Abstract
Parkinson's disease (PD) is a common neurodegenerative movement disorder. Whereas the majority of PD cases are sporadic, rare genetic defects have been linked to this prevalent movement disorder. Mutations in DJ-1 are associated with autosomal recessive early-onset PD. The exact biochemical function of DJ-1 has remained elusive. Here we report the generation of DJ-1 knockout (KO) mice by targeted deletion of exon 2 and exon 3. There is no observable degeneration of the central dopaminergic pathways, and the mice are anatomically and behaviorally similar to WT mice. Fluorescent Amplex red measurements of H2O2 indicate that isolated mitochondria from young and old DJ-1 KO mice have a 2-fold increase in H2O2. DJ-1 KO mice of 2–3 months of age have a 60% reduction in mitochondrial aconitase activity without compromising other mitochondrial processes. At an early age there are no differences in antioxidant enzymes, but in older mice there is an up-regulation of mitochondrial manganese superoxide dismutase and glutathione peroxidase and a 2-fold increase in mitochondrial glutathione peroxidase activity. Mutational analysis and mass spectrometry reveal that DJ-1 is an atypical peroxiredoxin-like peroxidase that scavenges H2O2 through oxidation of Cys-106. In vivo there is an increase of DJ-1 oxidized at Cys-106 after 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine intoxication of WT mice. Taken together these data indicate that the DJ-1 KO mice have a deficit in scavenging mitochondrial H2O2 due to the physiological function of DJ-1 as an atypical peroxiredoxin-like peroxidase.
Keywords: glutathione peroxidase, mitochondria, manganese superoxide dismutase, PARK7, Parkinson's disease
Parkinson's disease (PD) is a common progressive neurodegenerative movement disorder (1) caused by the selective loss of dopaminergic neurons in the substantia nigra, pars compacta (2, 3). Although in most cases the etiology of PD is not known, its pathogenesis may involve deficits in mitochondrial function, oxidative stress, excitotoxicity, inflammation, accumulation of aberrant or misfolded proteins (Lewy bodies), and ubiquitin–proteosome system dysfunction (2, 3). PD is essentially a sporadic disorder of the aging brain, but ≈10% of all cases are linked to a variety of genetic defects (4, 5). The identification of some of these genes has opened new areas of research (4, 5). In 2003, Bonifati et al. (6) found that loss-of-function mutations in the DJ-1 locus were associated with rare forms of autosomal recessive early-onset parkinsonism with psychiatric and behavioral disturbances, slow progression, and a good response to treatment with levodopa. DJ-1 mutations account for 1–2% of all early-onset PD (7–9), with a number of different pathogenic mutations, including exonic deletions, truncations, and homozygous and heterozygous point mutations.
DJ-1 is a highly conserved protein that belongs to the DJ-1/Thi/PfpI protein superfamily. In vertebrates it is expressed in a variety of tissues including brain (10), and at a subcellular level it is found in the matrix and the intermembrane space of the mitochondria (11). Although the biology of DJ-1 has only begun to be elucidated, it seems that DJ-1 operates as an antioxidant protein (12) and that its ablation in mice exacerbates, via an unknown mechanism, dopaminergic neurodegeneration caused by the parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) (13). Here we show that the absence of DJ-1 in mice does not lead to major behavioral, neurochemical, or anatomical deficits in the dopaminergic system, as previously described (14, 15). However, examinations of mitochondria from mutant mice deficient in DJ-1 coupled with biochemical and mass spectrometry analyses reveal that DJ-1 functions as an atypical peroxiredoxin-like peroxidase.
Results
Generation of DJ-1 Knockout (KO) Mice.
DJ-1 was disrupted by partial deletion of exon 2, deletion of exon 3, and the introduction of a stop codon and a neo selection cassette [supporting information (SI) Fig. 4A]. Heterozygous male and female mice were bred to generate homozygote mice. Southern blot analysis and PCR (SI Fig. 4B) confirm disruption of DJ-1. Mendelian frequencies are observed from the heterozygous matings for DJ-1 KO mice, consistent with a normal embryonic development. DJ-1 KO animals are fertile and show no differences in weight, gross anatomy, and longevity (data not shown). Northern blot and immunoblot analyses from DJ-1 KO mouse brains confirms the lack of endogenous DJ-1 transcript and DJ-1 protein (SI Fig. 4C).
Absence of Abnormalities in the Nigrostriatal Pathway in DJ-1 KO Mice.
We examined the total content of dopamine and its metabolites in striatal tissue and the morphology of the dopaminergic neurons in DJ-1 KO and WT age-matched littermate mice. No significant change in the levels of dopamine and its major metabolites 3,4-dihydroxyphenylactic acid and homovanillic acid are observed in DJ-1 KO mice compared with littermate age-matched WT mice (SI Fig. 4D). We next histologically examined the nigrostriatal dopaminergic system. In 18- to 24-month-old mice there is no noticeable difference in either the dopaminergic innervation of the striatum (SI Fig. 4 E and F) or the neuronal network in the SNpc (SI Fig. 4 G and H) as assessed by tyrosine hydroxylase immunoreactivity. Stereological quantification of tyrosine hydroxylase- and Nissl-positive neurons in SNpc was performed in 2- to 3-month-old and 18- to 24-month-old mice (SI Fig. 4I). There is no difference in dopaminergic neuron numbers in SNpc of DJ-1 KO mice compared with WT mice (SI Fig. 4I). To determine whether DJ-1 KO mice and WT mice exhibit motor behavior abnormalities, we carried out open-field analyses and found no difference in spontaneous horizontal and vertical locomotion between DJ-1 KO and WT in young and aged mice (SI Fig. 4J).
