Abstract
Postural sensitivity to moving visual environments in patients with anxiety disorders was studied. We hypothesized that patients with anxiety disorders would have greater sway in response to a moving visual environment compared to healthy adults, especially if they have space and motion discomfort (SMD). Twenty one patients with generalized anxiety without panic (NPA), and 38 patients with panic and agoraphobia (PAG) were compared to 22 healthy controls. SMD was evaluated in all subjects via questionnaire. Subjects stood on a force platform that was either fixed or rotating with the subject (i.e. sway referenced) during exposure to a sinusoidally moving visual surround. Center of Pressure (COP) data were computed from force transducers in the platform as a measure of sway. Results showed that patients swayed significantly more in response to the moving visual scene compared to control subjects, with no differences between the NPA and PAG groups. SMD was a predictor of sway response in the patients: patients with high SMD swayed significantly more than both Controls and anxiety patients with low SMD. These results indicate that patients with anxiety disorders, particularly those with SMD, are more visually dependent for balance. This subgroup of patients may be amenable to treatment used for patients with balance disorders (i.e. vestibular rehabilitation) that focuses on sensory re-integration processes that address visual sensitivity.
Keywords: Anxiety, Panic, Optic flow, Posture, Sway, Sensory Integration, Balance
Introduction
Anxiety is highly prevalent in patients with balance disorders. About 30 percent of patients with vestibular disorders report persistent panic and agoraphobic symptoms or generalized anxiety (Eagger, et al., 1992; Stein, et al., 1994; Sullivan, et al., 1993; Clark, et al., 1994). Conversely, vestibular dysfunction is common among patients with anxiety disorders (Jacob, Moller, et al, 1985; Sklare, et al., 1990; Hoffman, et al, 1994; Jacob Furman, Durrant, et al, 1996; Yardley, 1994, 1995; Perna, et al, 2001).
While many anxious patients have symptoms of imbalance and/or dizziness, few have a syndromal vestibular disorder (Furman & Jacob, 2001). For example, balance symptoms in agoraphobics only rarely include rotational vertigo (Jacob, Lillenfeld, et al., 1989). However, many patients with anxiety complain of discomfort in situations that are challenging for patients with balance disorders. These situationally specific symptoms include “supermarket syndrome” (Rudge and Chambers, 1982) “space phobia” (Marks, 1981), “visual vertigo” (Bronstein, 1995), and “height vertigo” (Brandt, et al, 1980). We have labeled this patterns of situationally specific symptom elicitation “Space and Motion Discomfort” (SMD) and developed a questionnaire to detect this condition (Jacob, Woody et al, 1993). SMD is commonly seen in patients with balance disorders, suggesting that it may be possible to identify a select group of patients with anxiety that has vestibular dysfunction.
Vestibular dysfunction can give rise to situational discomfort, i.e., SMD because three sensory channels (vision, proprioception and vestibular) are involved in balance control. When sensory conflict arises, the postural control system normally adjusts the sensory integration process toward an appropriate sensory channel or away from the channel providing misleading information. Thus, normal healthy adults, when exposed to misleading balance information, downweight information from the misleading sensory channel and upweight information from channels that provide correct information. Patients with vestibular disorders are prone to develop SMD because they become unusually dependent on information from non-vestibular channels and thus become sensitive to misleading information in these channels (Redfern and Yardley, 2001). For example, a person who is visually dependent due to vestibular dysfunction would be expected to experience particularly high levels of “height vertigo.” Visual information is degraded due to the absence of motion parallax and reduction of retinal slip resulting from the long visual distances characteristic of height and a lack of a reliable vestibular channel.
SMD is observed not only in patients with balance disorders, but also in individuals unable to flexibly adjust the above-mentioned inter-sensory integration process, e.g., those who are visually dependent or surface dependent. Some patients with anxiety disorders, particularly those with panic disorder and agoraphobia (Jacob,Lilienfeld et al 1989, Jacob Woody et al 1993) seem especially prone to developing SMD. In a previous study, we found that anxious patients with SMD swayed more in response to moving visual environments than did healthy adults. That is, they seemed to be visually dependent. (Jacob, Redfern, et al, 1995). That study however, did not include a non-SMD control group. Since it has been found that anxious patients in general are sensitive to visuo-vestibular sensory conflict (Viaud-Delmon, et al, 2000), we wanted to directly compare anxious patients with SMD to anxious patients without SMD. The motivation for this study was: (1) to investigate the central integration of visual information for balance (visual dependence) in an anxious population to determine if this mechanism was different in patients with anxiety as compared with healthy adults; and 2) to investigate if this mechanism was linked to phenomenological variables, such as the presence or absence of panic attacks, height phobia and SMD. In a previous study, we had shown that patients with panic disorder with agoraphobia were surface dependent, i.e., unusually sensitive to a sway referenced support surface. (Jacob et al. 1997). In that study, we also assessed visual dependence but used a different technique, i.e., sway-referencing the Equitest™ visual surround. Because this stimulus may have been too weak an intervention, we modified the Equitest™ balance test protocol for the present study to include an externally imposed motion of the visual surround.
We hypothesized that patients with anxiety disorders would have greater sway in response to a moving visual environment compared to healthy adults, especially in patients with SMD. There were two secondary analyses. The first examined the difference between two types of anxiety disorders; non-panic anxiety (NPA) and panic disorder and agoraphobia (PAG). Based on previous findings (Jacob, Furman et al 1997), we hypothesized that PAG patients would have greater visual dependence for balance and thus have greater sway in response to moving environments. The second supplementary analysis examined the role of height phobia in visual balance sensitivity. We suspected that visual dependence for balance may play a role in height phobia. Because it proved difficult to find patients with PAG who were completely free from fear of heights, this analysis was limited to anxiety patients in the NPA group. We hypothesized that patients with comorbid height phobia would have greater sway in response to optic flow environments.
