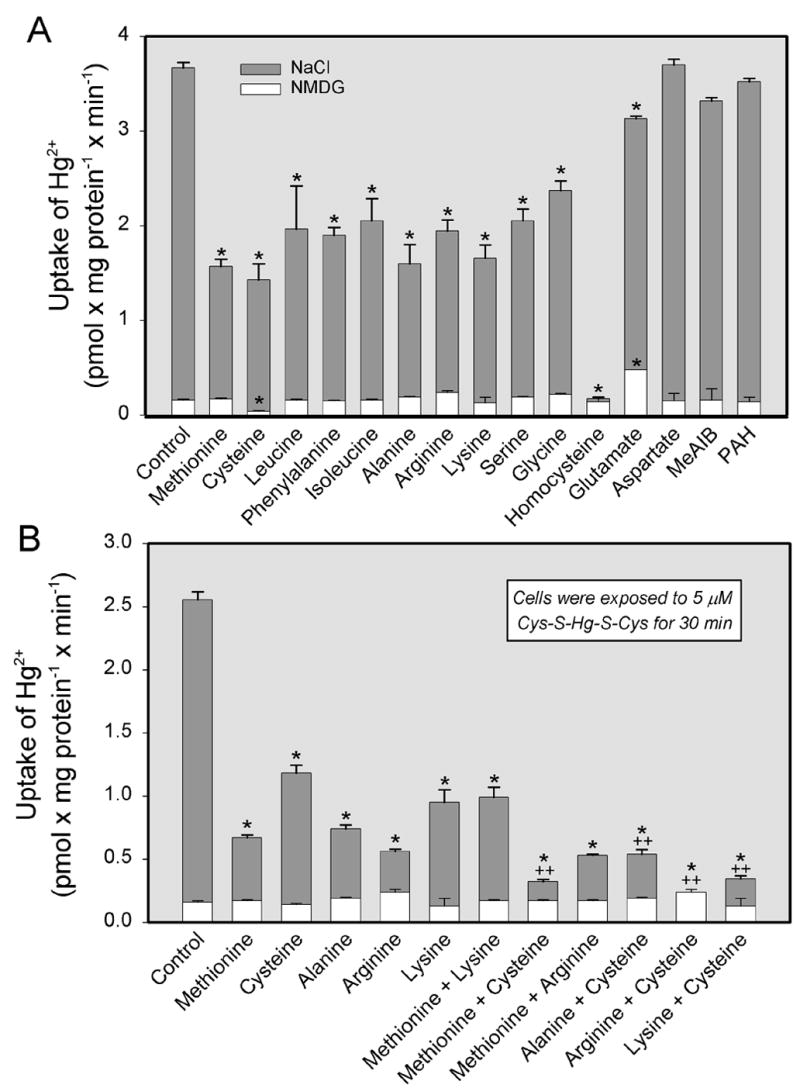
Substrate specificity of the transport of inorganic mercury (Hg2+) as a conjugate of cysteine (Cys-S-Hg-S-Cys) in ARPE-19 cells. Cells were exposed to 5 μM Cys-S-Hg-S-Cys for 30 min at 37°C in the presence of single amino acids (A) or in the presence of two amino acids (B) under Na+-dependent or independent conditions. Each amino acid was used at a concentration of 3 mM. Results are presented as means ± standard error. Data represent two experiments performed in triplicate. *, significantly different (p < 0.05) from the mean for the control group of cells. ++, significantly (p < 0.05) from the mean for each of the two groups of cells exposed to the corresponding individual amino acids.
Figure 6.
