Abstract
Background
Malignant gliomas recur even after extensive surgery and chemo-radiotherapy. Although a relatively novel chemotherapeutic agent, temozolomide (TMZ), has demonstrated promising activity against recurrent glioma, the effects last only a few months and drug resistance develops thereafter in most cases. Induction of O6-methylguanine-DNA methyltransferase (MGMT) in tumors is considered to be responsible for resistance to TMZ. Interferon-beta has been reported to suppress MGMT in an experimental glioma model. Here we report a patient with TMZ-refractory anaplastic astrocytoma (AA) who was treated successfully with a combination of interferon-beta and TMZ.
Case presentation
A patient with recurrent AA after radiation-chemotherapy and stereotactic radiotherapy was treated with TMZ. After 6 cycles, the tumor became refractory to TMZ, and the patient was treated with interferon-beta at 3 × 106 international units/body, followed by 5 consecutive days of 200 mg/m2 TMZ in cycles of 28 days. After the second cycle the tumor decreased in size by 50% (PR). The tumor showed further shrinkage after 8 months and the patient's KPS improved from 70% to 100%. The immunohistochemical study of the initial tumor specimen confirmed positive MGMT protein expression.
Conclusion
It is considered that interferon-beta pre-administration increased the TMZ sensitivity of the glioma, which had been refractory to TMZ monotherapy.
Background
Treatment modalities for recurrent glioma are limited. Since surgery or local radiotherapy can be applied only to limited patients, a more systemic approach such as chemotherapy is used in most cases. Until recently, however, chemotherapy has had only a limited impact for control of recurrent glioma. A relatively novel chemotherapeutic agent, temozolomide (TMZ), has demonstrated promising activity against recurrent glioma in some patients, however the effects last only a few months and drug resistance develops thereafter in most cases[1,2]. Resistance to TMZ is considered to be mediated, at least to some extent, by a DNA repair enzyme, MGMT (O6-methylguanine-DNA methyltransferase), which is induced in the tumor [3]. Interferon-beta has been reported to suppress MGMT in an experimental glioma model [4,5]. Here we report the successful use of a combination of interferon-beta and TMZ for treatment of recurrent anaplastic astrocytoma after failure of TMZ monotherapy.
Case presentation
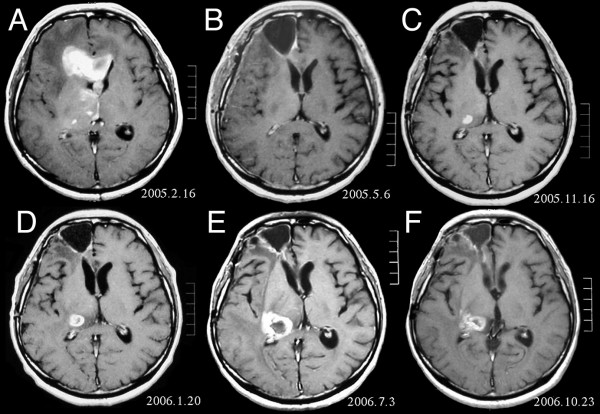
A 51-year-old man was found to have a diffusely infiltrative tumor in the bilateral frontal lobe and right thalamus (Figure 1A). The patient had undergone removal of a right frontal tumor, diagnosed as anaplastic astrocytoma (AA), on February 18, 2005. During local 60 Gy irradiation, chemotherapy consisting of procarbazine, nimustine hydrochloride (ACNU) and vincristine was given. After the radiation-chemotherapy, MRI showed complete disappearance of the lesion, including the thalamic tumor (Figure 1B). This combination chemotherapy was repeated every 3 months, but MRI on November 16, 2005, revealed recurrence in the right thalamus (Figure 1C). The patient received stereotaxic radiotherapy with 18 Gy (target volume 0.8 ml) for the recurrence in the thalamus, but follow-up MRI in January 2006 showed enlargement of the thalamic mass (Figure 1D).
Figure 1.
MRI of brain. (A) Initial MRI on February 16, 2005, shows a tumor in the right and left frontal lobe as well as the right thalamus. (B) MRI after surgery, radiation and chemotherapy. The tumor has completely disappeared except for slight enhancement adjacent to the surgical margin. (C) Recurrence of the thalamic tumor despite maintenance chemotherapy on November 16, 2005. (D) Increase in size of the thalamic tumor two months after stereotactic radiotherapy. (E) After 6 cycles of TMZ therapy, the thalamic lesion enlarged, and the patient developed dysarthria and hemiparesis. (F) After 2 courses of treatment with interferon-beta and TMZ, the tumor shows a partial response.
