Abstract
Novel, double-chained pyridinium compounds have been developed that display highly efficient DNA transfection properties. The transfection efficiency of several of these compounds is enhanced by an order of magnitude, when compared with the transfection efficiency accomplished with the widely used cationic lipid system, lipofectin. Most importantly, the pyridinium compounds were found to be essentially nontoxic toward cells. Using various reporter genes, such as β-galactosidase and pNEO (a gene construct that renders cells resistent to antibiotic derivatives of neomycin like G418), we demonstrate that the enhanced efficiency relates to the fact that a relative higher number of cells in the population is transfected (≈50% in the case of COS cells) by the pyridinium derivatives, whereas the delivery of DNA per cell is also enhanced. Furthermore, application of the pyridinium derivatives shows little cellular preference in their ability to transfect cells. By systematically modifying the structure of the pyridinium amphiphile, i.e., by changing either the headgroup structure or the alkyl chains, some insight was obtained that may lead to unraveling the mechanism of amphiphile-mediated transfection, and thus to protocols that further optimize the carrier properties of the amphiphile. Our results reveal that unsaturated alkyl chains enhance the transfection properties of the pyridinium-based amphiphiles. Preliminary experiments suggest that the structure-dependent improvement of transfection efficiency, when comparing pyridinium derivatives with lipofectin, likely relates to the mechanism of delivery rather than the packaging of the amphiphile/DNA complex.
Keywords: transfection, delivery, COS-7, carrier, synthetic amphiphile
Oligonucleotide and/or gene therapy are promising approaches not only in the treatment of diseases with a genetic defect (e.g., cystic fibrosis), but also in developing therapeutic strategies for diseases such as cancer, infectious diseases (e.g., AIDS, hepatitis), and acquired diseases (e.g., Parkinson disease, rheumatic arthritis). For gene therapy to be effective at least three conditions must be satisfied: (i) a functional gene must be placed in an appropriate vector, (ii) after internalization the gene must be transferred to the nucleus, and (iii) the gene must integrate in the endogenous genome where it has to be translated and transcribed. Under certain conditions, i.e., when applying for example herpes simplex virus or human papova BK virus (BKV) viral vectors, episomal replication can also be envisaged (1). Oligonucleotide-based therapeutic strategies, i.e., antisense or antigene DNA therapy, aimed at modulating the expression of genes having gone awry, require analogous needs to be met, including nucleotide stability and targeting.
So far, no general protocol has been developed for the efficacious treatment of any particular genetic defect (2, 3). One of the major problems shared in common by both approaches involves the delivery per se of the DNA or oligonucleotide into the cell, both in terms of intracellular delivery efficiencies and carrier-related toxicity. The methods used so far rely on either viral systems (reconstituted virus particles, adenoviruses) (4–6) or chemicophysical technology (polymers, electroporation, calcium–phosphate precipitation, and liposomes) (3, 7, 8).
During the last decade, vesicles composed of cationic synthetic amphiphiles have been developed for the delivery of genes and they have become particularly popular tools in vitro, as well as in vivo (9–13). Nucleic acids associate with amphiphiles in a highly efficient manner, and protocols for preparation and delivery are simple. Moreover, the transfection efficiencies are usually better than those obtained by procedures, as mentioned above. However, a major drawback in the application of synthetic amphiphiles has been the severe cellular toxicity of such compounds.
The mechanism by which these compounds, complexed with nucleic acids, interact with the cell membrane and thereby deliver their content into the cell is still not understood. Fusion (9, 14), endocytosis (15, 16), or a combination of these two (17) have been proposed as possible mechanisms, but irrefutable evidence for either of these mechanisms has yet to be presented. Also, structural requirements related to the ability of synthetic amphiphiles to transfect cells remain largely obscure. However, such insight is highly desirable for improving the transfection efficiency and in efforts to synthesize novel amphiphiles of lower toxicity without a loss of potency.
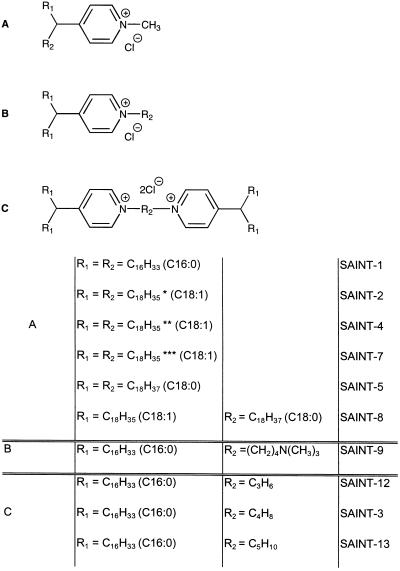
In 1944, pyridinium-derived amphiphiles were described that displayed some interesting features, such as having antiseptic and antibiotic properties. It was also noted that these amphiphiles were essentially nontoxic in vivo (18). More recently, Sudhölter et al. (19) showed that it was possible to form vesicles from simple fully synthetic pyridinium amphiphiles. In conjunction with our previous work (20) concerning the mechanism of amphiphile-mediated transfection, these findings prompted us to design and prepare several pyridinium-based amphiphiles, depicted in Fig. 1, which were systematically modified to determine molecular parameters that govern their transfection ability and their toxicity.
