Abstract
The estrogen receptor-α (ERα) is a critical transcription factor that regulates epithelial cell proliferation and ductal morphogenesis during postnatal mammary gland development. Tissue recombination and transplantation studies using the first generation of ERα knockout (ERKO) mice suggested that this steroid hormone receptor is required in the mammary stroma that subsequently exerts its effect on the epithelium through additional paracrine signaling events. A more detailed analysis revealed that ERKO mice produce a truncated ERα protein with detectable transactivation activity, and it is likely that this functional ERα variant has masked the biological significance of this steroid receptor in the mammary epithelium. In this article, we describe the generation a Cre-lox-based conditional knockout of the ERα gene to study the biological function of this steroid receptor in the epithelial compartment at defined stages of mammary gland development. The mouse mammary tumor virus (MMTV)-Cre-mediated, epithelial-specific ablation of exon 3 of the ERα gene in virgin mice severely impaired ductal elongation and side branching. The conditional knockout resulted in ablation of the ERα protein, and the progesterone receptor (PR), whose expression is under the control of ERα, was largely absent. The whey acidic protein (WAP)-Cre-mediated deletion of ERα during successive gestation cycles resulted in a loss of ductal side-branching and lobuloalveolar structures, ductal dilation, and decreased proliferation of alveolar progenitors. These abnormalities compromised milk production and led to malnourishment of the offspring by the second lactation. These observations suggest that ERα expression in the mammary epithelium is essential for normal ductal morphogenesis during puberty and alveologenesis during pregnancy and lactation.
Keywords: conditional knockout, mammary gland
Estrogen receptor (ER) is a transcription factor that regulates the genetic program of cell cycle progression and growth in healthy and cancerous mammary glands in response to circulating ovarian hormones. Of the two receptor forms (ERα and ERβ), ERα is considered the primary receptor for mammary gland development and function. The mammary gland is unique compared with every other organ of the body in that the bulk of its development and differentiation occurs postnatally. After birth and until puberty, the mammary gland is dormant and rudimentary. At puberty, the mammary gland develops rapidly in response to changes in circulating hormone levels. Terminal end buds (TEBs) that are composed of multiple layers of cuboidal epithelial cells begin to form at the termini of primitive ducts. The TEBs invade the fat pad and give rise to a branching network of ducts that end at the edge of the mammary fat pad. Growth of the ductal network ceases at this stage until further stimulation during pregnancy (1).
The role of ERα in mammary gland development was directly established by the seminal studies of Korach and colleagues in their genomic ERα knockout (ERKO) mouse model (2, 3). Later studies, however, showed that these genomic ERKO mice, in which the ERα gene was disrupted by the insertion of a neomycin resistance gene (neo) into the first coding exon, are hypomorphic for ERα in that substantial ERα function is retained (4). Circulating prolactin (PRL) levels were reduced in hypomorphic ERα females, and there was a lack of mammary gland development beyond the prepubertal stage (2). Restoration of PRL levels by pituitary isograft normalized mammary gland development, which could be prevented by ovariectomy. Likewise, exogenous estradiol and progesterone induced normal ductal elongation and TEB formation in hypomorphic ERα females. Thus, the observed phenotype was in part attributable to abnormal pituitary and ovarian hormone levels in the animals, whereas ERα function was largely retained in the mammary gland.