Methods
Subjects
Subjects between ages 18-55 were recruited either as normal volunteers or from psychiatric outpatients. All subjects provided informed consent to participate in this study, and the experimental protocol was approved by the Institutional Review Board at the University of Pittsburgh. Subjects included 59 patients with anxiety disorders (48 female; 53 Caucasian) and 22 healthy controls (19 female; 19 Caucasian). Twenty one patients had panic and agoraphobia (PAG) and 38 patients had non-panic anxiety (NPA). Of the NPA patients, 19 had comorbid height phobia and 19 did not have height phobia. Nineteen of the 21 PAG patients had comorbid height phobia. Assessment of Height phobia status (absent vs present) was based on: 1) clinician-rated fear of height on a scale of 0-8 (“no fear” to “very severe fear”), and 2) clinicianrated avoidance of heights on a scale of 0-8 (“no avoidance” to “always avoid”) from the “Specific Phobia” section of the ADIS (Brown, DiNardo and Barlow, 1994). To be accepted as a normal control, individuals had to have zero ratings on both fear and avoidance of heights. For the analysis of height, anxiety patients with fear and avoidance ratings indicating a mild severity level (3 or less on both scales) (N=5) were omitted. Descriptively, for the remaining NPA height phobic subjects, median fear rating was 6, indicating “severe fear”, and median avoidance rating was also 6 (corresponding to “often avoid”). For the PAG height phobic subjects, median fear was 7 and median avoidance was 8.
Patients were recruited from the outpatient clinic of the Western Psychiatric Institute and Clinic (WPIC) of the University of Pittsburgh Medical Center. The patients were recruited after an initial clinical diagnostic evaluation identified that the patient had an anxiety disorder (either panic disorder, with or without agoraphobia, generalized anxiety disorder, social phobia, or specific phobia). Patients with a history of drug or alcohol abuse, head injury, obsessive compulsive disorder, or psychotic disorder were excluded, as were patients with history of migraine headaches or orthopedic problems. Patients could not be taking benzodiazepines but were permitted to remain on antidepressant medications. Antidepressant use in the patients was 18.1 % and 38.1 % for the NPA and PAG groups, respectively. No Control subjects were taking antidepressants. Final diagnosis was based on a structured research interview using the Anxiety Disorders Interview Schedule (Brown & DiNardo, 1994) As part of their research assessment the patients filled out a battery of questionnaires that included the Situational Characteristics Questionnaire (Jacob, Woody 1993). Normal subjects were recruited from the community via flyers or word of mouth. Preference was given to individuals whose age (+/- 5 years), race and gender matched those same variables of a participant in the PAG group. The normal control subjects received the same structured interview as the patients.
Subjects were categorized as having abnormally high SMD if their score on part I of the Situational Characteristics Questionnaire was greater than or equal to 6.5. Note that for this study, all negative scores on individual questions on the Situational Characteristics Questionnaire were rescored as zero. The cutoff of 6.5 was based upon the normal subject data. No normal subject had an SMD score exceeding 6.5. For the NPA patients, 17 patients were abnormal for SMD and 21 patients were normal for SMD. For the PAG patients, 9 patients were abnormal for SMD and 13 patients were normal. Table 1 shows the characteristics of the subject populations examined in the study.
Table 1.
Characteristics of subjects
| Normal SMD | Abnormal SMD | |
|---|---|---|
| Controls | 22 | 0 |
| NPA | 21 (8 w/ height phobia) | 17 (11 w/ height phobia) |
| PAG | 12 (10 w/ height phobia) | 9 (9 w/ height phobia) |
All subjects underwent a vestibular examination including Computerized Dynamic Posturography (Equitest™, Neurocom Inc), Earth Vertical Axis Rotational Testing, Ocular Motor Screening, Positional Testing, and Caloric Testing. Results of these examinations are reported elsewhere. (Jacob, et al., 2006).
Protocol for visual dependence
Subjects stood on an Equitest™ posture platform in either a fixed or sway referenced platform condition during sinusoidal movement of the visual surround in the anterior-posterior direction. The visual surround filled the subject’s full field of view, with the front and sides of the surround about 1 m from the subject extending upwards 2 m from the floor. This scene is connected to a computer controlled rotary motor with its axis collinear with the subject’s ankle. Thus, the scene motion was a rotation about the subject’s ankle. The visual surround motion conditions were: no motion, 0.10 Hz sinusoid, or 0.30 Hz sinusoid. The magnitude of the motion was 10 degrees peak-to-peak about the ankle of the subject. The scene was modified from the standard clinical surround to include a “bullseye” pattern of six alternating black-and-white concentric rings (with each ring occupying 5° of visual arc), surrounded by a checkerboard of black-and-white squares (15cm per side). Previous studies have utilized similar patterns in moving visual environment paradigms to evoke postural responses in both healthy and patient populations (Redfern and Furman, 1994; Peterka and Benolken, 1995). Each trial consisted of 30 s of no scene movement, 60 s of optic flow, and 30 s of post-flow no scene movement. Thus, subjects were exposed to the flow conditions in the middle 60 s of the 120 s in each trial. The presentation order of the 6 trials for each subject was randomized. Center of Pressure (COP) data were computed from the force transducers in the support surface and recorded for each trial. Only the 60 s of COP data during the optic flow exposure were analyzed.