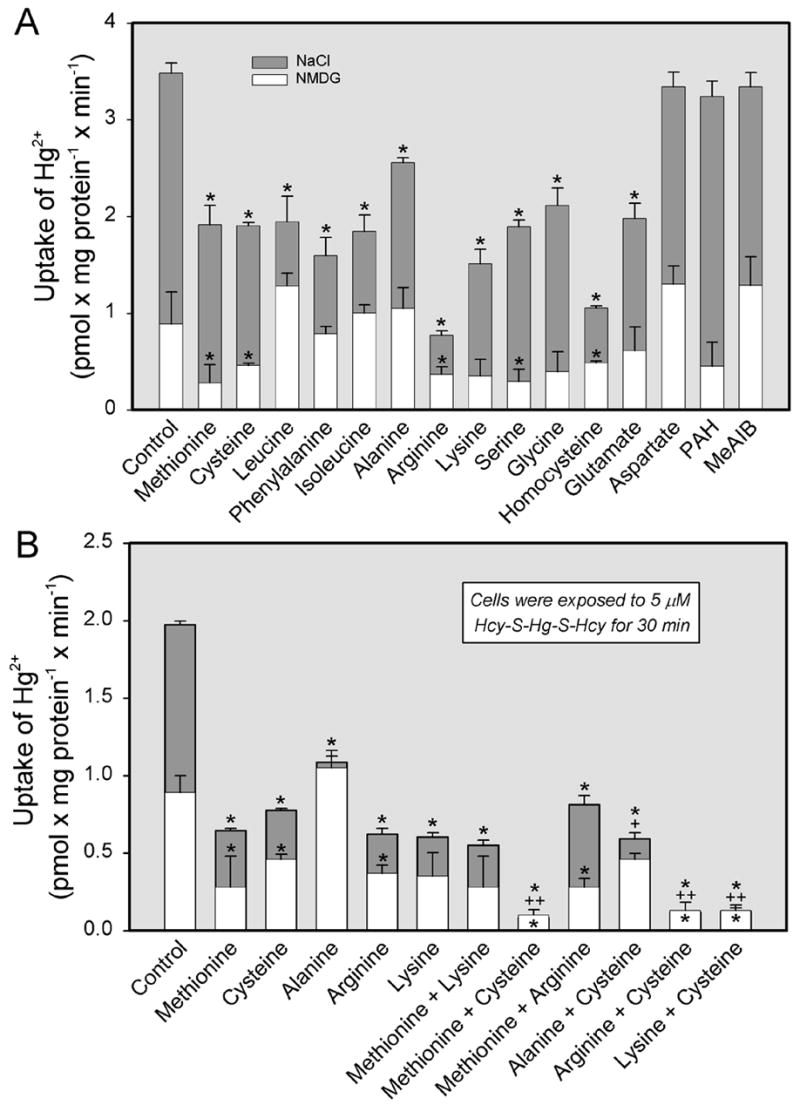
Substrate specificity of the transport of inorganic mercury (Hg2+) as a conjugate of homocysteine (Hcy-S-Hg-S-Hcy) in ARPE-19 cells. Cells were exposed to 5 μM Hcy-S-Hg-S-Hcy for 30 min at 37°C in the presence of single amino acids (A) or in the presence of two amino acids (B) under Na+-dependent or independent conditions. Each amino acid was used at a concentration of 3 mM. Results are presented as means ± standard error. Data represent two experiments performed in triplicate. *, significantly different (p < 0.05) from the mean for the control group of cells. ++, significantly (p < 0.05) from the mean for each of the two groups of cells exposed to the corresponding individual amino acids.
In separate experiments, cells were exposed to Cys-S-Hg-S-Cys (Figure 5B) or Hcy-S-Hg-S-Hcy (Figure 6B) in the presence of either a single amino acid or a combination of two amino acids. In the presence of Na+, the addition of methionine and cysteine, alanine and cysteine, arginine and cysteine, or lysine and cysteine reduced the transport of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy, in an additive manner, to levels significantly lower than those measured when these conjugates were exposed to a single amino acid. In the absence of Na+, there was little difference in the uptake of Cys-S-Hg-S-Cys among groups of cells. The Na+-independent uptake of Hcy-S-Hg-S-Hcy was significantly lower than control levels when cells were exposed to the following combinations: methionine and cysteine, methionine and arginine, arginine and cysteine, lysine and cysteine.
The effect of unlabeled cystine or homocystine on the uptake of Cys-S-Hg-S-Cys (Figure 7A) and Hcy-S-Hg-S-Hcy (Figure 8), respectively, was also measured in ARPE-19 cells. Surprisingly, the uptake of each conjugate increased over time as the concentration of corresponding disulfide was increased. Alternatively, when the uptake of radiolabeled cystine was analyzed in the presence of unlabeled Cys-S-Hg-S-Cys, the uptake of cystine was diminished significantly in a concentration-dependent manner (Figure 7B).
Figure 7.