DJ-1 KO Mice Show an Increase in Mitochondrial H2O2 Production.
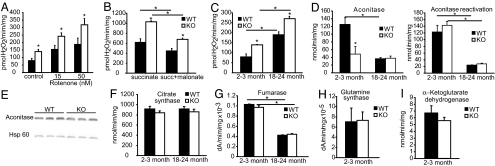
Because previous studies indicate that DJ-1 is expressed in the mitochondria (11, 16), we explored whether the absence of DJ-1 leads to mitochondrial dysfunction. Fluorescent Amplex red measurements of H2O2 were made with isolated brain mitochondria incubated in the presence or absence of rotenone in 2- to 3-month-old KO and WT mice (Fig. 1A). Isolated mitochondria from DJ-1 KO mice have an ≈2-fold increase in H2O2 production compared with control WT littermate mice. Rotenone has no statistically significant effect on the genotypic difference in Amplex red signal, suggesting that impairment in mitochondrial complex I activity does not account for the elevation in H2O2. Additionally, brain mitochondria from 2- to 3-month-old DJ-1 KO and WT mice were incubated in the presence or absence of succinate or succinate/malonate (17). Succinate-supported H2O2 production is elevated in DJ-1 KO mice compared with control mice (Fig. 1B). The presence of malonate comparably decreases the rate of the H2O2 generation in both DJ-1 KO and WT mice. Comparison of mitochondrial H2O2 production between young (2–3 months) and aged (18–24 months) mice reveals that the increase in H2O2 in the DJ-1 KO mice compared with WT is age-independent (Fig. 1C).
Fig. 1.
Increase in H2O2 production and deficits in aconitase activity. (A) Significant increase of mitochondrial H2O2 production in KO mice compared with WT littermates; brain-isolated mitochondria were incubated in the presence or absence of rotenone in young mice (2–3 months). (B) Brain mitochondria from KO and WT mice (2–3 months old) were incubated in the presence or absence of succinate or succinate plus malonate. (C) Comparison of the mitochondrial H2O2 production in young (2–3 months) and aged (18–24 months) mice. H2O2 production was measured by using Amplex red (Molecular Probes, Eugene, OR). H2O2 production was calculated from a standard curve generated from known concentrations of H2O2. (D) Mitochondrial aconitase activity in isolated mitochondria from brains of 2- to 3-month-old and 18- to 24-month-old WT and KO mice. Then mitochondrial aconitase was reactivated as described in Materials and Methods, and the activity was measured. (E) Mitochondrial proteins were subjected to immunoblot with densitometric analysis by using hsp70 as a loading control. No differences in protein expression were found. (F and G) Mitochondrial citrate synthase and fumarase activity of brains in 2- to 3-month-old and 18- to 24-month-old mice was measured. (H and I) No differences were found in glutamine synthase and α-ketoglutarate dehydrogenase activity between mitochondria isolated from 2- to 3-month-old KO mice and WT littermates. Significance was determined by a two-way ANOVA with the Student–Newman–Keuls test. *, P ≤ 0.05. Data are the means ± SEM (n = 3).
Inactivation of Mitochondrial Aconitase in DJ-1 KO Brain.
Previous reports show that the activity of mitochondrial aconitase is highly sensitive to reactive oxygen species (18, 19). We therefore assayed the activity of aconitase and the main enzymes in the Krebs cycle from mitochondria isolated from DJ-1 KO and WT mouse brains. There is no difference in the activity of mitochondrial citrate synthase, fumarase, glutamine synthase, and α-ketoglutarate dehydrogenase in 2- to 3-month-old and 18- to 24-month-old DJ-1 KO mice compared with control littermates (Fig. 1 F–I). In contrast, brain mitochondria isolated from 2- to 3-month-old DJ-1 KO mice reveal a ≥60% reduction in aconitase activity compared with WT mice (Fig. 1D), but there is no change in total aconitase enzyme activity (mitochondrial and cytosolic) in whole brain lysates (data not shown). Although no difference is noted in mitochondrial aconitase activity between 18- to 24-month-old mice, this enzymatic activity is reduced by >65% in the older mice compared with the younger animals (Fig. 1D) as a consequence of the age-dependent loss of aconitase activity (20, 21). There are no differences in the level of aconitase protein as assessed by immunoblot analysis in DJ-1 KO versus WT mice (Fig. 1E). To determine whether the loss of aconitase activity in the mitochondria was due to reversible inactivation, mitochondrial extracts were subjected to in vitro reactivation. After reactivation mitochondrial aconitase activity is restored only in the 2- to 3-month-old mice. These results suggest that the elevated H2O2 is inactivating mitochondrial aconitase, at least in young mice. To determine whether the elevated H2O2 could compromise other mitochondrial processes, we assessed the mitochondrial carbonyl content (SI Fig. 5), O2 consumption, ATP levels, and cytochrome c release in response to Bid, the MPTP metabolite 1-methyl-4-phenylpyridinium, and calcium (SI Fig. 6). No difference is found in the carbonyl content in 2- to 3-month-old and 18- to 24-month-old mice (SI Fig. 5A) and the oxyblot of whole brain lysates and mitochondrial fractions (SI Fig. 5 B and C). There is no difference in O2 consumption between the DJ-1 KO and control WT littermate animals, when the respiration was driven by either complex I or complex II (SI Fig. 6A). No difference is observed in the production of ATP in absence and presence of the complex I inhibitor rotenone between DJ-1 KO mice and WT littermate controls (SI Fig. 6B). Moreover, cytochrome c release is not different in response to Bid, 1-methyl-4-phenylpyridinium, or calcium in 2- to 3-month-old (SI Fig. 6C) and 18- to 24-month-old DJ-1 KO mice and WT littermate mice (data not shown). These results taken together indicate that the loss of DJ-1 leads to excess H2O2 and loss of aconitase activity, but these abnormalities are not sufficient to inflict overt mitochondrial damage or impair cell viability.