The mammary gland consists of multiple cell types including luminal and basal epithelial cells, stromal cells, adipocytes, and vascular endothelial and smooth muscle cells. In the mammary glands of rodents, both the epithelium and stroma express ERα (5–7). In an effort to dissect the complex paracrine and autocrine regulation of mammary gland development by estrogen, Cunha et al. pioneered a technique of tissue recombination in vivo consisting of various combinations of stromal and epithelial compartments from hypomorphic ERα and WT mice (8). The results from this and subsequent studies (9) suggested that stromal ERα was necessary for mammary gland development, whereas epithelial ERα was dispensable. This suggested role of ERα in the mammary gland of rodents appears to contradict the finding that tamoxifen treatment of breast cancer patients targets ERα in the cancer cells themselves and not the stroma of the breast. This issue is further substantiated by the fact that these targeted therapies are effective in the treatment of metastatic breast cancer cells, which are epithelial in origin and interact with stromal cells of distant organs. Thus, the conclusions drawn from the tissue recombination experiments using the hypomorphic ERα mice require reevaluation. Toward this end, a more recent ERα knockout (αERKO) mouse model has been developed in which exon 3 of the ERα gene was deleted without any detectable expression of ERα transcript (10). Mammary glands from genetic αERKO mice were normal before puberty. After the onset of puberty, however, TEBs remained absent and ducts failed to invade into the fatpad beyond the nipple area (11). In neither the ERα hypomorphic nor the αERKO mouse does the mammary gland develop beyond puberty. Although it was not reported, a reduction in circulating ovarian and pituitary hormones is likely in the αERKO mouse, similar to the ERα hypomorphic mouse (2, 12).
In this study, we sought to dissect the role of epithelial versus stromal ERα in mammary gland development, maturation, and lactation. We found that postnatal deletion of epithelial ERα arrests mammary gland development at the prepubertal stage. Strikingly, the deletion of mammary epithelial ERα during late pregnancy revealed that ERα is critically important for alveologensis and lactation during repeated gestational cycles.
Results
A targeting vector was generated from a mouse ERα genomic clone in which exon 3 was flanked by loxP recombination sites and electroporated into 129/SvOla ES cells. The targeted ERα allele was confirmed with Southern blot analysis of BamHI-digested ES cell genomic DNA, using 5′ and 3′ external probes (Fig. 1A). The floxed (loxP-flanked) phosphoglycerine kinase-Neo cassette was deleted from the ES clones and identified by 5′ and 3′ Southern blot analysis and genotyping PCR (SI Fig. 6 A and B). Targeted ES cells were injected into C57BL/6 blastocysts and returned to a pseudopregnant host of the same strain. Chimeric males were obtained that transmitted the mutation through crosses with C57BL/6 females, producing heterozygous ERαfl/+ mice (mice bearing one floxed allele in which exon 3 is flanked by loxP sites). Matings of ERαfl/+ yielded ERαfl/fl mice that were genotyped by using PCR on genomic DNA of tail biopsies (SI Fig. 6C).
Fig. 1.
Targeted and conditional disruption of the mouse ERα gene, using the Cre-loxP recombination system. (A) Components of the ERα exon 3 WT allele, the targeted allele after homologous recombination in ES cells, the floxed allele after deletion of the neomycin resistance gene (pGK-Neo) in ES cells, and the deleted ERα allele in Cre-recombinase transgenic mice. B, BamHI; N, NheI; E, Eco47III; H, HpaI; P, PmlI. (B) Genotyping PCR of tail clips of MMTV-Cre/ERαfl/fl mice (lane 1), virgin ERαfl/fl mice (lane 2), and WAP-Cre/ERαfl/fl mice (lane 3). Lane 4 contains extract of a mammary gland from a parous WAP-Cre/ERαfl/fl mouse. The MMTV promoter is active in skin, explaining the presence of the KO allele in the tail clip in lane 1, whereas WAP is specific to the mammary gland during late pregnancy, accounting for the 0.60-kb knockout allele in lane 4 but not lane 3.