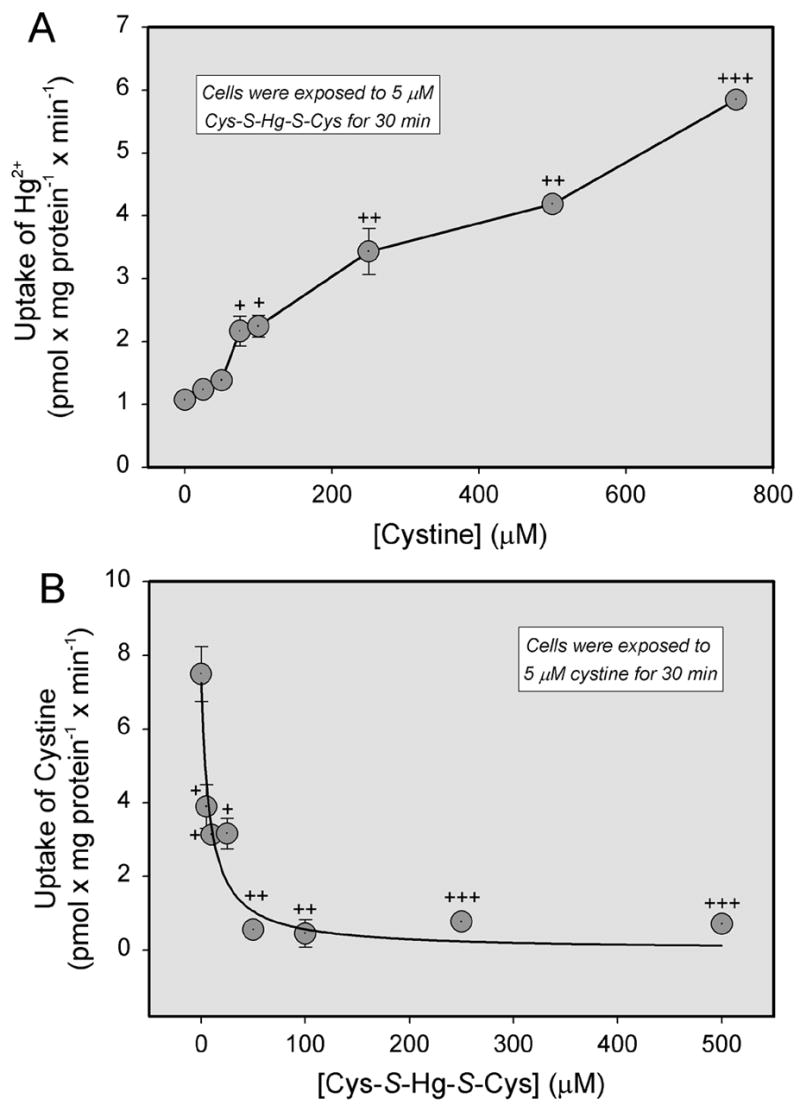
Uptake of inorganic mercury (Hg2+) as a conjugate of cysteine (Cys-S-Hg-S-Cys) in the presence of unlabeled cystine (A), or uptake of cystine in the presence of unlabeled Cys-S-Hg-S-Cys (B). ARPE-19 cells were exposed to 5 μM Cys-S-Hg-S-Cys or cystine for 30 min at 37°C in the presence of NaCl. Results are presented as means ± standard error. Data represent two experiments performed in triplicate. +, ++, +++, significantly (p < 0.05) from the mean for the cells at the preceding concentration.
Figure 8.
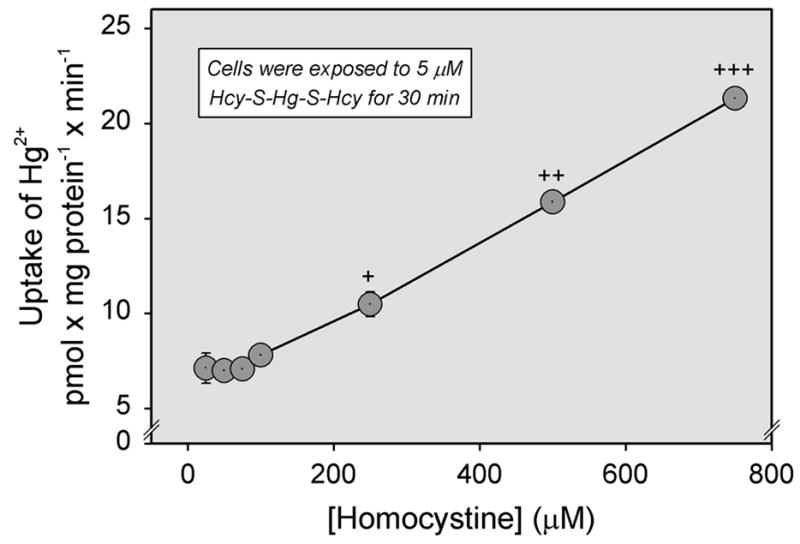
Uptake of inorganic mercury (Hg2+) as a conjugate of homocysteine (Hcy-S-Hg-S-Hcy) in the presence of unlabeled homocystine. ARPE-19 cells were exposed to 5 μM Hcy-S-Hg-S-Hcy for 30 min at 37°C in the presence of NaCl. Results are presented as means ± standard error. Data represent two experiments performed in triplicate. +, ++, +++, significantly (p < 0.05) from the mean for cells of the same treatment group at the preceding concentration.
