Abstract
The bacteriophage λ P and Escherichia coli DnaC proteins are known to recruit the bacterial DnaB replicative helicase to initiator complexes assembled at the phage and bacterial origins, respectively. These specialized nucleoprotein assemblies facilitate the transfer of one or more molecules of DnaB helicase onto the chromosome; the transferred DnaB, in turn, promotes establishment of a processive replication fork apparatus. To learn more about the mechanism of the DnaB transfer reaction, we investigated the interaction of replication initiation proteins with single-stranded DNA (ssDNA). These studies indicate that both P and DnaC contain a cryptic ssDNA-binding activity that is mobilized when each forms a complex with the DnaB helicase. Concomitantly, the capacity of DnaB to bind to ssDNA, as judged by UV-crosslinking analysis, is suppressed upon formation of a P·DnaB or a DnaB·DnaC complex. This novel switch in ssDNA-binding activity evoked by complex formation suggests that interactions of P or DnaC with ssDNA may precede the transfer of DnaB onto DNA during initiation of DNA replication. Further, we find that the λ O replication initiator enhances interaction of the P·DnaB complex with ssDNA. Partial disassembly of a ssDNA:O·P·DnaB complex by the DnaK/DnaJ/GrpE molecular chaperone system results in the transfer in cis of DnaB to the ssDNA template. On the basis of these findings, we present a general model for the transfer of DnaB onto ssDNA or onto chromosomal origins by replication initiation proteins.
Keywords: phage λ DNA replication, E. coli DNA replication, regulation of DNA helicase action
Biochemical studies of the initiation of Escherichia coli and phage λ chromosomal DNA replication in reconstituted multienzyme systems have illuminated the molecular events that bring about these complex biosynthetic reactions (see refs. 1–3 for recent reviews). Both initiation pathways involve the regulated assembly of large nucleoprotein structures at the replication origin that facilitate the transfer of the bacterial DnaB helicase (4) onto the chromosome (1, 5–12). The transfer of DnaB onto the DNA to initiate DNA unwinding is a key step in the overall replication process because it inaugurates the unregulated fork propagation phase of the reaction (4, 7, 10, 13–15).
The molecular mechanisms responsible for the transfer of DnaB helicase onto duplex DNA from preinitiation nucleoprotein structures at chromosomal origins remain ill defined. In the case of λ DNA replication, it is known that a nucleoprotein complex at oriλ that contains the phage O initiator protein, λ P replication protein, and DnaB helicase is the progenitor complex required for DNA unwinding (7, 10). This oriλ:O·P·DnaB preinitiation complex is assembled when multiple copies of λ P form a tight complex with the hexameric DnaB helicase (16) and the helicase is recruited to the viral origin through interactions of the P·DnaB complex with the O-some (7, 11, 12). This second-stage nucleoprotein structure is seemingly unreactive until it is partially disassembled by the action of the E. coli DnaJ, DnaK, and GrpE molecular chaperone system (8–10, 17). This disassembly reaction stimulates DnaB helicase action by freeing DnaB from its strong association with the P protein, an interaction which is known to suppress the ATPase and helicase activities of DnaB (16, 18). The DnaB helicase is believed to be loaded onto the DNA at the 40-bp A+T-rich region of oriλ, since this DNA segment acquires single-stranded character under the influence of negative DNA supercoiling and O-mediated bending of the four origin iterons (10, 11, 19, 20).
The initiation pathway at oriC, the E. coli chromosomal origin, shares many features with the λ reaction. The initial step involves the binding of multiple copies of the bacterial DnaA initiator protein to oriC to form a preinitiation complex, which in the presence of ATP and negative DNA supercoiling, destabilizes an A+T-rich element near the left boundary of oriC (21–25). Next, DnaC, the bacterial analogue of λ P, forms a DnaB6·DnaC6 complex with the bacterial DnaB helicase (26, 27), which in turn binds to the oriC:DnaA nucleoprotein structure to form a second-stage preinitiation complex. Transfer of DnaB onto DNA at oriC, presumably at the A+T-rich element, ensues and bidirectional DNA unwinding is initiated (13). The transfer step is believed to require ATP hydrolysis by DnaC (28) and is apparently accompanied by the release of DnaC (6).
We sought to learn more about the mechanisms involved in the transfer of DnaB helicase from nucleoprotein structures onto DNA. Our examination of the single-stranded DNA (ssDNA)-binding properties of both λ and E. coli replication initiation proteins indicates that the transfer of DnaB onto ssDNA is facilitated by a cryptic ssDNA-binding activity present in λ P and also in E. coli DnaC. Our findings suggest a general scheme for the transfer of DnaB helicase onto DNA by replication initiation proteins.
MATERIALS AND METHODS
Reagents.
Sources of reagents were as follows: adenosine 5′-[γ-thio]triphosphate (ATP[γS]) and 5′-adenylyl imidodiphosphate (AMP-PNP), Boehringer Mannheim; [γ-32P]ATP (6000 Ci/mmol; 1 Ci = 37 GBq), Amersham; 14C-labeled protein molecular weight standards, Life Technologies; poly(dI-dC)·poly(dI-dC), Pharmacia Biotech; and T4 polynucleotide kinase, New England Biolabs. Synthetic oligonucleotides were synthesized in this department.
Enzymes.
All proteins were >95% homogeneous. The λ O (29) and P (16) proteins and the E. coli DnaJ (30) and DnaK (30) proteins were purified as described previously. The E. coli DnaB and GrpE proteins were purified from overproducing strains by modifications (B.A.L. and R.M, unpublished results; A. Mehl and R.M., unpublished results) of previously described protocols (4, 31). As judged by HPLC analysis of acid-denatured DnaB, <2% of the polypeptides in our preparation of purified DnaB contained bound ATP or ADP. Purified E. coli DnaA and DnaC proteins were the generous gift of Kenneth Marians (Memorial Sloan-Kettering Cancer Center, New York). Protein concentrations, with the exception of DnaA and DnaC, were determined spectrophotometrically under native conditions (32).