To differentiate radiation necrosis from recurrence, a fluorodeoxy glucose (FDG) PET study was performed. As the FDG-PET findings strongly suggested recurrence, TMZ chemotherapy was started. The patient was treated with the usual 5-day protocol repeated in cycles every 28 days, i.e. TMZ 150 mg/m2 for the first 5 days, escalated to 200 mg/m2 in the following cycles. Although the TMZ chemotherapy seemed to have some effect, the tumor continued to grow. After 6 cycles, the patient developed dysarthria and hemiparesis, and Karnofsky performance status (KPS) decreased from 100% to 70%. From the clinical course, the serial MRI findings (Figure 1C, 1D, 1E) and the previous FDG-PET data, we considered this was due to progression of the recurrent disease, and therefore we abandoned TMZ monotherapy. After application to the IRB, and with the informed consent of the patient, a combination of interferon-beta and TMZ treatment was started. On July 12, 2006, 3 × 106 international unit (IU)/body interferon-beta (Feron®) was given intravenously followed by 5 days of 200 mg/m2 TMZ (Days 2 – 6).
The patient's neurological symptoms improved after this first cycle, and MRI after the second cycle showed shrinkage of the tumor (Figure 1F). The patient's neurological symptoms also showed further concomitant improvement. This treatment was repeated every 28 days. After 8 cycles, the tumor showed further shrinkage, and since then the patient's condition has been improving, with a KPS of 100 in April 2007. During this treatment, no steroid has been administered and there have been no significant side effects exceeding grade 3 in terms of hematological and other clinical parameters.
Immunohistochemical study to examine MGMT protein expression
The MGMT protein expression of the tumor specimen taken at the initial surgery was performed using immunohistochemical method as described previously [6]. Briefly, after antigen retrieval through autoclave treatment, the tissue sections were incubated in 0.3% hydrogen peroxidase for 30 min, then were incubated with the Ready-to Use anti-MGMT monoclonal antibody (clone MT3.1, Lab Vision, CA) overnight at 4°C. The sections were treated with a second biotinylated antibody (DAKO) then were incubated with streptavidin-biotin-peroxidase (SAB) complex (DAKO). Visualization was carried out with 3',3'-diaminobenzidine tetrahydrochloride (DAB).
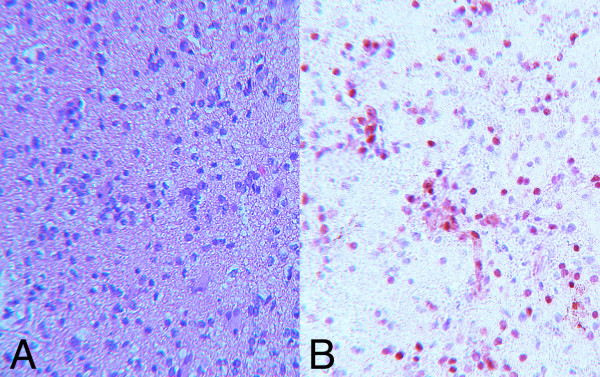
More than 40% of the tumor cells were positive for this staining thus it is confirmed that this tumor moderately[6]expressed MGMT protein (Figure 2).
Figure 2.
Photomicrograph. H&E staining (A) and immunohistochemical staining for MGMT protein (B) of the tumor resected at initial surgery. Forty-eight percent (± 3.7%) of the tumor cells express MGMT protein.
Discussion
We have reported a case of TMZ-refractory glioma that was treated successfully with interferon-beta and TMZ combination therapy. Although TMZ is one of the most effective chemotherapeutic agents for treatment of glioma recurrence, not all patients benefit from the drug. The response rate of glioblastoma (GBM) or AA to the standard TMZ protocol of 5 days in a 28-day cycle is 7–30% [1,2,7,8]. The median progression-free survival is only 2.1 months for GBM and 5.4 months for AA [2,7]. Once resistance has developed, there is no established management protocol. For TMZ, 21 consecutive days of treatment followed by 7 days of drug withdrawal ("21 days on and 7 days off") [9] or "7 days on and 7 days off" [10] or low-dose continuous treatment with TMZ have been tried [11]. Cyclophosphamide treatment has also been attempted [13]. However, all of these alternative treatments seem to have only limited effects. One of the mechanisms leading to TMZ resistance is the production of a DNA repair enzyme, MGMT [3,14]. High levels of MGMT activity in tumor cells create resistance by blunting the therapeutic effect of TMZ, leading to treatment failure. The tumor specimen of the present case taken at initial surgery showed that more than 40% of the cells expressed MTMT protein which might lead to the relative resistance to the TMZ monotherapy.