Cellular Viability Assays
Cellular viability of ARPE-19 cells was assessed following the exposure to different species of Hg2+ was evaluated to determine the relationship between cellular transport of Hg2+ and intoxication (Figure 9). After a 30-min exposure to 250 μM Cys-S-Hg-S-Cys or GSH-S-Hg-S-GSH, there was no significant reduction in the viability of ARPE-19 cells. In contrast, exposure to 250 μM Hcy-S-Hg-S-Hcy decreased cellular viability by about 18′. Exposure to HgCl2 was used as a positive control since it has been demonstrated to induce a substantial degree of cell death in cultured MDCK cells (Bridges et al., 2004). Cellular viability in ARPE-19 cells declined by approximately 18′ following exposure to 5 μM HgCl2. Finally, after exposure to 250 μM HgCl2, cellular viability decreased by about 65′.
Figure 9.
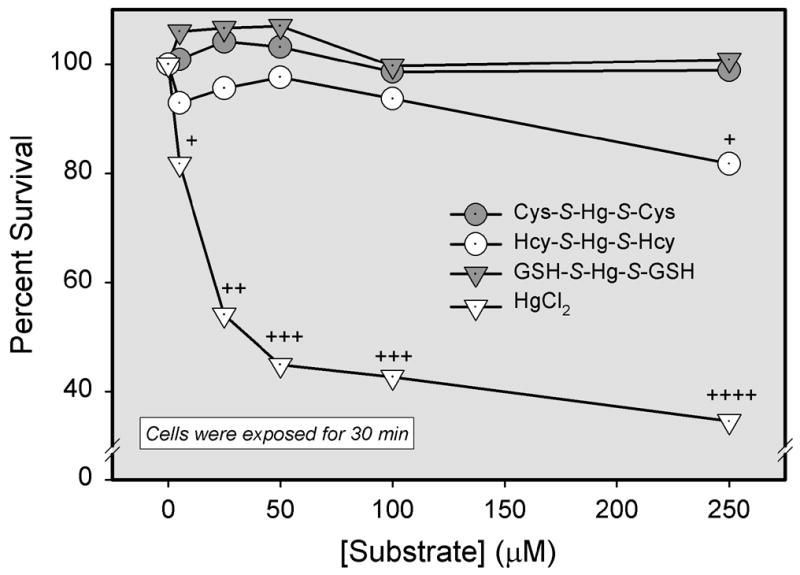
Analyses of cellular viability following exposure of ARPE-19 cells to inorganic mercury (Hg2+) as a conjugate of cysteine (Cys-S-Hg-S-Cys), homocysteine (Hcy-S-Hg-S-Hcy), glutathione (GSH-S-Hg-S-GSH) or HgCl2. Cells were exposed to various concentrations of each conjugate for 30 min at 37°C. Results are presented as means ± standard error. Data represent two experiments performed in quadruplicate. +, ++, +++, or ++++, significantly (p < 0.05) from the mean for cells of the same treatment group at the preceding concentration.
RT-PCR Analyses of Amino Acid Transporters in ARPE-19 cells
The presence of mRNA transcripts encoding systems B0,+ and ASC in ARPE-19 cells was examined using RT-PCR (Figure 10). RT-PCR products of the expected sizes were obtained for both of these transport systems.
Figure 10.
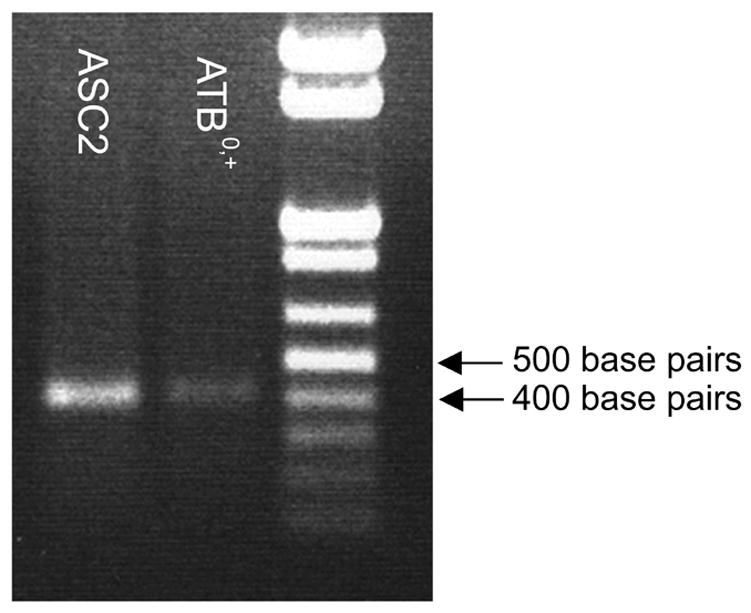
Agarose gel showing RT-PCR analyses of the expression of steady-state mRNA encoding ATB0,+ and ASC2 in ARPE-19 cells. The expected sizes of the RT-PCR products, as predicted from the position of the primers, were 425 base pairs (bp) for system B0,+ and 405 bp for system ASC. Lane 1, system ASC; Lane 2, system B0,+; Lane 3, DNA ladder.