Figure 1.
Structure of the novel pyridinium compounds.
This work describes the properties of novel amphiphiles, abbreviated as SAINT (Synthetic Amphiphile INTeraction). These compounds display superior transfection properties compared with lipofectin in terms of efficiency and toxicity.
MATERIALS AND METHODS
Synthesis of Amphiphiles.
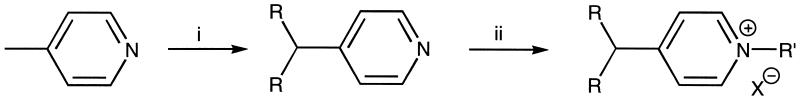
Alkyl halides that could not be purchased commercially were prepared by reduction of the corresponding esters or acids. The resulting alcohol was tosylated, followed by bromination or iodination with LiBr or NaI in acetone: (4-bromobutyl)-trimethylammonium bromide was synthesized as described (21). The general procedure is outlined in Fig. 2.
Figure 2.
General procedure for the synthesis of pyridinium amphiphiles A, B, and C. (i) Et2O, −15°C, LDA, RX. (ii) A: MeI, acetone, rt; B: EtOH, reflux, Br(CH2)4N+(CH3)3; C: 0.5 eq Br(CH2)nBr, neat 60°C.
The various pyridinium-derived amphiphiles (Fig. 1) have been synthesized according to established procedures (22), with minor modifications. Briefly, a solution of lithium diisopropylamide in dry ether (0.2 M; 2.1 eq) was added to a solution of freshly distilled 4-methylpyridine (0.1 M) in dry ether at −15°C. After stirring for 30 min at −15°C, the alkyl halide (bromide or iodide; 2 eq) was added. The mixture was stirred overnight at room temperature. Ether was added and the reaction mixture was washed twice with a 1 M NH4Cl solution, dried over Na2SO4, and evaporated to dryness. The product was either crystallized from acetone or purified by Al2O3 chromatography (neutral, activity II-III), using gradient elution, starting with hexane followed by hexane/ether (5:1, vol/vol). The resulting alkylpyridines were quaternized with an excess of CH3I in acetone for 1 h at room temperature to give product A. Product B was obtained by refluxing a mixture of the alkylpyridine (1.5 eq) with (4-bromobutyl)-trimethylammonium bromide (1 eq) in ethanol for 3 days. The product was purified by chromatography over Al2O3 (neutral, activity II-III), starting with chloroform as the eluent to remove the excess of alkylpyridine. Elution with 10% methanol in chloroform gave the pure compound, which was crystallized from acetonitrile.
Warming a mixture of alkyl dihalide (1 eq) at 60°C for 4 h with an excess of alkylpyridine (1.1 eq) followed by Al2O3 chromatography (neutral, activity II-III) using gradient elution, starting with dichloromethane and then 10% methanol in dichloromethane, yielded the pure compound C.
All bromide or iodide salts were converted into the chlorides by ion-exchange chromatography using Dowex 1 × 8 200–400 mesh (Cl− form). Compounds were eluted with CH3OH.
Cell Culture.
COS-7 and CV-1 cells were cultured in Costar flasks in Dulbecco’s modified Eagle’s medium (GIBCO), containing 7% fetal calf serum (FCS), 2 mM l-glutamine (GIBCO), 100 units per ml penicillin (GIBCO), and 100 mg/ml streptomycin (GIBCO) at 37°C in 5% CO2/95% air. Baby hamster kidney (BHK) cells were cultured in Costar flasks in Glasgow’s modified Eagle’s medium (GIBCO), containing 7% FCS, 2 mM l-glutamine (GIBCO), 100 units per ml penicillin (Gist-Brocades), 100 mg/ml streptomycin (Biochemie), 7.5% NaHCO3, 12.5% tryptose phosphate broth (GIBCO), and 2 M Hepes at 37°C in 5% CO2/95% air.
Preparation of Vesicles.
Chloroform solutions of N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA; synthesized as described in ref. 20) and dioleoyl phosphatidylethanolamine (1:1) (DOPE, Avanti Polar Lipids), or the pyridinium amphiphiles (Fig. 1) and DOPE (1:1), were dried under a stream of nitrogen. The residual solvent was removed under vacuum. The lipid film was then redissolved in water and sonicated to clarity in a bath sonicator in a closed vial. The small unilamellar vesicles thus obtained were sterilized by filtration.
Transfection Assay.
The cells were transfected by adding a complex of DNA [pCAT, pβ-galactosidase (β-gal), or pNEO; Promega] and vesicles, prepared by mixing lipid and nucleic acid at appropriate ratios, as described in the legends. Throughout this study, 1 μg of DNA was used and the concentration of amphiphile was varied. Chloramphenicol acetyltransferase (CAT) activity was determined as described (20).