Interferon-beta has been used in Japan as an adjuvant therapy for some patients with malignant glioma [15]. It has a direct tumor-suppressive effect [16], and also acts as an immunomodulator [12] and anti-angiogenetic agent [17,18]. Recently, Natsume and Park showed that interferon-beta lowers the activity of MGMT and enhances the effect of TMZ in vivo and in vitro [4,5]. We administered interferon-beta before administration of TMZ to a patient whose recurrent AA had become refractory to standard TMZ monotherapy. The tumor showed a partial response after the second cycle, and thereafter the patient's condition improved and remained good for 8 months. Although interferon-beta itself has an antitumor effect against glioma, its effect is relatively mild, and one or two administrations of 3 × 108 IU/body is far less than that needed for adequate control. However, this dose may allow attainment of a serum concentration about one fourth of that shown to suppress MGMT activity in an in vitro setting [5]. It is speculated that although the tumor was initially resistant to TMZ monotherapy because of positive MGMT protein expression, the interferon-beta suppressed MGMT expression by the tumor, thus rendering it sensitive to interferon-TMZ combination treatment.
The MRI enhancement after stereotactic radiotherapy might have been due to a radiation effect. MRI is often useless for differentiating recurrence from radiation necrosis [19], and false positivity with FDG-PET has also been reported [20]. However, most previously reported tumors showing false positivity by FDG-PET or by MRI have been weakly enhanced or accompanied by marked and extensive central necrosis on MRI [19-21], unlike the present case. No previously reported tumor has been as extensive and with such a thick rim after stereotactic radiotherapy with a target volume as small as 0.8 ml. Moreover this thalamic lesion regressed after interferon-TMZ combination chemotherapy, without the use of steroid. Therefore we considered that this thalamic lesion represented genuine recurrence.
This is the first clinical report of effective treatment using a combination of interferon-beta and TMZ against TMZ-refractory glioma. This combination warrants further testing in a larger-scale clinical study.
Conclusion
Combined treatment with interferon-beta and TMZ achieved a response in a case of TMZ-refractory recurrent AA. This approach may be a useful salvage therapy for patients with recurrent malignant glioma.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
TF: Involved in the diagnosis of the case, design of the treatment, submission of the protocol to the IRB, and drafting of the manuscript
HI: Involved in the treatment of the case
AM: Performed immunohistochemical study of the surgical specimen
HA: Involved in the surgery of the case
TN: Involved in the treatment of the case, and submission of the protocol to the IRB
Acknowledgments
Acknowledgements
Written consent was obtained from the patient for publication of this case report.
Contributor Information
Takamitsu Fujimaki, Email: tfujimak-tky@umin.ac.jp.
Hisato Ishii, Email: hisato-is@u01.gate01.com.
Akira Matsuno, Email: akirakun@med.teikyo-u.ac.jp.
Hajime Arai, Email: harai@med.juntendo.ac.jp.
Tadayoshi Nakagomi, Email: nsnaka@med.teikyo-u.ac.jp.