Mitochondrial Compensatory Mechanisms in DJ-1 KO Mice.
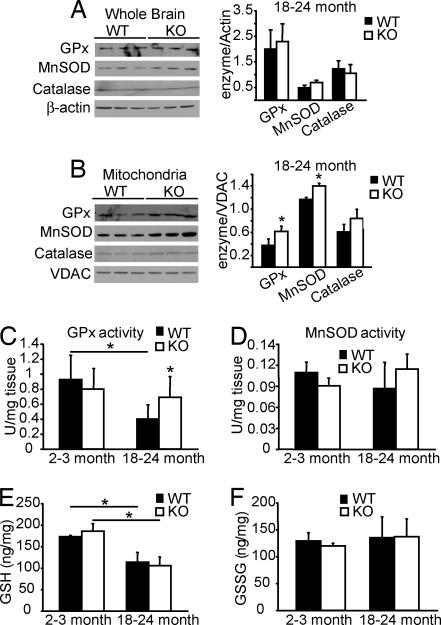
Although there is a 2-fold increase in the mitochondrial H2O2 production in DJ-1 KO brains (Fig. 1 A and C), the mice show no degenerative phenotype (SI Fig. 4), suggesting that a compensatory mechanism has been invoked. Normally, reactive oxygen species are removed by antioxidant enzymes such as catalase, glutathione peroxidase (GPx), catalase, copper zinc superoxide dismutase, and manganese superoxide dismutase (MnSOD) that are localized in the cytosol and in the mitochondria. We examined the possibility of whether an up-regulation of these enzymes could compensate for the elevated H2O2. In young (2–3 months) mice protein expression of the antioxidant enzymes in the mitochondria and cytosol is not different (data not shown). However, we find an up-regulation of MnSOD and GPx expression in the mitochondrial but not the cytosolic fractions from 18- to 24-month-old mice (Fig. 2 A and B). Concomitantly, we observe a 2-fold increase in GPx activity in isolated mitochondria from 18- to 24-month-old DJ-1 KO mice compared with WT littermates (Fig. 2C), whereas MnSOD activity is not changed (Fig. 2D). Consistent with the absence of alterations in protein expression of antioxidant enzymes in young mice, no change in GPx and MnSOD activity is observed in 2- to 3-month-old mice in mitochondria (Fig. 2 C and D) and in the cytosol fractions (data not shown). These compensatory increases in GPx levels and activity appear to compensate for lack of DJ-1 because the cytosolic levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) and the ratio GSSG/GSH in DJ-1 KO brains do not differ significantly compared with their respective WT littermates (Fig. 2 E and F). These results taken together suggest that the absence of DJ-1 leads to an increase in mitochondrial H2O2 and a compensatory increase in mitochondrial GPx activity in aged DJ-1 KO mice, suggesting that DJ-1 plays an important in vivo role in mitochondrial elimination of H2O2.
Fig. 2.
Compensatory mechanism in DJ-1 KO mice. Shown are immunoblot and densitometric analyses of the protein expression of GPx, MnSOD, and catalase in whole brain lysates with β-actin as a loading control (A) and VDAC as a loading control for the mitochondrial fraction (B) in aged mice (18–24 months old). Data are the means ± SEM (n = 3). Significance was determined by Student's t test. (C and D) Mitochondrial activity of antioxidant enzymes of young (2–3 months old) and aged (18–24 months old) WT and KO mice: GPx (C) and MnSOD (D). (E and F) Cytosolic levels of reduced (E) and oxidized (F) forms of glutathione in 2- to 3-month-old and 18- to 24-month-old mice. The enzyme activities are expressed in units per milligram of tissue whereas the reduced and oxidized forms of glutathione are expressed as nanograms per milligram of protein. Data represent the means ± SEM from samples of five different animals measured three times on different days. Significance was determined by ANOVA with the Student–Newman–Keuls test. *, P ≤ 0.05.