Oligonucleotides.
The sequence of the synthetic DNA oligonucleotide RM55 is 5′-TGACGAATAATCTTTTCTTTTTTCTTTTGTAATAGTGTCTTTT. RM55 corresponds to 43 nucleotides of the T-rich strand of the oriλ A+T-rich DNA sequence (positions 39165–39123 of the λ sequence). RM55 was end-labeled at its 5′ terminus using [γ-32P]ATP [6000 Ci/mmol] and T4 polynucleotide kinase and purified by polyacrylamide gel electrophoresis under denaturing conditions.
ssDNA-Binding Assays.
Reaction mixtures (20 μl) were assembled on ice and contained the following: 50 mM Tris·HCl, pH 7.6; 50 mM KCl; 10 mM magnesium acetate; 6 mM dithiothreitol; 50 μg/ml bovine serum albumin; 3 nM RM55 ssDNA oligonucleotide (5′ end-labeled with 32P); AMP-PNP, ATP[γS], or ADP, as indicated; and λ O (85 nM, as monomer), λ P (300 nM), DnaB (100 nM, as monomer), DnaA (200 nM) and/or DnaC (300 nM), as indicated. After a 1-hr incubation at 30°C, each reaction mixture was supplemented with competitor DNA, either 2 μg of poly(dI-dC)·poly(dI-dC) or 1 μg of unlabeled oligonucleotide, as indicated. Nucleoprotein complexes were analyzed either by gel-retardation analysis or with a UV DNA-protein crosslinking assay (see below).
Disassembly of nucleoprotein structures was initiated at 30°C, in the presence of excess unlabeled RM55 ssDNA competitor (1 μg), by the addition of ATP, DnaJ (to 60 nM), DnaK (to 1.9 μM), and GrpE (to 400 nM). Immediately following a 10-min (or 60-min) incubation, individual reaction mixtures were irradiated with UV light as described below. In certain experiments, disassembly reactions were modified to contain both AMP-PNP and ATP. In such instances, a mixture of DnaJ, DnaK, and GrpE was first preincubated with AMP-PNP for 40 min at 30°C to hydrolyze the small amount of ATP that contaminates commercial preparations of AMP-PNP. Preformed ssDNA:O·P·DnaB complexes were then mixed with the molecular chaperone/AMP-PNP mixture, so that the final concentrations of ssDNA substrate and individual proteins were as described above, and the final AMP-PNP concentration was 1 mM. Each combined mixture was equilibrated further for 15 min at 30°C, and disassembly of nucleoprotein complexes was initiated by the addition of unlabeled competitor ssDNA and ATP, as described above.
Gel-Retardation Assays.
Nucleoprotein complexes were formed, incubated for 5 min at 30°C in the presence of poly(dI-dC)·poly(dI-dC), and electrophoresed in nondenaturing 5% polyacrylamide gels equilibrated in 25 mM Tris/190 mM glycine/1 mM EDTA/5 mM magnesium acetate, essentially as described (33). Where indicated, polyacrylamide gels and PAGE buffers were supplemented with 10 μM ATP[γS] or ADP. Following electrophoresis, the gels were dried under vacuum and subjected to autoradiography.
UV ssDNA-Protein Crosslinking Assays.
Assembled nucleoprotein complexes were irradiated, using a hand-held UV source (model UVG-11, Ultraviolet Products, San Gabriel, CA), as follows: reaction mixtures were spotted onto plastic Petri dishes positioned in an ice bucket approximately 8 cm from the UV source, and irradiated (0.5 mJ/sec at 254 nm) for 5 min. Control experiments indicated that no net increase in crosslinking of protein to ssDNA occurred after 1 min of irradiation (data not shown). Following irradiation, reaction mixtures were denatured for 5 min at 100°C and electrophoresed in SDS/8% or 10% polyacrylamide gels as described (34). Gels were dried under vacuum and autoradiographed. Quantitation of radioactivity associated with protein–DNA complexes was performed using a Fuji BAS 1000 Phosphor Image Plate Scanner fitted with MacBAS computer software. The apparent molecular weight (Mr) of nucleoprotein complexes was determined from the relative electrophoretic mobility of each complex in SDS/PAGE as compared with the mobilities of 14C-labeled protein molecular weight standards (35). The crosslinked polypeptide-ssDNA complex migrates approximately at the position expected for a polypeptide whose Mr equals the sum of the Mrs of the individual components of the complex (35).
RESULTS
Formation of a ssDNA:O·P·DnaB Complex.
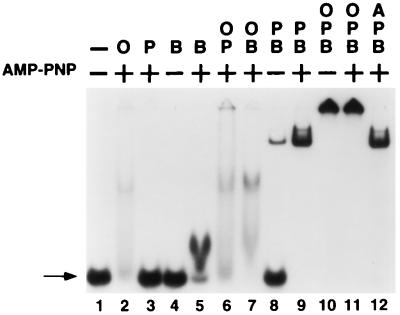
A critical step in the initiation of both E. coli chromosomal DNA replication and coliphage λ DNA replication is the transfer of one or more molecules of the E. coli DnaB helicase onto the genomic DNA at the respective bacterial and viral replication origins. The loading of DnaB at replication origins is facilitated by the action of specific replication initiator proteins—e.g., E. coli DnaA and DnaC for oriC and λ O and P for oriλ. We have taken advantage of the capacity of O and P to load DnaB onto ssDNA in a sequence-independent manner (36, 37) to examine the roles of these initiator proteins in the transfer reaction. Using gel-retardation (band-shift) analysis, we investigated the interactions of O, P, and DnaB, in various combinations, with a short (43-base) ssDNA oligonucleotide derived from the T-rich strand of the oriλ A+T-rich region. Both O and DnaB individually interact with ssDNA (Fig. 1, lanes 2 and 5, respectively), albeit weakly. As previously demonstrated (38, 39), the stable interaction of DnaB with ssDNA requires the continuous presence of ATP or an ATP analogue such as AMP-PNP (see also Fig. 4).