β-Gal staining was performed 2 days after transfection. Cells were washed twice with PBS and fixed with 0.5% glutardialdehyde (Merck) in PBS. After two more washes with PBS, the staining solution {0.02% Nonidet P-40/0.01% sodium deoxycholate/1 mM MgCl2/5 mM K3[Fe(CN6)]/5 mM K4[Fe(CN6)]/1 mg/ml 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (stock solution = 20 mg/ml dimethylformamide, Boehringer Mannheim)} was added and the cells were incubated overnight.
One day after transfection, cells transfected with pNEO were diluted (1:20) and replated. After another 24 h the cells were put on culture medium supplemented with 400 μg/ml geneticin (G418, Boehringer Mannheim). After 2 weeks, the number of cells was measured by protein determination (23).
Determination of Cell Toxicity.
The toxicity was determined as the number of cells surviving the transfection experiment by measuring the protein content. The control value (100% of surviving cells) was obtained from the amount of cellular protein remaining after the cells had undergone the transfection protocol, without the addition of the cationic amphiphile/DNA complex. Microscopic examination excluded the possibility of dead cells remaining attached to the plates. Only cells displaying a typical fracture plane of the membrane, which is indicative of living cells, were seen. To corroborate these results, the amount of lactate dehydrogenase (LDH) in the cells after they have been transfected was also measured. The LDH content was determined using Cytotox 96 228, a nonradioactive cytotoxicity assay (Promega). The 100% value is obtained from the amount of LDH measured in nontransfected cells.
Hemolysis of Erythrocytes.
Erythrocytes were isolated from whole blood samples. To 40 ml of KPN buffer solution (120 mM KCl/10 mM Na2HPO4·2H2O/30 mM NaCl, pH 7.4) 10 ml of A+ red blood cells were added. The cells were centrifuged at 5°C (5 min at 3000 rpm) and the pellet was resuspended in 40 ml of KPN buffer. Cells were recentrifuged until the supernatant was clear. The pellet was then resuspended in 30 ml of KPN buffer.
10E8 erythrocytes were added to the DNA/vesicle complex in a total volume of 0.6 ml. After various time intervals at 37°C, 0.4 ml of Hepes buffered saline was added and the cells were centrifuged immediately (3 min at maximal speed in an Eppendorf table centrifuge at 5°C). The supernatant was then screened for the presence of hemoglobin by measuring the absorbance at 540 nm. To determine the 100% value, the cells were lysed with Triton X-100 (1% vol/vol, final concentration).
DNA Binding Assay.
For DNA binding measurements, complexes of different amounts of vesicles and 1 μg of DNA were made as described. The complexes were incubated for 10 min at room temperature and then placed on top of a 0.8% agarose gel in 0.5 × TBE (100 mM EDTA/56 mM boric acid/56 mM Tris·HCl, pH 7.4). The charge ratio was calculated by measuring the amount of lipid needed to bind all the DNA, i.e., the amount of lipid at which (free) DNA no longer migrated into the gel.
RESULTS
Structural Requirements for Efficient Transfection by Pyridinium Amphiphiles.
An extensive synthesis protocol was developed to produce the compounds listed in Fig. 1. Subsequently, vesicles consisting of the indicated amphiphile and DOPE (1:1) were prepared as described. The ability of these vesicles to mediate transfection, as accomplished by DNA delivery, was determined by measuring the CAT activity in cell lysates. The lead for the synthesis of these amphiphiles was dictated by the initial observation that the pyridinium chloride, substituted with two C16:0 alkyl chains at the C4 position of the pyridinium ring structure (SAINT-1), displayed DNA carrier properties. A transfection efficiency compatible to that of the widely used lipofectin (9) was obtained. Of further interest was the observation that SAINT-1 was persistently less toxic than lipofectin, monitored by protein loss and LDH leakage. Consequently, additional pyridinium analogs were synthesized to determine the structural parameters relevant for transfection per se, and to investigate whether derivatives could be obtained with a higher efficiency and a lower toxicity.
First, an elongation of the alkyl chains to C18:0 (SAINT-5; Table 1) showed a reduction by a factor of about 2 in the transfection efficiency relative to that of SAINT-1, but also a decrease in the toxicity. Interestingly, when substituting one of the saturated C18:0 alkyl chains for an unsaturated C18:1 alkyl chain (SAINT-8, C18:0/C18:1), the transfection efficiency increased by an order of magnitude, compared with the disaturated compound (SAINT-5) and 2- to 3-fold compared with SAINT-1 and lipofectin. Because chain unsaturation drastically improved the transfection efficiency, the next obvious goal was be to test the diunsaturated compound. As shown in Table 1, such a compound (SAINT-2, C18:1/C18:1) was 10–30 times more efficient than the disaturated compound, indicating that an unsaturation in the hydrocarbon tail leads to an enhanced transfection efficiency. Most importantly, the improved transfection efficiency (see below) was not paralleled by a concomitant increase in toxicity.