Data Processing and Statistical Analyses
The sway of the subjects was quantified by processing the COP data in two ways. First, the root-mean-square (RMS) of the COP time series for each trial was calculated to obtain an overall measure of movement response to the visual condition. Second, a power analysis of the COP was performed to investigate the amount of movement obtained in response to a particular frequency of the scene movement. COP power within the frequency band of the scene movement was calculated via power spectrum techniques. Power within the frequency band from 0.05-0.15 Hz was computed for 0.10 Hz scene movements. For the 0.3 Hz scene movement, COP power was calculated within the 0.25-0.35 Hz frequency band. This technique measures sway in response to the scene movement (entrainment) and not generalized destabilization (Redfern & Furman, 1994; Loughlin & Redfern, 1996).
Statistical analyses were performed using ANOVA with a split-plot design. Subject was considered a random effect within each subject group. Subjects with anxiety (PAG and NPA) were grouped together and compared to healthy controls. Subsequent analyses were performed to compare the PAG and NPA patients. An analysis of sway responses comparing height phobia versus no height phobia in the NPA subjects was also performed. ANOVA’s were performed for each floor condition (fixed and sway referenced) separately. Data were log transformed to normalize the variance prior to analysis when necessary.
The relationship between SMD and sway responses to optic flow in patients was evaluated using ANOVA with the three groups being Controls, SMD-Abnormal, and SMD-Normal. Post hoc pair-wise comparisons were made to look at differences among the groups.
Results
Anxiety Patients versus Healthy Controls
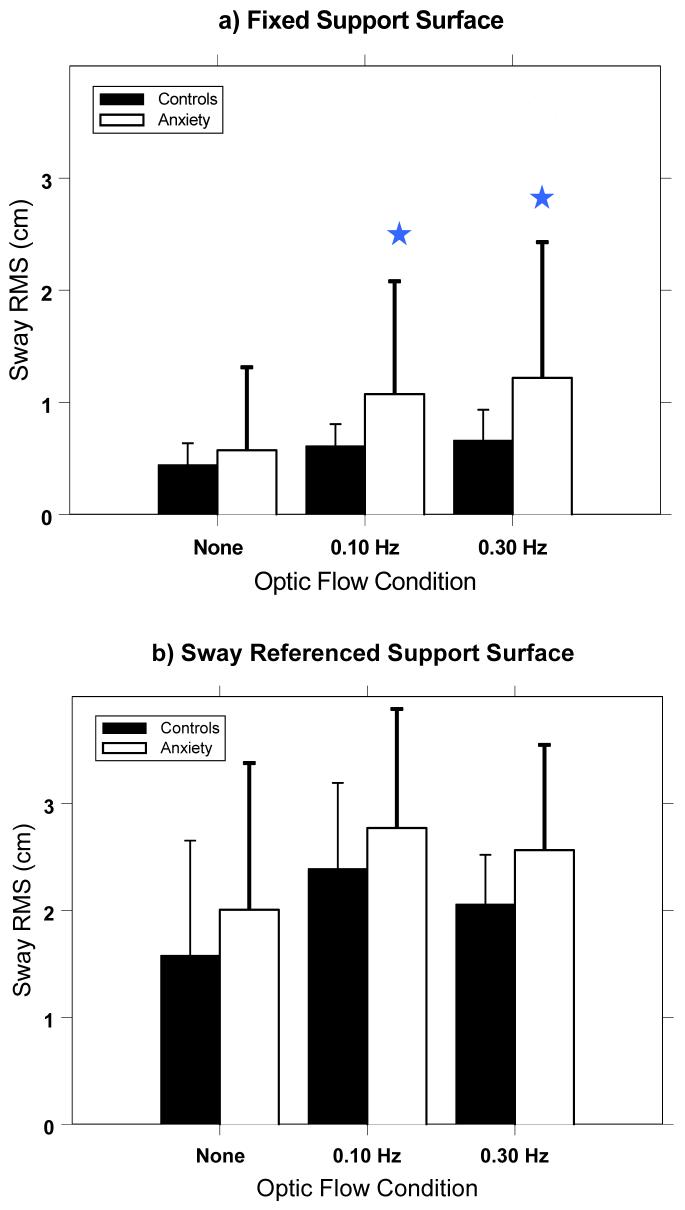
RMS sway was significantly different between the anxiety patients and healthy controls for the fixed platform condition, but not in the sway referenced condition. (Figure 1) For the fixed platform condition, significant frequency of optic flow (FREQ) (p<.001; F(2,156)=64.4), Group (p=.02; F(1,78)=5.57), and FREQ*Group (p=.01; F(2,156)=4.42) effects were found. Post hoc pair-wise t-test comparisons showed there were no differences between the groups for the no-flow condition; however, the patient group swayed significantly more than the controls in response to moving scenes at both the 0.10 Hz (p=.01) and the 0.30 Hz (p<.01) frequencies. A separate analysis of the fixed platform condition comparing the two patient populations (NPA, versus PAG) showed no significant difference in sway response for any stimulus frequency (F(1,57)=.061; p>.80).
Figure 1.
RMS sway for the Control subjects and Anxiety patients for each stimulus condition during: a) fixed platform and b) sway referenced platform conditions. (Error bars are one standard deviation across subjects.) Stars represent a significant difference (p<.05) between the patient group and the control group. Note that Anxiety patients sway significantly more than Controls during optic flow for the fixed platform, but not for the sway referenced platform.