Figure 1.
Formation of a ssDNA:O·P·DnaB nucleoprotein complex as assessed by band-shift analysis. 32P-labeled RM55 oligonucleotide was incubated with λ O (O), λ P (P), and E. coli DnaB (B) and/or DnaA (A) proteins, as indicated, in the presence or absence of AMP-PNP. Assembled nucleoprotein complexes were challenged with poly(dI-dC)·poly(dI-dC) and electrophoresed through a nondenaturing 5% polyacrylamide gel in the presence of 5 mM magnesium acetate. Arrow, free ssDNA. The slowest moving species in lane 9 is believed to represent two P·DnaB complexes bound to one DNA chain.
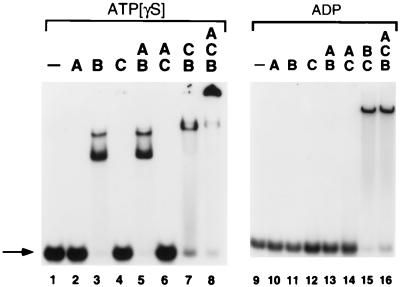
Figure 4.
Formation of ssDNA:DnaA·DnaB·DnaC nucleoprotein complexes. 32P-labeled RM55 oligonucleotide was incubated in the presence of 100 μM ATP[γS] (lanes 1–8) or 100 μM ADP (lanes 9–16) and DnaA (A), DnaB (B), and/or DnaC (C) proteins, as indicated. Assembled nucleoprotein complexes were challenged with poly(dI-dC)·poly(dI-dC) and electrophoresed in a nondenaturing 5% polyacrylamide gel in the presence of 5 mM magnesium acetate and either 10 μM ATP[γS] or 10 μM ADP. Arrow, free ssDNA.
P protein alone does not detectably interact with ssDNA. Unexpectedly, a stable and slowly migrating nucleoprotein structure was formed when a P·DnaB complex was incubated together with the labeled oligonucleotide (Fig. 1, lane 8). This result was surprising, since we had earlier demonstrated that the P·DnaB protein complex does not interact stably with ssDNA, as assessed with a filter binding assay using phage M13 genomic ssDNA (16). The nucleoprotein structure formed on the oligonucleotide in the presence of P and DnaB apparently is stabilized by the presence of AMP-PNP in the binding reaction (lane 9). Unlike free DnaB, however, a stable nucleoprotein complex is detected even when the nucleotide cofactor is absent. The nucleotide independence of the DNA interaction raises the possibility that DnaB polypeptides in the P·DnaB complex do not directly interact with DNA.
When the λ O and P initiators are both present together with DnaB and oligonucleotide, a very stable nucleoprotein complex is formed that does not enter the gel (Fig. 1, lanes 10 and 11). UV-crosslinking analysis of this complex (see below) indicates that it is extremely stable, even in the absence of ATP. We infer that specific protein–protein interactions between O and the P·DnaB complex are responsible for the greatly enhanced stability of this putative ssDNA:O·P·DnaB nucleoprotein structure. The specificity of O in this interaction is bolstered by the finding that the E. coli DnaA initiator fails to interact with the P·DnaB complex on ssDNA (compare lanes 11 and 12 in Fig. 1).
λ P Protein Contains a Cryptic ssDNA-Binding Activity.
To identify which proteins were in intimate contact with ssDNA in the various nucleoprotein complexes that are formed in this study, we made use of a UV-crosslinking approach. Nucleoprotein structures were assembled on a radiolabeled ssDNA oligonucleotide and irradiated with 254-nm light. Only proteins that directly contact DNA become crosslinked to the 32P-labeled oligonucleotide. The identity of the crosslinked proteins can be established from the apparent molecular weights of the polypeptide–ssDNA complexes, as determined from their relative mobilities under denaturing conditions during SDS/PAGE (35).
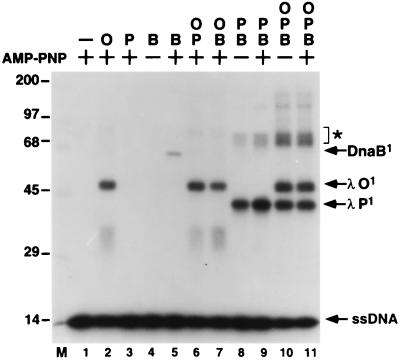
As expected from the results presented in Fig. 1, control experiments indicated that λ O (33.9 kDa) is readily crosslinked to the ssDNA substrate (Fig. 2, lanes 2, 6, and 7) and that DnaB (52.4 kDa) is crosslinked to oligonucleotide in the presence of AMP-PNP, but not in the absence of a nucleotide cofactor (Fig. 2, lanes 4 and 5). The crosslinking analysis of the P·DnaB complex on ssDNA, however, yielded an unanticipated result. The primary covalent polypeptide-ssDNA species obtained migrated in SDS/PAGE with a Mr ≈ 41,000, the size expected for a complex composed of a single molecule each of λ P and of the 43-base oligonucleotide (Fig. 2, lanes 8 and 9). This finding suggests that the λ P replication protein contains a cryptic ssDNA-binding activity that is mobilized as a consequence of its interaction with the DnaB helicase. Moreover, the ATP-independence of the binding of the P·DnaB complex to ssDNA (Figs. 1 and 2) is a strong indicator that DnaB does not directly participate in the DNA-binding event (4, 39). Our inability to detect crosslinking to ssDNA of DnaB when it is present in a ssDNA:P·DnaB complex or in a ssDNA:O·P·DnaB nucleoprotein complex (Fig. 2, lanes 8–11) is also consistent with this conclusion. On the other hand, crosslinks to P and O were readily observed with the latter complex (lanes 10 and 11). We infer, therefore, that interactions of these two λ replication proteins with ssDNA contribute prominently to the stability of the presumptive ssDNA:O·P·DnaB complex.