To study the role of ERα in the epithelium during different stages of mammary gland development, the ERαfl/fl mice were bred with the well characterized mouse mammary tumor virus (MMTV)-Cre mice (13–15), thus generating an ERα conditional knockout model (MMTV-Cre ERαfl/fl or MMTV-ERKO). As demonstrated in ref. 14, the expression of the MMTV-Cre transgene is limited to epithelial cells in the mammary gland and is not expressed in the mammary stroma. MMTV-ERKO mice were viable and developed to adulthood, but the mammary ductal outgrowth arrested at the prepubertal stage (Fig. 2). At the age of 6 months, the ductal network of the MMTV-ERKO mice had scarcely invaded into the fat pad, and side-branching was minimal (Fig. 2A). Few TEBs were detected in MMTV-ERKO mice (Fig. 2 C and E). Age-matched ERαfl/fl control females exhibited abundant TEBs, extensive ductal branching, and normal fat pad invasion (Fig. 2 B, D, and F). These observations clearly indicate that ERα is essential in the epithelial compartment of the murine mammary gland and not the stromal compartment as suggested in refs. 8 and 9.
Fig. 2.
ERα is required for mammary gland development. Whole mounts of mammary glands (A–D) and H&E staining (E and F) from mature virgin MMTV-ERKO mice (A, C, and E) and ERαfl/fl mice (B, D, and F). In ERαfl/fl, but not MMTV-ERKO mice, the no. 4 inguinal mammary fatpad was fully occupied by the developed epithelial network.
Next, we bred whey acidic protein (WAP)-Cre transgenic mice (13–15) with the ERαfl/fl mice to specifically ablate the ERα gene in the mammary epithelium during late pregnancy and lactation (WAP-Cre/ERαfl/fl or WAP-ERKO mice). The expression of the WAP-Cre transgene is largely limited to epithelial cells located at duct termini and within developing alveoli (15, 16). As expected, ductal morphogenesis was normal in nulliparous (virgin) WAP-ERKO mice and equivalent to ERαfl/fl control females (data not shown). As expected, deletion of the ERα-floxed locus was not detected in nulliparous WAP-ERKO mice (Fig. 1B, lane 3). Using PCR, we confirmed the deletion of ERα in WAP-ERKO mice after pregnancy (Fig. 1B, lane 4). Excision of ERα in WAP-ERKO mammary glands was first detected in primiparous females at lactation day 13.5. However, even during the second lactation, intact ERαfl/fl alleles were still detectable in WAP-ERKO females (data not shown), suggesting that (i) the WAP-Cre transgene exhibits a mosaic expression profile, and (ii) there is a negative selection pressure against ERα knockout cells. No ERα deletion was detected by genotyping PCR in other ERα-expressing organs, including uterus, ovary, skin, and brain collected from WAP-ERKO females, verifying the specific expression of Cre recombinase in the mammary glands of WAP-Cre mice (data not shown). Hypomorphic αERKO mice are characterized by increased circulating levels of estrogen and reduced levels of progesterone and PRL, all of which are required for normal mammary gland development, differentiation, and lactation (17). Using commercial ELISA kits, we found that serum estradiol and progesterone levels are within the normal physiological range in both virgin WAP-Cre/ERαfl/fl and pregnant WAP-ERKO mice compared with ERαfl/fl mice (n = 3 females per group, data not shown). In a cell-based assay, in which the proliferation of PRL-dependent mammary epithelial cells is quantified (18), we found that serum PRL levels were also normal in the conditional knockouts (data not shown).
To determine the impact of ERα conditional deletion on mammary gland development, we examined the inguinal mammary glands (no. 4) from WAP-ERKO and ERαfl/fl females at various stages of successive pregnancy and lactation cycles. During the first lactation, a mosaic phenotype was detected in the mammary glands of WAP-ERKO females. Many secretory alveoli were of normal size. However, alveolar growth was less extensive in various parts of the mammary gland of lactating WAP-ERKO females, and we also observed a mild defect in tertiary ductal branching during the first pregnancy and lactation (SI Fig. 7). In contrast, ductal structures were obscured and not directly visible because of the normal alveolar expansion in ERαfl/fl control mice during the first and also the second pregnancy (Fig. 3 A and B). However, during the second pregnancy cycle, WAP-ERKO females exhibited aberrantly dilated ducts with few side-branches that were filled with milk-like secretions (Fig. 3 C and D). We observed strikingly fewer lobular alveoli in WAP-ERKO mice compared with ERαfl/fl control females (Fig. 3C). The diameters of engorged WAP-ERKO ducts (Fig. 3D) were an order of magnitude larger than ERαfl/fl control ducts (Fig. 3B).