Table 1.
Transfection activity and toxicity of different amphiphiles examined in COS-7 cells
| Amphiphile | Concentration,* μM | Toxicity,† % of nonsurviving cells | Transfection efficiency,‡ arbitrary unit |
|---|---|---|---|
| DOTMA/DOPE | 36 –71 | 30 –40 | 1 |
| SAINT-1/DOPE | 38 –77 | 10 –20 | 1 |
| SAINT-2/DOPE | 15 –38 | 0 –25 | 8 |
| SAINT-3/DOPE | 8 –15 | 0 –20 | 2.5 |
| SAINT-4/DOPE | 15 –38 | 0 –30 | 12 |
| SAINT-5/DOPE | 15 –38 | 0 –10 | 0.6 |
| SAINT-7/DOPE | 5 –38 | 0 –40 | 10 |
| SAINT-8/DOPE | 5 –38 | 0 –30 | 3 |
| SAINT-9/DOPE | 38 –77 | 0 | 4 |
| SAINT-12/DOPE | 5 | 5 | 1.2 |
| SAINT-13/DOPE | 5 –10 | 25 –40 | 2.5 |
The concentration given is the concentration range at which the amphiphile is active.
The toxicities given here are the toxicities measured at the active concentration range, i.e., at the highest and lowest concentration given, respectively.
The different transfection efficiencies reflect the maximal efficiencies found within the amphiphile concentration range given and are expressed relative to the efficiency obtained with DOTMA/DOPE (=1). Transfection efficiencies were determined in at least three different experiments. Average values are given. (Error ± 10%.)
Upon analyzing SAINT-2 for the configuration of the double bonds, it was established by double resonance NMR that 85% was in the cis orientation. To investigate the relevance of a cis versus a trans orientation, unsaturated compounds, consisting of either pure trans or pure cis C18:1/C18:1 derivatives, were prepared. As is apparent from Table 1, the trans-orientation (SAINT-4) enhances the transfection efficiency compared with the transfection activity of the compound with cis-oriented alkyl chains (SAINT-7).
Apart from modulating the alkyl chains, it is equally possible that the headgroup structure (Figure 1C) significantly affects the transfection efficiency, particularly since the cationic charge is relevant for interaction with the negatively charged nucleic acid. This structural parameter was examined using disaturated C16:0 derivatives for reasons of synthetic convenience. Table 1 shows that an additional cationic charge improves the transfection efficiency, which is in agreement with other studies (24). The flanking group of the charged nitrogen does not seem to dominate the transfection efficiency (SAINT-3 versus SAINT-9). Rather, the intermolecular distances between the cationic charges appears to be more relevant. Thus, with a C4 spacing group (SAINT-9 and SAINT-3) the highest transfection efficiency is obtained when compared with SAINT-1. Lower transfection efficiencies are obtained with shorter (C3) or longer (C5) spacers. However, compared with the observed enhancement in transfection efficiencies obtained by modulating the alkyl chains, the described effects originating from variations in the headgroup structure are less prominent.
Pyridinium Amphiphiles Are only Slightly Toxic.
Although several types of synthetic amphiphile formulations are efficient carriers for delivering oligonucleotides and genes into cells, they have also been noted for their notorious cell toxicity. As already mentioned above, the pyridinium-derived amphiphiles appear to display little if any toxic effects toward the cells examined. As is apparent from the data summarized in Table 1, pyridinium-derived amphiphiles are only marginally toxic; often more than 90% of the cells survive the transfection experiments at the concentration of amphiphile needed for optimal transfection. Only SAINT-13 showed significant cell death (25–40%) after the transfection. This value is comparable to that for DOTMA/DOPE induced cell death.
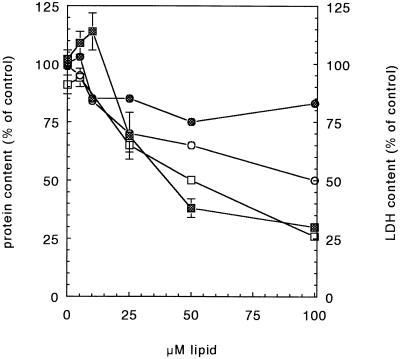
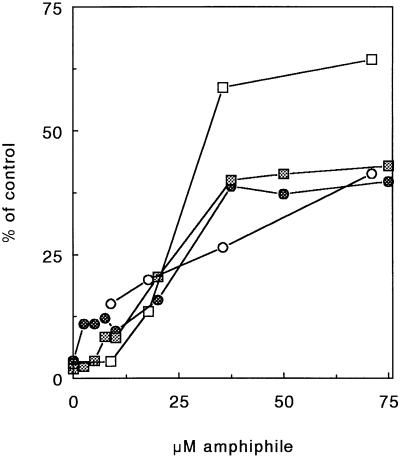
To corroborate these results, the amount of LDH in the cells after they have been transfected was also measured. Fig. 3 shows the LDH and protein content of cells transfected with SAINT-2/DOPE or with DOTMA/DOPE vesicles. Note that the LDH leakage (squares) is more pronounced than the decrease in protein content (circles) and that LDH loss does not parallel protein loss. The data suggest that both amphiphiles effectively permeabilize cells. However, the permeabilization does not necessarily lead to a similar extent of cell damage leading to cell death, as reflected by the dissimilarity in amphiphile concentration-dependent protein loss.