DISCUSSION
Since exposure to various forms of mercury can impair vision, it is important to understand the manner in which mercuric ions gain access to photoreceptor cells. These cells do not have a direct blood supply, but obtain all nutrients from the choroid via vectorial transport across the RPE. Although the transport of numerous substances across this cell-layer has been characterized, the mechanisms by which toxic compounds, such as metal complexes, cross the RPE are not yet understood. Therefore, the purpose of the current study was to test the hypothesis that transportable mercuric species, such as Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy, are taken up by ARPE-19 cells via amino acid transporters present in the plasma membrane. The results of the current study suggest that ARPE-19 cells are indeed capable of transporting Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy and that this transport is mediated, at least in part, by Na+-dependent amino acid transporters.
The ability of RPE cells to transport Cys-S-Hg-S-Cys, Hcy-S-Hg-S-Hcy, GSH-S-Hg-S-GSH, or NAC-S-Hg-S-NAC was examined in the current study. Of these conjugates, only Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy appear to be taken up by ARPE-19 cells. Similarly, these two conjugates have been identified as transportable forms of Hg2+ in other cell-types (Aslamkhan et al., 2003; Bridges et al., 2004; Bridges and Zalups, 2004; Zalups and Ahmad, 2004; Zalups et al., 2004). Moreover, the majority of the transport of these conjugates by RPE cells appears to require the presence of Na+. In contrast, the uptake of GSH-S-Hg-S-GSH and NAC-S-Hg-S-NAC was significantly lower than that of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy. This finding was not surprising since GSH-S-Hg-S-GSH and NAC-S-Hg-S-NAC are large and/or negatively charged at physiologic pH and have not been shown to be taken up by amino acid transporters in other types of cells.
To characterize the uptake of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy in ARPE-19 cells, analyses of temperature- and time-dependence of this transport were carried out in the presence and absence of NaCl. The uptake of either conjugate was significantly greater at 37°C than that at 4°C, which suggests that the transport of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy in ARPE-19 cells is mediated by carrier proteins. Also, a small fraction of this transport appears to be due to Na+-independent mechanisms. In addition, the transport of each conjugate increased over time at 37°C, although this transport did not appear to reach a plateau during the times studied. This lack of plateau may have been related to the intracellular bonding characteristics of Hg2+. Once Hg2+ gains access to the intracellular environment, it can move freely from one thiol to another and can bind readily to any thiol-containing molecule, including low-molecular-weight and protein thiols present within the cytoplasm and organelles. Therefore, once Hg2+ has gained access to the intracellular compartment of ARPE-19 cells, it may release the thiol (i.e. Cys or Hcy) to which it is bound in order to bond with other, more prevalent, free thiols. Cys and Hcy may then be utilized by the cell or oxidized to cystine or homocystine, respectively, and be transported back out into the extracellular space. Since these two disulfide amino acids are removed from the cell, the negative feedback loop that would halt their inward transport is not invoked. This may, therefore, result in the continued deposition of mercuric ions, via the uptake Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy, into the intracellular space of the cell.
Analyses of saturation kinetics for the transport of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy suggested that the uptake of these two conjugates occurs primarily by a Na+-dependent, carrier-mediated process. Based on Eadie-Hofstee analyses, we can conclude that there are at least two distinct transport mechanisms responsible for the Na+-dependent transport of each conjugate. Possible carriers involved in this transport include, but are not limited to, organic anion transporters (OAT), which are indirectly dependent upon Na+ for their activity, and amino acid transporters, such as systems B0,+, ASC, or A (Ganapathy et al., 2004; Kekuda et al., 1996; Sloan and Mager 1999). Interestingly, uptake of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy did not appear to plateau, even at a concentration of 100 μM, indicating a lack of transporter saturation. This may be related to the low affinity of the transporter(s) for each conjugate or the presence of multiple transport proteins involved in the uptake of these compounds. These transporters likely become saturated only after exposure to much higher concentrations of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy. In the present study, however, ARPE-19 cells were not exposed to either conjugate at concentrations greater than 100 μM because of observed decreases in cellular viability (Figure 9). Interestingly, plasma levels of Hg2+ in humans following acute exposure mercury vapor have been found to be as high as 293 μM (Houeto et al., 1994). Because of the lack of transporter saturation at 100 μM, it is likely that these carriers may contribute to mercury intoxication by their continual uptake of Hg2+.