Fig. 3.
Dilation and inadequate branching of ducts in WAP-ERKO mice during second lactation. Whole mounts (A and C) and H&E staining (B and D) were used to analyze the morphology of the mammary glands at second lactation day 1 of the WAP-ERKO females (C and D) and ERαfl/fl controls (A and B). Note different magnifications in B and D, emphasizing grossly enlarged ducts in mutant mice.
Unlike transplant models that are not suitable for lactation studies because of the lack of a nipple connection, the WAP-Cre-based, mammary-specific knockout of ERα allowed us to assess the importance of epithelial ERα for the growing offspring. Using the conditional knockout mice, we observed that ERα deficiency profoundly impaired the normal growth of the offspring. Approximately one-third of the pups nursed by WAP-ERKO mothers were malnourished during the first lactation period. The average body weight of 21-day-old pups was ≈17% less than that of pups from similar-sized litters nursed by ERαfl/fl females (10 g versus 12 g, n = 8, P < 0.001). The growth retardation of the pups was more severe during the second lactation. At this time, the average weight of 6-day-old pups nursing on a WAP-ERKO mother was ≈50% that of control pups nursing on ERαfl/fl dams (Fig. 4). The growth retardation of the offspring from WAP-ERKO females was fully rescued by fostering them with ERαfl/fl mothers (Fig. 4). Milk harvested from WAP-ERKO females contained normal concentrations of WAP (SI Fig. 8), which is critical for offspring nourishment (19). This suggests a defect in milk production and delivery attributable to abnormal glandular architecture rather than production of inferior-quality milk in WAP-ERKO nursing females. Accordingly, the volume of milk harvested from nursing WAP-ERKO females during the second lactation cycle was less than half that of ERαfl/fl females (n = 2 per group, data not shown).
Fig. 4.
Growth retardation in WAP-ERKO pups from the second litter is attributable to mother's genotype. (A) WAP-Cre/ERαfl/fl pups nursed by their WAP-ERKO mothers (WAPfl/fl–WAPfl/fl, n = 5) were significantly smaller than control ERαfl/fl pups nursed by ERαfl/fl mothers (fl/fl–fl/fl). Beginning on day 4 of lactation, a subset of WAP-Cre/ERαfl/fl pups was fostered with lactating ERαfl/fl mothers (WAP fl/fl-fl/fl). After 4 days of fostering by an ERαfl/fl dam (lactation day 8), there was no longer a statistically significant difference between WAP-Cre/ERαfl/fl and ERαfl/fl pup mass (n = 4). Data are presented as the mean ± 1 standard deviation. (B) Pairs of representative pups nursed for 10 days by birth mother or for 4 days by birth mother followed by 6 days of nursing by foster dam as in A.