DJ-1 Is an Atypical Peroxiredoxin-Like Peroxidase.
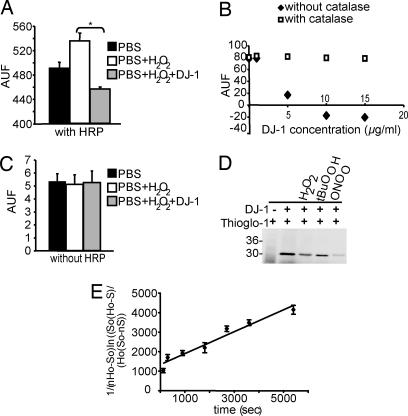
To explore whether DJ-1 directly scavenges H2O2 and the potential mechanism, we incubated recombinant DJ-1 with H2O2 and measured H2O2 concentration via the Amplex red assay. Recombinant DJ-1 significantly decreases H2O2 levels compared with the reaction without DJ-1 (Fig. 3A) consistent with prior reports (16, 22). DJ-1 abates the H2O2 Amplex red signal in a dose-dependent manner, and this reaction is abolished by purified catalase (Fig. 3B). To determine whether DJ-1 possesses peroxidase activity, we conducted the Amplex red assay in the absence of HRP. Amplex red is a coupled reaction that utilizes HRP, and in absence of HRP there is no signal (Fig. 3C). In the absence of HRP DJ-1 has no effect on the Amplex red assay (Fig. 3C), indicating that DJ-1 does not function as a peroxidase. To ascertain whether DJ-1 has catalase-like activity we measured O2 formation with a Clark electrode and found that DJ-1 has no catalase activity in the presence of H2O2 (data not show). These results taken together indicate that DJ-1 does not scavenge H2O2 in a manner similar to a peroxidase or a catalase.
Fig. 3.
DJ-1 consumes H2O2. (A) Recombinant DJ-1 (5 μg/ml) incubated with 10 nM H2O2 reduced the H2O2 concentration in the reaction shown by the Amplex red assay. (B) Recombinant DJ-1 consumes H2O2 in a dose-dependent manner. This reaction is abolished by purified catalase. DJ-1 does not exhibit catalase-like activity as assessed by Clark electrode (data not show). (C) In the absence of HRP there is no signal, indicating that DJ-1 is not peroxidase-like. (D) Representative in-gel detection of cysteine residues labeled with Thioglo-1. Lane 1, Thioglo-1 without protein; lane 2, human DJ-1; lane 3, DJ-1 reacted with 250 μM hydrogen peroxide for 60 min at 37°C; lane 4, DJ-1 reacted with 250 and 500 μM t-butyl hydroperoxide for 60 min at 37°C; lane 5, DJ-1 reacted with 250 μM peroxynitrite for 5 min at 37°C. (E) Second-order rate constant determination for the reaction between 250 μM H2O2 and DJ-1 at 37°C using the times indicated. Catalase was added to remove excess H2O2, and samples were assayed for nonreactive cysteine by using Thioglo-1 labeling. Data represent the mean and SD from three different analyses. Significance was determined by ANOVA with the Student–Newman–Keuls test. *, P ≤ 0.05.
To ascertain whether DJ-1 scavenges H2O2 similar to peroxiredoxins, we determined whether cysteines in DJ-1 account for its peroxidase-like activity. DJ-1 was incubated with H2O2 (250 μM) or t-butyl hydroperoxide (250 μM) or peroxynitrite/peroxynitrous acid (250 μM) and treated with Thioglo-1. The samples of Thioglo-1-labeled proteins were electrophoresed, and the fluorescent densitometric analysis of the fluorescence of Thioglo-1 revealed the loss of reduced cysteine labeling upon the reaction of DJ-1 with the different peroxides (Fig. 3D). The reaction of H2O2 with DJ-1 obeys second-order rate kinetics (Fig. 3E). The calculated second-order rate constant is 0.56 ± 0.05 M−1·s−1, which is typical for reactive cysteine residues. For example, the single cysteine residue in BSA has an apparent second-order rate constant of 1.14 M−1·s−1 upon reaction with H2O2 (23). By mass spectrometry analysis, we observe that Cys-106 is the principal target for H2O2 resulting in the formation of sulfinic acid (−SO2H) as revealed by the increase of 32 ± 1 atomic mass units after the reaction of DJ-1 with 25-fold excess H2O2 (SI Fig. 7 and Table 1). Under the same reaction conditions, sulfinic acid is observed in C53A and C46A but not in C106A mutant DJ-1, confirming that Cys-106 is modified primarily by H2O2. The oxidation to sulfinic acid was confirmed by treating the reacted protein with arsenite, which reduces sulfenic but not sulfinic acid to reduced cysteine. The arsenite-treated protein after the reaction with H2O2 showed an increase of 32 atomic mass units, indicating that indeed sulfinic acid was formed and not two sulfenic acid residues (Table 1). Excess of the tripeptide glutathione (GSH, 5 mM) partially prevents the oxidation of Cys-106 by H2O2 but did not result in glutathiolation of the protein because no other DJ-1 species were detected by mass spectrometry in the presence of GSH (Table 1). These data indicate that Cys-106 is selectively sensitive to H2O2 even in the presence of reducing molecules despite exhibiting a modest second-order rate constant. Because the surface exposure of Cys-106 is relatively less than the other two-cysteine