Substrate specificity experiments were carried out in order to determine a possible mechanism(s) for the transport of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy into RPE cells. Not surprisingly, the presence of various neutral and positively charged amino acids inhibited the Na+-dependent uptake of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy into RPE cells, indicating that amino acid transporters are responsible for at least a fraction of this uptake. Since MeAIB did not affect significantly the transport of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy, it can be concluded that system A does not play a role in this process. This conclusion is further supported by findings from isolated perfused rabbit proximal tubules where system A was ruled out as a carrier of Cys-S-Hg-S-Cys (Cannon et al., 2001). More likely candidates for this transport include systems B0,+ and ASC. Indeed, RNA encoding these amino acid transport systems was identified in ARPE-19 cells (Figure 10). System B0,+ transports cationic amino acids in addition to cysteine and other neutral amino acids (Sloan and Mager, 1999). Given that the cationic amino acids, lysine and arginine, significantly inhibited the uptake of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy in the ARPE-19 cells, it seems probable that system B0,+ is involved in this transport. Similarly, system ASC has been shown to transport alanine, serine, cysteine, and glutamate (Kekuda et al., 1996; Palacin et al., 1998). Since all of these amino acids had an inhibitory effect on the uptake of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy, one is led to believe that system ASC is also involved in the uptake of either mercuric species. Taken together, our data tend to indirectly implicate systems B°,+ and ASC in the uptake of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy.
When the Na+-independent uptake of Cys-S-Hg-S-Cys was measured in the presence of various amino acids, we found that the uptake of this conjguate was inhibited significantly by cysteine, suggesting that a small fraction of this uptake is due to a Na+-independent transporter. Furthermore, when the uptake of Hcy-S-Hg-S-Hcy was measured in the presence of various amino acids, we found that several neutral amino acids and a cationic amino acid, arginine, were able to inhibit the uptake of this conjugate. These data also suggest that there may be a small Na+-independent component of Hg2+ transport in RPE cells.
In a separate experiment, the Na+-dependent uptake of either Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy was measured in the presence of cysteine and a second amino acid. The uptake of either mercuric conjugate was reduced to levels significantly lower than those measured in the presence of each amino acid individually. These data suggest that one of the carriers responsible for the uptake of these conjugates has a high affinity for cysteine, as well as for neutral amino acids (e.g. system ASC), while another transporter (e.g. system B0,+) is less specific and prefers neutral and cationic amino acids. Interestingly, the presence of excess cystine or homocystine stimulated the uptake of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy, respectively. This stimulation is likely due to a trans-stimulatory effect in which cystine or homocystine within the cell is exchanged for Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy, respectively. Indeed this effect has been shown to occur with these conjugates at the sites of other transport proteins (Bridges et al., 2004; Bridges and Zalups, 2004).
In summary, the data from the current study provide indirect evidence suggesting that Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy are transported by RPE cells, possibly via the amino acid transporters systems B0,+ and ASC. Although additional studies are clearly needed to characterize more accurately the roles of individual transport proteins in the handling of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy by the RPE, the findings from the present study are important to human health in that they identify possible mechanisms that may play a role in allowing Hg2+ to access photoreceptor cells of the neural retina, where subsequent damage may occur.
Acknowledgments
We thank Dr. Delon Barfuss at Georgia State University, Atlanta, GA, for providing the 203Hg. We would also like to thank Ms. Lucy Joshee for technical assistance. This work was supported, in part, by the National Institutes of Health (National Institute of Environmental Health Sciences) grants ES05980 and ES11288 awarded to Dr. Zalups and a Ruth L. Kirschstein National Research Service Award, ES012556, awarded by the NIEHS to Dr. Bridges.