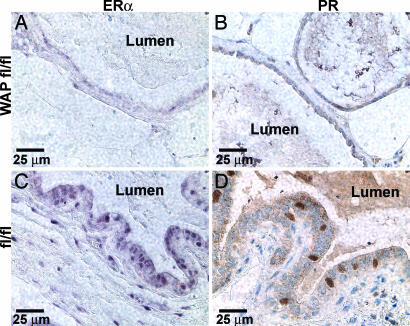
Based on the proposed role of WAP-Cre-expressing, parity-induced mammary epithelial cells (PI-MECs) that survive the first gestation cycle and function as alveolar progenitors during subsequent pregnancies, we reasoned that progressive loss of ERα- and downstream progesterone receptor (PR)-mediated signaling in the mammary gland with successive pregnancies was responsible for the observed mammary gland defects and the resulting offspring malnourishment. Progressive loss of ERα was verified by immunohistochemical staining of WAP-ERKO mammary glands during the second lactation (Fig. 5). Nuclear ERα was nearly undetectable in the epithelium of abnormally enlarged mammary ducts of biparous WAP-ERKO mice (Fig. 5A). Accordingly, expression of PR, which is a transcriptional target of ERα in mammary epithelial cells, was similarly absent (Fig. 5B). The loss of ERα and PR only occurred after lactation because WAP-Cre/ERαfl/fl virgin mice displayed robust nuclear staining throughout the mammary gland luminal epithelium (SI Fig. 9 A and B). Multiple nuclei of ductal and lobular epithelial cells of control ERαfl/fl expressed ERα and PR in the first day of the second lactation (Fig. 5 C and D). The expression of epithelial ERα in uteri of WAP-Cre/ERαfl/fl 2-month-old virgin and involuted primiparous 5-month-old mice was also analyzed. The uterine epithelial expression patterns of ERα in both groups of WAP-ERαfl/fl mice were similar to age-matched ERαfl/fl controls (data not shown), confirming the restriction of WAP-Cre recombinase expression to the mammary epithelium. Importantly, ERα expression in stromal cells was abundant in the mammary glands and uteri of lactating WAP-ERKO females (SI Fig. 10), and the pattern of stromal staining in both tissues was similar to control ERαfl/fl mice (data not shown). Next, we analyzed the expression of the proliferating cell nuclear antigen (PCNA) to determine the possible cause of the ductal dilation and loss of secretory alveoli. By day 1 of the second lactation, ductal and alveolar epithelial cells from control ERαfl/fl mammary glands robustly expressed PCNA. In contrast, PCNA expression was diminished in the enlarged ducts and alveoli of WAP-ERKO mammary glands. The number of nuclei of mammary epithelial cells in lactating WAP-ERKO mice that were PCNA-positive was approximately half of that observed in control ERαfl/fl mammary glands (data not shown). Thus, the dilation was not attributable to increased proliferation of ductal epithelial cells, whereas the loss of alveoli may be attributable in part to impaired ERα-dependent proliferation of luminal epithelial cells.
Fig. 5.
Loss of ERα and PR immunohistochemical staining in WAP-ERKO (WAP fl/fl) but not ERαfl/fl (fl/fl) mammary glands of the second lactation, day 1. Nuclei of multiple luminal epithelial cells in control mammary glands were positive for ERα and PR (C and D), which were absent in the enlarged ducts of WAP-ERKO mice (A and B).
Discussion
We found that progressive loss of ERα in mouse mammary glands during gestational cycles results in loss of lobuloalveoli, impaired ductal side-branching, and inadequate milk delivery. MMTV-Cre-mediated excision of ERα occurs in multiple organs shortly after birth. The resulting mammary gland phenotype of arrested ductal growth at the prepubertal stage was similar to the genomic ERα knockout (11). Our data indicate that early and complete loss of ERα throughout the mammary epithelium prevents the formation of TEBs and severely impairs ductal elongation. Studies that used tissue recombination and the ERα hypomorphic model (8, 9) suggested that ERα was dispensable in the mammary epithelium. The authors proposed a predominant role of ERα signaling in the stroma, which created much doubt in the scientific community about the legitimacy of mice to model ERα-based breast cancer prevention, because the mammary stroma in humans express little ERα. Then again, our findings in the Cre/lox-based conditional knockout model of the ER clearly indicate that ERα is essential in the epithelial compartment of the murine mammary gland. Therefore, our proposed model, which emphasizes the significance of ERα signaling in the epithelium, might also suggest that tamoxifen has a direct effect on the growth of premalignant lesions in selected ERα-positive murine mammary cancer models. Recent studies by Medina et al. demonstrate that tamoxifen had a profound impact on the prevention of mammary tumorigenesis in the p53 knockout transplant model (20). The ERα-expressing breast cancer models in combination with the ERα conditional knockout model will be invaluable for studying ER signaling in premalignant and cancerous lesions of the mammary gland.