Figure 2.
UV-crosslinking analysis of ssDNA:O·P·DnaB nucleoprotein complexes. Radiolabeled RM55 oligonucleotide was incubated in the presence of AMP-PNP, λ O (O), λ P (P), and/or E. coli DnaB (B) proteins as indicated. Assembled nucleoprotein complexes were mixed with unlabeled competitor, irradiated with UV light, and resolved by electrophoresis through an SDS/10% polyacrylamide gel. The migration positions of covalent protein-DNA complexes containing 1 molecule of ssDNA and 1 monomer of either O (λ O1), P (λ P1), or DnaB (DnaB1) proteins are indicated. M, 14C-labeled protein molecular weight standards (× 10−3).
For each nucleoprotein complex, single polypeptides of O, P, or DnaB were the primary species crosslinked to the radiolabeled ssDNA by UV irradiation. However, for the presumptive ssDNA:P·DnaB and ssDNA:O·P·DnaB nucleoprotein complexes, significant levels of higher-order crosslinked species were also detected (Fig. 2, lanes 8–11). One such species, labeled with an asterisk, has an electrophoretic mobility consistent with that expected for a complex containing two polypeptides of P crosslinked to the labeled oligonucleotide. Additional experimentation indicated that multiple subunits of P can bind the same oligonucleotide within a single P·DnaB complex, a complex that is apparently composed of six subunits of DnaB and three to six subunits of P (16).
Interaction of DnaB Helicase with ssDNA During Disassembly of the ssDNA:O·P·DnaB Complex by Molecular Chaperones.
Previous studies of λ DNA replication indicated that partial disassembly of preinitiation complexes by the DnaJ/DnaK/GrpE molecular chaperone system is required to complete the transfer of DnaB onto DNA (8–10, 12, 17, 36, 40). Taking these findings together with our present results, we considered it likely that molecular chaperone action will be required to permit DnaB polypeptides present in an oligonucleotide:O·P·DnaB complex to interact stably with the ssDNA. To test this idea, nucleoprotein complexes containing O, P, and DnaB were formed on a 32P-labeled oligonucleotide, challenged with a 1000-fold excess of unlabeled oligonucleotide, and incubated for 10 min in the presence of DnaJ, DnaK, and GrpE and 1 mM ATP prior to UV irradiation. Following the action of the molecular chaperones, neither O, P, nor DnaB was crosslinked to the labeled ssDNA (Fig. 3, lane 4), suggesting that few nucleoprotein complexes survived the chaperone treatment. Control experiments indicated that the ssDNA:O·P·DnaB structures were stable to ssDNA challenge for periods up to several hours in the absence of added chaperones (Fig. 3, lanes 1–3) or ATP (lane 5).
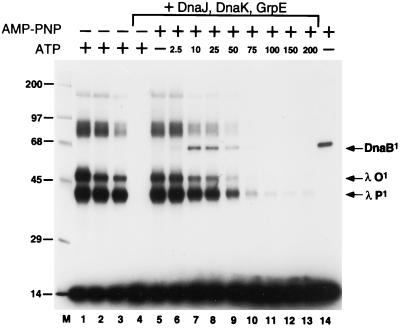
Figure 3.
Disassembly of the ssDNA:O·P·DnaB nucleoprotein complex by molecular chaperones transfers DnaB to ssDNA. Nucleoprotein ssDNA:O·P·DnaB complexes were assembled on 32P-labeled RM55 ssDNA in the presence of 1 mM ATP (lanes 1–4) or 1 mM AMP-PNP (lanes 5–13) as described in the text. Subsequently, reaction mixtures were supplemented with the following: (i) 1 μg of unlabeled RM55 ssDNA (lanes 2–13); (ii) 2 μg of poly(dI-dC)·poly(dI-dC) (lanes 1 and 14); (iii) DnaJ, DnaK, and GrpE proteins (lanes 4–13); and (iv) ATP, at the indicated final concentration in micromolar (lanes 6–13). After an additional incubation for 10 min at 30°C (60 min for the reaction mixture applied to lane 3), each reaction mixture was UV irradiated and subjected to SDS/PAGE as described in the legend of Fig. 2. For the lane 14 sample, purified DnaB alone was incubated in the presence of AMP-PNP with 32P-labeled RM55 ssDNA substrate and crosslinked by UV irradiation. The migration positions of certain crosslinked nucleoprotein complexes and of protein molecular weight standards (× 10−3) are indicated as described for Fig. 2.
The failure to detect the transfer of DnaB onto the oligonucleotide in the presence of the chaperones and ATP was predictable. We anticipated that the interaction of DnaB with an oligonucleotide might prove to be fleeting, because of the capacity of DnaB, when ATP is present, to directly dissociate from the DNA or translocate off the ends of short ssDNA fragments. We surmised that a lower concentration of ATP might slow the movement of DnaB helicase along a ssDNA fragment (4), yet still be adequate to support the chaperone-mediated nucleoprotein disassembly reactions required for the transfer of DnaB from the ssDNA:O·P·DnaB complex. The results of an ATP titration made in the presence of 1 mM AMP-PNP [a nucleotide cofactor that supports DNA binding but not DNA translocation by DnaB (4)] are depicted in Fig. 3 (lanes 5–13). Indeed, a band that comigrates with the oligonucleotide-DnaB standard (lane 14) is observed (lanes 7 and 8) when the nucleoprotein disassembly reaction is carried out in the presence of 10–25 μM ATP. This presumptive DnaB:ssDNA species is not detected at ATP concentrations above 100 μM, perhaps because the DnaB helicase molecules under these conditions no longer remain bound to λ P protein and, thus, are capable of rapid translocation or dissociation off the ssDNA oligonucleotide. We conclude that the “transfer” of DnaB to the ssDNA occurs in cis from an ssDNA:O·P·DnaB complex, since the transfer reaction to labeled ssDNA is ≈80% efficient (relative to DnaB alone, lane 14) despite the presence of a 1000-fold excess of unlabeled specific competitor DNA. Furthermore, we also conclude from the data presented in Fig. 3 that ATP concentrations that yield maximal levels of crosslinked DnaB support substantial disassembly of O and P from the starting ssDNA:O·P·DnaB complex, as judged by the significant reduction in those DNA species that contain a single crosslinked O or P polypeptide in such reaction mixtures.