References
- Nishikawa R, Shibui S, Maruno M, Sugiyama K, Sato S, Fujimaki T, Takahashi H, Wakabayashi T, Takahashi J, Kochi M, Nakamura H, Sawamura Y, Ikeda J, Hori T, Aoki T, Matsutani M. Efficacy and safety of monotherapy with temozolomide in patients with anaplastic astrocytoma at first relapse – a phase II clinical study. Gan To Kagaku Ryoho. 2006;33:1279–1285. [PubMed] [Google Scholar]
- Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O'Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, Yoshida J. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res. 2005;65:7573–7579. doi: 10.1158/0008-5472.CAN-05-0036. [DOI] [PubMed] [Google Scholar]
- Park JA, Joe YA, Kim TG, Hong YK. Potentiation of antiglioma effect with combined temozolomide and interferon-beta. Oncol Rep. 2006;16:1253–1260. [PubMed] [Google Scholar]
- Brell M, Tortosa A, Verger E, Gil JM, Vinolas N, Villa S, Acebes JJ, Caral L, Pujol T, Ferrer I, Ribalta T, Graus F. Prognostic significance of O6-Methylguanine-DNA Methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin Cancer Res. 2005;11:5167–5174. doi: 10.1158/1078-0432.CCR-05-0230. [DOI] [PubMed] [Google Scholar]
- Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. doi: 10.1023/A:1008382516636. [DOI] [PubMed] [Google Scholar]
- Groves MD, Puduvalli VK, Chang SM, Conrad CA, Gilbert MR, Tremont-Lukats IW, Liu TJ, Peterson P, Schiff D, Cloughesy TF, Wen PY, Greenberg H, Abrey LE, DeAngelis LM, Hess KR, Lamborn KR, Prados MD, Yung WK. A North American brain tumor consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81:271–277. doi: 10.1007/s11060-006-9225-y. [DOI] [PubMed] [Google Scholar]
- Brandes AA, Tosoni A, Cavallo G, Bertorelle R, Gioia V, Franceschi E, Biscuola M, Blatt V, Crino L, Ermani M. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO) Br J Cancer. 2006;95:1155–1160. doi: 10.1038/sj.bjc.6603376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W, Steinbach JP, Kuker WM, Dichgans J, Bamberg M, Weller M. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62:2113–2115. doi: 10.1212/01.wnl.0000127617.89363.84. [DOI] [PubMed] [Google Scholar]
- Kong DS, Lee JI, Kim WS, Son MJ, Lim do H, Kim ST, Park K, Kim JH, Eoh W, Nam DH. A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma. Oncol Rep. 2006;16:1117–1121. [PubMed] [Google Scholar]
- Kuroki M, Tanaka R, Suzuki Y, Moriyama M, Kuwabara Y, Kobayashi S. Antitumor effect of recombinant murine interferon-beta against mouse malignant glioma. J Interferon Res. 1987;7:301–311. doi: 10.1089/jir.1987.7.301. [DOI] [PubMed] [Google Scholar]
- Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with cyclophosphamide for recurrent temozolomide-refractory anaplastic astrocytoma. Cancer. 2006;106:172–179. doi: 10.1002/cncr.21582. [DOI] [PubMed] [Google Scholar]
- Kanzawa T, Bedwell J, Kondo Y, Kondo S, Germano IM. Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg. 2003;99:1047–1052. doi: 10.3171/jns.2003.99.6.1047. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Kajita Y, Mizuno M, Nagasaka T, Yoshida J. Efficacy of adjuvant therapy with procarbazine, MCNU, and vincristine for oligodendroglial tumors. Neurol Med Chir (Tokyo) 2001;41:115–119. doi: 10.2176/nmc.41.115. [DOI] [PubMed] [Google Scholar]
- Rosenblum MG, Yung WK, Kelleher PJ, Ruzicka F, Steck PA, Borden EC. Growth inhibitory effects of interferon-beta but not interferon-alpha on human glioma cells: correlation of receptor binding, 2',5'-oligoadenylate synthetase and protein kinase activity. J Interferon Res. 1990;10:141–151. doi: 10.1089/jir.1990.10.141. [DOI] [PubMed] [Google Scholar]
- Hong YK, Chung DS, Joe YA, Yang YJ, Kim KM, Park YS, Yung WK, Kang JK. Efficient inhibition of in vivo human malignant glioma growth and angiogenesis by interferon-beta treatment at early stage of tumor development. Clin Cancer Res. 2000;6:3354–3360. [PubMed] [Google Scholar]
- Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ. Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc Natl Acad Sci USA. 1995;92:4562–4566. doi: 10.1073/pnas.92.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera T, Kubota T, Kabuto M, Nakagawa T, Takeuchi H, Arishima H, Sato K, Kobayashi H, Kitabayashi M, Hirose S. Analysis of the proliferative potential of tumor cells after stereotactic radiosurgery for recurrent astrocytic tumors. Neurol Res. 2000;22:802–808. doi: 10.1080/01616412.2000.11740756. [DOI] [PubMed] [Google Scholar]
- Ross DA, Sandler HM, Balter JM, Hayman JA, Archer PG, Auer DL. Imaging changes after stereotactic radiosurgery of primary and secondary malignant brain tumors. J Neurooncol. 2002;56:175–181. doi: 10.1023/A:1014571900854. [DOI] [PubMed] [Google Scholar]
- Graves EE, Nelson SJ, Vigneron DB, Verhey L, McDermott M, Larson D, Chang S, Prados MD, Dillon WP. Serial proton MR spectroscopic imaging of recurrent malignant gliomas after gamma knife radiosurgery. Am J Neuroradiol. 2001;22:613–624. [PMC free article] [PubMed] [Google Scholar]