Figure 3.
Amphiphile-induced cell toxicity, monitored by protein loss and LDH leakage after transfection, as a function of the amphiphile concentration. Cells were transfected with a complex of 1 μg of DNA and different amounts of DOTMA/DOPE (open symbols) or with a complex of 1 μg of DNA and different amounts of SAINT-2/DOPE (cross-hatched symbols). After the transfection, protein content (circles) and LDH content (squares) were determined.
Cell Type Dependence.
It has been shown that not all cell types are equally susceptible to transfection with DOTMA/DOPE vesicles (9, 20). It was therefore of interest to examine the versatility of the SAINT derivatives in this respect. BHK cells, which are hardly transfected with DOTMA/DOPE, displayed a relatively high transfection susceptibility toward SAINT-2/DOPE (Table 2). Note that compared with lipofectin, the transfectability increased ≈20-fold. Also, 36C2.21 cells, an immortalized oligodendrocyte cell line, show a more efficient transfection when SAINT-2/DOPE is used as a carrier system in comparison with DOTMA/DOPE (Table 2). It must be noted however that the order of magnitude to which SAINT-2/DOPE exceeds DOTMA/DOPE is clearly cell type dependent. Nevertheless, in all cases, a much higher transfection efficiency was obtained when the transfection was carried out with SAINT-2/DOPE.
Table 2.
Transfection efficiency of different cell lines; comparison of DOTMA/DOPE vesicles and SAINT-2/DOPE vesicles
| Cell line | DOTMA/DOPE
|
SAINT-2/DOPE
|
||
|---|---|---|---|---|
| Concentration, μM | Transfection efficiency | Concentration, μM | Transfection efficiency | |
| COS-7 | 71 | 1 | 15 | 10 |
| CV-1 | 71 | 1 | 15 | 10 |
| BHK | 71 | 0.2 | 45 | 4 |
| 36C2.21 | 36 | 2 | 23 | 5 |
Different cell types were transfected with a complex of DOTMA/DOPE and 1 μg of DNA, and with a complex of SAINT-2/DOPE and 1 μg of DNA. The concentration of amphiphile given is the concentration at which the amphiphile exhibits the most efficient transfection. Note that the transfection efficiencies given are expressed relative to that obtained in COS-7 cells, using DOTMA/DOPE (=1). Average values are given as determined in at least three different experiments. (Error ± 10%.)
SAINT-2 Mediates Higher Transfection in Terms of Cell Number and Delivery per Cell.
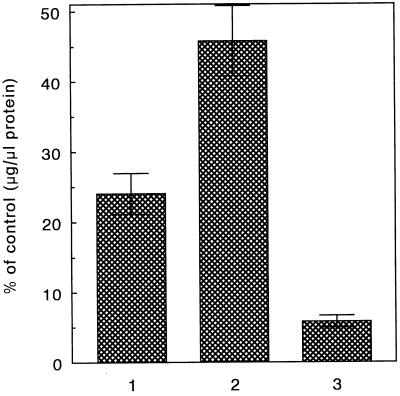
Of major importance is the question whether the higher efficiency of SAINT-2-mediated transfection is related to its ability to transfect a higher number of cells, or whether a higher amount of DNA per cell is delivered. Two approaches were taken to determine the efficiency of DNA delivery in terms of the number of cells transfected per population. First, the cells were transfected with a pNEO gene construct, which renders neomycin resistence toward cells expressing the gene. Second, cells were transfected with a gene construct expressing β-gal, allowing an estimation of the number of transfected cells based upon a blue-staining of cells expressing the gene. As shown in Fig. 4, when cells have been transfected with pNEO, using DOTMA/DOPE as a DNA carrier, 23% of the cells have taken up the DNA, as reflected by an efficient protection of that cell fraction against antibiotic treatment. However, when the cells have been transfected using SAINT-2/DOPE as a DNA carrier, more than 45% of the cell population showed resistence toward the neomycin treatment. Very similar results were obtained when carrying out analogous experiments, using β-gal as the reporter gene. Of the cells treated with DOTMA/DOPE, about 30% were positively (blue) stained, whereas of the cells treated with SAINT-2/DOPE, this number increased to around 45% (Table 3). Taken together, a higher number of cells per population have acquired the gene constructs when delivered via SAINT-2/DOPE vesicles. The enhancement of delivery is ≈1.5-fold when comparing lipofectin with SAINT-2, whereas in terms of CAT gene expression an increase of an order of magnitude is frequently seen (Table 1). Hence, these data also imply that the delivery per cell must be severalfold higher when using SAINT-2 as the nucleic acid carrier.