DnaC Contains a Cryptic ssDNA-Binding Activity That Is Elicited upon Interaction with DnaB Helicase.
The discovery that the λ P replication protein contains a cryptic ssDNA-binding activity that is revealed when it interacts with a molecule of DnaB helicase caused us to consider the possibility that the functionally homologous DnaC protein of E. coli contains a similar activity. To test this idea, various combinations of DnaC, DnaB, and DnaA were incubated with a labeled RM55 oligonucleotide, and the resulting nucleoprotein complexes were electrophoresed through nondenaturing polyacrylamide gels in the presence of ATP[γS] (Fig. 4, lanes 1–8) or ADP (Fig. 4, lanes 9–16). Of these proteins, when present alone, only DnaB formed a stable nucleoprotein complex with RM55, and then only when ATP[γS] (or AMP-PNP; data not shown) was present in both the binding reaction mixture and the electrophoresis running buffer (Fig. 4, lane 3). The two retarded bands produced by the interaction of DnaB with RM55 apparently represent oligonucleotides containing one and two bound hexamers of DnaB helicase. Consistent with this interpretation, just a single retarded protein-DNA species is formed on a shorter, (dT)20, oligonucleotide over a broad range of DnaB concentrations (1.2–600 nM monomer).
DnaC is capable of interacting with DnaB to form a new nucleoprotein structure on ssDNA (Fig. 4, lane 7). This complex migrates slightly behind the slower of the two DnaB-oligonucleotide species under native conditions, even though this species apparently represents just a single DnaB6·DnaC6 complex (27) bound to the oligonucleotide. Surprisingly, a similar DnaB·DnaC complex is formed on RM55 in the presence of ADP (Fig. 4, lane 15), whereas DnaB alone does not stably interact with this oligonucleotide either in the presence (Fig. 4, lane 11) or in the absence of ADP (data not shown). Since the presence of a rNTP is not required for stable DNA binding of the DnaB·DnaC complex, it is an indication that DnaC, rather than DnaB, may be responsible for the interaction of the complex with ssDNA. Further analysis indicated that DnaA is capable of interacting with the DnaB·DnaC complex to form a highly stable nucleoprotein complex that apparently contains all three E. coli replication proteins (Fig. 4, lane 8). The formation of this presumptive ssDNA:DnaA·DnaB·DnaC complex is strictly dependent upon the presence of ATP[γS]; it is notable that ADP does not serve as an effective nucleotide cofactor for formation of this higher-order nucleoprotein structure (Fig. 4, lane 16).
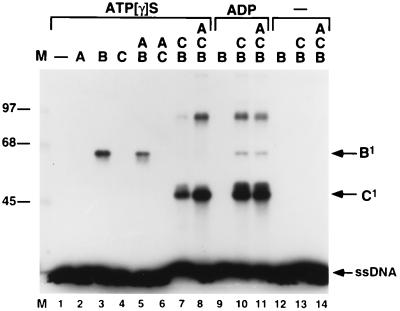
To determine which proteins directly interact with ssDNA in these nucleoprotein structures, we used UV-crosslinking analysis as described earlier for the studies with O, P, and DnaB. Consistent with the results depicted in Fig. 4, DnaB, but neither DnaA nor DnaC, was crosslinked to a labeled oligonucleotide when present alone with ATP[γS] (Fig. 5). Nevertheless, in the DnaB·DnaC nucleoprotein complex, our results indicate that it is DnaC, not DnaB, that is crosslinked to the DNA under these conditions (Fig. 5, lane 7). Complexes of oligonucleotide covalently coupled to one or to two DnaC polypeptides were readily detected. We conclude that DnaC acquires the capacity to interact with ssDNA when it is bound to DnaB. Concomitantly, the DnaB subunits present in the DnaB·DnaC nucleoprotein structure are apparently rendered incapable of interacting with the ssDNA chain, even when ATP[γS] is present. Interestingly, in the presence of ADP, a small fraction of the DnaB subunits in the DnaB·DnaC nucleoprotein structure become crosslinked to ssDNA (Fig. 5, lane 10). Finally, the presence of the DnaA initiator in the nucleoprotein complex had no distinctive effect on the UV-crosslinking pattern of either DnaC or DnaB regardless of the nucleotide cofactor, although in ATP[γS] the efficiency of DnaC crosslinking was reproducibly higher when DnaA was present (Fig. 5). These crosslinking results were obtained with an oligonucleotide derived from oriλ, but identical results were obtained with an oligonucleotide derived from the oriC A+T-rich region. This suggests that the DNA-binding interactions documented here are relatively sequence independent.
Figure 5.
UV-crosslinking analysis of ssDNA:DnaA·DnaB·DnaC and ssDNA:DnaB·DnaC nucleoprotein complexes. Nucleoprotein complexes were assembled on 32P-labeled RM55 ssDNA substrate, in the presence of 100 μM ATP[γS] (lanes 1–8) or 100 μM ADP (lanes 9–11), and DnaA (A), DnaB (B), and/or DnaC (C) as indicated. Following UV irradiation, nucleoprotein complexes were resolved in an SDS/8% polyacrylamide gel. The relative electrophoretic mobilities of covalent protein-DNA complexes containing 1 molecule of ssDNA and 1 monomer of DnaB (B1) or DnaC (C1) proteins are indicated. M, 14C-labeled protein molecular weight standards.