residues, the selectivity of oxidation could be explained by the ability of peroxides to access Cys-106 whereas even small tripeptides such as GSH may not have access. DJ-1 was also sensitive to oxidation by other peroxides such as t-butyl hydroperoxide and peroxynitrite/peroxynitrous acid. The kinetics of the reaction between DJ-1 and peroxynitrite/peroxynitrous acid were determined by stopped-flow spectroscopy at pH 7.4 and 25°C. The calculated apparent second order for the reaction of DJ-1 with peroxynitrite/peroxynitrous acid was 2.7 × 105 M−1·s−1, which is faster to the reactivity of the single cysteine residue in BSA but slower than bacterial and human peroxiredoxins (typical second-order rate constants in the range of 106 to 107 M−1·s−1) (24, 25). To examine DJ-1 peroxiredoxin activity in vivo, WT and KO mice were injected with MPTP (20 mg/kg every 2 h four times) or saline. Lysates of whole brain and the mitochondrial fraction were prepared from saline- or MPTP-treated mice. DJ-1 oxidized at Cys-106 was analyzed by immunoblot of whole brain lysates, after DJ-1 was immunoprecipitated with anti-DJ-1 antibody, and in the mitochondrial fraction. MPTP intoxication of mice leads to a 2-fold increase of DJ-1 oxidized at Cys-106/total DJ-1 ratio in the whole brain lysates (SI Fig. 8A) and a 3-fold increase of DJ-1 oxidized at Cys-106/VDAC ratio in the mitochondria fraction (SI Fig. 8B) in WT mice treated with MPTP compared with saline. Taken together these results indicate that DJ-1 is an atypical peroxiredoxin-like peroxidase that scavenges H2O2 through oxidation of Cys-106 to sulfinic acid.
Table 1.
Mass spectrometric evaluation of DJ-1 reacted with H2O2
| Protein | Detected mass | Δmass, atomic mass units |
|---|---|---|
| WT | 20,302 | |
| WT + H2O2 | 20,333 | +32 |
| 20,303 | ||
| C106A mutant | 20,270 | |
| C106A mutant + H2O2 | 20,270 | |
| C46A mutant | 20,268 | |
| 20,299 | +32 | |
| C46A mutant + H2O2 | 20,300 | +32 |
| C53A mutant | 20,301 | +32 |
| 20,270 | ||
| C53A mutant + H2O2 | 20,300 | +32 |
| 20,268 | ||
| WT + H2O2+ arsenite | 20,331 | +32 |
| 20,299 | ||
| C53A mutant + H2O2 + arsenite | 20,300 | +32 |
| 20,266 | ||
| WT + H2O2 + GSH (5 mM) | 20,303 | |
| 20,334 | +32 |
The protein was reacted with H2O2, and changes in protein mass were determined by ESI/mass spectrometry. Detected masses are listed in order of relative abundance within the same treatment (SI Fig. 7). The C46A and C53A mutant proteins contained oxidized protein molecules without treatment with H2O2, indicating an increased sensitivity to oxidation. Δmass reflects the difference in mass relative to the unmodified protein. All detected masses have errors of no more than ±2 atomic mass units.
Discussion
In the present study we show that DJ-1 has a functional role in scavenging mitochondrial H2O2 because of its physiological action as an atypical peroxiredoxin-like peroxidase. Furthermore, oxidative conditions induce the formation of sulfinic acid of Cys-106 of DJ-1. These findings further confirm and extend the results of in vitro studies and previous reports in cellular models (16, 22, 26). The mice do not show loss of dopaminergic neurons as previously observed (14), but the lack of DJ-1 in the mitochondria induces an increase of mitochondrial H2O2 production in the mice and, as a consequence, a reversible decrease in aconitase activity. The increase in mitochondrial H2O2 is detected in young and old mice whereas the decrease in aconitase activity is observed only in young mice. This may be because of the compensatory responses such as the increase in GPx expression in DJ-1 KO mice. GPx is one of the major defense molecules against cytotoxic reactive oxygen species (27, 28); increased expression of GPx provides neuroprotection against toxic stimuli (28, 29). The increase in GPx in the DJ-1 KO mice likely contributes to the normal mitochondrial function and tissue viability in these animals.
Based on structural and sequence homology the human DJ-1 protein belongs to the DJ-1/ThiJ/PfpI superfamily of proteins. Of the three cysteine residues in DJ-1, Cys-106 is conserved among the proteins of this superfamily (30–33). This conserved cysteine residue has been proposed as the catalytic nucleophile for the protease activity of the PfpI family (30, 34). In the crystal structure of DJ-1 a histidine residue (His-126) is within 3.7–5 Å from Cys-106, but the absence of an acidic residue in the vicinity of His-126 precludes DJ-1 from possessing protease activity like other members of this superfamily of proteins.