Footnotes
CONFLICT OF INTERST STATEMENT
There are no conflicts of interested associated with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substance and Disease Registry. Toxicological profile for mercury. US Department of Health and Human Services, Public Health Service, Centers for Disease Control; Atlanta, GA: 2003. [Google Scholar]
- Aposhian HV. DMSA and DMPS – water soluble antidotes for heavy metal poisoning. Ann Rev Pharmacol Toxicol. 1983;23:193–215. doi: 10.1146/annurev.pa.23.040183.001205. [DOI] [PubMed] [Google Scholar]
- Aslamkhan AG, Han YH, Yang XP, Zalups RK, Pritchard JB. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby Canine Kidney cells. Mol Pharmacol. 2003;63:590–596. doi: 10.1124/mol.63.3.590. [DOI] [PubMed] [Google Scholar]
- Belanger M, Westin A, Barfuss DW. Some health physics aspects of working with 203Hg in university research. Health Phys. 2001;80(Suppl 1):S28–S30. [PubMed] [Google Scholar]
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci. 1993;17(Suppl):189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Bauch C, Verrey F, Zalups RK. Mercuric conjugates of cysteine are transported by the amino acid transporter system b0,+: Implications of molecular mimicry. J Am Soc Nephrol. 2004;15:663–673. doi: 10.1097/01.ASN.0000113553.62380.F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Kekuda R, Prasad PD, Mehta P, Huang W, Smith SB, Ganapathy V. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001a;42:47–54. [PubMed] [Google Scholar]
- Bridges CC, Ola MS, Prasad PD, El-Sherbeny A, Ganapathy V, Smith SB. Regulation of taurine transporter expression by NO cultured human retinal pigment epithelial cells. Am J Physiol Cell Physiol. 2001b;281:C1825–C1836. doi: 10.1152/ajpcell.2001.281.6.C1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Homocysteine, system b0,+ and the renal epithelial transport and toxicity of inorganic mercury. Am J Pathol. 2004;165:1385–1394. doi: 10.1016/S0002-9440(10)63396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon VT, Zalups RK, Barfuss DW. Amino acid transporters involved in luminal transport of mercuric conjugates of cysteine in rabbit proximal tubule. J Pharmacol Exp Therap. 2001;298:780–789. [PubMed] [Google Scholar]
- Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, Roon P, Ganapathy V, Smith SB. Expression and differential polarization of the reduced- folate transporter-1 and the folate receptor α in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676–20684. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. Molecular and ionic mimicry of toxic metals. Ann Rev Biochem. 1993;32:545–571. doi: 10.1146/annurev.pa.33.040193.002553. [DOI] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, A human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Clarkson TW. Does mercury exhalation signal demethylation of methylmercury? Health Physiol. 1980;38:477–414. [PubMed] [Google Scholar]
- Dunn KC, Marmorstein AD, Bonilha VL, Rodriquez-Boulan E, Giordano F, Hjelmeland LM. Use of the ARPE-19 cell line as a model of RPE polarity: Basolateral secretion of FGF5. Invest Ophthalmol Vis Sci. 1998;39:2744–2749. [PubMed] [Google Scholar]
- Engqvist A, Colmsjo A, Skare I. Speciation of mercury excreted in feces from individuals with amalgam fillings. Arch Environ Health. 1998;53:205–213. doi: 10.1080/00039899809605697. [DOI] [PubMed] [Google Scholar]
- Erie JC, Butz JA, Good JA, Erie EA, Burritt MF, Cameron JD. Heavy metal concentrations in human eyes. Am J Ophthalmol. 2005;139:888–893. doi: 10.1016/j.ajo.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Evans HL, Garman RH. Scotopic vision as an indicator of neurotoxicity. In: Merigan WH, Weiss B, editors. Neurotoxicity of the Visual System. New York: Raven Press; 1980. pp. 135–147. [Google Scholar]
- Finocchio DV, Luschei ES, Mottet NK, Body R. Effects of methylmercury on the visual system of rhesus macaque (Macaca Mulatta). I. Pharmacokinetics of chronic methylmercury related to changes in vision and behavior. In: Merigan WH, Weiss B, editors. Neurotoxicity of the Visual System. New York: Raven Press; 1980. pp. 113–122. [Google Scholar]
- Fox DA, Sillman AJ. Heavy metals affect rod, but not cone, photoreceptors. Science. 1979;206:78–80. doi: 10.1126/science.314667. [DOI] [PubMed] [Google Scholar]
- Fuhr B, Rabenstein DL. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. The binding of cadmium, zinc, lead, and mercury by glutathione. J Am Chem Soc. 1973;95:6944–6950. doi: 10.1021/ja00802a013. [DOI] [PubMed] [Google Scholar]
- Gage JC. Distribution and excretion of methyl and phenylmercury salts. Br J Ind Med. 1964;21:197–202. doi: 10.1136/oem.21.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Inoue K, Prasad PD, Ganapathy ME. In Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition. Boca Raton, FL: CRC Press; 2004. Cellular uptake of amino acids: systems and regulation; pp. 63–78. [Google Scholar]