DISCUSSION
Our investigation of the ssDNA-binding properties of the λ P and E. coli DnaC proteins has revealed that each of these replication initiation proteins harbors a cryptic ssDNA-binding activity. For both P and DnaC, this DNA-binding activity is mobilized only when each protein forms a complex with the bacterial DnaB helicase. The discovery of this new DNA-binding activity in the P·DnaB and DnaB·DnaC complexes was initially surprising, since free DnaB itself was known to have an intrinsic ATP-dependent ssDNA-binding activity (4, 38, 41, 42). This DNA-binding capacity of DnaB was generally assumed to be both necessary and sufficient for the loading of DnaB onto DNA at replication origins. UV-crosslinking analysis of P·DnaB or DnaC·DnaB complexes bound to ssDNA fragments, however, identified crosslinks between DNA and P or between DNA and DnaC, respectively, but failed to detect any interaction of DnaB subunits with DNA, whether or not ATP was present. The absence of DnaB crosslinking is consistent with the ATP independence of the binding reaction as judged by band-shift analysis of the P·DnaB and DnaB·DnaC complexes (Fig. 2 and data not shown). Nevertheless, we cannot absolutely exclude the possibility that there are interactions between DnaB and ssDNA that cannot be detected by UV crosslinking.
These results suggest that the same P·DnaB and DnaB·DnaC protein–protein interactions responsible for mobilizing the DNA-binding activity of P or of DnaC also suppress the capacity of DnaB to bind to ssDNA. Because DnaB acts as a highly processive helicase in chromosomal DNA replication (4, 43), and because it has the capacity to drive priming and establishment of the replication fork apparatus once loaded onto duplex DNA, it presumably is critical to cellular viability that there be molecular mechanisms in place that preclude the loading of DnaB nonspecifically onto the chromosome. In vivo, it is likely that cytoplasmic DnaB exists primarily in a complex with DnaC (26, 44), or in a P·DnaB complex following infection by phage λ (16, 18, 45). Our data are consistent with the idea that P and DnaC each serve to modulate the activity of DnaB by restricting its capacity to bind to ssDNA.
Although the P·DnaB and DnaB·DnaC complexes are also capable of binding to ssDNA, this interaction is relatively weak. In fact, the interaction of the P·DnaB complex with long ssDNA chains, such as M13 viral DNA, cannot be detected by using standard filter binding assays (16). Stable interaction of the P·DnaB complex with ssDNA requires the presence of the λ O chromosomal initiator protein. Preliminary experiments indicate that a P·DnaB complex bound to oligonucleotide RM55 has a half-life of only 3 min in the presence or absence of nucleotide cofactor, whereas when the λ O initiator is present, the half-life of the P·DnaB interaction with ssDNA is increased to several hours. Thus, in vivo it is likely that stable interactions of the P·DnaB complex with ssDNA are restricted to oriλ sequences and occur only in the context of initiation of λ DNA replication. In this regard, we have recently discovered, in results to be published elsewhere, that the second-stage oriλ:O·P·DnaB nucleoprotein structure (7, 9), assembled on supercoiled oriλ templates during the initiation of λ DNA replication, has the unique capacity to form a stable pre-open complex that can trap the energy of DNA supercoiling. (L.H., B.A.L., D. S. Sampath, and R.M., unpublished results). It appears probable that interactions with oriλ DNA sequences of one or more of the P polypeptides that are present in this nucleoprotein assembly must play a critical role in the formation of this key replication intermediate.
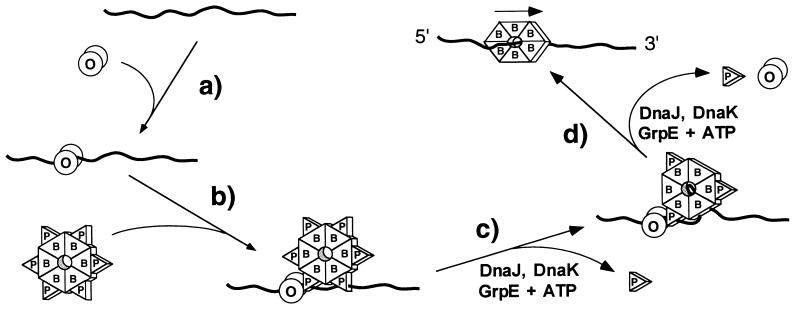
The foregoing results, when taken together with the findings presented in this report, suggest that P and DnaC carry out multiple functions during the initiation of λ and E. coli DNA replication, respectively. In addition to their known role as molecular matchmakers in recruiting DnaB helicase to an origin preinitiation nucleoprotein complex, P and DnaC apparently also actively participate in the transfer of DnaB from the complex onto template DNA. In Fig. 6, we depict one pathway for the O- and P-mediated transfer of DnaB onto ssDNA that is consistent with our results. In step a, O binds weakly and nonspecifically to the DNA fragment. After binding of P to DnaB in solution to form what is presumably a P6·DnaB6 complex (16), the P·DnaB complex interacts with the bound O protein (step b) to form a stable ssDNA:O·P·DnaB complex. The driving force for this assembly reaction is a specific interaction between O and P (7, 46–48). With ssDNA templates, it is possible that the first two steps occur in reverse order; the P·DnaB complex may initially bind weakly to ssDNA and then be stabilized by protein–protein interactions with O. Regardless, it is clear from the UV-crosslinking data that both the O and P polypeptides present in the ssDNA:O·P·DnaB complex interact directly with the ssDNA at this stage. Transfer of DnaB onto the DNA requires the partial disassembly of the nucleoprotein structure by the DnaJ, DnaK, and GrpE molecular chaperone system of E. coli (Fig. 6, steps c and d). We surmise that this reaction is mechanistically similar to the closely related nucleoprotein disassembly reaction required to establish DnaB as an active helicase at oriλ (8, 9, 12).
Figure 6.