For the sway referenced condition, there was a significant FREQ effect (p<.001; F(2,114)=30.8), but no significant Group (F(1,57)=1.76; p=.19) nor Group*FREQ (F(2,114)=2.70; p=.08) effects. Post hoc t-test comparisons confirmed that there were no significant group effects within any of the three flow conditions during sway referenced conditions. Thus, on fixed platform, anxiety patients swayed more in response to optic flow compared to healthy controls; and, there were no differences in sway response between the two patient groups (NAP versus PAG).
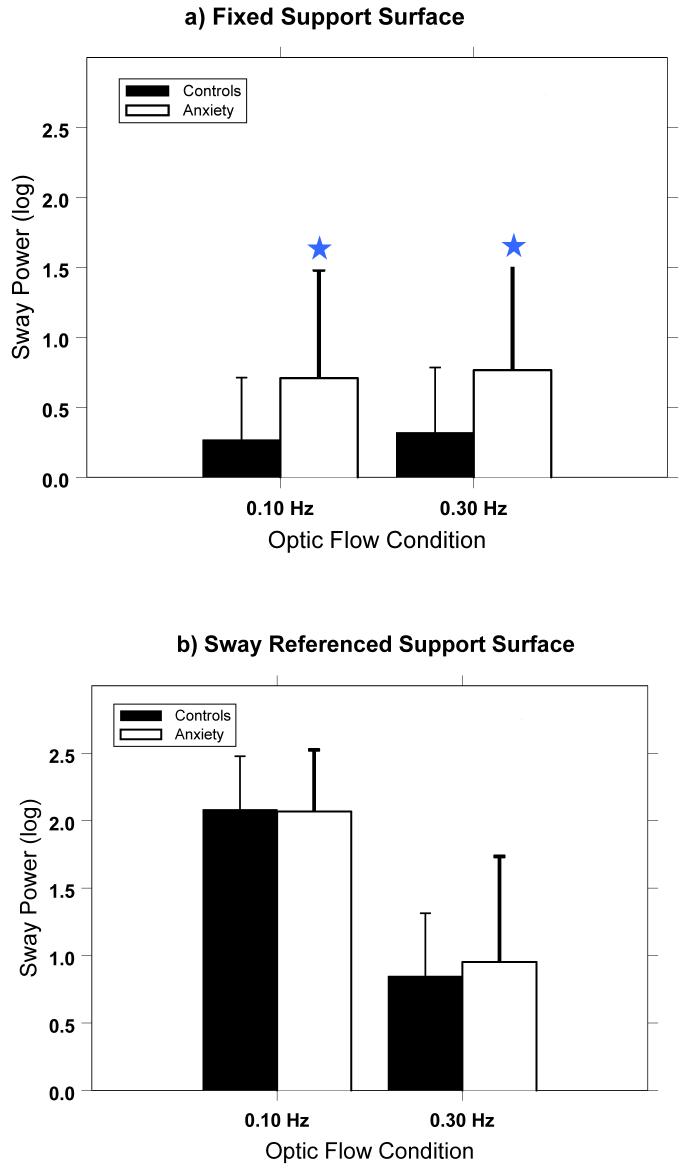
Analyses of the sway power in response to optic flow conditions confirmed the RMS analysis. During the fixed platform condition, patients swayed more than controls in response to optic flow stimuli at .10 Hz (F(1,78)=6.0; p=.02) and .30 Hz (F(1,78)=6.1; p=.01). (Figure 2) This was not true for the sway referenced condition, where anxiety patients did not respond differently than the controls for the stimuli at 0.1 Hz (F(1,78)=0.02; p=.89) or at 0.3 Hz (F(1,78)=0.35; p=.55). There were no differences between the sway power responses of the two patient groups (NPA vs PAG) for either optic flow stimulus in the fixed or sway referenced platform conditions.
Figure 2.
Sway power within the frequency band in response to the optic flow stimulus at that frequency. Note significant differences between the control subjects and patients during the fixed platform condition (denoted by the stars), but not for the sway referenced platform condition.
Sway responses did not significantly vary between anxiety patients with height phobia and patients without height phobia. ANOVA for the fixed and the sway referenced platform conditions did not reveal any significant effect of height phobia. An analysis of the NPA group only, where about half of the subjects (19 out of 38) were height phobic, also did not reveal any differences in sway response between the height phobic patients and non-height patients. Thus, height phobia was not found to be related to differences in postural response to optic flow.