Essential functions of ERα were primarily associated with ductal elongation rather than lobuloalveolar formation (8). Recent studies by Mallepell et al. (11) establish a role for ERα in mammary epithelial cells during puberty, and these observations are in complete agreement with the phenotypic analyses in the MMTV-Cre-based conditional knockout mice. However, the transplant model by Mallepell et al. (11) and the MMTV-ERKO mice provide limited insights into the role of ERα signaling at later stages of mammogenesis. Using WAP-ERKO mice, we were able to specifically ablate ERα in duct termini and alveolar units after ductal elongation was completed. Also, these mice permitted us to study the loss of ERα during multiple pregnancies and lactation cycles. Successive gestational cycles drove a progressive loss of ERα in the mammary epithelial cells (MECs). In summary, our observations clearly indicate that ERα signaling is required for ductal elongation. Furthermore, ERα is equally important for pregnancy-induced tertiary branching and the proliferation and maintenance of differentiating alveolar cells (i.e., WAP-Cre is first activated during the second half of pregnancy when alveolar cells assume an advanced differentiation profile).
Paracrine signaling between neighboring cells within the mammary gland can compensate for lack of ERα (and thus PR) in specific mammary epithelial subtypes. A 10:1 ratio of ERα-competent to ERKO transplanted MECs reconstituted normal mammary gland development and induced participation of ERKO MECs in all epithelial compartments: luminal and basal cells and cap and body cells of TEBs (11). A 1:1 ratio of PR-competent to PR-deficient transplanted MECs similarly rescued mammary gland development (21). We have found that alveolar abnormalities can emerge very early after initiation of ERα excision from MECs during the first lactation, uncovering a greater stringency for adequate ERα and PR signaling during reproduction that may have been overlooked or not feasible with previous transplantation approaches. More importantly, our observation of the critical role of ERα and PR during lactation was unexpected because estradiol and progesterone levels decline sharply after birth because of the loss of the corpus luteum.
Expression of the WAP gene continues throughout lactation, and the highest expression is restricted to differentiated luminal MECs. Expression declines rapidly with weaning, but a subpopulation of hormonally responsive alveolar MECs resists apoptosis and survives involution (15, 16). These PI-MECs are predominantly located within terminal ducts and alveolar units of involuted mammary glands (i.e., the murine equivalent of terminal duct lobular units in the human breast). Using genetic labeling, it has been shown that PI-MECs are able to self-renew, and they serve as alveolar progenitors in successive pregnancies. The increasing penetrance of the abnormal phenotype in multiparous WAP-ERKO females might suggest that PI-MECs critically require intrinsic ERα signaling to numerically expand during successive gestation cycles and that the ablation of ERα can inhibit the capacity of this epithelial subtype to self-renew. Because PI-MECs possess features of multipotent stem cells upon transplantation into the cleared fat pad of recipient mice (refs. 15 and 16 and L. A. Matulka and K.-U.W., unpublished data), the ERα conditional knockout mice might be a valuable tool to address in future studies the importance of ERα signaling in multipotent progenitors and mammary epithelial stem cells.
Materials and Methods
Generation of Conditional ERα Knockout Mice.