A cluster of acidic residues, Glu-15, Glu-16, Glu-18, and Asp-24, and two basic residues, Arg-48 and Arg-28, are within 8 Å from Cys-106, and they could potentially influence the reactivity of Cys-106. The kinetics of the reaction are considerably slower than the typical mammalian and bacterial peroxidase (second-order rate constants in the range of 2 × 105 to 1.8 × 108 M−1·s−1) (25, 35, 36). Cys-106 is sufficiently shielded, and access is limited, allowing reactivity toward peroxides but not to peptides. Whereas the lack of surface exposure provides selectivity toward small peroxides, the estimated local pKa of Cys-106 of 11.38 (using the propka algorithm) implies that at physiological pH Cys-106 will be protonated and therefore will likely react relatively more slowly with peroxides as previously reported (26). Indeed most of the reactive cysteine residues in peroxiredoxins have lower pKa values, which allows deprotonation to a more reactive thiolate anion, thus favoring fast second-order rate constants with peroxides (37). Collectively these data suggest that DJ-1 is likely an atypical peroxiredoxin that reacts exclusively with peroxides with cysteine residue Cys-106 representing the primary target (37, 38).
Furthermore, unlike other peroxiredoxins that are overoxidized to sulfinic acid, DJ-1 is not a substrate for the sulfenic acid reductase (39). Therefore, DJ-1 will not be functionally restored. This is an interesting observation because several reports provide evidence that DJ-1 functions as a cytoplasmic redox-sensitive molecular chaperone of α-synuclein, and this chaperone activity is stimulated by oxidation (40, 41). Thus, Cys-106-oxidized forms of DJ-1 could inhibit α-synuclein fibrillation, but this protective effect of DJ-1 against α-synuclein aggregation would be lost during overoxidization and inactivation of DJ-1. These data are consistent with the observations that oxidative damage of DJ-1 is linked to sporadic PD (42, 43).
In summary, we report that DJ-1 is an atypical peroxiredoxin-like peroxidase and that loss of DJ-1 expression leads to accumulation of mitochondrial H2O2. This increase in mitochondrial H2O2 is accompanied by a compensatory increase in the scavenging protein GPx. Identification of the biochemical function of DJ-1 and the consequences of the loss of DJ-1 function hold promise toward understanding the pathogenesis of familial and idiopathic PD.
Materials and Methods
Gene Targeting and Generation of DJ-1 Null Mice.
DJ-1 KO mice were generated by gene targeting of part of exon 2 and exon 3 with replacement by a loxP flanked neomycin selection cassette. Embryonic stem cells carrying the mutant allele were injected into blastocysts, and the resulting male chimeric mice were bred to C57BL/6 female mice to obtain heterozygous DJ-1 male and female mice, which were subsequently bred to generate DJ-1 KO mice. PCR genotyping was performed by using the following primers: forward 1, 5′-TTG GCT GTA TCC GTG ACT GCA GT-3′; forward 2, 5′-TGC TAA AGC GCA TGC TCC AGA CT; reverse, 5′-CAT CTC TAC AGC CCA GGT AGT GA-3′.
Southern and Northern Blot Analysis.
DNA extracted from kidney and RNA extracted from mouse brain were used to carry out Southern and Northern blot analysis as described previously (44) with visualization and documentation acquired by a phosphorimager system (Cyclone; Packard, Meriden, CT).
Immunoblot Analysis.
Primary antibodies used for immunoblot were anti-DJ-1 (11), anti-SOD, anti-GPx, anti-catalase, anti-Hsp70, anti-VDAC, and anti-aconitase (Abcam, Cambridge, MA), anti-DJ-1 oxidized at Cys-106 (AbD Serotec, Oxford, U.K.), and anti-actin (Sigma–Aldrich, St. Louis, MO). Immunoreactive bands were detected with HRP-conjugated anti-rabbit or anti-mouse secondary or anti-myc antibodies (Amersham) and visualized with SuperSignal West Pico or SuperSignal West Femto substrates (Pierce, Rockford, IL).
HPLC Electrochemical Measurement of Catecholamines.
Dopamine and its metabolites were assessed by HPLC analyses as previously described (44).
Immunohistochemistry and Stereological Cell Counts.
Free-floating 30-μm sections were incubated with antibody against tyrosine hydroxylase (rabbit polyclonal; Novus Biologicals, Littleton, CO) followed by incubation with biotin-conjugated anti-rabbit antibody (goat polyclonal; Jackson ImmunoResearch, West Grove, PA), ABC reagents (Vector Laboratories, Burlingame, CA), and SigmaFast DAB Peroxidase Substrate. Sections were counterstained with Nissl (0.09% thionin). Cell counts of tyrosine hydroxylase-positive and Nissl-positive neurons of the SNpc were counted by using optical fractionator STEREO INVESTIGATOR software (MicroBrightField, Williston, VT) (45, 46).
Behavioral Analysis.
An open-field test was performed as described previously in 2- to 3-month-old and 18- to 24-month-old male and female mice (44).
Isolation of Brain Mitochondria and Polarography.
Isolation of brain mitochondria and monitoring of mitochondrial oxygen consumption were performed as described (47, 48).
Measurements of Mitochondrial H2O2.
For information about mitochondrial H2O2 measurements see SI Materials and Methods.