Figure 4.
Stable transfection of COS-7 cells. Two weeks after transfection the cells were screened for their protein content, which is a measure of the number of cells that survived, and thus a measure of the number of transfected cells. The cells were transfected with a complex of DNA (pNEO) and the indicated amphiphiles. 1, 36 μM DOTMA/DOPE (1:1); 2, 15 μM SAINT-2/DOPE (1:1); 3, calcium phosphate precipitation method. The results are expressed as the mean of at least four experiments (±SD). All amphiphiles were significantly different from DOTMA/DOPE (P < 0.025).
Table 3.
Transfection of COS-7 cells with β-gal using different amphiphiles
| Amphiphile | Amount of positively stained cells % of total* |
|---|---|
| DOTMA/DOPE | 32.6 ± 2.3 |
| SAINT-2/DOPE | 45.3 ± 1.2 |
COS-7 cells were incubated with the optimal concentrations of the indicated amphiphiles complexed to 1 μg of DNA (β-gal). After 2 days, the number of cells with blue staining were counted, representing the number of cells transfected.
Amount ± SD; n = 4.
Pore Formation and Transfection Capacity.
In previous work (20) we observed that lipofectin effectively creates pores in cell membranes, as reflected by the release of hemoglobin when erythrocytes are incubated with the synthetic amphiphile. At relatively high DNA/amphiphile ratios the nucleic acid reduces amphiphile-induced hemolysis. At lower ratios a relatively enhanced and/or synergistic DNA effect on amphiphile-induced hemolysis is observed. Membrane permeabilization may be related to the mechanism of DNA delivery by synthetic amphiphiles (20). Di-dodecyl dimethylammonium bromide is ineffective in bringing about transfection (results not shown). Neither does the amphiphile trigger hemolysis (Fig. 5). Analogous to DOTMA (20), SAINT-2 is capable of triggering hemolysis. Interestingly, in contrast to the synergistic effect of DNA on hemolysis, seen in the case of DOTMA, such synergism is not immediately apparent for the SAINT-2/DNA complex, but some synergism may occur given the similarity in hemolysis of the free amphiphile and the complex. However, in the lipid concentration range required for optimal transfection efficiency, DOTMA triggers a substantially higher extent of hemolysis (around 50 μM) than SAINT-2 (around 20 μM). It would appear therefore that there is no direct correlation between the extent of hemolysis and transfection efficiency. However, the remarkable differences in their ability to trigger hemolysis may reflect differences in the structural nature of the two complexes. A direct interaction of amphiphile and the erythrocyte membrane is necessary for triggering hemolysis, and the presence of DNA, apart from displaying synergistic properties on amphiphile-induced hemolysis, can also sterically interfere, thereby inhibiting amphiphile-induced hemolysis. These properties are more prominently featured in the case of lipofectin than with SAINT-2 (Fig. 5). Therefore, some initial efforts were made to determine potential differences in DNA-complex formation with either amphiphile.
Figure 5.
Amphiphile/DNA membrane interaction induces membrane permeabilization. As a measure of membrane perturbation, the ability of SAINT-2 and DOTMA to induce hemolysis of erythrocytes was examined as a function of vesicle and DNA concentration. The 100% value was obtained by treating cells with 1% Triton X-100, which results in total cell lysis. Cells were treated either with amphiphiles alone (circles) or amphiphiles complexed to 5 μg of DNA (squares). Cells were treated with two different amphiphile preparations, DOTMA/DOPE (open symbols) and SAINT-2/DOPE (cross-hatched symbols).
Some Characteristics of DNA Complexes Formed with SAINT-2 and DOTMA.
To determine the binding efficiency of DNA to both amphiphiles, complex formation between DNA and amphiphile was analyzed by agarose gel chromatography, which allows the determination of the binding ratio of DNA/amphiphile. The complexes were prepared by mixing different amounts of SAINT-2/DOPE or DOTMA/DOPE and a fixed amount of DNA (1 μg). The samples were subsequently chromatographed on agarose gel, and the charge ratio was calculated after establishing the conditions at which free DNA no longer migrates into the gel. In this manner a charge ratio of 3.65 ± 0.05 (n = 3) was determined for SAINT-2/DOPE, whereas a ratio of 4.30 ± 0.05 (n = 3) was obtained for DOTMA/DOPE. Hence, with regard to the amount of DNA complexed to either SAINT-2 or DOTMA, there appears to be no major difference between both systems.