Proposed model for the transfer of DnaB onto DNA from a ssDNA:O·P·DnaB preinitiation complex. See text for details. For simplicity, all proteins are depicted as single shapes: λ O (O), λ P (P), and DnaB (B).
Although the precise molecular details of the DnaB transfer reaction remain to be determined, the UV-crosslinking data provide some clues. First, it is apparent (Fig. 2 and unpublished data) that multiple subunits of P (at least two and perhaps as many as three) in each ssDNA:P·DnaB complex and each ssDNA:O·P·DnaB complex interact with a single ssDNA fragment. This being the case, chaperone-mediated partial disassembly of the ssDNA:O·P·DnaB complex in the presence of ATP (Fig. 6, step c) may initially involve stepwise removal of a subset of the P subunits bound to DnaB. This would yield an intermediate in which some of the subunits of the presumptive DnaB hexamer are liberated from the inhibitory action of P protein and are now free to bind ssDNA in an ATP-dependent binding reaction. The ssDNA template would be expected to be present in high local concentration, since other subunits of the same DnaB hexamer would still be indirectly tethered to DNA by their interaction with DNA-bound P subunits. This model may explain why DnaB is apparently transferred in cis to a ssDNA fragment (Fig. 3) even in the presence of a 1000-fold excess of competitor ssDNA. Ultimately, the chaperone-mediated disassembly reaction completes the transfer of a hexamer of free DnaB helicase onto DNA. The mode of binding of ssDNA to DnaB is uncertain, but the high processivity of DnaB action once transferred to DNA may indicate that the ssDNA template passes through the center of the DnaB ring as depicted in Fig. 6 (49, 50).
We envision that the transfer of DnaB onto negatively supercoiled oriC DNA templates by DnaA and DnaC during the initiation of E. coli chromosomal DNA replication shares some of the mechanistic features described in Fig. 6. A DnaB6·DnaC6 complex interacts with an assembly of DnaA molecules bound to oriC to form an oriC:DnaA·DnaB·DnaC complex (51). This interaction requires the presence of ATP (Fig. 4) (52) and is presumably driven by specific protein interactions between DnaA and DnaC, or between DnaA and DnaB (51), or both. Our data indicate that these protein interactions mobilize the ssDNA-binding activity of DnaC and, concomitantly, suppress the capacity of DnaB to interact with DNA. We suggest that interactions of DnaC with unwound or partially unwound DNA in the A+T-rich element of oriC precedes the transfer of DnaB onto the chromosome and its activation as a helicase. We have not yet demonstrated the transfer of DnaB from the ssDNA:DnaA·DnaB·DnaC nucleoprotein structure onto the DNA chain, a reaction that may depend on DnaA being bound to a duplex recognition sequence (52). Previous biochemical studies of the oriC initiation pathway indicate that ATP hydrolysis by DnaC is needed to load DnaB onto DNA from a DnaB·DnaC complex (53). This transfer is seemingly accompanied by the dissociation of DnaC from both DnaB and DNA (6, 28).
Acknowledgments
We thank Dr. Ken Marians for his generous donation of purified DnaA and DnaC proteins. We are grateful to Rick Russell for HPLC analysis of the nucleotide content of DnaB and for advice; we thank the other members of this laboratory for many helpful discussions. This research was supported by Research Grant GM32253 from the National Institutes of Health. We acknowledge Center Grant ES-03819 from the National Institute of Environmental Health Sciences for its support of the oligonucleotide synthesis facility.
Footnotes
Abbreviations: ssDNA, single-stranded DNA; ATP[γS], adenosine 5′-[γ-thio]triphosphate; AMP-PNP, 5′-adenylyl imidodiphosphate.
References
- 1.Kornberg A, Baker T A. DNA Replication. New York: Freeman; 1992. [Google Scholar]
- 2.Marians K J. Annu Rev Biochem. 1992;61:673–719. doi: 10.1146/annurev.bi.61.070192.003325. [DOI] [PubMed] [Google Scholar]
- 3.Messer W, Weigel C. In: Initiation of Chromosome Replication. 2nd Ed. Neidhardt F C, Curtiss R III, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1579–1601. [Google Scholar]
- 4.LeBowitz J H, McMacken R. J Biol Chem. 1986;261:4738–4748. [PubMed] [Google Scholar]
- 5.Sekimizu K, Bramhill D, Kornberg A. J Biol Chem. 1988;263:7124–7130. [PubMed] [Google Scholar]
- 6.Funnell B E, Baker T A, Kornberg A. J Biol Chem. 1987;262:10327–10334. [PubMed] [Google Scholar]
- 7.Alfano C, McMacken R. J Biol Chem. 1989;264:10699–10708. [PubMed] [Google Scholar]
- 8.Alfano C, McMacken R. J Biol Chem. 1989;264:10709–10718. [PubMed] [Google Scholar]
- 9.Dodson M, McMacken R, Echols H. J Biol Chem. 1989;264:10719–10725. [PubMed] [Google Scholar]