SMD Group Analyses
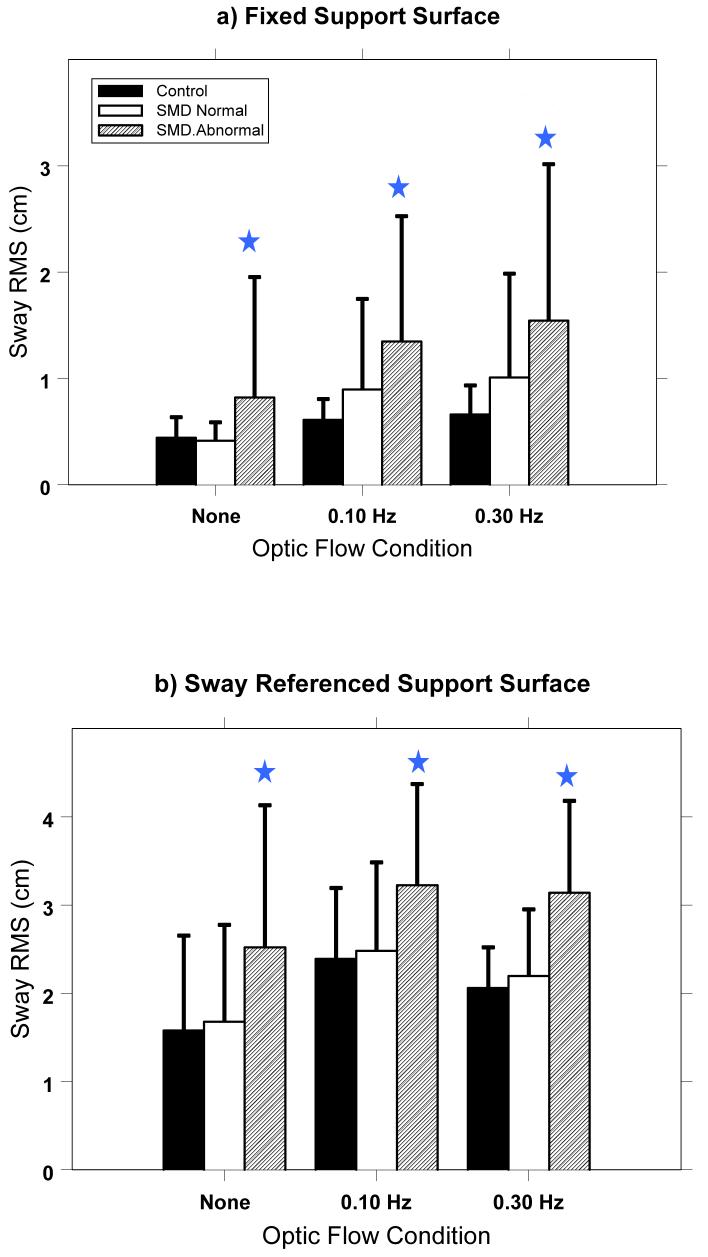
RMS sway was found to be significantly different among the three groups and post-hoc comparisons showed a significant difference between the two SMD categorized patient groups for fixed and sway referenced platform conditions. (Figure 3) For the fixed platform condition, significant FREQ (p<.001; F(2,153)=90.5) and Group (p=.002; F(2,78)=7.09) effects and a non-significant FREQ*Group (p=.06; F(4,153)=2.35) effect were found. Post hoc comparisons showed significant differences between the SMD-Abnormal and both the SMD-Normal and Control groups for each stimulus condition (p≤.001 in all comparisons). Note that this effect includes the NONE stimulus condition, which is quiet sway with eyes open and a fixed visual environment. Thus, patients with abnormal SMD have increased quiet sway responses as well as increased responses to optic flow. There were no significant differences between the SMD-Normal and Control groups (p>.10 in all comparisons).
Figure 3.
RMS sway for the Control group, Patients with Normal SMD, and Patients with Abnormal SMD for each stimulus condition during a) fixed platform and b) sway referenced platform conditions. (Error bars are one standard deviation across subjects.) Stars represent a significant difference (p<.05) between the Abnormal SMD patient groups and Control group. Note that patients with Abnormal SMD sway more than Controls and more than patients with Normal SMD under all conditions.
Results for the sway referenced surface condition were similar to those for the fixed platform condition, with increased RMS sway found in the SMD-Abnormal patients. ANOVA results showed a significant FREQ (p<.001; F(2,156)=56.9) and Group (p=.002; F(2,78)=6.7) effect, but no significant Group*FREQ effect (p=.71; F(4,156)=.53). Post hoc pairwise t-test comparisons showed significant differences between the SMD-Abnormal and both the SMD-Normal and Control groups for each stimulus condition (p≤.02 in all comparisons). There were no significant differences between the SMD-Normal and Control groups (p>.78 in all comparisons). As in the fixed platform condition, sway was greater in the SMD-Abnormal subjects in the quiet sway (none) condition as well as in the optic flow conditions.
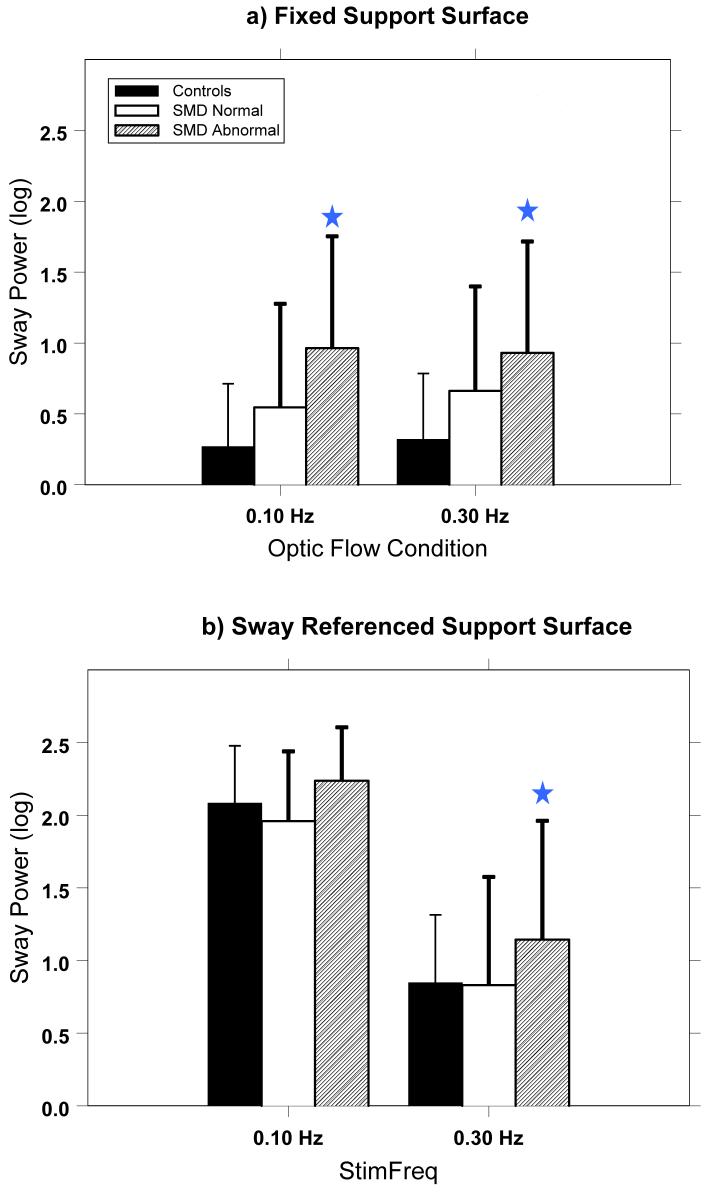
Analyses of the sway power in response to optic flow condition for the different SMD groups generally confirmed the RMS analysis. (Figure 4) During the fixed platform condition, there was a significant SMD group effect in response to optic flow stimuli at 0.10 Hz (F(2,78)=5.8; p<.01) and in response to flow at 0.30 Hz (F(2,78)=4.42; p=.02). Post hoc pair-wise t-test comparisons showed significant differences for the 0.1 Hz stimulus between the SMD-Abnormal patients and Controls (p=.001) and between the SMD-Abnormal and SMD-Normal patients (p=.03). A significant difference between the SMD-Abnormal patients and Controls for the 0.3 Hz stimulus was also found (p=.002). For the sway referenced support surface condition, there was a significant SMD group effect in response to optic flow stimuli at .10 Hz (F(2,78)=3.0; p=.05), and at .30 Hz (F(2,78)=4.05; p=.02). Post hoc analyses did not show significant differences between the SMD-Abnormal patients and Controls for the 0.1 Hz (p=.22), but did show a significant difference between the SMD-Abnormal patients and the SMD-Normal patients (p=.03). Sway power for the 0.3 Hz condition was significantly higher for the SMD-Abnormal patients compared with the Controls (p=.008) and for the SMD-Abnormal patients compared to the SMD-Normal patients (p=.03).
Figure 4.
Sway power for the SMD groups within the frequency band in response to the optic flow stimulus at that frequency. Significant differences (p<.05) between the SMD Abnormal patients and the Controls within optic flow conditions are noted by stars.
4. Discussion