Since DOTMA is more potent in triggering membrane perturbations, as reflected by an enhanced ability to induce hemolysis, the “shielding” of the amphiphilic molecules by DNA in the DOTMA complex might differ from that in the SAINT-2 complex, taking into account that the amphiphile’s direct accessibility to and interaction with the erythrocyte membrane is required for membrane perturbation (20). The “compactness” of the SAINT-2 and DOTMA complexes was therefore examined by determining the extent of DNA degradation after treatment with DNase I at 37°C. However, for both systems, significant degradation was not observed (not shown), implying that both amphiphiles effectively “shielded” the complexed DNA.
DISCUSSION
In this study, we have described the development of a series of novel pyridinium-based amphiphiles, which display a highly efficient ability to transfect cells. This efficiency relates to their ability (i) to transfect cells that are not easily transfected by other amphiphiles, such as lipofectin; (ii) to transfect a relative high number of cells per cell population (approximately in the order of 50%); and (iii) to deliver a relatively high number of DNA per cell. Compared with lipofectin, SAINT-2-mediated DNA delivery per cell is enhanced by about 3- to 6-fold, taking into account that the transfection efficiency is about an order of magnitude higher, whereas the relative number of transfected cells was increased by a factor of about 1.5.
Concerning the comparison with other amphiphiles with respect to improvement of transfection efficiency, it is of relevance to carry out comparable studies, using the various amphiphiles in parallel, since the net differences in efficiency are related to the cell type studied. For example, BHK cells are difficult to transfect with lipofectin. SAINT-2/DOPE, however, readily transfects such cells with an efficiency that is improved by a factor of about 20. The relative meaning of such a number is emphasized when analyzing the data in Table 2, showing that transfection with lipofectin of BHK cells approaches back ground level.
An important feature of these pyridinium-derived amphiphiles is their relatively low toxicity toward the cells tested, toxicity being a major drawback in the application of many amphiphiles thus far. At first sight, the far lower concentrations of the pyridinium amphiphiles needed to induce transfection (Table 1) may explain this improvement. Yet, the reduced toxicity appears to be an inherent property of these derivatives. At concentrations much higher than those required for transfection, toxicity is still marginal, compared with the toxicity observed when the cells were treated with lipofectin (Fig. 3). As noted before, the nontoxic properties of pyridinium salts, as well as their therapeutic properties per se, were already recognized more than 50 years ago (18). Obviously, these properties offer an additional attractive rationale for applying this class of amphiphiles as efficient carriers for nucleic acids in antisense and/or gene therapy.
A major issue of interest is obviously the underlying mechanism as to why SAINT-2 and similar analogs are more effective in the delivery of DNA to and into cells, than the compound used for comparison in this work, lipofectin. We have demonstrated that several structural parameters strongly affect the transfection efficiency. Alkyl chain “mobility” appears to be an important parameter in this regard. Thus, long-saturated alkyl chains reduce the transfection efficiency. Chain unsaturation may lead to more dynamic packing features, in terms of chain orientation in a bilayer. It should be noted that DOTMA also contains two C18:1 (oleyl) carbon chains. Preliminary experiments suggest that the complex formed is not aberrent in terms of DNA packing, since the nucleic acid packaged with an equal efficiency in both lipofectin and SAINT-2. Neither differed the accessibility to DNase in either system. These considerations suggest the relevance of structural features of the amphiphile in the actual delivery process of the DNA. This would also be in line with observations that the nonbilayer lipid DOPE frequently facilitates the entry of DNA into cells when employing amphiphiles. It is possible that the unsaturated alkyl chains facilitate a permeabilization of the cellular membranes, a feature typical for amphiphiles that are capable of causing cellular transfection. As noted above, the cationic di-dodecyl dimethylammonium bromide is incapable of transferring DNA into cells. Consistently, the amphiphile does not permeabilize red blood cells. Whether this inability relates to an effect of the chain length, since the longer chain analog dioctadecyl dimethylammonium bromide appears to have good transfection efficiencies (25), remains to be determined.
Our previous observations (20) suggested that permeabilization may be an important step in the translocation of DNA across membranes. This work reveals that although both types of amphiphiles (SAINT-2 and DOTMA) permeabilize cellular membranes (Figs. 3 and 5), there appears to exist no proportional relationship between membrane perturbation and transfection efficiency. In fact, the process is more subtle, which also becomes apparent when comparing the release of LDH (Fig. 3) with amphiphile-induced perturbation of erythrocyte membranes (Fig. 5). At the optimal concentration at which SAINT-2 brings about transfection (≈15 μM) LDH release is still marginal, whereas hemoglobin release numbers ≈10–15%.
In the case of lipofectin (optimum ≈ 50 μM), LDH release is ≈50%, whereas hemoglobin release has occurred to the same extent. This could indicate that the latter extent of permeabilization is not a priori required to obtain efficient delivery. Hence, the effects of DOTMA likely reflect a major toxic effect toward the cells, whereas SAINT-2 may reflect a more selective permeabilization allowing DNA translocation.