- 10.Dodson M, Echols H, Wickner S, Alfano C, Mensa-Wilmot K, Gomes B, LeBowitz J, Roberts J D, McMacken R. Proc Natl Acad Sci USA. 1986;83:7638–7642. doi: 10.1073/pnas.83.20.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodson M, Roberts J, McMacken R, Echols H. Proc Natl Acad Sci USA. 1985;82:4678–4682. doi: 10.1073/pnas.82.14.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zylicz M, Ang D, Liberek K, Georgopoulos C. EMBO J. 1989;8:1601–1608. doi: 10.1002/j.1460-2075.1989.tb03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker T A, Sekimizu K, Funnell B E, Kornberg A. Cell. 1986;45:53–64. doi: 10.1016/0092-8674(86)90537-4. [DOI] [PubMed] [Google Scholar]
- 14.Funnell B E, Baker T A, Kornberg A. J Biol Chem. 1986;261:5616–5624. [PubMed] [Google Scholar]
- 15.Wu C, Zechner E, Reems J, McHenry C, Marians K. J Biol Chem. 1992;267:4074–4083. [PubMed] [Google Scholar]
- 16.Mallory J B, Alfano C, McMacken R. J Biol Chem. 1990;265:13297–13307. [PubMed] [Google Scholar]
- 17.Liberek K, Georgopoulos C, Zylicz M. Proc Natl Acad Sci USA. 1988;85:6632–6636. doi: 10.1073/pnas.85.18.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wickner S H. Cold Spring Harbor Symp Quant Biol. 1979;43:303–310. doi: 10.1101/sqb.1979.043.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Zahn K, Blattner F R. EMBO J. 1985;4:3605–3616. doi: 10.1002/j.1460-2075.1985.tb04124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnos M, Zahn K, Inman R B, Blattner F R. Cell. 1988;52:385–395. doi: 10.1016/s0092-8674(88)80031-x. [DOI] [PubMed] [Google Scholar]
- 21.Fuller R S, Funnell B E, Kornberg A. Cell. 1984;38:889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- 22.Bramhill D, Kornberg A. Cell. 1988;52:743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- 23.Yung B Y-M, Kornberg A. J Biol Chem. 1989;264:6146–6150. [PubMed] [Google Scholar]
- 24.Woelker B, Messer W. Nucleic Acids Res. 1993;21:5025–5033. doi: 10.1093/nar/21.22.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gille H, Messer W. EMBO J. 1991;10:1579–1584. doi: 10.1002/j.1460-2075.1991.tb07678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickner S, Hurwitz J. Proc Natl Acad Sci USA. 1975;72:921–925. doi: 10.1073/pnas.72.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobori J A, Kornberg A. J Biol Chem. 1982;257:13770–13775. [PubMed] [Google Scholar]
- 28.Wahle E, Lasken R S, Kornberg A. J Biol Chem. 1989;264:2469–2475. [PubMed] [Google Scholar]
- 29.Roberts J D, McMacken R. Nucleic Acids Res. 1983;11:7435–7452. [PMC free article] [PubMed] [Google Scholar]
- 30.Karzai A W, McMacken R. J Biol Chem. 1996;271:11236–11246. doi: 10.1074/jbc.271.19.11236. [DOI] [PubMed] [Google Scholar]
- 31.Jordan R, McMacken R. J Biol Chem. 1995;270:4563–4569. doi: 10.1074/jbc.270.9.4563. [DOI] [PubMed] [Google Scholar]
- 32.Gill S C, von Hippel P H. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 33.Fried M, Crothers D M. Nucleic Acids Res. 1981;9:6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Hockensmith J W, Kubasek W L, Vorachek W R, Evertsz E M, von Hippel P H. Methods Enzymol. 1991;208:211–236. doi: 10.1016/0076-6879(91)08015-a. [DOI] [PubMed] [Google Scholar]
- 36.LeBowitz J H, Zylicz M, Georgopoulos C, McMacken R. Proc Natl Acad Sci USA. 1985;82:3988–3992. doi: 10.1073/pnas.82.12.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeBowitz J H, McMacken R. Nucleic Acids Res. 1984;12:3069–3088. doi: 10.1093/nar/12.7.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arai K, Kornberg A. J Biol Chem. 1981;256:5260–5266. [PubMed] [Google Scholar]
- 39.Arai K, Kornberg A. J Biol Chem. 1981;256:5253–5259. [PubMed] [Google Scholar]
- 40.Mensa-Wilmot K, Seaby R, Alfano C, Wold M S, Gomes B, McMacken R. J Biol Chem. 1989;264:2853–2861. [PubMed] [Google Scholar]
- 41.Wickner S, Wright M, Hurwitz J. Proc Natl Acad Sci USA. 1974;71:783–787. doi: 10.1073/pnas.71.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bujalowski W, Jezewska M J. Biochemistry. 1995;34:8513–8519. doi: 10.1021/bi00027a001. [DOI] [PubMed] [Google Scholar]
- 43.Mok M, Marians K J. J Biol Chem. 1987;262:16644–16654. [PubMed] [Google Scholar]
- 44.Allen G C, Jr, Kornberg A. J Biol Chem. 1991;266:22096–22101. [PubMed] [Google Scholar]
- 45.Klein A, Lanka E, Schuster H. Eur J Biochem. 1980;105:1–6. doi: 10.1111/j.1432-1033.1980.tb04467.x. [DOI] [PubMed] [Google Scholar]
- 46.Furth M E, McLeester C, Dove W F. J Mol Biol. 1978;126:195–225. doi: 10.1016/0022-2836(78)90359-5. [DOI] [PubMed] [Google Scholar]
- 47.Zylicz M, Gorska I, Taylor K, Georgopoulos C. Mol Gen Genet. 1984;196:401–406. doi: 10.1007/BF00436186. [DOI] [PubMed] [Google Scholar]
- 48.Wickner S H, Zahn K. J Biol Chem. 1986;261:7537–7543. [PubMed] [Google Scholar]
- 49.San Martin M C, Stamford N P J, Dammerova N, Dixon N E, Carazo J M. J Struct Biol. 1995;114:167–176. doi: 10.1006/jsbi.1995.1016. [DOI] [PubMed] [Google Scholar]
- 50.Yu X, Jezewska M J, Bujalowski W, Egelman E H. J Mol Biol. 1996;259:7–14. doi: 10.1006/jmbi.1996.0297. [DOI] [PubMed] [Google Scholar]
- 51.Marszalek J, Kaguni J M. J Biol Chem. 1994;269:4883–4890. [PubMed] [Google Scholar]
- 52.Masai H, Arai K I. Eur J Biochem. 1995;230:384–395. doi: 10.1111/j.1432-1033.1995.tb20573.x. [DOI] [PubMed] [Google Scholar]
- 53.Wahle E, Lasken R S, Kornberg A. J Biol Chem. 1989;264:2463–2468. [PubMed] [Google Scholar]