We found that anxiety patients responded differently than did control subjects when exposed to visual sensory conflict. When the platform was fixed, anxiety patients had increased sway responses to optic flow compared to healthy controls. Specifically, their baseline sway was normal; however, when optic flow was introduced, anxiety patients swayed more than did the control subjects. The increase in sway was not the result of a general destabilization; it was an ‘entrainment’ effect in which subjects swayed synchronously with the optic flow stimulus. It appears that the anxiety patients were less capable of ignoring (downweighting) the misleading visual information, i.e. they were more visually dependent. Alternatively, they may have been “attracted” to the optic flow stimuli as an effect of increased vigilance. However, increased sway was not observed in the sway referenced floor condition, when both vision and proprioception provided misleading information. The reason for the lack of an effect in the sway referenced condition may be related to a ‘dosing’ effect combined with a ceiling effect; given enough misleading spatial information even healthy controls show significant increases in sway.
Among specific anxiety symptoms previously identified as potential predictors of visual dependence, (i.e. SMD, Panic, and Height phobia) only SMD proved to be associated with imbalance. The increased sway in patients with abnormally high SMD was observed as a main effect across all conditions, both with and without sway-referencing. This SMD result is consistent with a companion study of the same patients on a standard clinical Equitest™ posturography protocol (Jacob, et al., 2006), where abnormal SMD was found to be associated with abnormal performance on Condition III, which consists of a sway referenced visual scene with a fixed platform surface and Condition IV, which consists of a fixed visual scene and a sway referenced support surface but not Conditions I and II (no sway referencing). Thus, both in the present study and the companion study abnormal sensory environments increased postural sway, presumably as a result of an inability to downweight the visual or somatosensory channels and/or upweight the vestibular channel. In light of these findings, our results with optic flow suggest that patients with SMD have deficiencies with the sensory integration process. We hypothesize that these patients have an inability to properly upweight somatosensation and/or vestibular information during inappropriate visual stimuli (i.e. optic flow) and downweight somatosensation during inappropriate support surface stimuli (i.e. sway referencing). We also found that patients with abnormal SMD had excessive sway when standing on a fixed platform with a stationary visual scene (see Figure 3a). The finding of differences in sway even in the absence of optic flow was unexpected. This finding may suggest a generalized balance abnormality in patients with excessive SMD rather than a problem limited to sensory integration in situations involving \sensory conflict.
There are potential limitations in the study. Inherent in any study that did not recruit randomly from the community is that of recruitment bias. For example, the proportion of female participants seems higher than population norms. This may suggest that the participants were not quite representative of anxiety patients in the community. However, the female predominance may be a consequence of the exclusion criterion of history of alcohol or drug abuse, which is more common in males. Another limitation applies to the negative finding concerning height phobia. Height phobia was only a comorbid condition and not the patient’s primary diagnosis. It is possible that the results would have been different in patients who have height phobia as their primary complaint. Further research on such individuals is needed.