Apart from a role of the alkyl chains in the processes as described above, it is also likely that the headgroup, containing the cationic charge necessary for interaction with the nucleic acid, is of considerable significance. Upon delivery, the DNA has to “dissociate” from the amphiphile (26). It is evident that the nature of the headgroup may modulate such a property and thereby affect transfection efficiency. In fact, while keeping the alkyl chain constant, subtle structural changes in the headgroup structure can change the transfection efficiency up to severalfold (Table 1).
Further work will be needed to more carefully define the various structural parameters involved. The current amphiphilic system should allow us to obtain such mechanistic insight. Nevertheless, this work has shown that the novel pyridinium-related amphiphiles have great potency in applications involving nucleotide therapies, both with respect to their efficiency and in terms of marginal toxicity.
Acknowledgments
This work was supported by Grant nr 3492758 from the Netherlands Foundation for Chemical Research (SON)/Netherlands Technology Foundation (STW).
Footnotes
Abbreviations: SAINT, Synthetic Amphiphile INTeraction; LDH, lactate dehydrogenase; CAT, chloramphenicol acetyltransferase; β-gal, β-galactosidase; BHK, baby hamster kidney.
References
- 1.Caputo A, Grossi M P, Bozzini R, Rossi C, Betti M, Marconi P C. Gene Ther. 1996;3:235–245. [PubMed] [Google Scholar]
- 2.Marshall E. Science. 1995;270:1751. doi: 10.1126/science.270.5243.1751. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson C P. Bio/Technology. 1995;13:222–225. doi: 10.1038/nbt0395-222. [DOI] [PubMed] [Google Scholar]
- 4.Anderson W F. Science. 1992;256:808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- 5.Gilboa E, Eglitis M A, Kantoff P W, Anderson W F. BioTechniques. 1986;4:504–512. [Google Scholar]
- 6.Rosenfeld M A, Siegfried W, Yoshimyra K, Yoneyama K, Fukuyama M, Stier L E, Paako P K, Gilardi P, Stradford-Perricaudet L D, Perricaudet M, Jallat S, Pavirani A, Lecocq J P, Crystal R G. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 7.Engberts J B F N, Hoekstra D. Biochim Biophys Acta. 1995;1241:323–340. doi: 10.1016/0304-4157(95)00008-9. [DOI] [PubMed] [Google Scholar]
- 8.Hug P, Sleight R G. Biochim Biophys Acta. 1991;1097:1–17. doi: 10.1016/0925-4439(91)90016-3. [DOI] [PubMed] [Google Scholar]
- 9.Felgner P L, Gadek T R, Holm M, Ronas R, Chan H W, Wenz M, Northrop J P, Ringold G M, Danielsen M. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akao T, Nakayama T, Takeshia K, Ito A. Biochem Mol Biol Int. 1994;34:915–920. [PubMed] [Google Scholar]
- 11.Behr J P, Demeneix B, Loeffler J P, Perex-Mutul J. Proc Natl Acad Sci USA. 1989;86:124–132. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Huang L. Biochem Biophys Res Commun. 1991;179:280–285. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- 13.Levantis R, Silvius J R. Biochim Biophys Acta. 1990;1023:124–132. doi: 10.1016/0005-2736(90)90017-i. [DOI] [PubMed] [Google Scholar]
- 14.Felgner P L, Ringold G M. Nature (London) 1989;337:387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 15.Zabner J, Fasbender A J, Moninger T, Poellinger K A, Welsh M J. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 16.Friend D S, Papahadlopoulos D, Debs R J. Biochim Biophys Acta. 1996;1278:41–50. doi: 10.1016/0005-2736(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y, Nomoto H, Takezawa R, Miyoshi N, Aikaike T. J Biochem. 1994;116:1220–1226. doi: 10.1093/oxfordjournals.jbchem.a124667. [DOI] [PubMed] [Google Scholar]
- 18.Barkovsky C. Ann de Chim. 1944;11:487–521. [Google Scholar]
- 19.Sudhölter E J R, Engberts J B F N, Hoekstra D. J Am Chem Soc. 1980;102:2467–2469. [Google Scholar]
- 20.van der Woude I, Visser H W, Ter Beest M B A, Wagenaar A, Ruiters M H J, Engberts J B N F, Hoekstra D. Biochim Biophys Acta. 1995;1240:34–40. doi: 10.1016/0005-2736(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 21.Sudhölter E J R, Engberts J B F N, de Jeu W H. J Phys Chem. 1982;86:1908–1913. [Google Scholar]
- 22.Thanei-Wyss P, Wasser P G. Helv Chim Acta. 1983;66:2198–2205. [Google Scholar]
- 23.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;293:265–275. [PubMed] [Google Scholar]
- 24.Remy J S, Sirlin C, Vierling P, Behr J P. Bioconjugate Chem. 1994;5:647–654. doi: 10.1021/bc00030a021. [DOI] [PubMed] [Google Scholar]
- 25.Philip R, Liggitt D, Philip M, Dazin P, Debs R. J Biol Chem. 1993;268:16087–16090. [PubMed] [Google Scholar]
- 26.Xu Y, Szoka F C., Jr Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]