The mouse ERα genomic clone from 129/sv ES cells containing exon 3 of ERα was a kind gift from J.-Å. Gustafsson (Karolinska Institute, Stockholm, Sweden). The 9.2-kb BamHI fragment containing exon 3 consists of nucleotides 655–845 and amino acids 156–218 and encodes the first zinc finger of the DNA binding domain (22). The floxed phosphoglycerine kinase-Neo cassette, a kind gift from P. Sanford and T. Doetschman (University of Cincinnati), was cloned into the Eco47III site, and a loxP site followed by a BamHI site was introduced at the NheI site. An HSV-TK expression cassette was subcloned into the HpaI site as the negative selection marker. The 12.2-kb targeting vector linearized at the PmlI site was electroporated into 129/SvOla ES cells (Fig. 1). ES cells containing a targeted ERα allele were identified by 5′ and 3′ outside PCR. The 5′ outside primers were: 5′-AGCAAGGGAAAACAAAAACCTGTGT-3′ (forward) and 5′-AGTCATAGCCGAATAGCCTCTCCAC-3′ (reverse). The 3′ outside primers were: 5′-CTATCAGGACATAGCGTTGGCTACC-3′ (forward) and 5′-AATGAGAGAGGACCAGCGATCTTAT-3′ (reverse). Genotyping on tail DNA was performed by PCR using the following primers: 5′-TGGGTTGCCCGATAACAATAAC-3′ (forward), and 5′-AAGAGATGTAGGGCGGGAAAAG-3′ (reverse). The size of the amplified DNA fragment was 1,280 bp in ERαfl/fl and 1,200 bp in WT mice as separated on a 1.3% agarose gel. ERαfl/fl mice were bred with transgenic mice expressing the Cre enzyme in the mammary tissue under the control of the MMTV long-terminal repeat and the WAP gene promoter as described in ref. 15. Cre-mediated excision of the ERα gene was verified by genotyping PCR of DNA extracted from tail clips, using the same primers described above.
Blood/Tissue Collection and Analyses.
Mice were killed in a CO2 chamber according to Institutional Animal Care and Use Committee and institutional protocols. Blood was collected from the ventricle of the heart with a 23-gauge needle. After clotting at room temperature for 2 h, the blood was centrifuged, and the serum was collected and stored at −80° C. Mammary glands were dissected, mounted on glass slides, and fixed and stained in carmine alum solution as described for whole-mount analysis (2). For immunohistochemical analysis, tissues were fixed overnight in 10% neutralized buffered formalin (Richard–Allan Scientific, Kalamazoo, MI; catalog no. 9400-5), processed, and embedded in paraffin according to standard procedures by the University of Cincinnati Comparative Pathology Core Laboratory. H&E staining was performed as described in ref. 23. Serum estradiol and progesterone levels were measured in collected serum according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI; catalog nos. 582251 and 582601). PRL levels were determined by a cell-based bioassay as described in ref. 18. SDS/PAGE and Coomassie blue staining of WAPs in the milk of lactating ERαfl/fl (+/+) and WAP-ERKO (−/−) females were performed as described in ref. 19.
Immunohistochemistry.
Slides were boiled in 1× citrate buffer (pH 6.0) for 20 min for antigen retrieval. Immunostaining with ERα (1:150; Santa Cruz Biotechnology, Santa Cruz, CA; catalog no. sc-542) and PR (1:250; Santa Cruz Biotechnology; catalog no. sc538) antibodies were performed as described in ref. 24. PCNA (1:1,000; Santa Cruz Biotechnology; catalog no. sc-56) IgG was used with the Histo-mouse-MAX kit (Zymed Laboratories, South San Francisco, CA; catalog no. 87-9551) to detect protein expression according to the manufacturer's instructions. Biotinylated secondary antibody (goat anti-rabbit IgG) and the avidin-biotin blocking kit were from Invitrogen (Carlsbad, CA; catalog nos. 50-235Z and 00-4303) and Vectastain ABC kit was from Vector Laboratories (Burlingame, CA). Digital photomicrographs were acquired with a Nikon (Tokyo, Japan) Microphot–FXA microscope and SPOT software, Version 4.0.9 (Diagnostic Instruments, Sterling Heights, MI).
Statistical Analysis.
Data were compared by a one-way ANOVA, followed by a one-tailed Student's t test to evaluate levels of significance at a 95% confidence interval. Differences were determined to be statistically significant when P < 0.01 unless otherwise noted.