Recent basic science studies have suggested a possible neuroanatomical / neurophysiological basis for the results found in the current study. Relationships between the vestibular system and circuitry involved in anxiety and panic disorders include: (1) connections between the locus coeruleus and the lateral vestibular nucleus (Scheurger and Balaban, 1993; (2) vestibular inputs to the raphae nuclei (Yates, Goto et al., 1993), (3) serotonergic effects on vestibular processes (Licata et al, 1993), and (4) vestibular-respiratory connections (Yates, Jakus, et al, 1993; Miller et al., 1995). Also, the nucleus parabrachialis receives vestibular and visceral input and is connected with the limbic system, including the central nucleus of the amygdala (Balaban, 1996). The amygdala and other parts of the limbic system are essential for the conditioning of fear responses (LeDoux, 1994). Of particular interest is the potential for these various connections to interact specifically with the lateral vestibular nuclei, which are known to have strong projections to the vestibulospinal system. Clearly, further work is required to further explore this potential mechanism for links between the vestibular and neural circuits involved in anxiety.
Finally, an interesting finding was that patients with normal SMD were not significantly different from control subjects in their optic flow responses. Under all conditions, it appears that SMD categorization captures those anxiety patients who are sensitive to optic flow. Thus, there may be a subgroup of anxious patients that has postural control abnormalities that are identifiable through assessing SMD. This subgroup of anxious patients with excessive SMD may be amenable to novel treatment not commonly considered for anxious patients, i.e. vestibular rehabilitation, perhaps in combination with cognitive behavioral therapy.
Acknowledgements
This work was supported by NIH grants P01-DC03417
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balaban CD. Vestibular nucleus projections to the parabrachial nucleus in rabbits: implications for vestibular influences on the autonomic nervous system. Experimental Brain Research. 1996;108(3):367–81. doi: 10.1007/BF00227260. [DOI] [PubMed] [Google Scholar]
- Brandt T, Arnold F, Bles W, Kapteyn TS. The mechanism of physiological height vertigo: 1. Theoretical approach and psychophysics. Acta Otolaryngologica. 1980;89:513–523. doi: 10.3109/00016488009127169. [DOI] [PubMed] [Google Scholar]
- Brown TA, DiNardo PABHH. Anxiety Disorders Interview Schedule for DSM-IV, Adult version. Graywind Publications; Oxford: 1994. [Google Scholar]
- Brown TA, DiNardo PA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV. Adult version. Client Interview Schedule. Graywind Publications Inc/The Psychological Corporation; HarcourtBrace & Company; San Antonio, TX: 1994. [Google Scholar]
- Clark DB, Hirsch BE, Smith MG, Furman JMR, Jacob RG. Panic in otolaryngology patients presenting with dizziness or hearing loss. American Journal of Psychiatry. 1994;151:1223–1225. doi: 10.1176/ajp.151.8.1223. [DOI] [PubMed] [Google Scholar]
- Clark MR, Sullivan MD, Fischl M, Katon WJ, Russo JE, Dobie RA, Voorhees R. Symptoms as a Clue to Otologic and Psychiatric-Diagnosis in Patients With Dizziness. Journal of Psychosomatic Research. 1994;38(5):461–470. doi: 10.1016/0022-3999(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Clark MR, Sullivan MD, Katon WJ, Russo JE, Fischl M, Dobie RA, Voorhees R. Psychiatric and Medical Factors Associated With Disability in Patients With Dizziness. Psychosomatics. 1993;34(5):409–415. doi: 10.1016/S0033-3182(93)71844-7. [DOI] [PubMed] [Google Scholar]
- Eagger S, Luxon LM, Davies RA, Coelho A, Ron MA. Psychiatric morbidity in patients with peripheral vestibular disorder: a clinical and neuro-otological study. Journal of Neurology, Neurosurgery and Psychiatry. 1992;55:383–387. doi: 10.1136/jnnp.55.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. Journal of Anxiety Disorders. 2001;15(12):9–26. doi: 10.1016/s0887-6185(00)00040-2. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Oleary DP, Munjack DJ. Autorotation Test Abnormalities of the Horizontal and Vertical Vestibuloocular Reflexes in Panic Disorder. Otolaryngology-Head and Neck Surgery. 1994;110(3):259–269. doi: 10.1177/019459989411000302. [DOI] [PubMed] [Google Scholar]
- Jacob RG, Furman JMR, Durrant JD, Turner SM. Panic, agoraphobia and vestibular dysfunction. American Journal of Psychiatry. 1996;153:503–512. doi: 10.1176/ajp.153.4.503. [DOI] [PubMed] [Google Scholar]
- Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosomatic Medicine. 1997;59(3):323–330. doi: 10.1097/00006842-199705000-00016. [DOI] [PubMed] [Google Scholar]
- Jacob RG, Lilienfeld SO, Furman JMR, Durrant JD, Turner SM. Panic disorder with vestibular dysfunction: further clinical observations and description of space and motion phobic stimuli. Journal of Anxiety Disorders. 1989;3:117–130. [Google Scholar]
- Jacob RG, Moller MB, Turner SM, Wall CW., III Otoneurological examination of panic disorder and agoraphobia with panic attacks: a pilot study. American Journal of Psychiatry. 1985;142:715–720. doi: 10.1176/ajp.142.6.715. [DOI] [PubMed] [Google Scholar]
- Jacob RG, Redfern MS, Furman JM. Optic flow-induced sway in anxiety disorders associated with space and motion discomfort. Journal of Anxiety Disorders. 1995;9:411–425. [Google Scholar]
- Jacob RG, Woody SR, Clark DB, Lilienfeld SO, Hirsch BE, Kucera GD, Furman JM, Durrant JD. Discomfort with space and motion: a possible marker of vestibular dysfunction assessed by the Situational Characteristics Questionnaire. Journal of Psychopathology and Behavioral Assessment. 1993;15(4):299–324. [Google Scholar]
- Jacob RG, Redfern MS, Furman JM. Space and Motion Discomfort and abnormal balance in patients with anxiety disorders. Journal of Neurology, Neurosurgery & Psychiatry. 2006 doi: 10.1136/jnnp.2007.136432. Submitted.