ATP Measurements, Cytochrome c Release Studies, and Measurements of Mitochondrial H2O2 Production.
For information about ATP measurements, the percentage of cytochrome c release, and mitochondrial H2O2 production see SI Materials and Methods.
Enzyme Assays and Glutathione Assay.
Assay conditions for tricarboxylic acid enzymes were based on established protocols (49–54). For information about aconitase and α-ketoglutarate dehydrogenase activity assay see SI Materials and Methods. GPx and superoxide dismutase activity were measured by using s Glutathione Peroxidase Assay Kit and s Superoxide Dismutase Assay Kit (Calbiochem, San Diego, CA) following the manufacturer's protocol. Glutathione levels were measured fluorimetrically following the method of Cohn and Lyle employing o-phthalaldehyde (55, 56).
Recombinant DJ-1 Expression and Purification.
WT DJ-1 and cysteine mutants were subcloned in frame with GST in pGEX-6P-1 expression vector and transformed into Escherichia coli BL21 cells. Protein expression was induced with the addition of isopropyl-β-d-thioglactopyranoside. Recombinant GST DJ-1 was purified and digested by using the manufacturer's protocol (Amersham Pharmacia Biotech, Piscataway, NJ).
DJ-1 Consumptions of Hydrogen Peroxide.
Treatment of DJ-1 and Labeling of Free SH Groups with Thioglo-1.
WT and mutant proteins were SDS-denatured (20 mM) for 1 h and then treated with Thioglo-1 (60 μM) for 90 min at 50°C. Thioglo-1-labeled proteins were electrophoresed and visualized with a Fluor MultiImager (Bio-Rad, Hercules, CA). The second-order rate constant was estimated by plotting (1/nHo − So)ln[So(Ho − S)/Ho(So − nS)] versus time as described (23). The second-order rate constant for the reaction with peroxynitrite was measured by stopped-flow analysis (23).
Mass Spectrometric Analysis.
Mass spectrometry was performed on an Agilent 1100 Series quadrupole mass spectrometer equipped with an electrospray ion source, and the spectrometer was operated in positive ion mode as described previously (57).
MPTP Injections.
All procedures involving animals were approved by and conformed to the guidelines of the Institutional Animal Care Committee of Johns Hopkins University. Animals received four injections i.p. of MPTP·HCl (20 mg/kg free base; Sigma–Aldrich, St. Louis, MO) in saline or saline alone at 2-h intervals. Animals were decapitated 7 days after the last MPTP injection, and brains were resected and processed for Western blot analysis.
Statistical Analysis.
Pooled results were expressed as means ± SEM. Significance was determined by one-way or two-way ANOVA followed by the Student–Newman–Keuls test or a two-tailed nonpaired Student t test. Significance was set at P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank Daniel Trinkaus for technical help. E.A.-M. has a Postdoctoral Fellowship from Ministerio de Educación y Ciencia, Spain. S.P. is the Page and William Black Professor of Neurology. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases. This work was supported by U.S. Public Health Service Grants NS38377, NS054817, NS16375, NS42269, NS38370, NS11766, AG 21617, ES013177, AG13966, and ES013508; National Institute on Environmental Health Sciences Center of Excellence in Environmental Toxicology/U.S. Department of Defense Grant DAMD-17-03-1; the National Parkinson Foundation; the American Parkinson Disease Association; and the Parkinson's Disease Foundation.
Abbreviations
- PD
Parkinson's disease
- KO
knockout
- GPx
glutathione peroxidase
- MnSOD
manganese superoxide dismutase
- GSH
reduced glutathione
- MPTP
1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703219104/DC1.
References
- 1.Fahn S, Przedborski S. In: Merritt's Neurology. Rowland LP, editor. New York: Lippincott Williams and Wilkins; 2005. pp. 828–846. [Google Scholar]
- 2.Dauer W, Przedborski S. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 3.Moore DJ, West AB, Dawson VL, Dawson TM. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 4.Dawson TM, Dawson VL. J Clin Invest. 2003;111:145–151. doi: 10.1172/JCI17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vila M, Przedborski S. Nat Med. 2004;10(Suppl):S58–S62. doi: 10.1038/nm1068. [DOI] [PubMed] [Google Scholar]
- 6.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, et al. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. Ann Neurol. 2003;54:283–286. doi: 10.1002/ana.10675. [DOI] [PubMed] [Google Scholar]
- 8.Hague S, Rogaeva E, Hernandez D, Gulick C, Singleton A, Hanson M, Johnson J, Weiser R, Gallardo M, Ravina B, et al. Ann Neurol. 2003;54:271–274. doi: 10.1002/ana.10663. [DOI] [PubMed] [Google Scholar]
- 9.Hedrich K, Djarmati A, Schafer N, Hering R, Wellenbrock C, Weiss PH, Hilker R, Vieregge P, Ozelius LJ, Heutink P, et al. Neurology. 2004;62:389–394. doi: 10.1212/01.wnl.0000113022.51739.88. [DOI] [PubMed] [Google Scholar]