Supplementary Material
Acknowledgments
We thank Dr. Keith Stringer (Cincinnati Children's Hospital Medical Center) and Dr. Greg Boivin and Rita Angel (University of Cincinnati Comparative Pathology Core) for assistance with immunostaining, Drs. Eric Hugo and Nira Ben-Jonathan (University of Cincinnati) for the PRL bioassay, and Dr. Nelson Horseman (University of Cincinnati) for helpful discussions. This work was supported by National Institutes of Health Grants CA72039, HD297731, K12 HD051953, and T32 HD07463; Center for Environmental Genetics Grant ES06096; U.S. Department of Defense Grant BC981038; and a Barrett Cancer Center Pilot grant.
Abbreviations
- Cre
Cre-recombinase
- ER
estrogen receptor
- ERKO
ER knockout
- MEC
mammary epithelial cell
- MMTV
mouse mammary tumor virus
- P
progesterone
- PCNA
proliferating cell nuclear antigen
- PI-MEC
parity-induced mammary epithelial cell
- PR
progesterone receptor
- PRL
prolactin
- TEB
terminal end bud
- WAP
whey acidic protein.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706933104/DC1.
References
- 1.Russo IH, Russo J. Environ Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, Korach KS. Endocrinology. 2000;141:2982–2994. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- 3.Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, et al. Recent Prog Horm Res. 1996;51:159–188. [PubMed] [Google Scholar]
- 4.Kos M, Denger S, Reid G, Korach KS, Gannon F. J Mol Endocrinol. 2002;29:281–286. doi: 10.1677/jme.0.0290281. [DOI] [PubMed] [Google Scholar]
- 5.Edery M, McGrath M, Larson L, Nandi S. Endocrinology. 1984;115:1691–1697. doi: 10.1210/endo-115-5-1691. [DOI] [PubMed] [Google Scholar]
- 6.Haslam SZ, Nummy KA. J Steroid Biochem Mol Biol. 1992;42:589–595. doi: 10.1016/0960-0760(92)90449-s. [DOI] [PubMed] [Google Scholar]
- 7.Haslam SZ, Shyamala G. Endocrinology. 1981;108:825–830. doi: 10.1210/endo-108-3-825. [DOI] [PubMed] [Google Scholar]
- 8.Cunha GR, Young P, Hom YK, Cooke PS, Taylor JA, Lubahn DB. J Mammary Gland Biol Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- 9.Mueller SO, Clark JA, Myers PH, Korach KS. Endocrinology. 2002;143:2357–2365. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]
- 10.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Development (Cambridge, UK) 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 11.Mallepell S, Krust A, Chambon P, Brisken C. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couse JF, Korach KS. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 13.Wagner KU, Krempler A, Qi Y, Park K, Henry MD, Triplett AA, Riedlinger G, Rucker IE, Hennighausen L. Mol Cell Biol. 2003;23:150–162. doi: 10.1128/MCB.23.1.150-162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- 15.Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. Development (Cambridge, UK) 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- 17.Couse JF, Yates MM, Walker VR, Korach KS. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 18.Liby K, Neltner B, Mohamet L, Menchen L, Ben-Jonathan N. Breast Cancer Res Treat. 2003;79:241–252. doi: 10.1023/a:1023956223037. [DOI] [PubMed] [Google Scholar]
- 19.Triplett AA, Sakamoto K, Matulka LA, Shen L, Smith GH, Wagner KU. Genesis. 2005;43:1–11. doi: 10.1002/gene.20149. [DOI] [PubMed] [Google Scholar]
- 20.Medina D, Kittrell FS, Hill J, Shepard A, Thordarson G, Brown P. Cancer Res. 2005;65:3493–3496. doi: 10.1158/0008.5472.CAN-04-3869. [DOI] [PubMed] [Google Scholar]
- 21.Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA. Proc Natl Acad Sci USA. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White R, Lees JA, Needham M, Ham J, Parker M. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- 23.Manka D, Spicer Z, Millhorn DE. Cancer Res. 2005;65:11689–11693. doi: 10.1158/0008-5472.CAN-05-3091. [DOI] [PubMed] [Google Scholar]
- 24.Cheng G, Weihua Z, Warner M, Gustafsson JA. Proc Natl Acad Sci USA. 2004;101:3739–3746. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.