- LeDoux JE. Emotion, memory and the brain. Scientific American. 1994;270(6):50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- Licata F, Li Volsi G, Maugeri G, Ciranna L, Santangelo F. Serotonin-evoked modifications of the neuronal firing rate in the superior vestibular nucleus: a microiontophoretic study in the rat. Neuroscience. 1993;52(4):941–949. doi: 10.1016/0306-4522(93)90541-m. [DOI] [PubMed] [Google Scholar]
- Loughlin PJ, Redfern MS, Furman JM. Time-varying characteristics of visually induced postural sway. IEEE Transaction on Rehabilitation Engineering. 1996;4(4):416–424. doi: 10.1109/86.547944. [DOI] [PubMed] [Google Scholar]
- Marks I. Space “phobia”: A pseudo-agoraphobic syndrome. Journal of Neurological and Neurosurgical Psychiatry. 1981;44:387–391. doi: 10.1136/jnnp.44.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Yamaguchi T, Siniaia MS, Yates BJ. Ventral respiratory group bulbospinal inspiratory neurons participate in vestibular-respiratory reflexes. Journal of Neurophysiology. 1995;73(3):1303–1307. doi: 10.1152/jn.1995.73.3.1303. [DOI] [PubMed] [Google Scholar]
- Perna G, Dario A, Caldirola D, Stefania B, Cesarani A, Bellodi L. Panic disorder: the role of the balance system. Journal of Psychiatric Research. 2001;35(5):279–286. doi: 10.1016/s0022-3956(01)00031-0. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Experimental Brain Research. 1995;105(1):101–110. doi: 10.1007/BF00242186. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Furman JM. Postural sway of patients with vestibular disorders during optic flow. Journal of Vestibular Research. 1994;4:221–230. [PubMed] [Google Scholar]
- Rudge R, Chambers BR. Physiological basis for enduring vestibular symptoms. Journal of Neurology Neurosurgery & Psychiatry. 1982;45(2):126–130. doi: 10.1136/jnnp.45.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerger RJ, Balaban CD. Immunohistochemical demonstration of regionally selective projections from locus coeruleus to the vestibular nuclei in rats. Experimental Brain Research. 1993;92(3):351–359. doi: 10.1007/BF00229022. [DOI] [PubMed] [Google Scholar]
- Sklare DA, Stein MB, Pikus AM, Uhde TW. Dysequilibrium and audiovestibular function in panic disorder: symptom profiles and test findings. American Journal of Otology. 1990;11(5):338–341. [PubMed] [Google Scholar]
- Stein MB, Asmundson GJG, Ireland D, Walker JR. Panic Disorder in Patients Attending a Clinic for Vestibular Disorders. American Journal of Psychiatry. 1994;151(11):1697–1700. doi: 10.1176/ajp.151.11.1697. [DOI] [PubMed] [Google Scholar]
- Sullivan MD. Psychosomatic clinic or pain clinic. Which is more viable? General Hospital Psychiatry. 1993;15(6):375–380. doi: 10.1016/0163-8343(93)90005-9. [DOI] [PubMed] [Google Scholar]
- Viaud-Delmon I, Berthoz A, Jouvent R. Multisensory integration for spatial orientation in trait anxiety subjects: absence of visual dependence. European Psychiatry. 2002;17(4):194–199. doi: 10.1016/s0924-9338(02)00667-3. [DOI] [PubMed] [Google Scholar]
- Viaud-Delmon I, Ivanenko YP, Berthoz A, Jouvent R. Adaptation as a sensorial profile in trait anxiety: a study with virtual reality. Journal of Anxiety Disorders. 2000;4(6):583–601. doi: 10.1016/s0887-6185(00)00052-9. [DOI] [PubMed] [Google Scholar]
- Viaud-Delmon I, Siegler I, Israel I, Jouvent R, Berthoz A. Eye deviation during rotation in darkness in trait anxiety: an early expression of perceptual avoidance? Biological Psychiatry. 2000;47(2):112–118. doi: 10.1016/s0006-3223(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Yardley L, Britton J, Lear S, Bird J, Luxon LM. Relationship between balance system function and agoraphobic avoidance. Behaviour Research and Therapy. 1995;33:435–439. doi: 10.1016/0005-7967(94)00060-w. [DOI] [PubMed] [Google Scholar]
- Yardley L, Luxon L, Lear S, Britton J, Bird J. Vestibular and posturographic test results in people with symptoms of panic and agoraphobia. Journal of Audiological Medicine. 1994;3:48–65. [Google Scholar]
- Yates BJ, Goto T, Bolton PS. Responses of neurons in the rostral ventrolateral medulla of the cat to natural vestibular stimulation. Brain Research. 1993;601(12):255–64. doi: 10.1016/0006-8993(93)91718-8. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Goto T, Kerman I, Bolton PS. Responses of caudal medullary raphe neurons to natural vestibular stimulation. Journal of Neurophysiology. 1993;70(3):938–946. doi: 10.1152/jn.1993.70.3.938. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Jakus J, Miller AD. Vestibular effects on respiratory outflow in the decerebrate cat. Brain Research. 1993;629(2):209–17. doi: 10.1016/0006-8993(93)91322-j. [DOI] [PubMed] [Google Scholar]