- Houeto P, Sandouk P, Baud FJ, Levillain P. Elemental mercury vapour toxicity: Treatment and levels in plasma and urine. Human Exp Toxicol. 1994;13:848–852. doi: 10.1177/096032719401301205. [DOI] [PubMed] [Google Scholar]
- Kekuda T, Prasad PD, Fei YJ, Torres-Zamorano V, Sinha S, Yang-Feng TL, Leibach FH, Ganapathy V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter B0 from a human placental choriocarcinoma cell line. J Biol Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- Kershaw TG, Clarkson TW, Dhahir P. The relationship between blood levels and dose of methylmercury in man. Arch Environ Health. 1980;35:28–36. doi: 10.1080/00039896.1980.10667458. [DOI] [PubMed] [Google Scholar]
- Magos L, Clarkson TW, Hudson AR. The effects of dose of elemental mercury and first-pass circulation time on exhalation and organ distribution of inorganic mercury in rats. Biochim Biophys Acta. 1989;991:85–89. doi: 10.1016/0304-4165(89)90032-9. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872. doi: 10.1034/j.1600-0854.2001.21202.x. [DOI] [PubMed] [Google Scholar]
- Naggar H, Fei YJ, Ganapathy V, Smith SB. Regulation of reduced-folate transporter-1 (RFT-1) by homocysteine and identity of transport systems for homocysteine uptake in retinal pigment epithelial (RPE) cells. Exp Eye Res. 2003;77:687–697. doi: 10.1016/j.exer.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Norseth T, Clarkson TW. Studies on the biotransformation of 203Hg-labeled methylmercury chloride in rats. Arch Environ Health. 1970a;21:717–727. doi: 10.1080/00039896.1970.10667325. [DOI] [PubMed] [Google Scholar]
- Norseth T, Clarkson TW. Biotransformation of methylmercury salts in the rat studied by specific determination of inorganic mercury. Biochem Pharmacol. 1970b;19:2775–2783. doi: 10.1016/0006-2952(70)90104-8. [DOI] [PubMed] [Google Scholar]
- Ogata M, Aikoh H. The oxidation mechanism of metallic mercury in vitro by catalase. Physiol Chem Phys Med NMR. 1983;15:89–91. [PubMed] [Google Scholar]
- Omata S, Sata M, Sakimura K, Sugano H. Time-dependent accumulation of inorganic mercury in subcellular fractions of kidney, liver, and brain or rats exposed to methylmercury. Arch Toxicol. 1980;44:231–241. doi: 10.1007/BF00278031. [DOI] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Ruprecht J. Scientific Monograph Dimaval (DMPS) Heyltex Corp; Houston, TX: 1997. [Google Scholar]
- Saldana M, Collins CE, Gale R, Backhouse O. Diet-related mercury poisoning resulting in visual loss. British J Ophthalmol. 2006;90:1432–1434. doi: 10.1136/bjo.2006.094821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CM, Mottet NK, Chen WJ. Effects of methylmercury on the visual system of rhesus macaque (Macaca Mulatta). II. Neuropathological findings (with emphasis on vascular lesions in the brain) In: Merigan WH, Weiss B, editors. Neurotoxicity of the Visual System. New York: Raven Press; 1980. pp. 123–134. [Google Scholar]
- Sichak SP, Mavis RD, Finkelstein JN, Clarkson TW. An examination of the oxidation of mercury vapor by rat brain homogenate. J Biochem Toxicol. 1986;1:53–68. doi: 10.1002/jbt.2570010107. [DOI] [PubMed] [Google Scholar]
- Sloan JL, Mager S. Cloning and functional expression of a human Na+ and Cl−-dependent neutral and cationic amino acid transporter B0,+ J Biol Chem. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- Smith SB, Kekuda R, Gu X, Chancy C, Conway SJ, Ganapathy V. Expression of folate receptor α in the mammalian retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999;40:840–848. [PubMed] [Google Scholar]
- Tessier-Lavigne M, Mobbs P, Attwell D. Lead and mercury toxicity and the rod light response. Invest Ophthalmol Vis Sci. 1985;26:1117–1123. [PubMed] [Google Scholar]
- Warfvinge K, Bruun A. Mercury accumulation in the Squirrel monkey eye after mercury varpour exposure. Toxicol. 1996;107:189–200. doi: 10.1016/0300-483x(95)03257-g. [DOI] [PubMed] [Google Scholar]
- Warfvinge K, Bruun A. Mercury distribution in the squirrel monkey retinal after in utero exposure to mercury vapor. Environ Res. 2000;83:102–109. doi: 10.1006/enrs.1999.4029. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1. J Am Soc Nephrol. 2004;15:2023–2031. doi: 10.1097/01.ASN.0000135115.63412.A9. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Handling of the homocysteine S-conjugate of methylmercury by renal epithelial cells: role of organic anion transporter 1 and amino acid transporters. J Pharmacol Exp Ther. 2005a;315:896–904. doi: 10.1124/jpet.105.090530. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Transport of N-acetylcysteine s-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J Pharmacol Exp Therap. 2005b;314:1158–1168. doi: 10.1124/jpet.105.086645. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Aslamkhan AG, Ahmad S. Human organic anion transporter 1 mediates cellular uptake of cysteine-S-conjugates of inorganic mercury. Kidney Int. 2004;66:251–261. doi: 10.1111/j.1523-1755.2004.00726.x. [DOI] [PubMed] [Google Scholar]