- 10.Olzmann JA, Bordelon JR, Muly EC, Rees HD, Levey AI, Li L, Chin LS. J Comp Neurol. 2007;500:585–599. doi: 10.1002/cne.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 12.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, et al. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Cagniard B, Mathews T, Jones S, Koh HC, Ding Y, Carvey PM, Ling Z, Kang UJ, Zhuang X. J Biol Chem. 2005;280:21418–21426. doi: 10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi H, Shen J. Mol Neurodegener. 2007;2:10. doi: 10.1186/1750-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Fiskum G, Schubert D. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 18.Gardner PR, Fridovich I. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 19.Tretter L, Adam-Vizi V. J Neurosci. 2000;20:8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarian CS, Toroser D, Sohal RS. Mech Ageing Dev. 2006;127:79–84. doi: 10.1016/j.mad.2005.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan LJ, Levine RL, Sohal RS. Proc Natl Acad Sci USA. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinumi T, Kimata J, Taira T, Ariga H, Niki E. Biochem Biophys Res Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- 23.Radi R, Beckman JS, Bush KM, Freeman BA. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 24.Bryk R, Griffin P, Nathan C. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 25.Dubuisson M, Vander Stricht D, Clippe A, Etienne F, Nauser T, Kissner R, Koppenol WH, Rees JF, Knoops B. FEBS Lett. 2004;571:161–165. doi: 10.1016/j.febslet.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 26.Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y. Free Radical Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 27.Desagher S, Glowinski J, Premont J. J Neurosci. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishida Y, Nagai A, Kobayashi S, Kim SU. J Neuropathol Exp Neurol. 2006;65:66–77. doi: 10.1097/01.jnen.0000195941.48033.eb. [DOI] [PubMed] [Google Scholar]
- 29.Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Richfield EK, Buckley B, Mirochnitchenko O. J Biol Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Kim SJ, Kim IK, Ko J, Jeong CS, Kim GH, Park C, Kang SO, Suh PG, Lee HS, Cha SS. J Biol Chem. 2003;278:44552–44559. doi: 10.1074/jbc.M304517200. [DOI] [PubMed] [Google Scholar]
- 31.Honbou K, Suzuki NN, Horiuchi M, Niki T, Taira T, Ariga H, Inagaki F. J Biol Chem. 2003;278:31380–31384. doi: 10.1074/jbc.M305878200. [DOI] [PubMed] [Google Scholar]
- 32.Tao X, Tong L. J Biol Chem. 2003;278:31372–31379. doi: 10.1074/jbc.M304221200. [DOI] [PubMed] [Google Scholar]
- 33.Wilson MA, Collins JL, Hod Y, Ringe D, Petsko GA. Proc Natl Acad Sci USA. 2003;100:9256–9261. doi: 10.1073/pnas.1133288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honbou K, Suzuki NN, Horiuchi M, Taira T, Niki T, Ariga H, Inagaki F. Acta Crystallogr D. 2003;59:1502–1503. doi: 10.1107/s090744490301271x. [DOI] [PubMed] [Google Scholar]
- 35.Harrison WH, Whisler WW, Hill BJ. Biochemistry. 1968;7:3089–3094. doi: 10.1021/bi00849a010. [DOI] [PubMed] [Google Scholar]
- 36.Baker LM, Raudonikiene A, Hoffman PS, Poole LB. J Bacteriol. 2001;183:1961–1973. doi: 10.1128/JB.183.6.1961-1973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee SG, Chae HZ, Kim K. Free Radical Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Wood ZA, Schroder E, Robin Harris J, Poole LB. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 39.Woo HA, Jeong W, Chang TS, Park KJ, Park SJ, Yang JS, Rhee SG. J Biol Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 40.Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. J Mol Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, Gearing M, Levey AI, Chin LS, Li L. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Proc Natl Acad Sci USA. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Proc Natl Acad Sci USA. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM. Proc Natl Acad Sci USA. 1999;96:5774–5779. doi: 10.1073/pnas.96.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West MJ. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 47.Tieu K, Perier C, Caspersen C, Teismann P, Wu D-C, Yan S-D, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, Vila M. Proc Natl Acad Sci USA. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hausladen A, Fridovich I. Methods Enzymol. 1996;269:37–41. doi: 10.1016/s0076-6879(96)69007-7. [DOI] [PubMed] [Google Scholar]
- 50.Lai JC, Cooper AJ. J Neurochem. 1986;47:1376–1386. doi: 10.1111/j.1471-4159.1986.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 51.Levintow L, Meister A. J Biol Chem. 1954;209:265–280. [PubMed] [Google Scholar]
- 52.Robinson JB, Jr, Inman L, Sumegi B, Srere PA. J Biol Chem. 1987;262:1786–1790. [PubMed] [Google Scholar]
- 53.Srere PA. J Biol Chem. 1966;241:2157–2165. [PubMed] [Google Scholar]
- 54.Srere PA, Brooks GC. Arch Biochem Biophys. 1969;129:708–710. doi: 10.1016/0003-9861(69)90231-8. [DOI] [PubMed] [Google Scholar]
- 55.Cohn VH, Lyle J. Anal Biochem. 1966;14:434–440. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- 56.Thomas B, Mohanakumar KP. J Pineal Res. 2004;36:25–32. doi: 10.1046/j.1600-079x.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 57.Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.